Abstract
Amniotes, regardless of genetic sex, develop two sets of genital ducts: the Wolffian and Müllerian ducts. For normal sexual development to occur, one duct must differentiate into its corresponding organs, and the other must regress. In mammals, the Wolffian duct differentiates into the male reproductive tract, mainly the vasa deferentia, epididymides, and seminal vesicles, whereas the Müllerian duct develops into the four components of the female reproductive tract, the oviducts, uterus, cervix, and upper third of the vagina. In males, the fetal Leydig cells produce testosterone, which stimulates the differentiation of the Wolffian duct, whereas the Sertoli cells of the fetal testes express anti-Müllerian hormone, which activates the regression of the Müllerian duct. Anti-Müllerian hormone is a member of the transforming growth factor-beta (TGF-beta) family of secreted signaling molecules and has been shown to signal through the BMP pathway. It binds to its type II receptor, anti-Müllerian hormone receptor 2 (AMHR2), in the Müllerian duct mesenchyme and through an unknown mechanism(s); the mesenchyme induces the regression of the Müllerian duct mesoepithelium. Using tissue-specific gene inactivation with an Amhr2-Cre allele, we have determined that two TGF-beta type I receptors (Acvr1 and Bmpr1a) and all three BMP receptor-Smads (Smad1, Smad5, and Smad8) function redundantly in transducing the anti-Müllerian hormone signal required for Müllerian duct regression. Loss of these genes in the Müllerian duct mesenchyme results in male infertility due to retention of Müllerian duct derivatives in an otherwise virilized male.
Keywords: AMH, AMHR2, female reproductive tract, male reproductive tract, Müllerian ducts, signal transduction, Wolffian duct
INTRODUCTION
The development and differentiation of the reproductive systems are crucial for the transmittal of genes to subsequent generations. In mice, the female reproductive tract consists of the oviducts, uterus, cervix and vagina, whereas the male reproductive tract includes the epididymides, vasa deferentia and seminal vesicles. Regardless of their genetic sex, amniotes develop two sets of embryonic genital ducts, the Wolffian and Müllerian ducts. The former gives rise to the male reproductive tract, whereas the latter gives rise to the female reproductive tract. The Wolffian duct forms from the intermediate mesoderm and, in the mouse, is a complete epithelial tube by Embryonic Day 10.5 (E10.5) [1, 2]. Formation of the Müllerian duct occurs in three separate and distinct phases. The first phase consists of a specification of the rostral most coelomic epithelium to a Müllerian duct fate [3, 4]. These cells under the control of Wnt4 will invaginate caudally into the mesonephros toward the Wolffian duct [5–7]. Upon contact with the Wolffian duct, the Müllerian duct will elongate caudally along the length of the Wolffian duct, where it will contact the urogenital sinus. The forming Müllerian duct has been identified as a simple mesoepithelial tube with a surrounding mesenchyme [4]. Recently, the primary mechanism for Müllerian duct elongation has been identified. A small group of cells at the caudal most tip of the Müllerian duct under the control of Wnt9b lay the foundation of the Müllerian duct through cell proliferation [4, 8, 9].
For correct sexual development to occur, one duct must differentiate into its corresponding organs, whereas the other must regress. In males, the Leydig cells of the fetal testes produce testosterone, which leads to the maintenance and differentiation of the Wolffian duct [10]. The Sertoli cells of the fetal testes express anti-Müllerian hormone (AMH), which induces the regression of the Müllerian duct [11, 12]. Female embryos produce neither testosterone nor AMH, which allows for the passive regression of the Wolffian duct and differentiation of the Müllerian duct, respectively. Anti-Müllerian hormone is a member of the transforming growth factor-β (TGF-β) superfamily of secreted signaling ligands and is responsible for the regression of the Müllerian duct [12, 13]. Anti-Müllerian hormone secreted by the Sertoli cells of the testes binds to its type II receptor, AMH receptor 2 (AMHR2), in the Müllerian duct mesenchyme and regulates downstream targets. Expression of Wnt7a by the mesoepithelium and along with Sf1 and Wt1 expression in the coelomic epithelium activates expression of Amhr2 in the coelomic epithelium [14–16]. Zhan et al. have shown that early expression of Amh leads to a sexually dimorphic pattern of Amhr2-expressing cells in the rat [17]. In vitro evidence suggests that AMH activates an epithelial to mesenchymal transition of the Amhr2-expressing coelomic epithelial cells. These cells migrate from the coelomic epithelium to completely surround the mesoepithelium [17]. Continuous expression of Amh directs the active regression of the mesoepithelium via apoptosis [18, 19], and it has been hypothesized to also induce a cell fate change of some of the Müllerian duct mesoepithelial cells [20]. Similarly to Müllerian duct development, regression broadly occurs in a rostral to caudal manner and is dependent upon the expression of both Amh and Amhr2 [13, 21].
In humans, mutations in either AMH or AMHR2 lead to persistent Müllerian duct syndrome (PMDS), a condition characterized by the presence of Müllerian duct derivatives in an otherwise virilized male. Persistent Müllerian duct syndrome is an autosomal recessive disorder, and genetic studies of 76 families with members having PMDS found that 45% had mutations in the AMH gene, and 39% in AMHR2. In the remaining families, no mutations were found in either gene, and it was concluded that the syndrome was caused by mutations in other genes responsible for transducing the AMH signal [22]. Knockout studies of Amh and Amhr2 in the mouse mimic the human phenotype, a male mouse with Müllerian duct derivatives [13, 21, 23]. TGF-β family molecules transduce their signal to the nucleus by first binding their corresponding type II receptor. The type II receptor will then form heteromeric complexes with a Type I receptor(s) and phosphorylate that receptor(s) [24]. Type I receptors phosphorylate receptor-Smads (r-Smads), and these phosphorylated r-Smads can then bind to the common Smad (Smad4). This complex will translocate to the nucleus and regulate transcription of downstream target genes; however, Smad4 is not required for all TGF-β family-induced signaling [24, 25]. Using XVent2-luciferase and 3TP-luciferase reporter genes, which can discriminate between BMP-like and TGF-β/activin-like signaling, respectively, AMH was shown to stimulate BMP-responsive constructs, but not those of TGF-β [26].
Two possible type I receptors have been identified by both their expression patterns and through biochemical studies. In an in vitro signaling system, Acvr1 (Alk2) was shown to produce the strongest AMH-induced signal, and it is also expressed in the coelomic epithelium and Müllerian duct mesenchyme [17, 27, 28]. Dominant-negative forms of ACVR1 blocked the AMH-induced response [27], and in vitro culture of the urogenital system with antisense Acvr1 nucleotides showed that loss of Acvr1 blocked Müllerian duct regression [28]. In vitro analysis of Bmpr1a (Alk3) initially suggested it had no role in mediating the AMH signal. It is expressed in the Müllerian duct mesenchyme, but dominant-negative forms do not block the AMH-stimulated BMP-responsive constructs [26, 27]. However, in vivo conditional inactivation of Bmpr1a using an Amhr2-Cre allele resulted in retention of the Müllerian duct in ~50% of the mutant males generated [29]. Overexpression of a human AMH transgene in Bmpr1a conditional mutant males resulted in regression of the Müllerian ducts in all males produced [30], and in a mouse immature Sertoli cell line, Bmpr1a mediates the AMH signal by phosphorylation of SMAD1. In the same cell line, Acvr1 in the absence of Bmpr1a can transduce the AMH signal and lead to phosphorylation of SMAD1 [31]. These in vitro and in vivo studies suggest that both Acvr1 and Bmpr1a can function in transducing the AMH signal.
The heteromeric complex of the type II and type I receptors results in the phosphorylation of r-Smads. Three Smads have been identified as possible r-Smads responsible for activating Müllerian duct regression, Smad1, Smad5, and Smad8. Both Smad1 and Smad8 show strong expression in the Müllerian duct mesenchyme, and expression of Smad8 is much more robust in the mesenchyme of the male compared with that of the female [27, 32]. Smad5 is also expressed in the Müllerian duct mesenchyme; however, expression is very low, and no sexually dimorphic differences are seen [27]. Anti-Müllerian hormone induces the phosphorylation of SMAD1 in vitro, but phosphorylation of SMAD5 and SMAD8 has not been determined [26, 31]. Knockout studies show embryonic lethality for Smad1 and Smad5, whereas Smad8 mutants are viable and fertile [33, 34]. To date, no in vivo studies have been performed to determine the role of these Smads in mediating Müllerian duct regression.
In this study, we investigated the genetics of Müllerian duct regression. We investigated the in vivo role of various TGF-β family type I receptors and r-Smads by conditional inactivation using the Cre/loxP system. We used a Cre allele specifically expressed in the Müllerian duct mesenchyme and generated double-conditional knockout males of the Acvr1 and Bmpr1a genes. We demonstrate that in the mouse, Acvr1 and Bmpr1a function redundantly as type I receptors in mediating Müllerian duct regression. Furthermore, we generated triple-conditional knockout males of the Smad1, Smad5, and Smad8 genes. We reveal that these three r-Smads function redundantly in activating AMH-induced Müllerian duct regression.
MATERIALS AND METHODS
Mice
Amhr2-Cre [29], Acvr1+/− [35], Bmpr1a+/− [36], and Acvr1fx/fx [37]; Bmpr1afx/fx [38] mice were maintained on a C57BL/6; 129/SvEv mixed genetic background. Smad1fx/fx [39]; Smad5fx/fx [40]; Smad8−/− (J.F. Martin, unpublished observations) mice were maintained on a C57BL/6; 129/SvEv; FVB mixed genetic background. Amhr2-Cre; Acvr1+/−; Bmpr1a+/− males were bred to Acvr1fx/fx; Bmpr1afx/fx females to generate males that were conditionally null for Acvr1 alone, Bmpr1a alone, or both Acvr1 and Bmpr1a. Similarly, Amhr2-Cre; Smad1fx/+; Smad5fx/+; Smad8+/− males were bred to Smad1fx/fx; Smad5fx/fx; Smad8−/− females. This breeding scheme allowed for the generation of single-conditional mutant males null for Smad1 and Smad5, and double-conditional mutants for Smad1/Smad5, Smad1/Smad8, and Smad5/Smad8, and 1 of every 16 males was a triple-conditional mutant. All phenotypes were analyzed in adult mice between the ages of 5 and 8 wk. All experimental procedures using mice were approved by the University of Texas M.D. Anderson Cancer Center Animal Care and Use Committee.
Tissue Preparation and Immunohistochemistry
Tissue was fixed in 4% paraformaldehyde in PBS at 4°C overnight, dehydrated through a series of ethanols, and embedded in paraffin. The paraffin-embedded tissue was sectioned at 5 μm and processed for hematoxylin and eosin staining or immunohistochemistry. Immunohistochemistry was performed as previously described [4]. Goat anti-AMH (sc-6886; Santa Cruz Biotechnology) was used at a dilution of 1:200 and incubated at 4°C overnight. A biotinylated anti-goat immunoglobulin G was used at a dilution of 1:250 and incubated for 10 min at room temperature. Enzymatic detection was performed with AEC solution and counterstained with Harris hematoxylin.
RESULTS
Acvr1 and Bmpr1a Function Redundantly for Müllerian Duct Regression
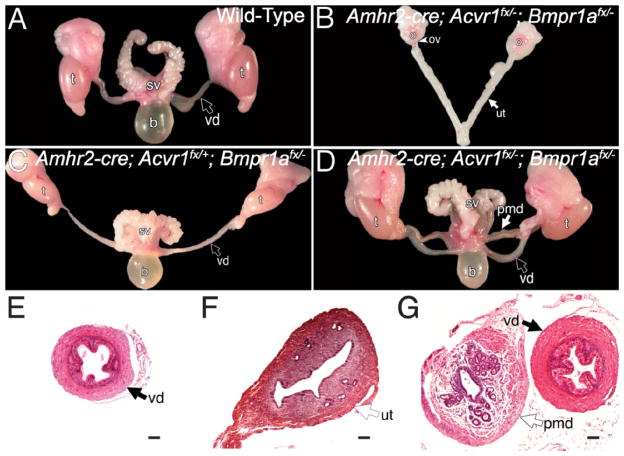
Previous data suggest that both Acvr1 and Bmpr1a transduce the AMH signal. Therefore, we tested the hypothesis that Acvr1 and Bmpr1a function redundantly as AMH type I receptors for Müllerian duct regression. Utilization of an Amhr2-Cre allele allowed us to generate males with genes conditionally inactivated for Acvr1 or Bmpr1a as well as Acvr1/Bmpr1a double-conditional mutants in the Müllerian duct mesenchyme. Males with Acvr1 conditionally inactivated properly regressed the Müllerian duct in 100% of the males generated (n = 21; Table 1). Conditional inactivation of Bmpr1a using the Amhr2-Cre allele blocked Müllerian duct regression in ~50% of the males generated [29]. Our current data corroborate these results, and when Bmpr1a is conditionally inactivated, ~55% of the males generated retained the Müllerian duct, whereas the other 45% properly regressed the Müllerian duct (Fig. 1C and Table 1). Of the 11 males generated, 5 correctly regressed the Müllerian duct, whereas it persisted in the other 6 males. Similar to the Amh knockouts, the males that retained the Müllerian duct were infertile due to the presence of the female reproductive tract (data not shown). These results suggest that Bmpr1a is the primary BMP type I receptor used by the AMH pathway and is required for Müllerian duct regression, but Acvr1 in the absence of Bmpr1a is capable of transducing the AMH signal. We therefore analyzed the phenotypes of males with both Acvr1 and Bmpr1a conditionally inactivated in the Müllerian duct mesenchyme. When both Acvr1 and Bmpr1a were conditionally inactivated, 100% of the males generated completely retained the Müllerian duct derivatives, the oviducts, and uterus (n = 10; Fig. 1D and Table 1). Using the Fisher exact test to compare the loss of Bmpr1a alone to the loss of both Acvr1 and Bmpr1a, the difference is statistically significant (P = 0.01).
TABLE 1.
Müllerian duct (MD) regression in Acvr1/Bmpr1a conditionally mutant males.
| Conditional allelea | No. generated | Regressed MD | Retained MD |
|---|---|---|---|
| Acvr1 CKO | 21 | 21 | 0 |
| Bmpr1a CKO | 11 | 5 | 6 |
| Acvr1/Bmpr1a CKO | 10 | 0 | 10 |
CKO, conditional knockout.
FIG. 1.
Phenotypic analysis of adult Acvr1/Bmpr1a conditionally mutant reproductive tracts in whole mount (A–D) and histological sections (E–G). The reproductive system of an adult wild-type male consists of the testes, vas deferens, and seminal vesicles (A). The reproductive system from an Acvr1/Bmpr1a double-conditional mutant female consists of the ovaries, oviducts, and uterine horns (B). C) Correctly differentiated reproductive system from a Bmpr1a conditionally mutant male. The Müllerian duct persisted in males conditionally mutant for both Acvr1 and Bmpr1a (D). Section of the vas deferens from a wild-type adult male (E) and uterus of a wild-type female (F). Section of the vas deferens and persisted Müllerian duct in an Acvr1/Bmpr1a double-conditional mutant male (G). b, bladder; o, ovary; ov, oviduct; pmd; persisted Müllerian duct; sv, seminal vesicles; t, testes; ut, uterus; vd, vas deferens. Bar = 100 μm.
Females with both Acvr1 and Bmpr1a conditionally inactivated correctly developed the reproductive tract and were fertile (n = 3; Fig. 1B and data not shown). Based on the fact that Acvr1/Bmpr1a double-conditional mutant females are fertile, these data suggest that the differentiation of the Müllerian duct into the female reproductive tract does not require Acvr1 and Bmpr1a. Therefore, Acvr1 and Bmpr1a function redundantly in the Müllerian duct mesenchyme to mediate regression of the Müllerian duct mesoepithelium in males.
We also performed histology and immunohistochemistry on wild-type and mutant animals. The vas deferens of Acvr1/Bmpr1a double mutants appears phenotypically normal when compared to wild-type males (Fig. 1, E and G). However, the persisted Müllerian duct derivatives of the Acvr1/Bmpr1a conditionally mutant males do not appear phenotypically normal compared with the uterus of a wild-type female. There is a defined luminal epithelial layer with glands, but there are fewer surrounding stroma compared with that of the female (Fig. 1, F and G). The phenotype of these males (persistence of Müllerian duct derivatives) is similar to the phenotype found in Amh knockout males, and it is possible that the results are due to the loss of Amh expression in the animals generated. We therefore analyzed Amh expression in Acvr1/Bmpr1a double-conditional mutants by immunohistochemistry for AMH. Strong expression of Amh can be seen in the Sertoli cells of males at E13.5 [41]. We therefore chose this embryonic stage for analysis of AMH in conditionally mutant males. At E13.5, males conditionally null for both Acvr1/Bmpr1a expressed AMH comparable to wild-type embryos (Fig. 2, A and B). Therefore, the phenotype seen in males with conditional loss of both Acvr1 and Bmpr1a is not due to a lack of AMH expression.
FIG. 2.
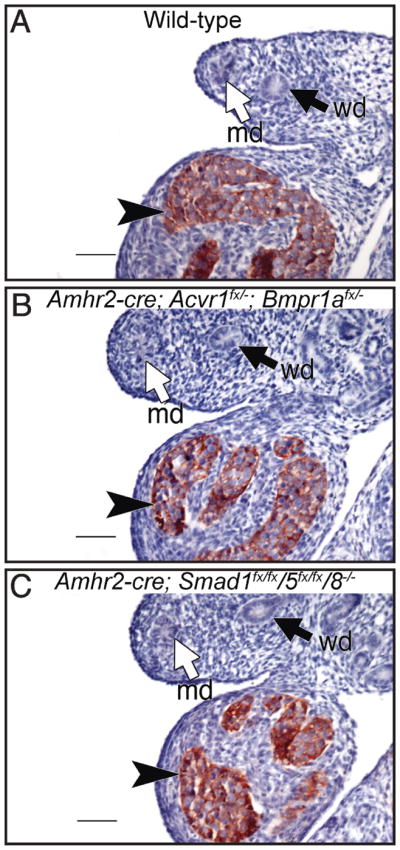
Anti-Müllerian hormone immunostaining in male embryos at E13.5. Wild-type male (A), Acvr1/Bmpr1a double-conditional male (B), and Smad1/Smad5/Smad8 triple-conditional mutant male (C). Anti-Müllerian hormone immunostaining is comparable in all three samples, indicating the phenotype seen is not due to loss of Amh expression. md, Müllerian duct; wd, Wolffian duct. Black arrowheads indicate AMH-producing Sertoli cells. Bar = 100 μm.
BMP r-Smads Function Redundantly in Mediating Regression of the Müllerian Duct
The activation of the TGF-β family type I receptors (Acvr1 and Bmpr1a) will lead to the phosphorylation of r-Smads. Smad1, Smad5, and Smad8 are the r-Smads used by the BMP signaling pathway, and all three are expressed in the Müllerian duct mesenchyme [27, 28, 32]. SMAD1 can be phosphorylated by either ACVR1 or BMPR1A [26, 31], but no studies have been performed with SMAD5 or SMAD8. We again used the Amhr2-Cre allele to conditionally inactivate these genes in the Müllerian duct mesenchyme. Our genetic strategy allowed for the generation of all combinations of conditional alleles, from single knockouts to double- and triple-conditional knockouts. Mice conditionally mutant for one gene were heterozygous for the other two genes, and double-conditional mutants were heterozygous for the third gene.
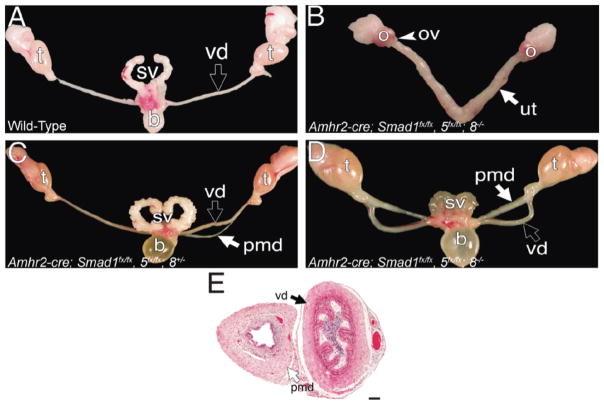
Loss of Smad1 or Smad1/Smad8 had no affect on Müllerian duct regression. In the males generated (n =5) for Smad1, all of the males generated properly regressed the Müllerian duct (Table 2). Also, in Smad1/Smad8 double-conditional mutants, the Müllerian duct regressed (n = 6; Table 2). Loss of Smad5 in any combination, Smad5 (n = 8), Smad1/Smad5 (n = 4), and Smad5/Smad8 (n = 6) resulted in a partial Müllerian duct retention phenotype (Fig. 3C and Table 2). In Figure 3C, a case is shown with only one Müllerian duct that was partially retained. We defined a partial Müllerian duct retention as the incomplete retention of Müllerian duct derivatives. In this case, the Müllerian duct on one side of the animal properly regressed, whereas the derivatives of the duct on the other side were partially retained. The partially retained duct was retained either rostrally or caudally, but the whole duct on that side was never completely retained. Furthermore, there was no sidedness to the partial retention phenotype. Complete Müllerian duct retention occurred only when all three genes Smad1/Smad5/Smad8 were conditionally inactivated (n = 4; Fig. 3D and Table 2). The reproductive tracts of females with Smad1, Smad5, and Smad8 all conditionally inactivated developed properly, and these females were also fertile and could carry a litter through parturition (n = 3; Fig. 3B and data not shown). However, after 3–4 mo of age, females conditionally null for Smad1/Smad5 and Smad1/Smad5/Smad8 became infertile and developed granulosa cell tumors [42].
TABLE 2.
Müllerian duct (MD) regression in Smad1/Smad5/Smad8 conditionally mutant males.
| Conditional allelea | No. generated | Regressed MD | Complete retention | Partial retention |
|---|---|---|---|---|
| Smad1 CKO | 5 | 5 | 0 | 0 |
| Smad5 CKO | 8 | 0 | 0 | 8 |
| Smad1/5 CKO | 4 | 0 | 0 | 4 |
| Smad1/8 CKO | 6 | 6 | 0 | 0 |
| Smad5/8 CKO | 6 | 5 | 0 | 1 |
| Smad1/5/8 CKO | 4 | 0 | 4 | 0 |
CKO, conditional knockout.
FIG. 3.
Analysis of adult Smad1, Smad5, and Smad8 conditionally mutant reproductive systems in whole mount (A–D) and histological section (E). The reproductive system of an adult wild-type male consists of the testes, vas deferens, and seminal vesicles (A). The reproductive system from a Smad1/Smad5/Smad8 triple-conditional mutant female consists of the ovaries, oviducts, and uterine horns (B). Partially retained Müllerian duct derivatives in the reproductive system of a Smad1/Smad5 double-conditionally mutant male (C). The Müllerian duct persisted in males conditionally mutant for Smad1, Smad5, and Smad8 (D). Section of the vas deferens and persisted Müllerian duct in a Smad1/Smad5/Smad8 triple-conditional mutant male (E). b, bladder; o, ovary; ov, oviduct; pmd; persisted Müllerian duct; sv, seminal vesicles; t, testes; ut, uterus; vd, vas deferens. Bar = 100 μm.
We also performed histology on the reproductive tracts of adult males conditionally null for Smad1, Smad5, and Smad8. The persisted Müllerian duct derivatives of these males were also not phenotypically normal compared with the uterus of a wild-type female (Figs. 1F and 3E). Furthermore, the persisted Müllerian duct of these males was different compared with that of the Acvr1/Bmpr1a double-conditional males (Figs. 1G and 3E). In the Smad1, Smad5, and Smad8 triple-conditional mutant males, the persisted Müllerian duct has a defined luminal epithelium and stroma, but lacks glands (Fig. 3E). These data, along with the Acvr1 and Bmpr1a conditional males, suggest that the differentiation of the Müllerian duct into the adult female reproductive tract is not a “default” mechanism, but rather requires another female specific signal.
We performed immunohistochemistry on male embryos conditionally null for Smad1, Smad5, and Smad8 at E13.5. In these embryos, AMH production by the Sertoli cells of the fetal testes was comparable to that in wild-type males (Fig. 2, A and C). Thus, the BMP r-Smads, Smad1, Smad5, and Smad8, function redundantly in transducing the AMH signal and mediating regression of the Müllerian duct in males.
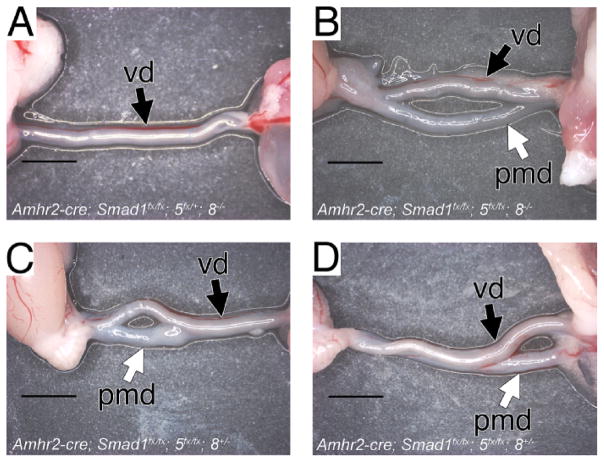
We were intrigued by the expression patterns of the BMP r-Smads as shown by Clarke et al. [27] and Visser [32]. Smad1 is expressed in the Müllerian duct mesenchyme and coelomic epithelium, whereas Smad8 expression occurs in a sexually dimorphic pattern and is much more robust in the male [27]. Expression of Smad5 is weaker than that of Smad1 and Smad8 and does not show a sexually dimorphic pattern of expression [32]. We therefore investigated the nature and genetics of the partial Müllerian duct retention phenotype. We defined this phenotype as the partial retention of one Müllerian duct and the complete regression of the other, whereas the complete duct persists only in Smad1, Smad5, and Smad8 triple-conditional mutant males (Fig. 3, C and D, and Fig. 4, B–D). We find that the Müllerian duct is partially retained in Smad5, Smad1/Smad5, and Smad5/Smad8 conditionally mutant males (Table 2). Smad5 and Smad1/Smad5 conditionally mutant males, which contain one allele of Smad1/Smad8 and one allele of Smad8, respectively, all partially retained the Müllerian duct. However, Smad5/Smad8 conditionally mutant males who contained one functional allele of Smad1 properly regressed the Müllerian duct in all but one male (Table 2). Although an abnormal phenotype was observed in these animals, it was not one that affected fecundity, as the males were fertile (data not shown). These results suggest that Smad5 is the r-Smad preferentially used in mediating AMH-induced Müllerian duct regression; however, in the absence of Smad5, Smad1 is capable of transducing the AMH signal and, to a lesser extent, Smad8.
FIG. 4.
Analysis of the persisted Müllerian duct in Smad1/Smad5/Smad8 conditionally mutant males at 5 wk of age. Wild-type male containing only the vas deferens (A). The Müllerian duct was completely retained in males conditionally mutant for Smad1, Smad5, and Smad8 (B). The Müllerian duct was only partially retained in males conditionally mutant for Smad1/Smad5 (C, D). The partial retention could occur rostrally (C) or caudally (D). pmd, persisted Müllerian duct; vd, vas deferens. The testes are located to the left of the photo. Bar = 1000 μm.
DISCUSSION
AMH and Müllerian Duct Regression
Anti-Müllerian hormone is most well known from the experiments performed by Alfred Jost, who identified the testes as the tissue responsible for producing a “hormone” that induced the regression of the Müllerian duct mesoepithelium [12]. It has also been referred to as Müllerian inhibiting substance, Müllerian inhibiting factor, and X-factor. However, the activity of AMH in inducing the regression of the Müllerian duct is one that has evolved in higher vertebrates. Anti-Müllerian hormone and its type II receptor are found in lower vertebrates, such as zebrafish and Medaka, which do not form the Müllerian duct [43–45]. Anti-Müllerian hormone signaling suppresses germ cell proliferation in medaka fish, and loss of this signaling results in gonadal malformation and sex reversal in XY males [44]. In the mouse, AMH signaling has an inhibitory effect on the recruitment of primordial follicles, reviewed by Visser et al. [46]. This evidence suggests the original function of AMH signaling evolved in the gonad.
Amniotes of both sexes form the Müllerian duct, and AMH signaling induces its regression in males. The precise mechanism(s) involved in the regression of the Müllerian duct mesoepithelium has not been completely defined. Lineage tracing experiments, using the lipophilic dye, Dil, have shown that in the alligator, the Müllerian duct mesoepithelium under the control of AMH signaling to the mesenchyme will migrate to the mesonephros and become nephric tubule epithelium [20]. In higher amniotes, such as the mouse, apoptosis has been shown to contribute to the active regression of the Müllerian duct [18, 47]. In mice, cells of the seminal vesicles have been known to take on the fate of uterine endometrial cells and express uterine proteins when exposed to pharmacological conditions [48]. Furthermore, in the rat, analysis of the structure of the Müllerian duct mesoepithelial cells and the breakdown of its extracellular matrix suggests that some cells may undertake a cell fate change [49–51]. These data suggest that in the mouse, rat, and human, regression of the Müllerian duct can occur both by apoptosis and cell fate change, but the exact fate(s) of the regressing male Müllerian duct mesoepithelium has not yet been determined.
Acvr1 and Bmpr1a Function Redundantly in Mediating the AMH Signal
In vivo and in vitro evidence has been conflicting in determining which TGF-β type I receptors are required for transducing the AMH signal in the Müllerian duct mesenchyme for regression. In vitro evidence suggests that Acvr1 is required, whereas in vivo conditional inactivation of Bmpr1a results in the retention of the Müllerian duct in males [17, 27–30]. We show that Acvr1 and Bmpr1a can both activate the regression of the Müllerian duct, and both genes must be lost to see a fully penetrant phenotype. We hypothesize that in the mouse, Acvr1 is used to activate the epithelial to mesenchymal transition of the Amhr2-expressing coelomic epithelial cells. Cells of the coelomic epithelium express Amhr2, and under the control of AMH they migrate from the coelomic epithelium to surround the Müllerian duct mesoepithelium [16, 17]. Furthermore, in vitro loss of Acvr1 in the coelomic epithelium leads to retention of the Müllerian duct [17, 27, 28]. These Müllerian duct mesenchymal cells migrate away from the coelomic epithelium and induce the regression of the mesoepithelium, as seen by the “swirling” of the mesenchymal cells around the mesoepithelium. We suggest that AMH uses Bmpr1a as its primary type I receptor to induce regression. However, in the absence of Bmpr1a, Acvr1 is capable of transducing the signal and inducing regression of the mesoepithelium. Furthermore, our Amhr2-Cre allele does not appear to be expressed in the coelomic epithelium, but only in the Müllerian duct mesenchymal cells (S.P. Jamin and R.R. Behringer, unpublished observations), which would account for the regression of the Müllerian duct in Acvr1 conditionally mutant males.
Utilization of BMP r-Smads in Activating Müllerian Duct Regression
Activation of the type I receptors leads to the phosphorylation of r-Smads, and we demonstrate that the BMP r-Smads, Smad1, Smad5, and Smad8, can all activate Müllerian duct regression, but to different degrees. The functional redundancy of these Smads has also been demonstrated in the gonads of both male and female mice [42]. We hypothesize that, similar to the type I receptors, Smad1 and Smad5 function differently during AMH-induced Müllerian duct regression. In vitro cell culture experiments have shown that SMAD1 can be phosphorylated by AMH signaling, but these experiments used cell lines not originating from the Müllerian duct mesenchyme, but rather gonadal tissue [26, 31]. Anti-Müllerian hormone signaling in the gonad is perhaps the most ancient, and the first amniotes may have used Smad1 as the r-Smad. Smad1 may be responsible for the cell fate change of the Müllerian duct, as seen in the alligator [20]. However, higher amniotes, such as the mouse, have evolved a second mechanism of Müllerian duct regression, inducing programmed cell death [18]. We hypothesize that Smad1 activates the transcription of Smad8 and the epithelial to mesenchymal cell fate change of the coelomic epithelium. Smad8 expression is seen in a sexually dimorphic pattern [27], and this evidence suggests it may be a transcriptional target of AMH signaling. Furthermore, Smad8 may be involved in a negative feedback to temper Smad1 function. This would explain the milder phenotype observed in the Smad5/Smad8 double-conditional knockout. Smad5 then activates the paracrine signal(s) responsible for the death of the mesoepithelial cells. However, in the absence of Smad5, Smad1 and Smad8 are capable of inducing partial Müllerian duct regression. Therefore, Smad1, Smad5, and Smad8 function redundantly in activating regression of the Müllerian duct mesoepithelium.
AMH Signaling
We provide new genetic evidence defining the AMH signaling pathway for Müllerian duct regression during male sexual development in the mouse. Anti-Müllerian hormone secreted by Sertoli cells of the fetal testes binds AMHR2 expressed by the Müllerian duct mesenchyme. AMHR2 complexes with BMPR-1A and ACVR1 to engage the BMP class receptor-specific Smads, SMAD1, SMAD5, and SMAD8, to regulate the transcription of target genes (Mmp2 and perhaps a Wnt) to modify Müllerian duct mesenchyme signals to the Müllerian duct mesoepithelium. The mesoepithelium then responds by apoptosis and/or cell fate changes, leading to its regression (Fig. 5). Future studies will emphasize the identification of AMH-regulated target genes that mediate mesenchyme to mesoepithelium signaling for regression.
FIG. 5.
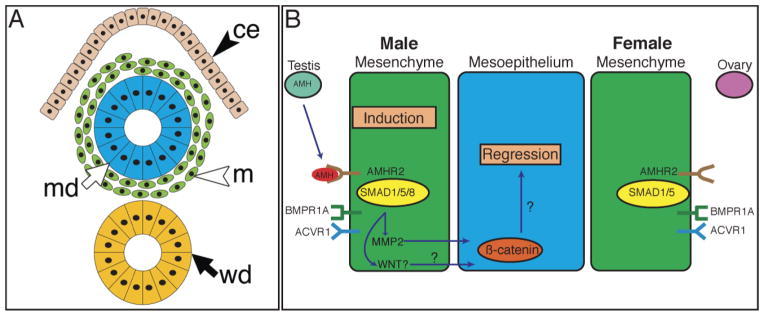
TGF-β family signaling for Müllerian duct regression. Diagram of transverse section of the mouse urogenital ridge at E14.5 (A). Summary of AMH signaling pathway in the Müllerian duct of male and female embryos (B). In males, AMH produced by the fetal testis signals through AMHR2 expressed by the mesenchyme, which activates ACVR1 and BMPR1A, which in turn activates SMAD1 and SMAD5 to induce the expression of SMAD8, MMP2, and a possible WNT. The mesoepithelium regresses in response to these signals. Females, which do not produce AMH at this time, do not activate the TGF-β type I receptors or r-Smads and therefore do not regress the Müllerian duct. ce, coelomic epithelium; m, Müllerian duct mesenchyme; md, Müllerian duct mesoepithelium; wd, Wolffian duct.
Acknowledgments
We thank Ying Wang for assistance with immunohistochemistry and Henry Adams for advice on microscopic imaging.
Footnotes
Supported by National Institutes of Health (NIH) grant HD30284 and the Ben F. Love Endowment to R.R.B., NIH DE/HD12324 to J.F.M., and NIH DEO13085 to V.M.K. G.D.O. was supported by the National Cancer Institute CA09299 Training Program in the Molecular Genetics of Cancer. S.P.J. was supported in part by a Lalor Foundation Postdoctoral Fellowship.
References
- 1.Jacob M, Christ B, Jacob HJ, Poelmann RE. The role of fibronectin and laminin in development and migration of the avian Wolffian duct with reference to somitogenesis. Anat Embryol (Berl) 1991;183:385–395. doi: 10.1007/BF00196840. [DOI] [PubMed] [Google Scholar]
- 2.Obara-Ishihara T, Kuhlman J, Niswander L, Herzlinger D. The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development. 1999;126:1103–1108. doi: 10.1242/dev.126.6.1103. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi A, Shawlot W, Kania A, Behringer RR. Requirement of Lim1 for female reproductive tract development. Development. 2004;131:539–549. doi: 10.1242/dev.00951. [DOI] [PubMed] [Google Scholar]
- 4.Orvis GD, Behringer RR. Cellular mechanisms of Mullerian duct formation in the mouse. Dev Biol. 2007;306:493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruenwald P. The relation of the growing Müllerian duct to the Wolffian duct and its importance for the genesis of malformations. Anat Rec. 1941;81:1–19. [Google Scholar]
- 6.Jacob M, Konrad K, Jacob HJ. Early development of the mullerian duct in avian embryos with reference to the human. An ultrastructural and immunohistochemical study. Cells Tissues Organs. 1999;164:63–81. doi: 10.1159/000016644. [DOI] [PubMed] [Google Scholar]
- 7.Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- 8.Guioli S, Sekido R, Lovell-Badge R. The origin of the Mullerian duct in chick and mouse. Dev Biol. 2007;302:389–398. doi: 10.1016/j.ydbio.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Drews U. Direct and mediated effects of testosterone: analysis of sex reversed mosaic mice heterozygous for testicular feminization. Cytogenet Cell Genet. 1998;80:68–74. doi: 10.1159/000014959. [DOI] [PubMed] [Google Scholar]
- 11.Josso N, Lamarre I, Picard JY, Berta P, Davies N, Morichon N, Peschanski M, Jeny R. Anti-mullerian hormone in early human development. Early Hum Dev. 1993;33:91–99. doi: 10.1016/0378-3782(93)90204-8. [DOI] [PubMed] [Google Scholar]
- 12.Jost A. Problems of fetal endocrinology. The gonadal and hypophyseal hormones. Recent Prog Horm Res. 1953;8:379–418. [Google Scholar]
- 13.Behringer RR, Finegold MJ, Cate RL. Mullerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 14.Klattig J, Sierig R, Kruspe D, Besenbeck B, Englert C. Wilms’ tumor protein Wt1 is an activator of the anti-Mullerian hormnewyone receptor gene Amhr2. Mol Cell Biol. 2007;27:4355–4364. doi: 10.1128/MCB.01780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hossain A, Saunders GF. Synergistic cooperation between the beta-catenin signaling pathway and steroidogenic factor 1 in the activation of the Mullerian inhibiting substance type II receptor. J Biol Chem. 2003;278:26511–26516. doi: 10.1074/jbc.M300804200. [DOI] [PubMed] [Google Scholar]
- 16.Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395:707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- 17.Zhan Y, Fujino A, MacLaughlin DT, Manganaro TF, Szotek PP, Arango NA, Teixeira J, Donahoe PK. Mullerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Mullerian duct regression. Development. 2006;133:2359–2369. doi: 10.1242/dev.02383. [DOI] [PubMed] [Google Scholar]
- 18.Allard S, Adin P, Gouedard L, di Clemente N, Josso N, Orgebin-Crist MC, Picard JY, Xavier F. Molecular mechanisms of hormone-mediated Mullerian duct regression: involvement of beta-catenin. Development. 2000;127:3349–3360. doi: 10.1242/dev.127.15.3349. [DOI] [PubMed] [Google Scholar]
- 19.Xavier F, Allard S. Anti-Mullerian hormone, beta-catenin and Mullerian duct regression. Mol Cell Endocrinol. 2003;211:115–121. doi: 10.1016/j.mce.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Austin HB. DiI analysis of cell migration during mullerian duct regression. Dev Biol. 1995;169:29–36. doi: 10.1006/dbio.1995.1123. [DOI] [PubMed] [Google Scholar]
- 21.Mishina Y, Whitworth DJ, Racine C, Behringer RR. High specificity of Mullerian-inhibiting substance signaling in vivo. Endocrinology. 1999;140:2084–2088. doi: 10.1210/endo.140.5.6705. [DOI] [PubMed] [Google Scholar]
- 22.Picard JY, Belville C. Genetics and molecular pathology of anti-Mullerian hormone and its receptor. J Soc Biol. 2002;196:217–221. [PubMed] [Google Scholar]
- 23.Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, Behringer RR. Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996;10:2577–2587. doi: 10.1101/gad.10.20.2577. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 25.He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MA, Massague J. Hematopoiesis controlled by distinct TIF1gamma and Smad4 branches of the TGFbeta pathway. Cell. 2006;125:929–941. doi: 10.1016/j.cell.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 26.Gouedard L, Chen YG, Thevenet L, Racine C, Borie S, Lamarre I, Josso N, Massague J, di Clemente N. Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Mullerian hormone and its type II receptor. J Biol Chem. 2000;275:27973–27978. doi: 10.1074/jbc.M002704200. [DOI] [PubMed] [Google Scholar]
- 27.Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, Donahoe PK. Mullerian inhibiting substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces SMAD6 expression. Mol Endocrinol. 2001;15:946–959. doi: 10.1210/mend.15.6.0664. [DOI] [PubMed] [Google Scholar]
- 28.Visser JA, Olaso R, Verhoef-Post M, Kramer P, Themmen AP, Ingraham HA. The serine/threonine transmembrane receptor ALK2 mediates Mullerian inhibiting substance signaling. Mol Endocrinol. 2001;15:936–945. doi: 10.1210/mend.15.6.0645. [DOI] [PubMed] [Google Scholar]
- 29.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 30.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Genetic studies of the AMH/MIS signaling pathway for Mullerian duct regression. Mol Cell Endocrinol. 2003;211:15–19. doi: 10.1016/j.mce.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Belville C, Jamin SP, Picard JY, Josso N, di Clemente N. Role of type I receptors for anti-Mullerian hormone in the SMAT-1 Sertoli cell line. Oncogene. 2005;24:4984–4992. doi: 10.1038/sj.onc.1208686. [DOI] [PubMed] [Google Scholar]
- 32.Visser JA. AMH signaling: from receptor to target gene. Mol Cell Endocrinol. 2003;211:65–73. doi: 10.1016/j.mce.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development. 1999;126:1631–1642. doi: 10.1242/dev.126.8.1631. [DOI] [PubMed] [Google Scholar]
- 34.Lechleider RJ, Ryan JL, Garrett L, Eng C, Deng C, Wynshaw-Boris A, Roberts AB. Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion. Dev Biol. 2001;240:157–167. doi: 10.1006/dbio.2001.0469. [DOI] [PubMed] [Google Scholar]
- 35.Mishina Y, Crombie R, Bradley A, Behringer RR. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol. 1999;213:314–326. doi: 10.1006/dbio.1999.9378. [DOI] [PubMed] [Google Scholar]
- 36.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 37.Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev. 2004;121:173–182. doi: 10.1016/j.mod.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- 39.Huang S, Tang B, Usoskin D, Lechleider RJ, Jamin SP, Li C, Anzano MA, Ebendal T, Deng C, Roberts AB. Conditional knockout of the Smad1 gene. Genesis. 2001;32:76–79. doi: 10.1002/gene.10059. [DOI] [PubMed] [Google Scholar]
- 40.Umans L, Vermeire L, Francis A, Chang H, Huylebroeck D, Zwijsen A. Generation of a floxed allele of Smad5 for cre-mediated conditional knockout in the mouse. Genesis. 2003;37:5–11. doi: 10.1002/gene.10219. [DOI] [PubMed] [Google Scholar]
- 41.Gao F, Maiti S, Alam N, Zhang Z, Deng JM, Behringer RR, Lecureuil C, Guillou F, Huff V. The Wilms tumor gene, Wt1, is required for Sox9 expression and maintenance of tubular architecture in the developing testis. Proc Natl Acad Sci U S A. 2006;103:11987–11992. doi: 10.1073/pnas.0600994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez CC, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol. 2008;8:248–257. doi: 10.1128/MCB.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kluver N, Pfennig F, Pala I, Storch K, Schlieder M, Froschauer A, Gutzeit HO, Schartl M. Differential expression of anti-Mullerian hormone (amh) and anti-Mullerian hormone receptor type II (amhrII) in the teleost medaka. Dev Dyn. 2007;236:271–281. doi: 10.1002/dvdy.20997. [DOI] [PubMed] [Google Scholar]
- 44.Morinaga C, Saito D, Nakamura S, Sasaki T, Asakawa S, Shimizu N, Mitani H, Furutani-Seiki M, Tanaka M, Kondoh H. The hotei mutation of medaka in the anti-Mullerian hormone receptor causes the dysregulation of germ cell and sexual development. Proc Natl Acad Sci U S A. 2007;104:9691–9696. doi: 10.1073/pnas.0611379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Hofsten J, Larsson A, Olsson PE. Novel steroidogenic factor-1 homolog (ff1d) is coexpressed with anti-Mullerian hormone (AMH) in zebrafish. Dev Dyn. 2005;233:595–604. doi: 10.1002/dvdy.20335. [DOI] [PubMed] [Google Scholar]
- 46.Visser JA, de Jong FH, Laven JSE, Themmen AP. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 47.Dyche WJ. A comparative study of the differentiation and involution of the Mullerian duct and Wolffian duct in the male and female fetal mouse. J Morphol. 1979;162:175–209. doi: 10.1002/jmor.1051620203. [DOI] [PubMed] [Google Scholar]
- 48.Newbold RR, Pentecost BT, Yamashita S, Lum K, Miller JV, Nelson P, Blair J, Kong H, Teng C, McLachlan JA. Female gene expression in the seminal vesicle of mice after prenatal exposure to diethylstilbestrol. Endocrinology. 1989;124:2568–2576. doi: 10.1210/endo-124-5-2568. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi A, Donahoe PK, Budzik GP, Trelstad RL. Periductal and matrix glycosaminoglycans in rat Mullerian duct development and regression. Dev Biol. 1982;92:16–26. doi: 10.1016/0012-1606(82)90146-4. [DOI] [PubMed] [Google Scholar]
- 50.Ikawa H, Trelstad RL, Hutson JM, Manganaro TF, Donahoe PK. Changing patterns of fibronectin, laminin, type IV collagen, and a basement membrane proteoglycan during rat Mullerian duct regression. Dev Biol. 1984;102:260–263. doi: 10.1016/0012-1606(84)90190-8. [DOI] [PubMed] [Google Scholar]
- 51.Trelstad RL, Hayashi A, Hayashi K, Donahoe PK. The epithelial-mesesnchymal interface of the male rat Mullerian duct: loss of basement membrane integrity and ductal regression. Dev Biol. 1982;92:27–40. doi: 10.1016/0012-1606(82)90147-6. [DOI] [PubMed] [Google Scholar]