Abstract
Germline mutation in the adenomatous polyposis coli (APC) gene causes the majority (80%) of familial adenomatous polyposis (FAP), an autosomal dominantly inherited form of colorectal cancer (CRC). Mutation in 5′end of exon 9 of APC usually results in an attenuated form of FAP (aFAP), characterized by later age of onset and fewer polyps. The presence of exon 9a, an in-frame isoform with exon 8 spliced to 3′end of exon 9, modulates any deleterious effect of the mutation. A third lowly expressed isoform that completely skips exon 9 is present in both healthy individuals and FAP patients. We report here an interesting case of a proband with an APC mutation in 5′end of exon 9 that presented with six synchronous advanced CRCs at age 37. The novel insertion–deletion (indel) at codon 409, c.1226-1229delTTTTinsAAA, caused upregulation of the ‘skip exon 9' isoform, r934-1312del, resulting in a premature stop codon at exon 10 and a truncated protein that removed all of the β-catenin (CTNNB1) binding motifs, thus activating the downstream T-cell transcription factor (Tcf) pathway. Exon 9a isoform was concomitantly downregulated. This finding emphasizes the necessity of examining the various isoforms of exon 9 to avoid clinical mismanagement and counseling based on just the mutation site by genomic DNA sequencing alone.
Keywords: colorectal cancer, FAP, APC splicing isoforms, exonic splicing enhancer, indel
INTRODUCTION
Familial adenomatous polyposis (FAP) (MIM: 175100), an autosomal dominantly inherited form of colorectal cancer (CRC), is characterized by up to thousands of adenomatous polyps in the colon and the rectum, and afflicts an affected individual by the second or the third decade of life. The risk of cancer is virtually 100% if the polyps are not removed. The adenomatous polyposis coli (APC) tumor suppressor gene (MIM: 611731) is a large gene spanning about 100 kb of genomic region that causes the majority of FAP.1, 2
Multiple APC mRNA isoforms including isoforms with extra exon in the coding region of the APC have been reported.3, 4 An in-frame physiologically relevant isoform, exon 9a, has an exon 8-3′exon 9 connection that removes 101 residues from 5′end of exon 9. Mutation in residues 5′ to exon 9a often results in an attenuated form of FAP (aFAP), with later age of onset (usually in the fourth and the fifth decade of life) and fewer (<100) polyps.5, 6 This is due to the splicing-out of the mutation site in a fraction of mRNA molecules and in the residual production of shorter, in-frame transcripts from the mutant APC allele. Hence, exon 9a modulates the effect of any mutation in the 5′end of exon 9, resulting in the aFAP phenotype. Nevertheless, there are exceptions where mutation carriers from the same family can present with profuse polyposis, possibly due to other modifiers.7, 8, 9
We have previously identified another very lowly expressed isoform that skips exon 9 completely (unpublished data). In this study, we report a novel indel at the 5′end of exon 9 that upregulates this ‘skip exon 9' isoform and concomitantly downregulates exon 9a, resulting in very severe polyposis and six synchronous advanced cancers in a 37-year-old female proband.
MATERIALS AND METHODS
Sample selection
Blood was collected from members 8, 19, and 21 of the family with informed consent. Pedigree was retrieved from the Singapore Polyposis Registry. This study was approved by the Institutional Review Board of Singapore General Hospital.
DNA/RNA extraction
DNA was obtained from peripheral blood lymphocytes by a simple salting procedure.10 RNA was extracted from blood lysate using the Promega (Madison, WI, USA) Total RNA extraction kit according to the manufacturer's protocol.
Protein truncation test
The APC coding region (NM 000038.4) was amplified by six overlapping PCR/RT-PCR segments as previously described.11 The unpurified PCR product was in vitro transcribed and translated into protein with the TNT/T7 coupled reticulocyte lysate system (Promega).
PCR amplification of exons 8–10
Exons 8–10 were amplified by nested PCR with 1 μl of RT-PCR product from Segment 1 (exons 1–14) and 10 pmol of primers U281 (5′-ACTACACGAATGGACCAT-3′) and R464 (5′-GCATGTCTATGCTCTTCA-3′) corresponding to sequences in exons 8 and 10, respectively. Amplification conditions were 35 cycles of 95 °C/30 s, 55 °C/30 s, and 72 °C/60 s. The PCR products were electrophoresed on 10% polyacrylamide precast minigel and stained with Ethidium Bromide.
Sequence analysis
The size of the truncated protein was used to estimate the mutation site. Relevant PCR fragment was identified and primers made for PCR-based BigDye dideoxy sequencing using an ABI 310 automated sequencer (Applied Biosystems, Foster City, CA, USA).
RESULTS
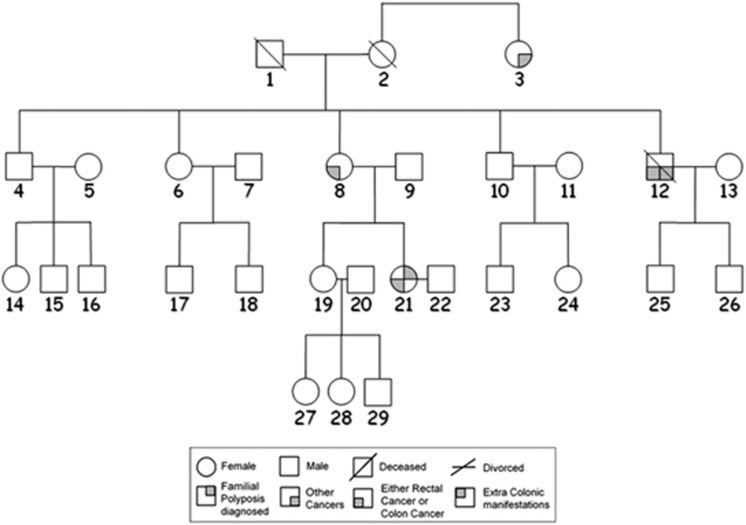
The proband (patient 21) of FAP family 92, a 37-year-old female, presented with severe polyposis and six synchronous tumors that have metastasized to lungs, liver, and bone and was deceased at age 39 (Figure 1). The mother (patient 8), however, had CRC in her early sixties and no documentation of polyps. An uncle had prostate cancer and CRC in his early sixties. His pathological records were not available.
Figure 1.
Pedigree of FAP family 92.
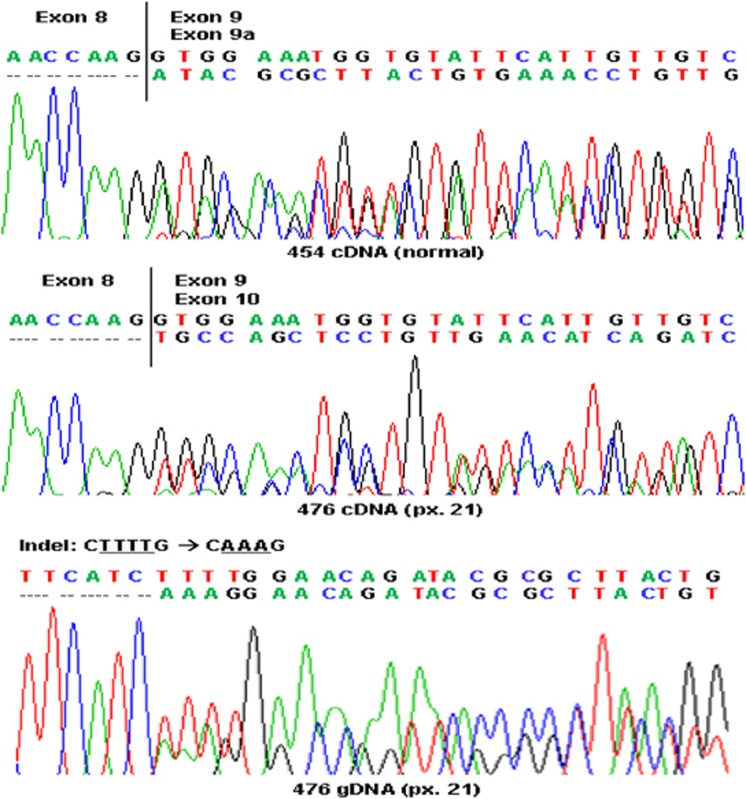
Protein truncation test (PTT) performed on the proband revealed an extra lower band in segment 1.2 of APC that is indicative of truncation in exon 10. cDNA sequencing analysis of exon 9 of APC normally revealed a second allele representing the in-frame exon 9a isoform (Figure 2). However, in patient 21, the exon 9a allele was missing. Instead, the second allele had an exon 8–10 connection. Genomic DNA sequencing of exon 9 of APC identified a novel indel at codon 409 (c.1226-1229delTTTTinsAAA), which would result in a premature stop codon (UAA) in exon 10. The variant was submitted to the Leiden Open Variation Database (LOVD) at http://chromium.liacs.nl/LOVD2/colon_cancer/variants.php?select_db=APC&action=view&view=1013090.
Figure 2.
Electropherogram of sequence analysis from cDNAs of normal individual (454) and patient 21 (476) and from genomic DNA of patient 21. 454 and 476 refer to lymphocyte samples of the normal individual and patient 21, respectively; px, patient.
It is predicted that the effect of any mutation 5′ to exon 9a, that is, between codons 312 and 412, would be modulated by exon 9a, a physiologically present isoform, which is in-frame and functional.5, 6 Consequently, any mutation 5′ to exon 9a would most likely result in an aFAP phenotype. It was thus very surprising to note that the colonic manifestation in the proband was very severe, compared with that of the mother and the uncle. Moreover, the patient had relative sparing of polyps in the proximal colon, a feature in contrast to most mutations at the 5′end of exon 9 that typically results in aFAP.5, 8, 12 The six synchronous tumors were also predominantly on the left (two transverse, two sigmoid, and two rectal). The exon 8–10 connection reminded us of our previous unpublished finding of a ‘skip exon 9' isoform that is present in low abundance in the lymphocytes of all 22 FAP patients and 21 healthy individuals screened with an age range of 14–70 years.
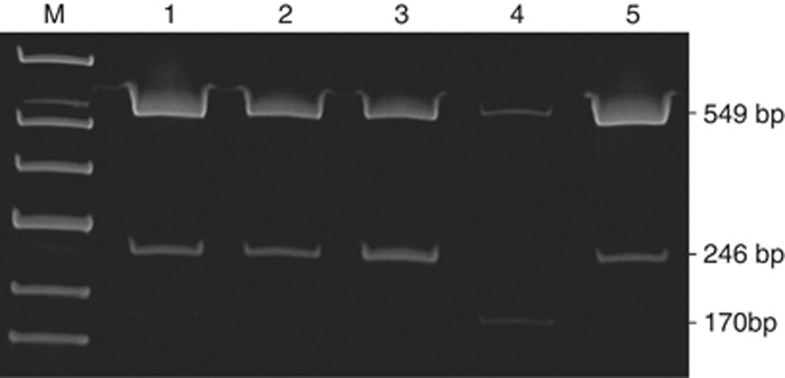
We amplified segment 1 (exons 1–14) of the APC gene of patient 21, her mother (affected member 8) and sister (unaffected member 19; Figure 1) together with a normal individual and another FAP patient whose germline mutation was in segment 3 of APC.11 The sequences of the 549-bp and 246-bp fragments corresponded to the published alternatively spliced APC sequences containing exons 9 and 9a, respectively,1 while that of the 170-bp fragment lacked exon 9 completely (r934_1312del) and had an exon 8–10 connection (Figure 3). Densitometric analysis showed that the intensity of the 549-bp band in patient 21 (lane 4) was 18% of the normal individual (lane 1). Further, the 246-bp band and the 170-bp band in this patient were 6 and 200% of the normal individual, respectively. This corroborates with the cDNA sequencing results, which revealed that the exon 9a allele was missing in patient 21 compared with that of a normal individual (Figure 2). The analyses also suggest that the indel was de novo and was shared neither by the affected mother nor by the sister. Genomic DNA sequencing analysis confirmed that both mother and sister did not harbor this mutation.
Figure 3.
RT-PCR products of APC exons 8–10 from a normal individual (lane 1), members 8 (lane 2), 19 (lane 3), and 21 (lane 4) of FAP 92 and another FAP patient with germline mutation in segment 3 of APC (lane 5).
DISCUSSION
We report on an interesting case of a young Chinese female proband who had a de novo indel at 5′end of exon 9 of APC that resulted in a severe form of FAP rather than aFAP as predicted by the mutation site. The de novo mutation could be due to mosaicism occurring in the germline of the patient's parents (especially the father). If a mutation occurs in the sperm of the father, who is phenotypically normal, then the mutation would appear as a constitutional de novo variant in the daughter.13 Unfortunately, no sample is available from the father for testing.
The severity of the disease would not have been anticipated if the mutation screening has not begun with the PTT followed by cDNA sequencing, which showed an exon 8–10 connection as a second allele and the absence of the exon 9a isoform. The downregulation of the exons 8–9, exon 8–9a wild-type isoforms, and the concomitant upregulation of the exon 8–10 out-of-frame variant indicates that the severity of the phenotype is most likely due to the relative dosage of these transcripts (Figure 3). A recent study has shown that perturbation of isoforms at the alternatively spliced region of exon 9 contributed to the attenuated phenotype of an FAP patient.14
The ‘skip exon 9' isoform leads to a frameshift and termination of the reading frame in exon 10. This results in a truncated protein that lacks all functional domains and retains only the homo-dimerization domain of wild-type APC. Such an isoform could conceivably bind to and remove the full-length APC from the available pool for interaction with other proteins such as Asef, β-catenin, or axin thus interfering with all APC functions.15 This could possibly explain the severity of the phenotype in the proband.
The finding of this study reiterates the importance of proper mutation screening (encompassing in vitro protein and transcript analysis, not just genomic sequencing) techniques. This would avoid any clinical mismanagement and counseling based on just the mutation site from genomic DNA sequencing alone.
Acknowledgments
We thank Ms Michelle Lo from Department of Colorectal Surgery and Dr Zhao Yi from Department of Clinical Research, SGH for technical support.
The authors declare no conflict of interest.
References
- Groden J, Thliveris A, Samowitz W, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su LK, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Xia L, Denis KAS, Bapat B. Evidence for a novel exon in the coding region of the Adenomatous Polyposis Coli (APC) gene. Genomics. 1995;28:581–591. doi: 10.1006/geno.1995.1195. [DOI] [PubMed] [Google Scholar]
- Sulekova Z, Ballhausen WG. A novel coding exon of the human adenomatous polyposis coli gene. Hum Genet. 1995;96:469–471. doi: 10.1007/BF00191808. [DOI] [PubMed] [Google Scholar]
- van der Lujit RB, Vasen HFA, Tops CMJ, Breukel C, Fodde R, Khan PM. APC mutation in the alternatively spliced region of exon 9 associated with late onset familial adenomatous polyposis. Hum Genet. 1995;96:705–710. doi: 10.1007/BF00210303. [DOI] [PubMed] [Google Scholar]
- Curia MC, Esposito DL, Aceto G, et al. Transcript dosage effect in familial adenomatous polyposis: model offered by two kindreds with exon 9 APC gene mutations. Hum Mut. 1998;11:197–201. doi: 10.1002/(SICI)1098-1004(1998)11:3<197::AID-HUMU3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Soravia C, Berk T, Madlensky L, et al. Genotype-phenotype correlations in attenuated adenomatous polyposis coli. Am J Hum Genet. 1998;62:1290–1301. doi: 10.1086/301883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt RW, Leppert MF, Slattery ML, et al. Genetic testing and phenotype in a large kindred with attenuated familial adenomatous polyposis. Gastroenterology. 2004;127:444–451. doi: 10.1053/j.gastro.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis MH, Vasen HF. Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit Rev Oncol Hematol. 2007;61:153–161. doi: 10.1016/j.critrevonc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:739. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Eu KW, Seow-Choen F, Cheah PY. Germline mutations are frequent in the APC gene but absent in the β-catenin gene in Familial Adenomatous Polyposis patients. Genes Chromosomes Cancer. 1999;25:396–398. doi: 10.1002/(sici)1098-2264(199908)25:4<396::aid-gcc13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Young J, Simms LA, Tarish J, et al. A family with attenuated familial adenomatous polyposis due to a mutation in the alternatively spliced region of APC exon 9. Hum Mut. 1998;11:450–455. doi: 10.1002/(SICI)1098-1004(1998)11:6<450::AID-HUMU5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Biesecker GL, Spinner NB. A genomic view of mosaicism and human disease. Nat Rev Genet. 2013;14:307–320. doi: 10.1038/nrg3424. [DOI] [PubMed] [Google Scholar]
- Florentia F, Yannoukakos D. A distinct mutation on the alternative splice site of APC exon 9 results in attenuated familial adenomatous polyposis phenotype. Fam Cancer. 2010;9:395–400. doi: 10.1007/s10689-009-9317-x. [DOI] [PubMed] [Google Scholar]
- Sieber OM, Tomlinson IP, Lamlum H. The adenomatous polyposis coli (APC) tumor suppressor—genetics, function and disease. Mol Med Today. 2000;6:462–469. doi: 10.1016/s1357-4310(00)01828-1. [DOI] [PubMed] [Google Scholar]