Abstract
Neural tube defects (NTDs) are severe congenital malformations affecting around 1 in every 1000 pregnancies. Here we review recent advances and currently unsolved issues in the NTD field. An innovation in clinical management has come from the demonstration that closure of open spina bifida lesions in utero can diminish neurological dysfunction in children. Primary prevention by folic acid has been enhanced through introduction of mandatory food fortification in some countries, although not yet in UK. Genetic predisposition comprises the majority of NTD risk, and genes that regulate folate one-carbon metabolism and planar cell polarity have been strongly implicated. The sequence of human neural tube closure events remains controversial, but study of mouse NTD models shows that anencephaly, open spina bifida and craniorachischisis result from failure of primary neurulation, while skin-covered spinal dysraphism results from defective secondary neurulation. Other ‘NTD’ malformations, such as encephalocele, are likely to be post-neurulation disorders.
Introduction
Neural tube defects (NTDs) affect an average of 1 in every 1000 established pregnancies world wide 1, although variations in NTD prevalence have been reported from 0.2-10 per 1000 in specific geographical locations. Higher frequencies occur in miscarriage material 2. NTDs rank amongst the commonest of birth defects, alongside congenital heart anomalies and genito-urinary defects 3. NTDs have a long history: reports of fetuses and infants with anencephaly, myelomeningocele and craniorachischisis extend back to Ancient Egyptian times 4. Moreover, throughout the past 60 years, a striking progression of diverse bio-medical advances has impacted the NTD field, meaning that this topic has rarely been ‘out of the news’.
Record and McKeown’s landmark paper 5 in 1949 first raised awareness of the complex epidemiological features of NTDs. These include marked variations in NTD prevalence between geographical locations and ethnic groups, variation with socioeconomic status, pregnancy order in multiparous women and the striking female preponderance of anencephaly 6. Carter’s analysis in 1971 of the epidemiological data 6 led to our persisting view of a multi-factorial NTD aetiology. Modern findings support a multi-gene predisposition, together with a role for ‘environmental’ factors such as the diabetic milieu or folate status.
In this Review we consider the clinical management and primary prevention of NTDs, advances in the understanding of NTD causation, and NTD pathogenesis. We note a number of unsolved questions and controversial topics.
Clinical features and management
Clinical severity of NTDs varies greatly (Table 1). Open lesions affecting the brain (anencephaly, craniorachischisis) are invariably lethal before or at birth. Encephalocele can also be lethal depending on the extent of brain damage during herniation. Open spina bifida is generally compatible with postnatal survival, although the resulting neurological impairment below the level of the lesion can lead to lack of sensation, inability to walk and incontinence. Associated conditions include hydrocephalus, which often requires cerebrospinal fluid shunting, vertebral deformities, and genitourinary and gastrointestinal disorders. Closed spinal lesions are generally less severe and can be asymptomatic, as with spina bifida occulta which is considered a variant of normal. However, lumbosacral spinal cord tethering may be present in spinal dysraphism, and can lead to lower limb motor and sensory deficits, and a neuropathic bladder.
Table 1. Characteristics of the main types of NTDs.
| Anencephaly | Myelomeningocele (open spina bifida; SB) | Craniorachischisis | Spinal dysraphism | Encephalocele | |
|---|---|---|---|---|---|
| Relative frequency * | 40% | 50% | 3% | Unknown | 7% |
| Epidemiological features | Typical of NTDs as a whole; mostly sporadic | Typical of NTDs as a whole; mostly sporadic | Unknown; high prevalence noted in North China 106 | Unknown | Usually sporadic, but can be syndromic (e.g. in Meckel syndrome) |
| Sex ratio (F:M) | Marked female excess (3:1) | Variable in different populations; approximately equal overall | Female excess, as for anencephaly | Equal | Female excess among occipital lesions |
| Clinical presentation | Lack of brain and cranial vault; fetal loss or stillbirth | Open spinal cord covered by meningeal sac (SB cystica) or exposed (SB aperta); most commonly thoraco-lumbar, lumbar or lumbo-sacral; usually live birth; frequently associated with hydrocephalus post-natally | Anencephaly continuous with complete open spina bifida; fetal loss or stillbirth | Skin covered lesion involving 2 or more vertebrae, apparent only on radiography; hair tuft, lipoma or other cutaneous feature often co-exists | Meningeal sac, often containing brain tissue, protrudes from skull; commonly in occipital, parietal or fronto-ethmoidal locations |
| Prenatal diagnosis | Ultrasound from first trimester; elevated serum AFP | Ultrasound from first trimester; elevated serum AFP | Ultrasound from first trimester; elevated serum AFP | No | Ultrasound, depending on size of lesion |
| Surgical treatment | None: lethal beyond birth | Surgical closure post-natally or in utero in some centres; insertion of cerebro-spinal fluid shunt for hydrocephalus | None: lethal beyond birth | Untethering of spinal cord, usually in childhood | Repair by removal of sac and closure |
| Non-surgical treatment | None | Long-term treatment of hydrocephalus, skeletal, renal, gut and other secondary disorders | None | Treatment of genito-urinary disorders as common sequelae | Treatment of epilepsy and learning disorders as common sequelae |
| Genetic causation | Genes as for NTDs as a whole (see text) | Genes as for NTDs as a whole (see text) | Planar cell polarity genes are only positive findings | Unknown | MKS1-3, RPGRIP1L, CEP290 genes all identified as causal for Meckel syndrome (occipital encephalocele) |
| Non-genetic causation | Increased risk in diabetic pregnancy; no specific associations | Valproic acid exposure increases risk 10X; increased risk in diabetic pregnancy | None known | None known | None known |
| Primary prevention | FA, as for NTDs as a whole | FA, as for NTDs as a whole; inositol prevention of open spinal bifida in Grhl3 mouse model | FA may prevent: North China frequency declined after FA introduced | FA-resistant? Lipomyelomeningocele frequency shows no decline after food fortification with FA107 | FA may prevent: some evidence of decline after food fortification with FA |
| Embryonic origin | Failure of cranial neural tube closure | Failure of caudal neuropore closure | Failure of Closure 1 | Defective secondary neurulation | Post-neurulation disorder? |
| Pathogenesis | Originates after failed closure as exencephaly; converted to anencephaly by degeneration of neural tissue and absence of cranial vault formation | Degeneration of exposed neural tissue following failed closure; origin of meningeal sac in SB cystica is not clear | Combined anencephaly and myelomeningocele | Unknown | Herniation of meninges with or without brain tissue through defect in skull |
Before the 1970s, management of open spina bifida consisted solely of palliative surgical and medical support. While children generally survived if their lesion was closed surgically, thereby avoiding ascending infection, neurological outcome varied markedly with the vertebral level of lesion (i.e. higher defects had greater neurological handicap). This led to suggestions that surgery should be offered only in cases with a better prognosis 7. An ethical debate ensued, around whether surgical treatment should be withheld, but this was superseded in the 1970s when methods for prenatal diagnosis of open NTDs were developed. Initially, diagnosis was based on measurement of alphafetoprotein (AFP) concentration in the amniotic fluid and maternal blood 8,9, but later technological improvements enabled ultrasound to replace AFP measurement as the mainstay of prenatal diagnosis 10. Today, most fetuses with NTDs are diagnosed prenatally in developed countries, and many are aborted therapeutically. In contrast, large numbers of babies with NTDs continue to be born in developing countries where prenatal diagnosis is not routine, as well as in countries where therapeutic abortion is either illegal, or not practised owing to religious or cultural views.
In human and mouse embryos, the persistently open spinal cord undergoes relatively normal neuronal differentiation during the embryonic period, including development of spinal motor and sensory function even below the lesion level 11. This demonstrates that neural tube closure is not required for subsequent events of neuronal differentiation. As gestation progresses, however, neurons die within the exposed spinal cord, revealing that the amniotic fluid environment is toxic for cells that would normally be contained within the closed neural tube. Axonal connections are interrupted, and function is lost 11,12. Hence, neurological disability in open spina bifida is a ‘two-hit’ process: failed neural tube closure followed by neurodegeneration in utero. This has encouraged attempts to arrest or prevent further neurodegeneration by covering the persistently open neural tube as early as possible during fetal development.
Surgical repair in utero for early open spina bifida has been practised in several centres in the USA for the past 15 years 13. An important recent development was the report of a controlled clinical trial to evaluate the success of this procedure. The Management of Myelomeningocele Study (MOMS)14 randomly assigned fetuses with prenatally diagnosed myelomeningocele to either in utero surgery or standard post-natal repair. The trial showed that fetal surgery brings significant short-term benefits for the newborn child, including a 50% reduction in shunting for hydrocephalus and a significant improvement in spinal neurological function. Against this was a significantly higher rate of premature birth and maternal complications such as uterine dehiscence at the operation site in the in utero group. While long-term outcomes for children following this surgical intervention remain unknown, these pioneering studies will no doubt encourage other centres to consider implementation of in utero surgery.
Primary prevention of NTDs
In the 1970s, Smithells and colleagues noted that several vitamins (folate, riboflavin and vitamin C) had reduced concentrations in the serum of mothers pregnant with NTD fetuses 15. They performed an intervention trial of peri-conceptional Pregnavite Forte F, a multi-vitamin supplement containing 0.36 mg folic acid (FA), to assess its possible effect in preventing NTD recurrence in high-risk women with a previous affected pregnancy. The ethics committees denied requests for randomisation at this time, so the findings of significant prevention by Pregnavite Forte F 16 were not universally accepted as definitive evidence of prevention 17. In due course, a randomised, double blind study (the MRC trial) was performed which specifically assessed 4 mg FA separately from multi-vitamins and demonstrated that FA is the essential factor for significant prevention of NTD recurrence 18. Subsequently, a randomised clinical trial in Hungary of a multivitamin supplement containing 0.8 mg FA was shown to significantly prevent the first occurrence of NTDs 19, an important finding considering that 95% of all NTD cases are first occurrences in a family. A further clinical trial, based in China, demonstrated a fall in NTD prevalence subsequent to introduction of FA supplementation 20.
The MRC trial led to the recommendation that all women planning a pregnancy should consume 0.4 mg FA per day (the dose used in the Smithells trials), and that women at high risk of NTD should receive 4-5 mg per day. However, despite widespread public health education efforts in the UK and other countries, the prevalence of NTDs did not decrease during the decade following the MRC trial publication 21. In the USA, a campaign of governmental lobbying 22 eventually achieved its aims and mandatory fortification of bread flour with FA was introduced in 1998. Fortification was introduced soon after in Canada, and then throughout South America, South Africa, Australia, and other countries. Although NTD rates were falling in several countries prior to this period 23, most authorities agree that the decision to fortify with FA, ensuring a more favourable folate status in women who become pregnant, has contributed significantly to reducing the number of pregnancies affected by NTDs 24. It is notable that no European countries, including the UK, have yet to implement food fortification. Other strategies for improving supplementation include incorporation of folate within an oral contraceptive 25, and the use of supplements that contain a more ‘bioavailable’ form of folate. Compounds such as 5-methyltetrahydrofolate (6S-5-MTHF) may offer a means of enhancing folate status more effectively than FA in some individuals 26. However, an appropriate format to retain stability in food is required 27, and a formal trial for prevention of NTDs has not yet been performed.
Surveys of NTD prevalence show that the decline following food fortification 24 has been smaller than expected from the MRC trial. While some have advocated increasing doses of FA, in order to prevent a larger proportion of total NTDs 28, most authorities accept that a proportion of cases, perhaps 0.7-0.8 per 1,000 pregnancies, are likely to persist regardless of FA usage, and that little additional benefit will accrue from a further increase in dose level 29,30. In other words, a proportion of NTDs are likely to be ‘FA-resistant’, and perhaps of different aetiology from the FA-sensitive subgroup. This concept is well established in mouse models of NTDs where some genetic types are FA-preventable while others are FA-resistant 31. One potential adjunct therapy that has arisen from the mouse studies is the use of inositol, which is effective in preventing a large proportion of spinal NTDs in the Grhl3 (curly tail mutant) mouse, where FA is ineffective 32. Uniquely among vitamins, inositol deficiency leads to NTDs in rodent embryos 33. A randomised clinical trial to evaluate inositol for prevention of human NTD recurrence is currently underway in the UK.
Controversies and unsolved questions in NTD prevention
Are NTDs a vitamin-deficiency disorder?
The finding that FA can prevent many NTD cases is often interpreted as showing NTDs to be a ‘vitamin-deficiency’ condition 34. Indeed, both folate and vitamin B12 deficiency are statistical risk factors for NTDs 35. However, maternal folate levels in most affected pregnancies are within the ‘normal’ range 35,36, arguing against a simple folate-deficiency model. In mice, severely FA-deficient wild-type mothers do not have embryos affected by NTDs, although intrauterine growth retardation is routinely observed 37,38. The frequency of cranial NTDs is exacerbated by maternal folate deficiency in mutant splotch (Pax3) embryos, whereas wild-type littermates are never affected by NTDs 39. Similarly, in the Shmt knockout mouse, the development of NTDs is seen solely in mothers that are folate deficient 40. Clearly, folate deficiency is a risk factor for NTDs, but only in the presence of a predisposing genotype.
Could FA ‘prevent’ NTDs by killing affected embryos?
This concept, termed ‘terathanasia’ is theoretically possible, as the disappearance of a proportion of NTD cases, owing to early pregnancy loss, might be interpreted as primary prevention 41. There was a small excess of miscarriages in the multivitamin-treated group of the Hungarian randomised trial 19, but this has been interpreted as FA encouraging survival of some pregnancies to a stage when their loss can be recognised (as miscarriage). In several mouse NTD models, embryos whose genotype destines them to develop NTDs can be normalised by FA 42,43. Moreover, in the splotch (Pax3) model, folate-deficiency exacerbates cranial NTDs, consistent with true primary prevention by FA 39. However, exposure of several mouse NTD strains to dietary FA supplementation has produced a more diverse range of responses, with apparent exacerbation of NTDs in some cases 44. This variation in response may result in part from the widely varying FA dose levels used in different mouse strains, often amounting to a 100-fold excess compared with human supplementation 45. Interestingly, however, serum folate concentrations are generally similar in supplemented mice and humans 44. A further consideration is that various pathogenic mechanisms are known to underlie NTDs, in mice and probably also in humans; hence, the interaction of FA supplementation with these mechanisms is also likely to be heterogeneous.
How does FA promote normal neural tube closure?
This fundamental question has received little attention compared with the hundreds of publications on clinical and epidemiological aspects of FA supplementation. So far, we know that FA has a direct effect on the neurulation stage embryo, as treatment of genetically predisposed mouse embryos in vitro can normalise neural tube closure 42. FA enters one-carbon metabolism, which has two main outputs: production of pyrimidines and purines for DNA replication during cell proliferation, and donation of methyl groups to macromolecules including DNA, proteins and lipids (Figure 1). Cell multiplication plays a key role in neural tube closure 46,47, encouraging the hypothesis that enhanced cell proliferation may be a key effect of FA. On the other hand, methylation of genomic DNA and histones is increasingly being implicated in the (epigenetic) regulation of gene expression 48, and could underlie the action of FA in preventing NTDs. Detailed analysis of FA prevention of NTDs in animal models may resolve this issue in the coming years.
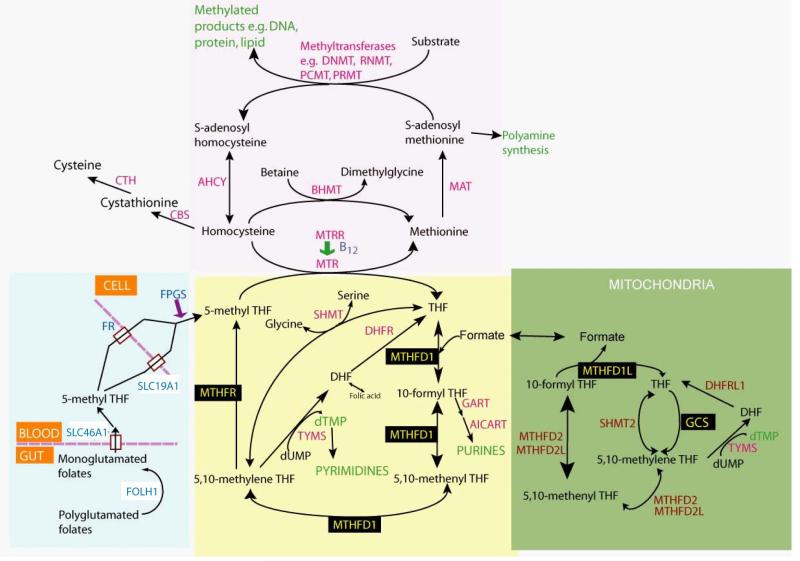
Figure 1. Summary of folate one-carbon metabolism showing the main pathways and reactions.
Blue shading: processing of folates in the digestive tract, transport and cellular retention (by addition of glutamates). Yellow shading: transfer of one-carbon groups between folate molecules for purine and pyrimidine biosynthesis. Pink shading: reactions of the methylation cycle that generate SAM, the universal methyl group donor. Green: mitochondrial reactions that generate formate via cleavage of glycine. Enzymes whose genetic variation have been implicated in human NTDs are indicated in black boxes. Figure modified from Greene et al, 2009 52.
Causes of NTDs
Both genetic and non-genetic factors are involved in the aetiology of NTDs, with up to 70% of the variance in NTD prevalence due to genetic factors 49. Evidence for genetic causation includes an increased recurrence risk for siblings of index cases (2-5%) compared with the 0.1% risk in the general population, together with a gradually decreasing frequency in more distant relatives. Women with two or more affected pregnancies have a higher risk (~ 10%) of further recurrence 50. NTD prevalence is greater in like-sex twins (assumed to include all monozygotic cases) compared with unlike-sex pairs, consistent with a significant genetic component. Nevertheless, NTDs rarely present as multiple cases in families; instead a sporadic pattern is usually observed. Taken together with the relatively high prevalence of NTDs across the world, this is consistent with a multifactorial polygenic or oligogenic pattern of inheritance, and an important role for non-genetic factors.
The genomics revolution of the last 15 years has impacted NTD research in two major ways: first, in providing the tools necessary to evaluate candidate genes for NTD causation and, second, in providing a wealth of mouse genetic NTD models (see Pathogenesis, below) which have stimulated many studies of human disease aetiology. In the near future, a full genome-wide assessment of interacting variants, including coding, regulatory and epigenetic marks, will be possible to determine an individual’s risk of developing a NTD. Indeed it may well be necessary to adopt such a strategy, if we are to unravel the complex multifactorial causation of these conditions. However, in view of the likely causal heterogeneity amongst sporadic NTDs, the implementation of this approach is highly demanding, requiring large numbers of samples for analysis. To date, genes in two main areas of biology have yielded positive findings with regard to NTD aetiology: folate one-carbon metabolism and non-canonical Wnt signalling (the planar cell polarity pathway).
Folate-related genes
Given the historical relationship between FA and NTDs, it is not surprising that folate pathway genes have been most intensively studied (Figure 1). Positive associations have been reported between specific folate-related gene variants and NTDs in a number of case-control studies 51-53. For example, methylenetetrahydrofolate reductase (MTHFR) encodes a key cytoplasmic enzyme of folate metabolism, that generates 5-methyl tetrahydrofolate for homocysteine remethylation. The MTHFR polymorphism C677T (rs1801133) is associated with a roughly 1.8-fold increased risk of NTDs, although the predisposition is detectable only in non-Hispanic populations 54. A further significant risk factor is the R653Q variant (rs2236225) of MTHFD1, a trifunctional enzyme that catalyses the conversion of tetrahydrofolate to 5,10 methylenetetrahydrofolate 53,55.
Most recently, genes that encode enzymes functioning in mitochondrial one-carbon metabolism have also been implicated in NTD aetiology. An intronic polymorphism in MTHFD1L, the gene for mitochondrial 10-formyl-THF synthetase, is associated with increased risk of NTD 56, while two genes encoding enzymes of the glycine cleavage system, AMT and GLDC, have been found to harbour a number of missense (i.e. amino acid-changing) genomic alterations in NTD cases, but not in unaffected controls 57. In the case of GLDC, these variants diminish enzyme activity indicating a functional effect on folate metabolism. Each of these enzymes markedly affects flux of formate from the mitochondrion into the cytoplasm, which accounts for approximately 75% of one-carbon units entering folate metabolism 58.
Hence, genetic variants that reduce the ‘efficiency’ of folate one-carbon metabolism increase the risk of NTDs. These findings are consistent with a study of cell lines derived from NTD fetuses, in which a subset were found to exhibit apparent inborn errors of folate metabolism, as indicated by diminished thymidylate biosynthesis 59. Strikingly, however, under folate-replete dietary conditions, only the mitochondrial enzymes Mthfd1l and Amt have been found to cause NTDs in knockout or gene trap mice 57,60. Disruption of the cytoplasmic enzymes Mthfr and Mthfd1 do not cause mouse NTDs 61,62, while exencephaly occurs in Shmt null embryos under folate-deficient conditions 40. This may indicate that the mitochondrial contribution to folate metabolism is particularly relevant and/or sensitive in terms of mammalian neural tube closure.
Planar cell polarity genes
At the onset of neurulation, the embryo undergoes lengthening and narrowing of the initially disc-shaped neural plate in order to ensure that the neural folds are spaced sufficiently close together for closure to begin 63,64. This elongation of the neural plate and underlying mesoderm requires a lateral to medial displacement and intercalation of cells, termed convergent extension 65. At the molecular level, convergent extension cell movements are dependent on non-canonical Wnt signalling: the planar cell polarity (PCP) pathway (Figure 2), which signals via frizzled membrane receptors and cytoplasmic dishevelled, but does not involve downstream stabilisation of beta-catenin 66.
Figure 2. Summary of non-canonical Wnt signalling in a mammalian cell.
Black arrows indicate the signalling pathway necessary for establishment of planar cell polarity (PCP). Known biochemical interactions are indicated by blue arrows and genetic interactions are shown by red arrows. Genes that have been implicated in human NTDs are indicated by asterisks. Figure modified from Greene et al, 2009 52.
Indications of a possible involvement of the PCP pathway in human NTDs came from the discovery of PCP gene involvement underlying severe NTDs in several mouse mutants. Mutations in the trans-membrane proteins Vangl2, Celsr1, Ptk7 and Fzd3/6 (double mutant), and the cytoplasmic proteins Dvl1/2/3 and Scrib, all result in craniorachischisis, a severe NTD (Table 1) in which closure fails along most of the body axis, yielding an open neural tube from midbrain to low spine 67. An increasing number of studies have subsequently reported unique and predominantly missense variants in PCP genes of NTD patients as likely causal alleles (Table 2). However, the finding of non-synonymous amino acid changes in affected humans but not in controls, whilst suggestive, does not prove their causal role in the NTDs. Functional evidence is needed to demonstrate whether the specific human ‘mutations’ actually cause protein dysfunction or, better still, reproduce the NTD phenotype in an animal model.
Table 2. PCP genes and their mutant phenotypes in mice and humans.
| PCP genes | Phenotype in mouse mutant homozygotes | Phenotype associated with ‘mutations’ in human gene | References |
|---|---|---|---|
| Celsr1 | CRN * | CRN | 70,110 |
| Dv1/2 or Dvl2/3 | CRN | DVL2: Varying types of NTDs, not CRN | 111,112 |
| Fuz | Exencephaly | Varying types of NTDs, not exencephaly | 113 |
| Fz3/6 | CRN | FZD6: Varying types of NTDs, not CRN | 114,115 |
| Pk1 | None | Varying types of NTDs, not CRN | 85 |
| Ptk7 (CCK-4) | CRN | No reports | 116 |
| Scrib | CRN | CRN | 70,117 |
| Sec24b | CRN | No reports | 118 |
| Vangl1 | None | Varying types of NTDs, not CRN | 68,119,120 |
| Vangl2 | CRN | Varying types of NTDs, not CRN | 69,84,121,122 |
CRN: craniorachischisis
To date, assays of PCP protein function including interaction with Dishevelled 68,69 and translocation to the plasma membrane 70 have identified functional defects in NTD-associated variants of VANGL1, VANGL2, CELSR1 and SCRIB. Several VANGL1 missense variants block the rescuing effect of Vangl1 mRNA on the Vangl2 (trilobite) mutant phenotype in zebrafish 71. It remains to be determined whether any of the putative NTD-causing variants in human PCP genes would also reproduce an NTD phenotype in an experimental mammal, such as a knock-in mouse strain.
Genetic causes of encephalocele
In contrast to open NTDs, occipital encephalocele is often syndromic, most commonly as part of Meckel syndrome. A number of genes have been identified in the causation of this condition: MKS1, MKS2 (TMEM216), MKS3 (TMEM67), RPGRIP1L, CEP290 [3]. MKS proteins play a key role in the structure and function of primary cilia, protrusions of the cell surface that are rooted in the centrosome and undergo a disassembly and reassembly cycle as the cell proliferates. Primary cilia are essential for signalling pathways, for example downstream of hedgehog proteins. Many, often individually rare, disorders are now known to be causally associated with genes required for ciliary structure and function, which has led to the concept of ‘ciliopathies’. How encephalocele results from disordered ciliary function is currently unknown.
Environmental factors
Few non-genetic factors have been definitively associated with human NTDs. This contrasts with the wide variety of teratogenic agents known to cause the defects in rodents 72. Of particular clinical significance is valproic acid (VPA), an anticonvulsant that increases risk of spinal NTDs by approximately 10-fold when taken during early in pregnancy 73. Although the teratogenic mechanisms underlying anticonvulsant action may involve anti-folate effects, particularly for carbamazepine 74, recent findings with VPA suggest a potent histone deacetylase (HDAC) inhibitory activity. This could disturb the balance of protein acetylation versus deacetylation, similar to the action of the HDAC inhibitor, trichostatin-A, which causes NTDs in mice 75. A further interesting environmental teratogen with proven effect in humans is the fungal product fumonisin, which was responsible for a 2-fold increase in NTD prevalence along the Texas-Mexico border in the early 1990s 76. Fumonisin is a potent NTD-causing teratogen in mice, with marked effects on spingolipid metabolism, that likely disturbs downstream embryonic gene expression 77. Other ‘environmental’ factors implicated in the aetiology of NTDs include maternal diabetes 78, maternal obesity 79, and exposure to high temperatures during early pregnancy 80.
While environmental causes of birth defects are perhaps the most preventable of predisposing factors, it is important to note that only a very small proportion of all congenital disorders have a known environmental cause: estimated at 0.12 cases per 1000 births (0.5% of all defects) in a recent survey of European pregnancies 3. Moreover, genetic variation is likely to play an important role in determining the susceptibility of a particular pregnancy to non-genetic factors. For example, marked differences are routinely observed between different inbred mouse strains for many teratogenic factors including VPA and fumonisin 77,81. In humans, single nucleotide polymorphisms in genes associated with types I and II diabetes mellitus have been associated with NTDs 82,83.
Controversies and unsolved questions
Can FA supplementation be targeted to genetically-defined ‘sensitive’ pregnancies?
Inheritance of a ‘predisposing’ folate enzyme variant, perhaps affecting MTHFR or MTHFD1, might be predicted to affect an individual’s response to exogenous FA supplementation. Carriers could require a larger dose of FA than wild-type individuals, to be ‘protected’ from NTDs. Hence, genotyping for known (or novel) risk variants might provide a means of FA targeting. A multivariate analysis of NTD cases (or mother-fetus pairs) is required to examine this possibility, with assessment of all known folate-related genetic variants, indices of folate supplementation and serum/red cell folate, homocysteine and other metabolites.
Do human and mouse phenotypes correspond in cases of shared genetic aetiology?
Despite the finding of PCP gene mutations in both mice and human NTDs, the phenotypes of humans and mice do not always correspond closely. For example, the human NTDs associated with heterozygosity for VANGL2 variants have included anencephaly, holoprosencephaly (not a defect of neural tube closure) and closed spina bifida 69,84. By contrast, mouse Vangl2 mutants exhibit craniorachischisis in homozygotes and low open spina bifida in compound heterozygotes with other gene mutations 52. Even more puzzling, human ‘mutations’ have been found in VANGL1 and PK1 68,85 despite there being no NTD phenotype in homozygous mouse mutants for these genes (Table 2). Only two PCP genes, CELSR1 and SCRIB, have been associated with the same NTD phenotype, craniorachischisis, in both mice and humans 70, whereas VANGL2 mutations were not identified in humans with craniorachischisis 86. Based on a polygenic model of NTD causation, we would predict that current genetic findings are incomplete, with additional ‘interacting’ genetic variants remaining to be discovered. The precise combination of predisposing variants may determine whether an individual develops anencephaly, spina bifida or craniorachischisis.
NTD pathogenesis
NTDs comprise a diverse set of birth defects that are usually considered to arise during the third and fourth weeks post-fertilisation. However, there remain many questions about the precise timing of origin of specific anomalies that are included under the umbrella of ‘NTDs’. Moreover, we have a limited understanding of the cellular and molecular mechanisms by which human NTDs arise during embryonic development. In fact, there has been a strong tendency for investigators of the clinical, epidemiological and FA-prevention aspects of NTDs to consider embryonic development as a ‘black box’ that can be left firmly closed. On the other hand, as genetic risk factors start to emerge from modern genomics research, it is vital to be able to understand when and how such gene variants might exert their effects. Similarly, in attempting to optimise FA-mediated prevention, and introduce new preventive therapies, it is important to appreciate the precise embryonic mechanisms that might be the targets of therapeutic interventions.
Advances in developmental studies of mouse neurulation
The availability of more than 250 different models of open NTDs in mice 87 is enabling increasingly sophisticated analysis of the neurulation events, at tissue, cellular and molecular levels. Because this large range of mouse models covers the majority of NTD types seen in humans, it has also been possible to build a picture of the events that are specific to each stage of neural tube formation, and therefore to the NTDs that arise from defects at particular stages. One potential limitation to mammalian research is the relative inaccessibility of the developing embryo within the uterus. However, access to neurulation stages is greatly facilitated by use of whole mouse embryo culture, in which intact mid-gestation embryos encased within their extraembryonic membranes, can be grown in vitro during the entire period of neurulation 88. Moreover, as live imaging techniques based on confocal microscopy become increasingly sophisticated and adaptable, a new era is beginning of real-time information on the dynamic events of neurulation in mammals through the imaging of cultured mouse embryos 89. Non-mammalian models, especially chick, frog and zebrafish, continue to provide insight into some of the key pathways and cellular mechanisms of neural tube formation 90-92.
Events of mouse neural tube formation
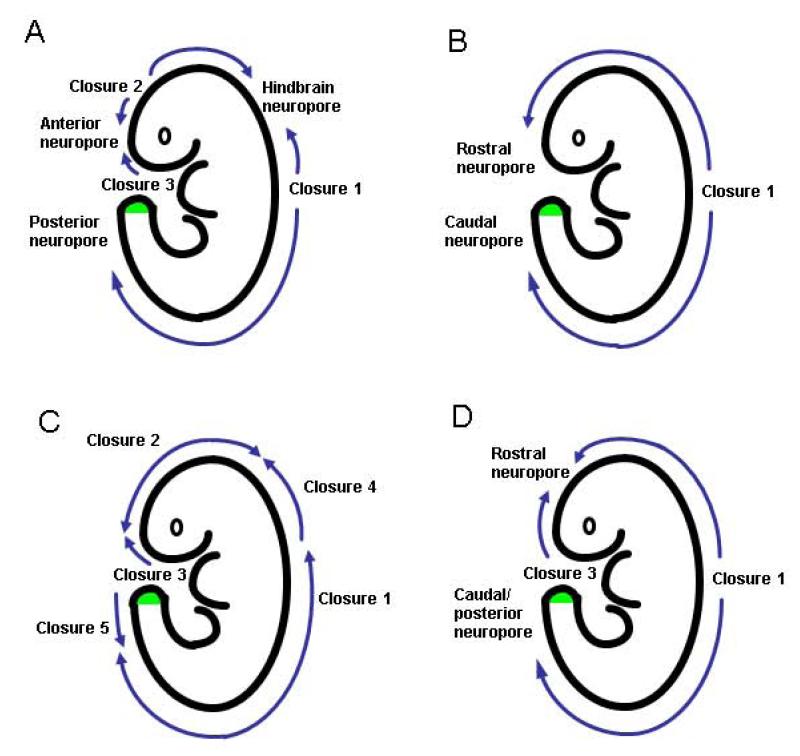
Neural tube formation is divided into primary (closure) and secondary (canalisation) phases. Primary closure is initiated at several discrete points along the rostro-caudal axis (Figure 3A): first, at the boundary between future hindbrain and cervical spine (termed Closure 1), then around 12 hours later at the boundary between future forebrain and midbrain (Closure 2) and soon afterward at the rostral extremity of the future forebrain (Closure 3)31,93. The open regions of neural folds between the sites of initial closure are termed ‘neuropores’, and these close progressively as the neural tube ‘zips up’ bi-directionally from the sites of Closures 1 and 2, and in a caudal direction from the site of Closure 3. The anterior and hindbrain neuropores complete closure within a few hours of Closures 2 and 3, whereas spinal neurulation continues zipping caudally along the growing spinal region until the posterior neuropore finally closes during embryonic day 10. This marks the end of primary neurulation, a process that has taken approximately 48 hours to be completed in mice.
Figure 3. Schematic showing different concepts of how mouse (A) and human (B-D) embryos undergo primary neurulation.
The site of secondary neurulation in the tailbud is indicated by green shading. (A) Pattern of mouse neural tube closure, as experimentally verified in multiple mouse strains 31. (B) The original concept of human closure in which bidirectional zippering occurs from an initiation site (Closure 1) towards the rostral and caudal extremities. (C) Modified concept based on mouse multi-site closure, as used for retrospective interpretation of human NTDs 105. (D) Pattern of human neural tube closure based on embryo observation 100.
Secondary neurulation follows on seamlessly from primary neurulation, and is the process by which the neural tube forms in the lower sacral and coccygeal regions 94,95. The caudal end of the embryo comprises the tail bud, which contains self-renewing stem cells whose derivatives condense into longitudinal cell masses. The most dorsal of these undergoes ‘canalisation’, converting the solid neural precursor into a hollow secondary neural tube. The stem cell population within the tail bud appears multipotent 96, giving rise to all non-epidermal tissues of the post-lumbar body, including neural tube and vertebrae. Probably for this reason, malformations and tumours (e.g. teratomas) of the sacral and coccygeal regions often embrace several tissue types.
Origin of NTDs during neural tube formation
Analysis of the mouse mutant loop-tail (Vangl2 gene) has shown that craniorachischisis, the most severe NTD, results from failure of Closure 1 97. Most commonly, however, embryos complete Closure 1 but fail in later neurulation, presenting NTDs as separate open lesions of the cranial neural tube (exencephaly, progressing to anencephaly) and/or spinal neural tube (open spina bifida). The wave of ‘zippering’ closure down the body axis can arrest at any stage, yielding an open spina bifida of varying length. Hence, Zic2 mutant mice fail early in spinal neurulation, owing to lack of dorsolateral neural plate bending 98. These mice exhibit a large spina bifida from thoracic level downwards. In contrast, spinal closure in the curly tail (Grhl3) mutant fails later, due to enhanced axial curvature of the body axis 99. This produces a spina bifida confined to the lumbo-sacral region. When secondary neurulation is disturbed, closed defects occur at sacro-coccygeal levels (‘spinal dysraphism’) in which the spinal cord is characteristically ‘tethered’ to adjacent tissues, reflecting faulty tissue separation during tailbud development.
Controversies and unsolved questions on neurulation
Do similar neurulation events occur in humans and mice?
The human neural tube begins to close at 17-18 days post-fertilisation and is discontinuous as in the mouse (Figure 3D). The site of closure initiation occurs at the same level as Closure 1 in mice, and the onset of closure from the extreme rostral end of the neural plate appears comparable to mouse Closure 3 100. Whether a Closure 2-like event exists in human embryos is controversial, but it seems increasingly likely that there is no independent closure initiation event in the midbrain or forebrain 100-102. Unlike mice, therefore, human brain formation is achieved by neurulation progressing directly between Closures 1 and 3, with completion of a single cranial (rostral) neuropore (Figure 3D). Interestingly, Closure 2 is also absent in the SELH/Bc mouse strain 103 and yet more than 80% of embryos successfully complete brain formation, demonstrating that Closure 2 is not obligatory, even in mice.
Do all NTDs result from defective neural tube closure?
A variety of malformations are included under the overall description of NTDs (Table 1) but do they all arise directly from abnormalities of primary or secondary neurulation? Historically, neurulation was depicted in text books as starting half-way along the embryonic body and progressing by ‘zippering’ towards the cranial and caudal ends, with closure of anterior and posterior neuropores respectively (Figure 3B). A major change in this view occurred when the idea of ‘multi-site closure’ was introduced, based on observations of neurulation stage mouse embryos 104. The origin of human NTDs 105 was re-interpreted, based on appearance of late fetuses and the ‘closure events’ that were thought to have been defective (Figure 3C). However, extrapolation from a late stage fetus to its embryonic origins is largely guesswork and subsequent studies showed that Closures 4 and 5 exist in neither mouse nor human neurulation 31,100. We have seen that Closure 2 likely does not exist in humans either. What remains is a relatively ‘simple’ pattern of human neurulation (Figure 3D), most akin to the original model (Figure 3B). While it is possible to explain craniorachischisis, anencephaly, open spina bifida and closed secondary neurulation lesions based on key embryonic events (Figure 4), other ‘NTDs’ including encephalocele, meningocele and iniencephaly are unlikely to arise directly from failure of neural tube formation, but more likely as post-neurulation disorders. This is consistent with the finding of a distinct aetiology for encephalocele as part of Meckel syndrome.
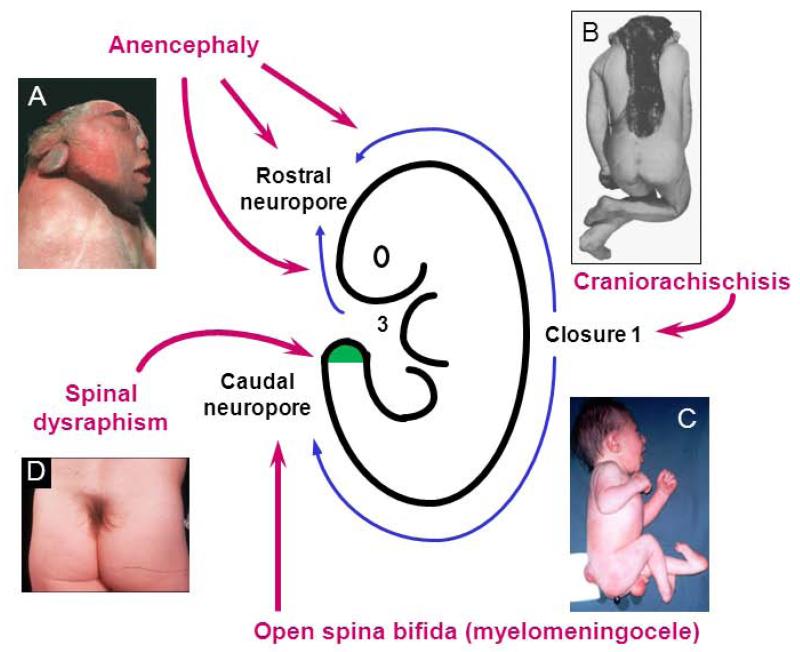
Figure 4. Sites of origin of NTDs in the human embryo directly resulting from disturbance of primary or secondary neurulation.
(A) Anencephaly is the consequence of faulty cranial closure events; (B) Craniorachischisis arises when Closure 1 fails; (C) Open spina bifida results from failure of caudal neuropore closure; (D) Skin-covered spinal ‘dysraphism’ arises through disturbance of the secondary neurulation process. Figure modified from: Copp, 2005. In: R. A. Meyers (Ed.), Encyclopedia of Molecular Cell Biology and Molecular Medicine, 9, 119-138. Wiley-VCH, Weinheim (B,C); Copp, 2008. In: eLS. John Wiley & Sons Ltd, A20913, Chichester. http://www.els.net (D).
Conclusions
NTDs continue to provide a multifaceted challenge to epidemiologists, clinicians, and developmental biologists alike. Although their imminent eradication was predicted when prenatal diagnosis was introduced, and again after the discovery of the preventive effects of folic acid, in fact NTDs remain one of the commonest categories of birth defects worldwide. Their clinical severity and uncertain cause make them priorities for further research, whether to better target primary preventive measures, to improve in-utero surgery for prenatal repair, or to identify the causative genes to provide an objective basis for individual genetic counselling. In this Review, we provide evidence that NTDs are not vitamin-deficiency disorders in the way that rickets results from early vitamin D deficiency. Rather, folate one-carbon metabolism is a key mechanism in the development of NTDs that is affected by, and interacts with, both genetic and environmental factors. The application of new genomic technologies to NTDs should herald the identification of many further risk factors, enabling understanding of the entire range of causative factors that affect the mother and her neurulation stage embryo. Being accurate about exactly how the neural tube is formed during embryogenesis is important, and we have shown how extrapolation backwards from late fetal appearance to presumed early embryonic events is hazardous and, in the case of NTDs, has led to misconceptions about the developmental origin of these disorders.
Acknowledgments
Funding source: This work was supported by the Wellcome Trust (grants 087259 and 087525), the Medical Research Council (grants G0801124, G0802163 and J003794), Sparks (grants 04IMP03, 06ICH06, 08ICH03 and 09ICH01) and Newlife (grant 11/12-06).
Footnotes
Conflicts of interest: We declare that we have no conflicts of interest.
References
- (1).Mitchell LE. Epidemiology of neural tube defects. Am J Med Genet C Semin Med Genet. 2005;135:88–94. doi: 10.1002/ajmg.c.30057. [DOI] [PubMed] [Google Scholar]
- (2).Creasy MR, Alberman ED. Congenital malformations of the central nervous system in spontaneous abortions. J Med Genet. 1976;13:9–16. doi: 10.1136/jmg.13.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Adv Exp Med Biol. 2010;686:349–64. doi: 10.1007/978-90-481-9485-8_20. [DOI] [PubMed] [Google Scholar]
- (4).Obladen M. Cats, frogs, and snakes: early concepts of neural tube defects. J Child Neurol. 2011;26:1452–61. doi: 10.1177/0883073811411191. [DOI] [PubMed] [Google Scholar]
- (5).Record RG, McKeown T. Congenital malformations of the central nervous system. I. A survey of 930 cases. Brit J Soc Med. 1949;3:183–219. [Google Scholar]
- (6).Carter CO. Clues to the aetiology of neural tube malformations. Dev Med Child Neurol. 1974;16(Suppl.32):3–15. doi: 10.1111/j.1469-8749.1974.tb03442.x. [DOI] [PubMed] [Google Scholar]
- (7).Lorber J. Results of treatment of myelomeningocele. An analysis of 524 unselected cases with special reference to possible selection for treatment. Dev Med Child Neurol. 1971;13:279–303. [PubMed] [Google Scholar]
- (8).Brock DJH, Sutcliffe RG. Early prenatal diagnosis of anencephaly. Lancet. 1972;2:1252–3. doi: 10.1016/s0140-6736(72)92306-9. [DOI] [PubMed] [Google Scholar]
- (9).Seller MJ, Campbell S, Coltart TM, et al. Early termination of anencephalic pregnancy after detection by raised alpha-fetoprotein levels. Lancet. 1973;2:73. doi: 10.1016/s0140-6736(73)93264-9. [DOI] [PubMed] [Google Scholar]
- (10).Cameron M, Moran P. Prenatal screening and diagnosis of neural tube defects. Prenatal Diag. 2009;29:402–11. doi: 10.1002/pd.2250. [DOI] [PubMed] [Google Scholar]
- (11).Stiefel D, Shibata T, Meuli M, et al. Tethering of the spinal cord in mouse fetuses and neonates with spina bifida. J Neurosurg. 2003;99:206–13. doi: 10.3171/spi.2003.99.2.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wood LR, Smith MT. Generation of anencephaly: 1. Aberrant neurulation and 2. Conversion of exencephaly to anencephaly. J Neuropath exp Neurol. 1984;43:620–33. [PubMed] [Google Scholar]
- (13).Adzick NS, Sutton LN, Crombleholme TM, et al. Successful fetal surgery for spina bifida. Lancet. 1998;352:1675–6. doi: 10.1016/S0140-6736(98)00070-1. [DOI] [PubMed] [Google Scholar]
- (14).Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Smithells RW, Sheppard S, Schorah CJ. Vitamin deficiencies and neural tube defects. Arch Dis Child. 1976;51:944–50. doi: 10.1136/adc.51.12.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Smithells RW, Sheppard S, Schorah CJ, et al. Apparent prevention of neural tube defects by periconceptional vitamin supplementation. Arch Dis Child. 1981;56:911–8. doi: 10.1136/adc.56.12.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wald NJ, Polani PE. Neural-tube defects and vitamins: the need for a randomized clinical trial. Br J Obstet Gynaecol. 1984;91:516–23. doi: 10.1111/j.1471-0528.1984.tb04796.x. [DOI] [PubMed] [Google Scholar]
- (18).MRC Vitamin Study Research Group Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–7. [PubMed] [Google Scholar]
- (19).Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–5. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- (20).Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China. N Engl J Med. 1999;341:1485–90. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- (21).Abramsky L, Botting B, Chapple J, et al. Has advice on periconceptional folate supplementation reduced neural-tube defects? Lancet. 1999;354:998–9. doi: 10.1016/S0140-6736(99)03248-1. [DOI] [PubMed] [Google Scholar]
- (22).Oakley GP, Jr., Erickson JD, Adams MJ., Jr. Urgent need to increase folic acid consumption. JAMA. 1995;274:1717–8. [PubMed] [Google Scholar]
- (23).Honein MA, Paulozzi LJ, Mathews TJ, et al. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981–6. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- (24).Eichholzer M, Tonz O, Zimmermann R. Folic acid: a public-health challenge. Lancet. 2006;367:1352–61. doi: 10.1016/S0140-6736(06)68582-6. [DOI] [PubMed] [Google Scholar]
- (25).Taylor TN, Farkouh RA, Graham JB, et al. Potential reduction in neural tube defects associated with use of Metafolin-fortified oral contraceptives in the United States. Am J Obstet Gynecol. 2011;205:460–8. doi: 10.1016/j.ajog.2011.06.048. [DOI] [PubMed] [Google Scholar]
- (26).Prinz-Langenohl R, Bramswig S, Tobolski O, et al. [6S]-5-methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild-type 677C-->T polymorphism of methylenetetrahydrofolate reductase. Br J Pharmacol. 2009;158:2014–21. doi: 10.1111/j.1476-5381.2009.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Green TJ, Liu Y, Dadgar S, et al. Wheat Rolls Fortified with Microencapsulated L-5-Methyltetrahydrofolic Acid or Equimolar Folic Acid Increase Blood Folate Concentrations to a Similar Extent in Healthy Men and Women. J Nutr. 2013;143:867–871. doi: 10.3945/jn.113.174268. [DOI] [PubMed] [Google Scholar]
- (28).Wald NJ, Law MR, Morris JK, et al. Quantifying the effect of folic acid. Lancet. 2001;358:2069–73. doi: 10.1016/s0140-6736(01)07104-5. [DOI] [PubMed] [Google Scholar]
- (29).Heseker HB, Mason JB, Selhub J, et al. Not all cases of neural-tube defect can be prevented by increasing the intake of folic acid. Br J Nutr. 2009;102:173–80. doi: 10.1017/S0007114508149200. [DOI] [PubMed] [Google Scholar]
- (30).Mosley BS, Cleves MA, Siega-Riz AM, et al. Neural tube defects and maternal folate intake among pregnancies conceived after folic acid fortification in the United States. Am J Epidemiol. 2009;169:9–17. doi: 10.1093/aje/kwn331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Copp AJ, Greene NDE, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–93. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- (32).Greene NDE, Copp AJ. Inositol prevents folate-resistant neural tube defects in the mouse. Nature Med. 1997;3:60–6. doi: 10.1038/nm0197-60. [DOI] [PubMed] [Google Scholar]
- (33).Cockroft DL. Changes with gestational age in the nutritional requirements of postimplantation rat embryos in culture. Teratology. 1988;38:281–90. doi: 10.1002/tera.1420380312. [DOI] [PubMed] [Google Scholar]
- (34).Vandevijvere S, Amsalkhir S, Van Oven H, et al. Determinants of folate status in pregnant women: results from a national cross-sectional survey in Belgium. Eur J Clin Nutr. 2012;66:1172–7. doi: 10.1038/ejcn.2012.111. [DOI] [PubMed] [Google Scholar]
- (35).Kirke PN, Molloy AM, Daly LE, et al. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med. 1993;86:703–8. [PubMed] [Google Scholar]
- (36).Scott JM. Folate and vitamin B12. Proc Nutr Soc. 1999;58:441–8. doi: 10.1017/s0029665199000580. [DOI] [PubMed] [Google Scholar]
- (37).Heid MK, Bills ND, Hinrichs SH, et al. Folate deficiency alone does not produce neural tube defects in mice. J Nutr. 1992;122:888–94. doi: 10.1093/jn/122.4.888. [DOI] [PubMed] [Google Scholar]
- (38).Burgoon JM, Selhub J, Nadeau M, et al. Investigation of the effects of folate deficiency on embryonic development through the establishment of a folate deficient mouse model. Teratology. 2002;65:219–27. doi: 10.1002/tera.10040. [DOI] [PubMed] [Google Scholar]
- (39).Burren KA, Savery D, Massa V, et al. Gene-environment interactions in the causation of neural tube defects: folate deficiency increases susceptibility conferred by loss of Pax3 function. Hum Mol Genet. 2008;17:3675–85. doi: 10.1093/hmg/ddn262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Beaudin AE, Abarinov EV, Noden DM, et al. Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice. Am J Clin Nutr. 2011;93:789–98. doi: 10.3945/ajcn.110.002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Hook EB, Czeizel AE. Can terathanasia explain the protective effect of folic-acid supplementation on birth defects? Lancet. 1997;350:513–5. doi: 10.1016/S0140-6736(97)01342-1. [DOI] [PubMed] [Google Scholar]
- (42).Fleming A, Copp AJ. Embryonic folate metabolism and mouse neural tube defects. Science. 1998;280:2107–9. doi: 10.1126/science.280.5372.2107. [DOI] [PubMed] [Google Scholar]
- (43).Barbera JP, Rodriguez TA, Greene ND, et al. Folic acid prevents exencephaly in Cited2 deficient mice. Hum Mol Genet. 2002;11:283–93. doi: 10.1093/hmg/11.3.283. [DOI] [PubMed] [Google Scholar]
- (44).Marean A, Graf A, Zhang Y, et al. Folic acid supplementation can adversely affect murine neural tube closure and embryonic survival. Hum Mol Genet. 2011;20:3678–83. doi: 10.1093/hmg/ddr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Harris MJ. Insights into prevention of human neural tube defects by folic acid arising from consideration of mouse mutants. Birth Defects Res A Clin Mol Teratol. 2009;85:331–9. doi: 10.1002/bdra.20552. [DOI] [PubMed] [Google Scholar]
- (46).Smith JL, Schoenwolf GC. Cell cycle and neuroepithelial cell shape during bending of the chick neural plate. Anat Rec. 1987;218:196–206. doi: 10.1002/ar.1092180215. [DOI] [PubMed] [Google Scholar]
- (47).Copp AJ, Brook FA, Roberts HJ. A cell-type-specific abnormality of cell proliferation in mutant (curly tail) mouse embryos developing spinal neural tube defects. Development. 1988;104:285–95. doi: 10.1242/dev.104.2.285. [DOI] [PubMed] [Google Scholar]
- (48).Greene ND, Stanier P, Moore GE. The emerging role of epigenetic mechanisms in the aetiology of neural tube defects. Epigenetics. 2011;6:875–83. doi: 10.4161/epi.6.7.16400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Leck I. Causation of neural tube defects: clues from epidemiology. Br Med Bull. 1974;30:158–63. doi: 10.1093/oxfordjournals.bmb.a071187. [DOI] [PubMed] [Google Scholar]
- (50).Rampersaud E, Melvin EC, Speer MC. Nonsyndromic neural tube defects: genetic basis and genetic investigations. In: Wyszynski DF, editor. Neural Tube Defects: From Origin to Treatment. Oxford University Press; Oxford: 2006. pp. 165–75. [Google Scholar]
- (51).Boyles AL, Hammock P, Speer MC. Candidate gene analysis in human neural tube defects. Am J Med Genet C Semin Med Genet. 2005;135C:9–23. doi: 10.1002/ajmg.c.30048. [DOI] [PubMed] [Google Scholar]
- (52).Greene NDE, Stanier P, Copp AJ. Genetics of human neural tube defects. Hum Mol Genet. 2009;18:R113–R129. doi: 10.1093/hmg/ddp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Etheredge AJ, Finnell RH, Carmichael SL, et al. Maternal and infant gene-folate interactions and the risk of neural tube defects. Am J Med Genet A. 2012;158A:2439–46. doi: 10.1002/ajmg.a.35552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Amorim MR, Lima MA, Castilla EE, et al. Non-Latin European descent could be a requirement for association of NTDs and MTHFR variant 677C > T: a meta-analysis. Am J Med Genet A. 2007;143A:1726–32. doi: 10.1002/ajmg.a.31812. [DOI] [PubMed] [Google Scholar]
- (55).Brody LC, Conley M, Cox C, et al. A polymorphism, R653Q, in the trifunctional enzyme methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase/formyltetrahydrofolate synthetase is a maternal genetic risk factor for neural tube defects: report of the Birth Defects Research Group. Am J Hum Genet. 2002;71:1207–15. doi: 10.1086/344213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Parle-McDermott A, Pangilinan F, O’Brien KK, et al. A common variant in MTHFD1L is associated with neural tube defects and mRNA splicing efficiency. Hum Mutat. 2009;30:1650–6. doi: 10.1002/humu.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Narisawa A, Komatsuzaki S, Kikuchi A, et al. Mutations in genes encoding the glycine cleavage system predispose to neural tube defects in mice and humans. Hum Mol Genet. 2012;21:1496–503. doi: 10.1093/hmg/ddr585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Pike ST, Rajendra R, Artzt K, et al. Mitochondrial C1-tetrahydrofolate synthase (MTHFD1L) supports the flow of mitochondrial one-carbon units into the methyl cycle in embryos. J Biol Chem. 2010;285:4612–20. doi: 10.1074/jbc.M109.079855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Dunlevy LPE, Chitty LS, Doudney K, et al. Abnormal folate metabolism in foetuses affected by neural tube defects. Brain. 2007;130:1043–9. doi: 10.1093/brain/awm028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Momb J, Lewandowski JP, Bryant JD, et al. Deletion of Mthfd1l causes embryonic lethality and neural tube and craniofacial defects in mice. Proc Natl Acad Sci U S A. 2013;110:549–54. doi: 10.1073/pnas.1211199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Chen ZT, Karaplis AC, Ackerman SL, et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–43. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- (62).Beaudin AE, Perry CA, Stabler SP, et al. Maternal Mthfd1 disruption impairs fetal growth but does not cause neural tube defects in mice. Am J Clin Nutr. 2012;95:882–91. doi: 10.3945/ajcn.111.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–25. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- (64).Ybot-Gonzalez P, Savery D, Gerrelli D, et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789–99. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Keller R, Shook D, Skoglund P. The forces that shape embryos: physical aspects of convergent extension by cell intercalation. Phys Biol. 2008;5:015007. doi: 10.1088/1478-3975/5/1/015007. [DOI] [PubMed] [Google Scholar]
- (66).Montcouquiol M, Crenshaw EB, III, Kelley MW. Noncanonical Wnt signaling and neural polarity. Annu Rev Neurosci. 2006;29:363–86. doi: 10.1146/annurev.neuro.29.051605.112933. [DOI] [PubMed] [Google Scholar]
- (67).Greene NDE, Gerrelli D, Van Straaten HWM, et al. Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech Dev. 1998;73:59–72. doi: 10.1016/s0925-4773(98)00029-x. [DOI] [PubMed] [Google Scholar]
- (68).Kibar Z, Torban E, McDearmid JR, et al. Mutations in VANGL1 associated with neural-tube defects. N Engl J Med. 2007;356:1432–7. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- (69).Lei YP, Zhang T, Li H, et al. VANGL2 mutations in human cranial neural-tube defects. N Engl J Med. 2010;362:2232–5. doi: 10.1056/NEJMc0910820. [DOI] [PubMed] [Google Scholar]
- (70).Robinson A, Escuin S, Doudney K, et al. Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum Mutat. 2012;33:440–7. doi: 10.1002/humu.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Reynolds A, McDearmid JR, Lachance S, et al. VANGL1 rare variants associated with neural tube defects affect convergent extension in zebrafish. Mech Dev. 2010;127:385–92. doi: 10.1016/j.mod.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Copp AJ, Greene NDE. Genetics and development of neural tube defects. J Pathol. 2010;220:217–30. doi: 10.1002/path.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Lammer EJ, Sever LE, Oakley GP. Teratogen update: valproic acid. Teratology. 1987;35:465–73. doi: 10.1002/tera.1420350319. [DOI] [PubMed] [Google Scholar]
- (74).Hernández-Díaz S, Werler MM, Walker AM, et al. Neural tube defects in relation to use of folic acid antagonists during pregnancy. Am J Epidemiol. 2001;153:961–8. doi: 10.1093/aje/153.10.961. [DOI] [PubMed] [Google Scholar]
- (75).Menegola E, Di Renzo F, Broccia ML, et al. Inhibition of histone deacetylase activity on specific embryonic tissues as a new mechanism for teratogenicity. Birth Defects Res B Dev Reprod Toxicol. 2005;74:392–8. doi: 10.1002/bdrb.20053. [DOI] [PubMed] [Google Scholar]
- (76).Missmer SA, Suarez L, Felkner M, et al. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ Health Perspect. 2006;114:237–41. doi: 10.1289/ehp.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Gelineau-van Waes J, Rainey MA, Maddox JR, et al. Increased sphingoid base-1-phosphates and failure of neural tube closure after exposure to fumonisin or FTY720. Birth Defects Res A Clin Mol Teratol. 2012;94:790–803. doi: 10.1002/bdra.23074. [DOI] [PubMed] [Google Scholar]
- (78).Soler NG, Walsh CH, Malins JM. Congenital malformations in infants of diabetic mothers. Q J Med. 1976;45:303–13. [PubMed] [Google Scholar]
- (79).Rasmussen SA, Chu SY, Kim SY, et al. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198:611–9. doi: 10.1016/j.ajog.2008.04.021. [DOI] [PubMed] [Google Scholar]
- (80).Moretti ME, Bar-Oz B, Fried S, et al. Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiology. 2005;16:216–9. doi: 10.1097/01.ede.0000152903.55579.15. [DOI] [PubMed] [Google Scholar]
- (81).Finnell RH, Bennett GD, Karras SB, et al. Common hierarchies of susceptibility to the induction of neural tube defects in mouse embryos by valproic acid and its 4-propyl-4-pentenoic acid metabolite. Teratology. 1988;38:313–20. doi: 10.1002/tera.1420380403. [DOI] [PubMed] [Google Scholar]
- (82).Davidson CM, Northrup H, King TM, et al. Genes in glucose metabolism and association with spina bifida. Reprod Sci. 2008;15:51–8. doi: 10.1177/1933719107309590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Lupo PJ, Canfield MA, Chapa C, et al. Diabetes and obesity-related genes and the risk of neural tube defects in the national birth defects prevention study. Am J Epidemiol. 2012;176:1101–9. doi: 10.1093/aje/kws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Kibar Z, Salem S, Bosoi CM, et al. Contribution of VANGL2 mutations to isolated neural tube defects. Clin Genet. 2010;80:76–82. doi: 10.1111/j.1399-0004.2010.01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Bosoi CM, Capra V, Allache R, et al. Identification and characterization of novel rare mutations in the planar cell polarity gene PRICKLE1 in human neural tube defects. Hum Mutat. 2011;32:1371–5. doi: 10.1002/humu.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Doudney K, Ybot-Gonzalez P, Paternotte C, et al. Analysis of the planar cell polarity gene Vangl2 and its co-expressed paralogue Vangl1 in neural tube defect patients. Am J Med Genet A. 2005;136:90–2. doi: 10.1002/ajmg.a.30766. [DOI] [PubMed] [Google Scholar]
- (87).Harris MJ, Juriloff DM. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res A Clin Mol Teratol. 2010;88:653–69. doi: 10.1002/bdra.20676. [DOI] [PubMed] [Google Scholar]
- (88).Sadler TW. Culture of early somite mouse embryos during organogenesis. J Embryol Exp Morphol. 1979;49:17–25. [PubMed] [Google Scholar]
- (89).Massarwa R, Niswander L. In toto live imaging of mouse morphogenesis and new insights into neural tube closure. Development. 2013;140:226–36. doi: 10.1242/dev.085001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Garcia-Lopez R, Pombero A, Martinez S. Fate map of the chick embryo neural tube. Dev Growth Differ. 2009;51:145–65. doi: 10.1111/j.1440-169X.2009.01096.x. [DOI] [PubMed] [Google Scholar]
- (91).Suzuki M, Morita H, Ueno N. Molecular mechanisms of cell shape changes that contribute to vertebrate neural tube closure. Dev Growth Differ. 2012;54:266–76. doi: 10.1111/j.1440-169X.2012.01346.x. [DOI] [PubMed] [Google Scholar]
- (92).Tawk M, Araya C, Lyons DA, et al. A mirror-symmetric cell division that orchestrates neuroepithelial morphogenesis. Nature. 2007;446:797–800. doi: 10.1038/nature05722. [DOI] [PubMed] [Google Scholar]
- (93).Copp AJ. Neurulation in the cranial region - normal and abnormal. J Anat. 2005;207:623–35. doi: 10.1111/j.1469-7580.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Schoenwolf GC. Histological and ultrastructural studies of secondary neurulation of mouse embryos. Am J Anat. 1984;169:361–74. doi: 10.1002/aja.1001690402. [DOI] [PubMed] [Google Scholar]
- (95).Lemire RJ. Variations in development of the caudal neural tube in human embryos (Horizons XIV-XXI) Teratology. 1969;2:361–70. doi: 10.1002/tera.1420020410. [DOI] [PubMed] [Google Scholar]
- (96).Cambray N, Wilson V. Axial progenitors with extensive potency are localised to the mouse chordoneural hinge. Development. 2002;129:4855–66. doi: 10.1242/dev.129.20.4855. [DOI] [PubMed] [Google Scholar]
- (97).Copp AJ, Checiu I, Henson JN. Developmental basis of severe neural tube defects in the loop-tail (Lp) mutant mouse: Use of microsatellite DNA markers to identify embryonic genotype. Dev Biol. 1994;165:20–9. doi: 10.1006/dbio.1994.1230. [DOI] [PubMed] [Google Scholar]
- (98).Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, et al. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of BMP signalling. Development. 2007;134:3203–11. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- (99).Van Straaten HWM, Copp AJ. Curly tail: a 50-year history of the mouse spina bifida model. Anat Embryol. 2001;203:225–37. doi: 10.1007/s004290100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).O’Rahilly R, Müller F. The two sites of fusion of the neural folds and the two neuropores in the human embryo. Teratology. 2002;65:162–70. doi: 10.1002/tera.10007. [DOI] [PubMed] [Google Scholar]
- (101).Sulik KK, Zuker RM, Dehart DB, et al. Normal patterns of neural tube closure differ in the human and the mouse. Proceedings of the Greenwood Genetic Center. 1998;18:129–30. [Google Scholar]
- (102).Nakatsu T, Uwabe C, Shiota K. Neural tube closure in humans initiates at multiple sites: evidence from human embryos and implications for the pathogenesis of neural tube defects. Anat Embryol. 2000;201:455–66. doi: 10.1007/s004290050332. [DOI] [PubMed] [Google Scholar]
- (103).MacDonald KB, Juriloff DM, Harris MJ. Developmental study of neural tube closure in a mouse stock with a high incidence of exencephaly. Teratology. 1989;39:195–213. doi: 10.1002/tera.1420390211. [DOI] [PubMed] [Google Scholar]
- (104).Golden JA, Chernoff GF. Intermittent pattern of neural tube closure in two strains of mice. Teratology. 1993;47:73–80. doi: 10.1002/tera.1420470112. [DOI] [PubMed] [Google Scholar]
- (105).Van Allen MI, Kalousek DK, Chernoff GF, et al. Evidence for multi-site closure of the neural tube in humans. Am J Med Genet. 1993;47:723–43. doi: 10.1002/ajmg.1320470528. [DOI] [PubMed] [Google Scholar]
- (106).Moore CA, Li S, Li Z, et al. Elevated rates of severe neural tube defects in a high-prevalence area in northern China. Am J Med Genet. 1997;73:113–8. [PubMed] [Google Scholar]
- (107).McNeely PD, Howes WJ. Ineffectiveness of dietary folic acid supplementation on the incidence of lipomyelomeningocele: pathogenetic implications. J Neurosurg. 2004;100:98–100. doi: 10.3171/ped.2004.100.2.0098. [DOI] [PubMed] [Google Scholar]
- (108).Dolk H, De Wals P, Gillerot Y, et al. Heterogeneity of neural tube defects in Europe: The significance of site of defect and presence of other major anomalies in relation to geographic differences in prevalence. Teratology. 1991;44:547–59. doi: 10.1002/tera.1420440508. [DOI] [PubMed] [Google Scholar]
- (109).Johnson KMK, Suarez L, Felkner MM, et al. Prevalence of craniorachischisis in a Texas-Mexico border population. Birth Defects Res Part A Clin Mol Teratol. 2004;70:92–4. doi: 10.1002/bdra.10143. [DOI] [PubMed] [Google Scholar]
- (110).Curtin JA, Quint E, Tsipouri V, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- (111).Etheridge SL, Ray S, Li S, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).De Marco P, Merello E, Consales A, et al. Genetic Analysis of Disheveled 2 and Disheveled 3 in Human Neural Tube Defects. J Mol Neurosci. 2013;49:582–588. doi: 10.1007/s12031-012-9871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Seo JH, Zilber Y, Babayeva S, et al. Mutations in the planar cell polarity gene, Fuzzy, are associated with neural tube defects in humans. Hum Mol Genet. 2011;20:4324–33. doi: 10.1093/hmg/ddr359. [DOI] [PubMed] [Google Scholar]
- (114).Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).De Marco P, Merello E, Rossi A, et al. FZD6 is a novel gene for human neural tube defects. Hum Mutat. 2012;33:384–90. doi: 10.1002/humu.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Lu X, Borchers AG, Jolicoeur C, et al. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–8. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- (117).Murdoch JN, Henderson DJ, Doudney K, et al. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- (118).Wansleeben C, Feitsma H, Montcouquiol M, et al. Planar cell polarity defects and defective Vangl2 trafficking in mutants for the COPII gene Sec24b. Development. 2010;137:1067–73. doi: 10.1242/dev.041434. [DOI] [PubMed] [Google Scholar]
- (119).Torban E, Patenaude AM, Leclerc S, et al. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc Natl Acad Sci U S A. 2008;105:3449–54. doi: 10.1073/pnas.0712126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Kibar Z, Bosoi CM, Kooistra M, et al. Novel mutations in VANGL1 in neural tube defects. Hum Mutat. 2009;30:E706–E715. doi: 10.1002/humu.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Murdoch JN, Doudney K, Paternotte C, et al. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- (122).Kibar Z, Vogan KJ, Groulx N, et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nature Genet. 2001;28:251–5. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]