Abstract
Objective:
The aim of this study was to compare color stability and surface topography of three different feldspathic porcelains both qualitatively and quantitatively after exposure to routinely consumed beverages over different time periods using a Spectrophotometer, Stereomicroscope and Surface roughness tester, respectively.
Materials and Methods:
A total of 90 plastic discs were casted to obtain metal dies for three different newer ceramic applications each on thirty samples. The color and surface roughness of these samples were measured using stereomicroscope and surface roughness tester following which they were kept in different test solutions for different durations and revaluated for color changes and surface roughness in the similar manner.
Results and Conclusion:
Among all the five test solutions, Coffee showed the maximum staining of the ceramic whereas maximum surface roughness was shown by the Duceram Kiss (1.48 μm) by Orange Juice which could be due to its high titratable acidity.
Keywords: Color stability, feldspathic porcelains, spectrophotometer
INTRODUCTION
Restoration of smile is one of the most challenging and esthetically satisfying services a prosthodontist can render to a patient. Dental porcelain is used extensively as a restorative material in a variety of dental restorations, including all ceramic restorations, metal ceramic crowns, and fixed partial dentures because of because of its esthetic properties, durability, and biocompatibility. Discoloration of porcelain restoration may be endogenous or exogenous. Chemical instability of the material may lead to endogenous color change. The exogenous staining may occur due to the ability of the restoration to adsorb or absorb stains in the oral cavity which may be potentialized by surface conditions such as roughness.
Studies have been undertaken to evaluate the color stability of composite restorations and it has been concluded that composite resins are unable to retain the color they possess at the time of insertion.[1] Recently spectrophotometer has been used to evaluate the amount of color change in restorative materials. “A spectrophotometer is scientific standardized colorimetric equipment for matching and measuring color that gives information about reflectance curve as a function of wavelength in entire range”.[2]
The quality of color is measured by CIELAB (Commission Internationale de I’Eclairage) coordinates. Spectrophotometer measures the amount of light reflected by a surface as a function of wavelength to produce a reflectance spectrum. The reflectance spectrum of a sample can be used in conjunction with the CIE standards. CIE system provides information about location of object color in a uniform three-dimensional color space. It quantifies the color in terms of three coordinate values i.e., L*, a* and b*.
Color change (DE) mathematically expresses the amount of difference between the Commission Internationale de I’Eclairage (CIE) L*a*b* coordinates of different specimens or the same specimen at different instances. The L* value is a measure of the lightness, the * value is a measure of redness (positive a*) or greenness (negative a*) and the b* value is the measure of yellowness (positive b*) or blueness (negative b*) of an object. CIELAB color difference formula is designed to provide numeric data (ΔE) that represents the magnitude of the color difference between two objects.
ΔE = [ΔL2+ Δa2+ Δb2]1/2
Beside color, surface topography can also alter the ultimate outcome of a restoration. Kim, et al.[2] stated that surface topography influenced the color of porcelain, especially the CIE L* value. Although glazed surfaces appeared whiter, the CIE L* value measured with the Specular Component Excluded (SCE) geometry was lower than that of polished surfaces. Porcelain has been found to be resistant to surface corrosion, abrasion and dissolution even in an acidic environment. However, one of the studies has shown that the highly glazed surface of porcelain restorations when subjected to repeated exposure of carbonated beverages can lead to roughened and etched surface texture.[3] The simplest way to assess this surface texture visually is by using a Scanning Electron Microscope.[4]
To evaluate its discoloration potential of porcelain, it is essential to consider the type of diet which is consumed by the larger population. The commonly consumed dietary food includes the soft drinks, beverages and the fruit juices. It is interesting to note that literature shows most studies dealing with color stability of porcelain restoration but, there is very little evidence regarding qualitative and quantitative evaluation of color stability of porcelain. Thus the present study was undertaken to compare color stability and surface topography of three different feldspathic porcelains both qualitatively and quantitatively after exposure to Coffee, Coca Cola, Orange Juice, Tea and Water over different time periods using a Spectrophotometer, Stereomicroscope and Surface roughness tester, respectively.
MATERIALS AND METHODS
The study evaluated the color stability of Vita (VMK 95), Ceramco-3, Duceram Kiss against discoloration caused by commonly consumed beverages using a Spectrophotometer. Furthermore, it also evaluated the surface topography of above porcelains after exposure to commonly consumed beverages using a Stereomicroscope and Surface roughness tester.
A total of 90 porcelains samples in disc form were fabricated which were divided into three groups, each group consisting of 30 samples i.e. Group I (Vita), Group II (Ceramco-3) and Group III (Duceram Kiss). Each group is further sub-divided into five subgroups of six samples on the basis solutions used for immersion [Table 1].
Table 1.
Sample distribution according to the type of materials and beverages used
Metal disc preparation
Ninety plastic discs of 30 mm diameter and 0.7 mm thickness were obtained using metal die for uniform cutting [Figure 1]. Plastic discs were sprued at the center of prepared pattern for investing and casting using phosphate-bonded investment material strictly following the manufacturer's instructions. The castings was divested and the residual surface investment removed by sandblasting with 100 μm aluminum oxide abrasion particles. The metal discs were finished with the help of carborundum discs, metal trimmers and were sandblasted to achieve a uniform thickness with final dimensions being 30 mm × 0.5 mm for each sample.
Figure 1.
Die used for cutting of plastic strips
Ceramic application
The cast specimens prepared then were taken up for ceramic application. Thirty samples were prepared using Vita (VMK 95) porcelain over cast specimens. Two coats of paste opaque were applied. Ceramic firing was done according to manufacturer's instructions. For the application of dentine, layer powder and liquid were mixed according to manufacturer's instructions and applied over the specimen using a metallic jig [Figures 2 and 3]. Enamel layer then was applied using the same technique as for dentine layer, and then samples were fired. Firing was done according to manufacturer's instructions and samples were finished with a diamond bur to achieve a uniform thickness of 2.5 mm. The samples were finally glazed. Using the same technique, 30 samples was prepared with Ceramco-3 porcelain and 30 samples with Duceram Kiss porcelain. The samples were evaluated for color and surface roughness and immersed in 300 ml different test solutions, which were prepared in the following manner [Figure 4];
Figure 2.
(a) Metallic jig used for ceramic application base (b) Plate for dentine application (c) Plate for enamel application
Figure 3.

Assembled jig
Figure 4.

Beverages used for sample immersion
Coffee: 30 g of Coffee powder was added in 1 L of boiling water simmered for 5 min and then filtered through a filter paper.
Coca Cola: 300 mL of Coca Cola was taken in an airtight container.
Orange Juice: 300 mL of Orange Juice is taken in an airtight container.
Tea: 30 g of Tea was added to 1 L of boiling water, simmered for 5 min. and then filtered through a filter paper.
Water: 300 mL of Water was taken in a container.
Coffee, Tea and Water were kept at a constant temperature of 50°±1°C in an incubator. Coca Cola and Orange Juice were kept at room temperature in an incubator. Test solutions were changed every day. Base line readings for color were taken at 0 day and then color changes were measured after 45 days and 90 days. Similarly, base line readings for surface topography were taken at 0 day and changes in surface topography were measured after 90 days [Figure 5].
Figure 5.
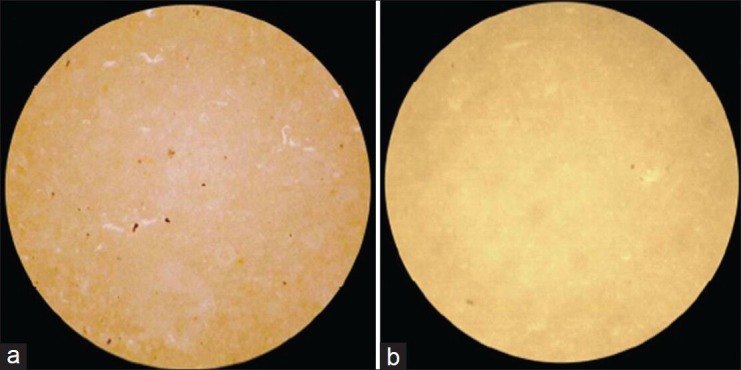
Stereomicroscopic view of Duceram Kiss sample with surface roughness (Ra) 0.54 μm, before (a) and after immersion (b), at ×40
Statistical analysis
Data obtained was entered into MS Excel spreadsheet and analyzed using SPSS statistical software. Descriptive statistics was calculated for each variable for the three groups. The values of color change and surface roughness were compared using one-way ANOVA test/Kruskak Wallis test followed by post hoc comparison by Bonferroni method.
RESULTS
Descriptive analysis of Group I (Vita VMK 95) samples showed a decrease in value of L* after a period of 45 days (i.e. samples become darker), while an increase in value of L* was shown after a period of 90 days (i.e. samples become lighter). The value of a* showed an increase from 0th to 90th day (indicating a shift towards red axis). The value of b* showed an increase from 0th to 90th day (indicating a shift towards yellow axis [Table 2], whereas the samples of Group II (Ceramco-3) showed a decrease in value of L* from 0th to 90th day (i.e. samples become darker). Here the value of a* showed an increase from 0th to 45th day (indicating a shift towards red axis), and decreased from 45th to 90th day while the value of b* showed an increase from 0th to 45th day (indicating a shift towards yellow axis), and decreased from 45th to 90th day [Table 3]. Group III (Duceram kiss) samples that showed an increase in value of L* was shown after a period of 45 days (i.e. samples become lighter), while a decrease in value of L*was shown after a period of 90 days (i.e. samples become darker) [Table 4]. Comparison of color change of Vita VMK 95, Ceramco-3 and Duceram Kiss was analyzed after exposure to Coffee, Coca Cola, Orange Juice, Tea and Water after a time interval of 45 days and 90 days and it revealed variable results for different porcelain samples and color solutions [Tables 5–9, Graphs 1 and 2]. Comparison of surface roughness of Vita VMK 95, Ceramco-3 and Duceram Kiss was evaluated and compared after exposure to Coffee, Coca Cola, Orange Juice, Tea and Water for a time interval of 90 days.
Table 2.
Evaluation of color change in Group I (Vita VMK 95) after exposure to Coffee, Coca Cola, Orange juice, Tea and Water after a time interval of 45 days and 90 days (n = 30)
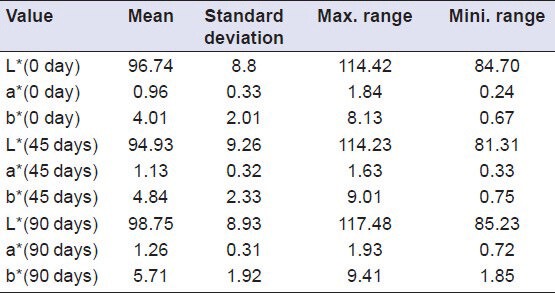
Table 3.
Evaluation of color change in Group II (Ceramco-3) after exposure to Coffee, Coca Cola, Orange juice, Tea and Water after a time interval of 45 days and 90 days (n = 30)
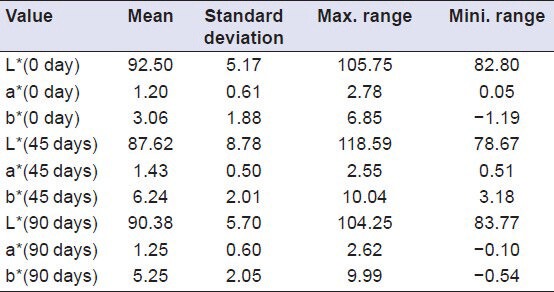
Table 4.
Evaluation of color change in Group III (Duceram kiss) after exposure to Coffee, Coca Cola, Orange juice, Tea and Water after a time interval of 45 days and 90 days (n = 30)
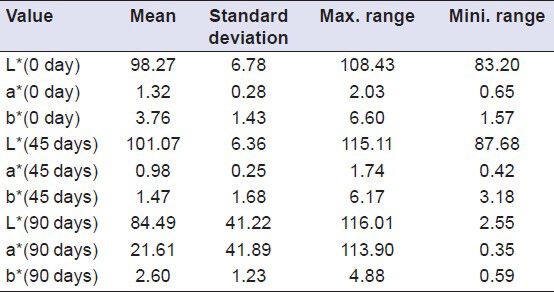
Table 5.
Comparison of color change of Group I (Vita VMK 95), Group II (Ceramco-3) and Group III (Duceram Kiss) after exposure to Coffee for a time interval of 45 days and 90 days (n = 18)
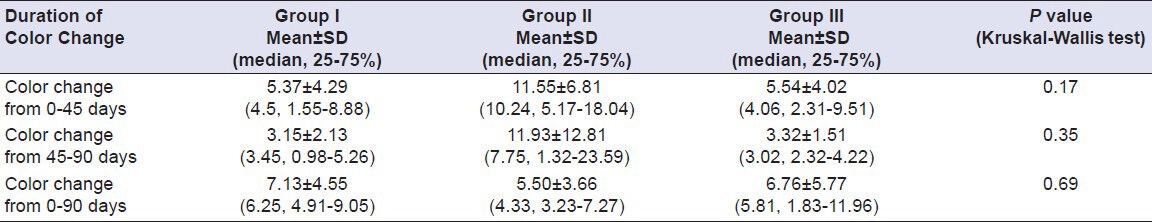
Table 9.
Comparison of color change of Group I (Vita VMK 95), Group II (Ceramco-3) and Group III (Duceram Kiss) after exposure to Water for a time interval of 45 days and 90 days (n = 18)
Graph 1.
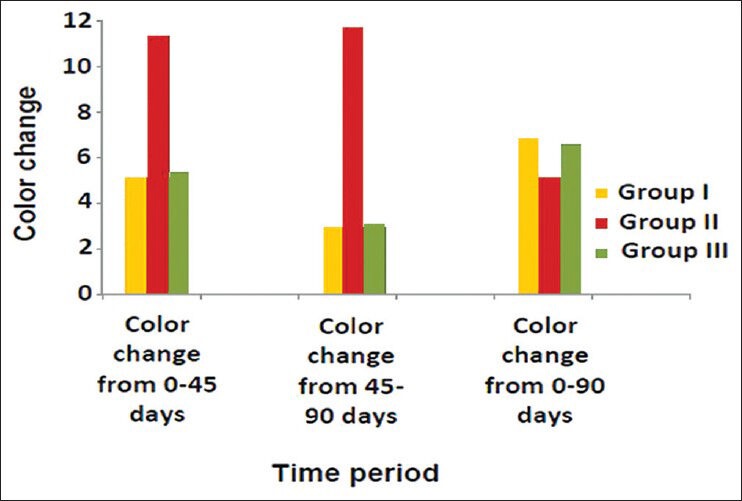
Comparison of color change of Group I (Vita VMK 95), Group II (Ceramco-3) and Group III (Duceram Kiss) after exposure to Coffee for a time interval of 45 days and 90 days (n = 18)
Graph 2.
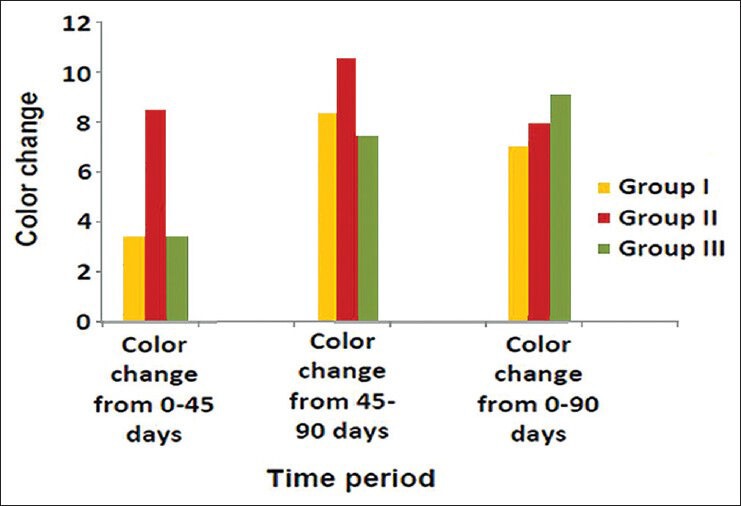
Comparison of Color change of Group I (Vita VMK 95), Group II (Ceramco-3) and Group III (Duceram Kiss) after exposure to tea for a time interval of 45 days and 90 days (n = 18)
Table 6.
Comparison of color change of Group I (Vita VMK 95), Group II (Ceramco-3) and Group III (Duceram Kiss) after exposure to Coca Cola for a time interval of 45 days and 90 days (n = 18)
Table 7.
Comparison of color change of Group I (Vita VMK 95), Group II (Ceramco-3) and Group III (Duceram Kiss) after exposure to Orange juice for a time interval of 45 days and 90 days (n = 18)
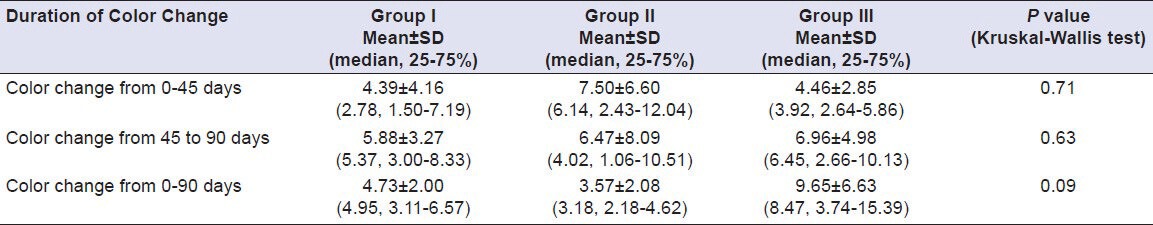
Table 8.
Comparison of color change of Group I (Vita VMK 95), Group II (Ceramco-3) and Group III (Duceram Kiss) after exposure to Tea for a time interval of 45 days and 90 days (n = 18)
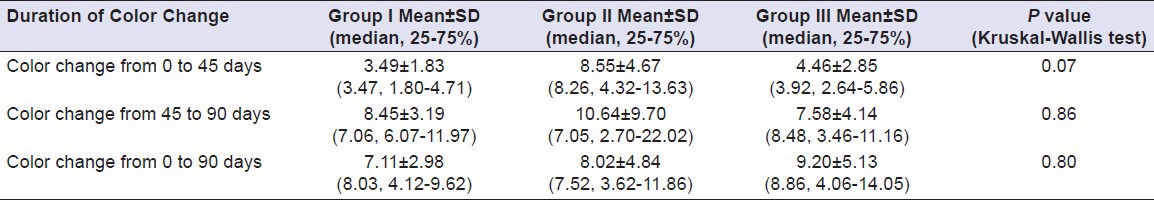
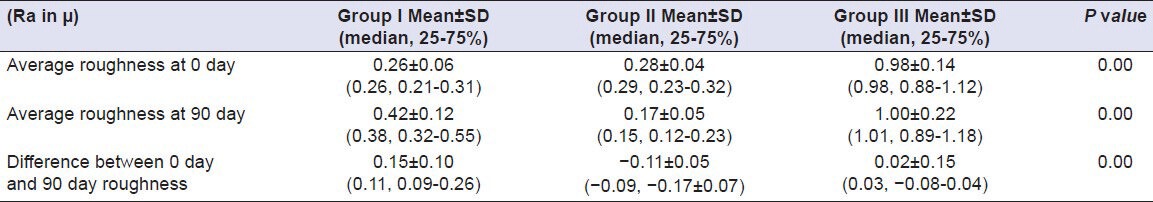
On comparing values for average surface roughness at 0th day of immersion in Coffee for all samples, it was found that the difference was statistically significant (P < 0.05). A mean difference of 0.29, 0.41 and 1.10, respectively, was observed. On comparing values for average surface roughness at 90th day for Group I, II and III, the difference was statistically significant (P < 0.05). A mean difference of 0.53, 0.20 and 1.04, respectively, was observed whereas the comparing values for difference of average surface roughness at 0th day and 90th day for Group I, II and III, it was found that the difference was statistically significant (P < 0.05). A mean difference of 0.24, –0.20 and –0.05, respectively, was observed. In Coffee at 90th day, Group III showed maximum surface roughness and Group II showed minimum surface roughness. Difference from 0th to 90th day was maximum in Group I and minimum in Group II [Table 10]. Results of comparison of surface roughness of porcelain in other test solutions are compiled in Tables 11–14.
Table 10.
Comparison of surface roughness of Group I (Vita VMK 95), Group II (Ceramco-3) and Group III (Duceram Kiss) after exposure to Coffee, Coca Cola, Orange juice, Tea and Water after a time interval of 90 days
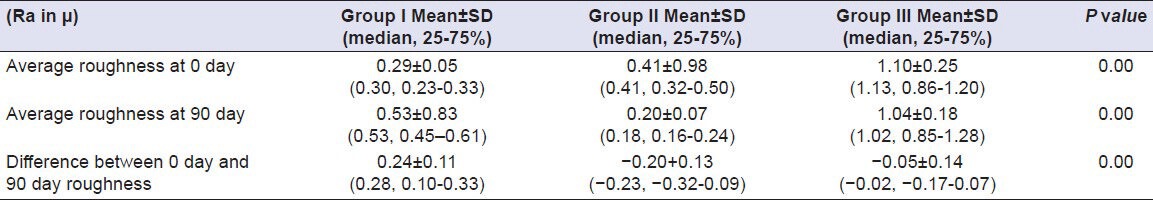
Table 11.
Comparison of surface roughness of Group I (Vita VMK 95), Group II (Ceramco-3) and Group III (Duceram Kiss) after exposure to Coca Cola for a time interval of 90 days (n = 18)
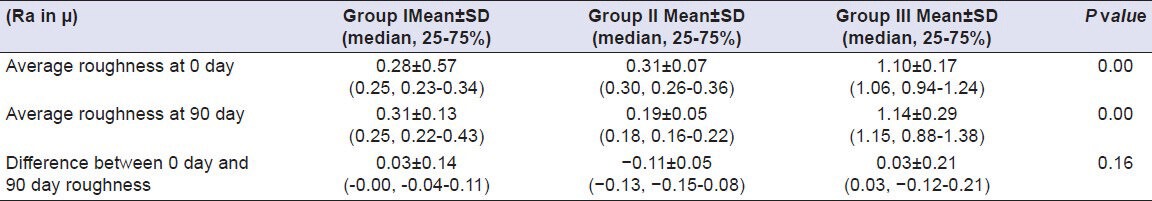
Table 14.
Comparison of surface roughness of Group I (Vita VMK 95), Group II (Ceramco-3) and Group III (Duceram Kiss) after exposure to Water for a time interval of 90 days (n = 18)
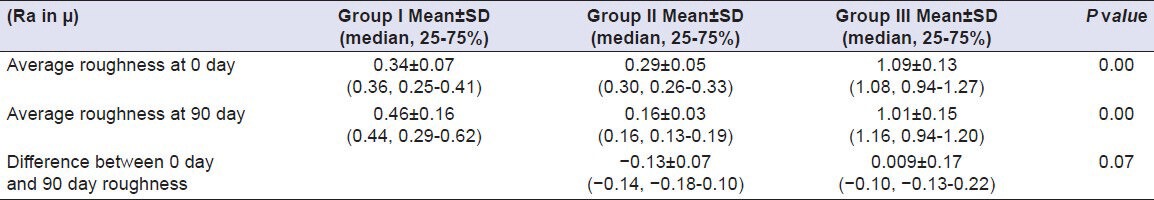
Table 12.
Comparison of surface roughness of Group I (Vita VMK 95), Group II (Ceramco-3) and Group III (Duceram kiss) after exposure to Orange juice for a time interval of 90 days (n = 18)
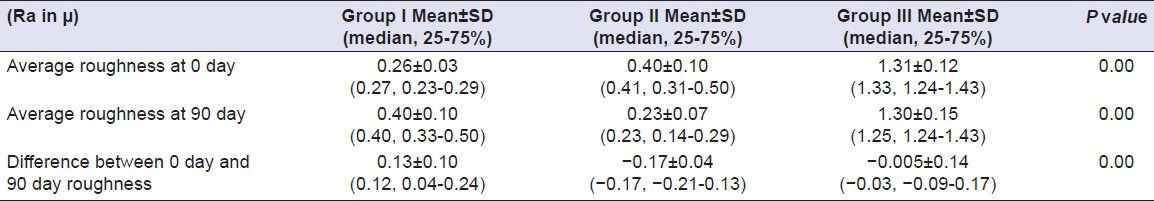
Table 13.
Comparison of surface roughness of Group I (Vita VMK 95), Group II (Ceramco-3) and Group III (Duceram kiss) after exposure to Tea for a time interval of 90 days (n = 18)
DISCUSSION
Porcelain has been established as an ultimate anterior esthetic restorative material because of its natural appearance, good wear resistance and color stability. Porcelain has color rendering and optical properties that simulate natural teeth. Though porcelain restorations are considered to be color stable, yet discoloration is one of the primary factors for failure of esthetic restorations. Discoloration of porcelain may be due to intrinsic or extrinsic factors. Intrinsic factors involve changes within the material itself and extrinsic factors involve adsorption or absorption of stains in the oral cavity. In this study, the samples that were immersed in Coca Cola and Orange Juice were stored at room temperature, while the samples that were immersed in Tea, Coffee and Water were stored at 50°±1°C, which was in accordance with the study done by Gupta, Parkash, Shah and Jain.[2]
The three different porcelains used in the study showed a mean color change ranging from 0.72 to 20.92 ΔE units after a period of 90 days. The overall color change was very high to that reported by Razzog et al.,[1] in which they compared two porcelain systems viz. Ceramco-3 and Procera and concluded that there was a color change in the range of 0.5 to 1.5 ΔE units after an accelerated aging process of 900 h.[5]
Ghahramanoh, et al. conducted a similar study in which they compared color change of GC Gradia (composite) and Vita VMK 95 (porcelain) after immersion in Tea, Coca Cola, Orange Juice and distilled water and concluded that all the three factors studied i.e. type of material, solution and time factor had a significant effect on each of the three parameters of color i.e. (L*a*b*).[6] Vita VMK 95 after immersion in the test solution showed a small amount of color change ranging from 0.21 to 0.51 ΔE units; this value is quite low from our observations. The reason for this difference in our results could possibly be attributed to the extended time period of immersion (90 days) and also to the nature of other immersion solutions used in our study (i.e. Coffee). Further they were of the view that there is probability of a slow breakdown at matrix filler interface because of water sorption over an extended period of time.
In the present study the results showed a significant change in the values of L* (i.e. brightness) in all the three materials. Group I (Vita VMK 95) and Group II (Ceramco-3) showed a reduction in L* values after a period of 45 days which is in accordance with the studies done by Gupta, et al. and Ghahramanho, et al. who also reported a decrease in L* values after a period of 1 month.[2,6] Group III (Duceram Kiss) showed an increase in the values of L* after a period of 45 days. When an overall change in the values of L* was seen after 90 days, it was observed that Group I (Vita VMK 95) showed an increase in the values of L* (i.e. samples became lighter) and Group II (Ceramco-3) and Group III (Duceram Kiss) showed a decrease in the values of L* (i.e. samples became darker). Δa* i.e. change along red-green axis for all the groups was positive, indicating a shift towards red axis, Δb* i.e. change along yellow-blue axis was positive indicating a shift towards yellow axis for Group I (Vita VMK 95) and Group II (Ceramco-3).
These results are also in agreement with those suggested by Gupta, et al. and Ghahramanho, et al.[2,6] Group III (Duceram Kiss) showed a negative Δb* i.e. shift towards the blue axis. The reason for this color change has been explained on the basis of basic components of the porcelain i.e. glass, which has been reported to discolor with test solutions used in the present study, which is supported by Gross and Moser who reported that materials containing glass beads as filler were stained with beverages like Coffee and Tea.[7,8,9,10,11,12] The clinical relevance of the present study depends on how much color change (ΔE) is considered perceptible. It is shown that a ΔE < 1 is not considered perceptible to most subjects with normal color vision and the restorations with a ΔE as high as 3.3 and 3.7 required replacement as it is clinically perceptible color change.[12] Considering the mean staining intensity of all the solutions used for the present study, Coffee was found to cause more discoloration than Tea, Coca Cola, Orange Juice and Water. This is also in general concurrence with the results of previous studies conducted by Gross and Moser and Chan, Fuller and Harmati; they concluded that staining intensity of Coffee was higher than that of Tea, Coca Cola and Water.[7,8]
The three different porcelains used in the present study showed an average surface roughness ranging from 0.26 to 1.48 μm, which was in accordance with the results contributed by Kamala and Annapurni.[3] In our study, Group I (Vita VMK 95) and Group II (Ceramco-3) showed a surface roughness (Ra) ranging from 0.18 to 0.44 μm and 0.20 to 0.55 μm, respectively, before immersion in test solutions.
All the materials used in the present study showed a difference in average surface roughness ranging (Ra) from –0.14 to 0.13 μm after immersion in test solutions for 90 days. A study was done by Jones et al. in which they concluded that the majority of patients could detect difference of about 0.3μm in mean roughness.[9] Considering the erosive potential of test solutions, Orange Juice was found to cause maximum amount of surface roughness, which differs from the findings of Jensdottir, et al. who had evaluated the immediate erosive potential of Cola drinks and Orange Juice and concluded that Cola drinks had more than 10-fold higher erosive potential than Orange Juices within the first minute after exposure.[10] The probable cause of this difference in results might be the elongated time period (90 days) used in the present study, as it is the titratable acidity that comes into play when any drink/solution is kept in contact with teeth. Titratable acidity is the buffering capacity of a solution. Greater the titratable acidity of the drink, the longer time will be taken by the saliva to neutralize it. Since there are no salivary contents in the test solutions used in our study, so titratable acidity of the solution is bound to cause dissolution of the surface layer of the porcelain to neutralize itself. It has been proved that Orange Juice has greater titratable acidity in comparison to Cola drinks.[11,12]
Though all the three porcelains used in the present study were feldspathic and more or less have same composition and firing cycles but all have shown different amount of color change and surface roughness. This variation may be attributed to the difference in percentage of basic individual composition. Also it is not known, whether it is the glaze layer which undergoes disruption due to acidic solutions and cause retention of stains or these are absorbed within the body of the porcelain. Furthermore, in the oral cavity, salivary proteins decrease the erosive potential of the acidic solutions and it is difficult to entirely correlate laboratory findings with clinical behavior of any restoration. The oral cavity is in a constant dynamic change. The pH changes, temperature changes, abrasive action of food, titratable acidity of solution, role of saliva etc., are all subjecting the ceramic to a fluctuating environment. The exact behavior of the ceramic, the color change and change in surface roughness can probably be explained in an in vivo study. Hence this study does highlight the effect of commonly consumed beverages in causing surface roughness and staining of the feldspathic porcelain. Therefore the exact role of feldspathic and glass ceramics, the compositional and structural changes occurring post firing and glazing, and their effect on the ΔE needs further research.
CONCLUSION
The following conclusions were arrived from this study:
Vita VMK 95 after immersion in test solutions showed minimum color change (i.e. maximum color stable) in water (3.50 + 0.79 ΔE units), and maximum color change in Coffee (7.13 + 4.55 ΔE units) whereas Ceramco-3 showed minimum color change (i.e. maximum color stable) in Orange Juice (3.57 + 2.08 ΔE units), and maximum color change in water (10.08 + 6.97 ΔE units). Additionally Duceram Kiss after immersion in test solutions demonstrated minimum color change (i.e. maximum color stable) in water (4.42 + 3.29 ΔE units) and maximum color change in Tea (9.65 + 6.63 ΔE units).
Ceramco-3 showed maximum color change (10.08 + 6.97 ΔE units) after a period of 90 days, irrespective of test solution whereas among all the 5 test solutions, Coffee showed the maximum staining (11.93 + 12.81 ΔE units) in Group II (Ceramco-3) between 45 and 90 days.
Maximum surface roughness was shown by the samples of Duceram Kiss (1.30 + 0.15 μm); however, among all test solutions, maximum surface roughness was caused by Orange Juice (1.30 + 0.15 μm), which probably is due to its high titratable acidity, and minimum surface roughness was caused by water (0.16 + 0.03 μm).
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Douglas R. Color stability of new generation indirect resins for Prosthodontic application. J Prosthet Dent. 2000;83:166–70. doi: 10.1016/s0022-3913(00)80008-6. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R, Prakash H, Shah N, Jain V. Spectrophotometric evaluation of color changes of various tooth colored veneering materials after exposure to commonly consumed beverages. J Indian Prosthodont Soc. 2005;5:72–8. [Google Scholar]
- 3.Kamala KR, Annapurni H. Evaluation of surface roughness of glazed and polished ceramic surface on exposure to fluoride gel, bleaching agent and aerated drink: An in vitro study. J Indian Prosthodont Soc. 2006;6:128–32. [Google Scholar]
- 4.Villata P, Lu H, Okte Z, Godoy GF, Powers MJ. Effect of staining and bleaching on color changes of dental composite resins. J Prosthet Dent. 2006;95:137–42. doi: 10.1016/j.prosdent.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Caul HJ, Schoonover IC. Color stability of direct filling resins. J Am Dent Assoc. 1953;47:448–54. doi: 10.14219/jada.archive.1953.0198. [DOI] [PubMed] [Google Scholar]
- 6.Ghahramanloo A, Madani SA, Sohrabi K, Sabzevari S. An evaluation of color stability of reinforced composite resin compared with dental porcelain in commonly consumed beverages. J Can Dent Assoc. 2008;136:673–80. [PubMed] [Google Scholar]
- 7.Gross MD, Moser JB. Colorimetric study of coffee and tea staining of four composite resins. J Oral Rehab. 1977;4:318–22. doi: 10.1111/j.1365-2842.1977.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 8.Chan CK, Fuller LJ, Hormati AA. The ability of foods to stain two composite resins. J Prosthet Dent. 1980;43:542–5. doi: 10.1016/0022-3913(80)90328-5. [DOI] [PubMed] [Google Scholar]
- 9.Koidis PT, Schroeder K, Johnston W, Campagni W. Color consistency, plaque accumulation, and external marginal surface characteristics of the collarless metal-ceramic restoration. J Prosthet Dent. 1991;65:391–400. doi: 10.1016/0022-3913(91)90231-k. [DOI] [PubMed] [Google Scholar]
- 10.Jensdottir T, Holbrook P, Nauntofte B, Buchwald C, Bardow A. Immediate erosive potential of cola drinks and orange juices. J Dent Res. 2006;85:226–30. doi: 10.1177/154405910608500304. [DOI] [PubMed] [Google Scholar]
- 11.Zero TD, Lussi A. Erosion- chemical and biological factors of importance to the dental practitioner. Int Dent J. 2005;55:285–90. doi: 10.1111/j.1875-595x.2005.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 12.Yannikakis AS, Zissis JA, Polyzois LG, Caroni C. Color stability of provisional resin restorative materials. J Prosthet Dent. 1998;80:533–9. doi: 10.1016/s0022-3913(98)70028-9. [DOI] [PubMed] [Google Scholar]


