Abstract
Background
Lassa fever (LF) is an acute viral haemorrhagic infection, endemic in West Africa. Confirmatory diagnosis and treatment (ribavirin) is difficult, expensive, and restricted to specialised hospitals. Among confirmed and suspected LF cases, we report on clinical and laboratory features, timing and administration of ribavirin and the relationship with case fatality.
Methods
We conducted an audit of patient files of suspected LF cases admitted to a pediatric and obstetric referral hospital in rural Sierra Leone (April 2011 to February 2012).
Results
There were 84 suspected LF cases; 36 (43%) were laboratory-confirmed cases, of whom only 20 (56%) received ribavirin after a median duration of eight days (IQR 314 days) of hospital admission. Of 16 patients who did not receive ribavirin, 14 (87%) died before ribavirin treatment could be commenced. Starting ribavirin within six days of admission was associated with a case fatality of 29% (2/7), while starting ribavirin later than six days was associated with a case fatality of 50% (6/12). Among the 48 suspected LF cases without laboratory confirmation, there were 21 (44%) deaths.
Conclusions
These findings highlight shortcomings in LF management, including diagnostic and treatment delays. More research and development efforts should be devoted to this ‘neglected disease’.
Keywords: Case fatality, Children, Lassa fever, Operational research, Ribavirin, Sierra Leone
Introduction
Lassa fever (LF) is an acute haemorrhagic viral infection caused by the Lassa virus, which is endemic in the LF belt of West Africa (Nigeria, Sierra Leone, Guinea and Liberia).1,2 It is a zoonotic infection, with the main reservoir being rodents of the species Mastomys natalensis, which are commonly present in sub-Saharan Africa.3 Transmission to man occurs from exposure to excreta (urine and faeces) and blood of the rat, through eating contaminated food or water, and using rats as food. There may also be seasonal variations.4 Infection also occurs through contact with any body fluid from an infected patient.5,6 It is estimated that 100 000 to 500 000 individuals are infected each year in the LF belt.7,8 The case fatality rate in symptomatic, hospitalized patients ranges from 15–20%, but could be as high as 90% for pregnant women.7 In severe cases, death usually occurs within two weeks following onset of symptoms. LF is also well-documented as an imported disease, with sometimes fatal outcomes.9–13
The clinical manifestations of LF are non specific (fever, sore throat, headache, muscle pain, vomiting) in the early stage of the illness and may mimic common conditions like flu and malaria. Diagnostic confirmation is difficult, requiring sophisticated laboratory facilities and specific treatment relies on the antiviral drug ribavirin,8,14–17 which is more effective if administered within a few days after onset of clinical illness. Available data suggest that mortality might be higher in children and during pregnancy.3 For resource-limited settings, the drug is also expensive, at approximately €5000 per course of treatment.
Since 2003, Médecins Sans Frontières (MSF) has been running a 200 bed secondary level referral hospital (MSF Gondama Referral Hospital [GRC]) in the rural Bo district of Sierra Leone. The hospital is situated in an area where there are sporadic cases of LF. When LF is suspected on clinical grounds, the patient is isolated, a blood specimen taken and sent to a LF reference laboratory located 60 km from Bo (Kenema GovernmentHospital). If the reference laboratory confirms the diagnosis (results are generally made available in 24–48 hours), the patient is transferred by ambulance to Kenema Government Hospital, where treatment with ribavirin is then started.
In resource-limited areas, lacking of laboratory diagnostic capacity for LF, the World Health Organization (WHO) has developed a case definition of LF (Box 1), based on clinical symptoms, to assist with the diagnosis. However, anecdotal experience suggests that there are problems with its use, especially for children, and this may delay the suspicion and diagnosis of LF cases. In addition the transfer of patients to a centralized facility for confirmatory diagnosis and eventual treatment with ribavirin is likely to cause additional delays. These factors will probably negatively influence the early start of effective ribavirin therapy, compromise the chances of a favourable outcome, and decrease the likelihood of survival.
Box 1. Adapted WHO Case Definition for Lassa fever at GRC referral hospital.20 A patient with fever >38°C not responding to effective anti-malarial and broad spectrum antibiotics within 48 h if artemisinin is used or 72 h if quinine is used, with no obvious localizing signs of infection and at least two major, or one major and at least two minor criteria. This patient must either be living in, or have travelled to, an endemic zone in the past 6–21 days. This definition is for a conventional referral hospital setting with adequate laboratory and diagnostic facilities.
| Major criteria | Abnormal bleeding Swollen neck or face Conjunctivitis or sub-conjunctival haemorrhage Spontaneous abortion Unexplained tinnitus or altered hearing during a febrile illness Persistent low systolic blood pressure Known exposure to a suspect, probable or confirmed patient with Lassa fever or participated in funeral practices in the last 21 days Readmitted within three weeks of inpatient care for illness with fever Markedly elevated aspartate aminotransferase (AST)/alanine aminotransferase (ALT) Positive coagulation test |
| Minor criteria | Headache Sore throat Persistent vomiting Diffuse abdominal pain/tenderness Retro-sternal pain Diarrhoea Generalized myalgia and arthralgia Profuse weakness Proteinuria WBC count <4000 μL |
In order to better understand the operational challenges posed by LF in these settings, we thus report on: clinical and laboratory features; timing and administration of ribavirin; and their relationships with case fatality among confirmed and suspected cases of LF.
Materials and methods
Study design, setting and population
This was an audit of case files of patients with suspected or confirmed LF conducted in the MSF GRC, Bo district, in rural Sierra Leone. The hospital is located close to Bo town, 250 km from the capital city, Freetown, and offers free services to a target population of about 600 000 inhabitants. The hospital is a referral hospital for children younger than 15 years and women with obstetric emergencies. Patients admitted to this hospital are referred from peripheral health centres and in 2011, 10 369 children and 2003 pregnant women were admitted to the GRC. At the time of analysis, the hospital had 291 national clinical staff (consisting of approximately 38 clinical heath officers, the remainder being nurses) and six international experts (five medical doctors and one midwife). Available laboratory tests included haematology, routine biochemistry, HIV, malaria and hepatitis B testing and microscopy. There was also a blood bank on site.
The study population consisted of all children and women admitted with obstetric emergencies with either suspected or confirmed LF from April 2011 to February 2012.
Diagnosis and management of Lassa fever
Patients referred with fever and admitted to the GRC first undergo malaria rapid diagnostic test (RDT) (SD Bioline, HRP-2 Standard Diagnostics, Kyonggi, Republic of Korea) and are assessed clinically. Those found to be malaria positive on testing receive a course of anti-malarial drugs, and antibiotics if clinically indicated.
The adapted WHO case definition of LF used in the GRC includes a patient with fever >38°C not responding to effective anti-malarial drugs and broad spectrum antibiotics within 48 hours if artemisinin is used or 72 hours if quinine is used, with no obvious localizing signs of infection and at least two major, or one major and at least two minor criteria (Box 1). The patient must also either be living in, or have travelled to, an LF endemic zone in the past 6–21 days. A ‘suspected’ LF case is one that fits this definition without laboratory confirmation (either no laboratory test done or a negative laboratory result for LF). A confirmed LF case is one which has a positive laboratory test for LF (Lassa Ag or anti-Lassa IgM detected).18 Results of laboratory testing done in Kenema Government hospital are transmitted to the GRC through phone or fax, or collected by the GRC ambulance.
A diagnosis of acute LF is made by an ELISA against viral antigens (Ag) or IgM antibodies against Lassa virus, which in Sierra Leone is only available at the Kenema Government Hospital (the national reference and diagnostic centre for LF). Combined ELISA for Lassa virus Ag and IgM has a sensitivity of 88% and a specificity of 90% for an acute LF infection.18 All confirmed cases are referred to the Kenema Government Hospital for treatment. Treatment consists of the administration of intravenous ribavirin according to international guidelines (loading dose of 30 mg/kg, followed by 15 mg/kg four times daily from day 1 to 4 and 7.5 mg/kg three times/day from day 5 to 10). In general, ribavirin is only given to patients with laboratory confirmed LF due to treatment related side effects and high cost (approximately 5000 €/patient). Standard infection control procedures are in place in the hospital and once a case is diagnosed, the person is isolated according to standard LF guidelines.3
All services in the GRC and Kenema Government Hospital (including LF diagnosis and treatment) are provided free of charge.
Data collection and statistical analysis
Data were extracted from patient medical records. Variables included clinical and laboratory features at presentation, treatment received and hospital outcomes. Data were collected using a proforma sheet and single entered into EpiData software 2.1 (EpiData Association, Copenhagen, Denmark). Data analysis was done using the EpiData analysis software (The EpiData Association, Odense, Denmark) and Stata® 11 software (StataCorp LP, College Station, TX, USA).
Ethical considerations
This study met the MSF Ethics Review Board (Geneva, Switzerland) and the Union Ethics Advisory Group (International Union against Tuberculosis and Lung Disease, Paris, France) ethics criteria for studies using routinely-collected data. It also received approval from the Sierra Leone ethics review board.
Results
Characteristics of the study population
A total of 84 patients with suspected LF, including 36 (43%) laboratory confirmed and 48 (57%) laboratory negative cases, were included in the study. There were 73 children, 10 pregnant women referred from the obstetric ward, and one adult male part of the medical team. Among the 84 patients with suspected LF, there were 62 (73%) children aged less than five years and 38 (45%) of all cases were females (Table 1).
Table 1.
Characteristics of suspected and confirmed Lassa fever cases in Gondama referral hospital, Bo, Sierra Leone (April 2011- February 2012)
| All suspects n (%) | Laboratory confirmed cases n (%) | Laboratory negative cases n (%) | |
|---|---|---|---|
| Total | 84 | 36 | 48 |
| Gender | |||
| Female | 38 (45) | 16 (44) | 22 (46) |
| Age, years (n=83)a | |||
| <2 | 34 (40) | 13 (36) | 21 (44) |
| 2–5 | 28 (33) | 11 (31) | 17 (35) |
| >5–15 | 10 (12) | 5 (14) | 5 (10) |
| >15 | 11 (13) | 7 (19) | 4 (8) |
| Diagnosis at admission | |||
| Malariab (all cases) | 42 (50) | 20 (56) | 22 (46) |
| Malaria only | 23 (27) | 9 (25) | 14 (29) |
| Malaria and pneumonia | 19 (23) | 11 (31) | 8 (17) |
| Pneumonia only | 3 (4) | 3 (8) | 0 |
| Obstetric related | 10 (12) | 7 (19) | 3 (6) |
| Others | 10 (12) | 6 (17) | 4 (8) |
| Missing | 19 (23) | 0 | 19 (40) |
| Most common symptoms/abnormalities at the time of suspicion of Lassa feverc | |||
| Abnormal bleeding | 44 (52) | 18 (50) | 26 (54) |
| Swollen neck or face | 27 (32) | 12 (33) | 15 (31) |
| Conjunctivitis or sub-conjunctival hemorrhage | 8 (10) | 5 (14) | 3 (6) |
| Proteinuria | 34 (40) | 17 (47) | 17 (35) |
| Laboratory testing for Lassa fever | |||
| Agc,d(+)/IgM (-) | 14 (39) | 0 | |
| Ag (+)/IgM (+) | 12 (33) | 0 | |
| IgM (+)/Ag (-) | 10 (28) | 0 |
a One missing data.
b Including 3 patients with unknown RDT results.
c Symptoms not mutually exclusive.
d Includes one case where diagnosis was made using Lassa fever RDT.
Clinical and laboratory features
The two main clinical features that lead to suspicion of LF in the hospital ward were abnormal bleeding (52%; 44/84) and swollen face and/or neck (32%; 27/84). Among the 10 women referred from the obstetric ward post-partum with suspected LF, six (60%) had severe post-partum haemorrhage or bleeding. Seventy-six (90%) of all 84 patients with suspected LF were prescribed at least one antibiotic course at admission and 27 (32%) received two or more additional courses with different antibiotics during their hospital stay.
Of 36 laboratory-confirmed LF cases, 20 (56%) also had an RDT-confirmed malaria diagnosis on admission. Similarly, 14 (39%) confirmed cases had clinical pneumonia (with or without confirmed malaria) at the time of admission. Minor criteria in the WHO case definition were not reported in patient files and some laboratory tests such as liver function and white blood count were only requested for one patient, while coagulation tests were never performed. Of all 36 patients with confirmed LF, 26 (72%) had a positive LF antigen test implying acute infection (Table 1).
Among laboratory confirmed cases, the median delay from admission to laboratory testing was eight days (IQR 2–13) for all patients, 11 (7–16) for those who subsequently died and six (0–10) for patients who were discharged alive.
Timing and administration of ribavirin
Out of the 36 patients with laboratory confirmed LF, only 20 (56%) received ribavirin. Seven of those (35%; 7/20) started treatment in less than six days after admission. The median delay from admission to starting treatment was eight days (IQR 3–14) for all patients, 11 days (2–16) for those who died and seven days (0–13) for patients discharged alive. The reasons for not starting ribavirin were: death before transfer to Kenema Government Hospital (7), death during transfer (2) and death shortly after reaching Kenema Government Hospital (2). Two patients survived without ribavirin treatment and treatment data were missing for three cases.
Case fatality rate
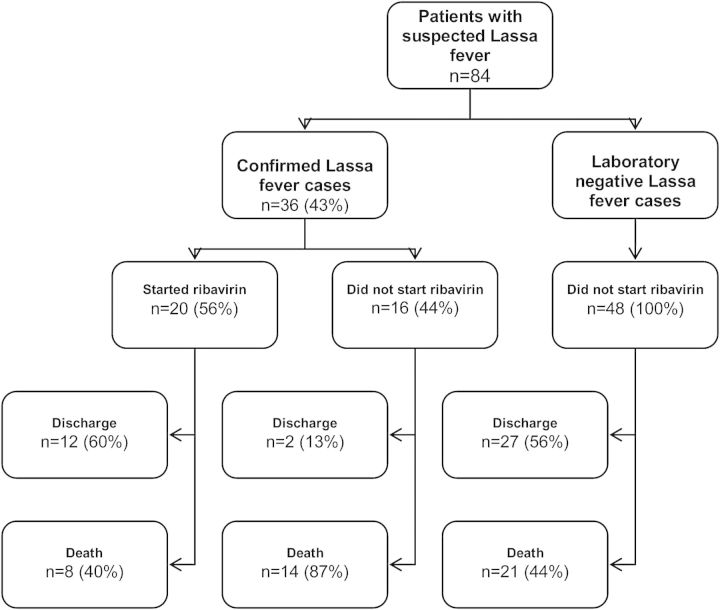
Figure 1 shows the LF cases and their relation to laboratory confirmed diagnosis, ribavirin treatment and outcome. Table 2 shows mortality related to the patients' characteristics. Among laboratory confirmed LF cases, the case fatality was 61% (22/36) overall, 40% (8/20) for those treated with ribavirin, and 87% (14/16) for those who did not receive ribavirin (because they died before or during transfer to Kenema Government Hospital. The case fatality rate was 29% (2/7) when ribavirin was started within six days of admission and 50% (6/12) when the treatment started after this threshold. We did not notice striking differences according to gender, age or diagnosis at admission, but numbers were small. The case fatality rate was higher in patients presenting with abnormal bleeding, swollen neck, sub-conjunctival haemorrhage and proteinuria when compared to those presenting without these conditions. There was no difference in the distribution of these severe LF symptoms among patients treated with ribavirin or not.
Figure 1.
Flowchart of all patients with suspected and confirmed Lassa fever admitted to the Médecins Sans Frontières Gondama referral hospital, Bo district in Sierra Leone, between April 2011 and February 2012.
Table 2.
Proportion of deaths according to patient characteristics, Gondama referral hospital, Bo, Sierra-Leone (April 2011–February 2012)
| Laboratory confirmed cases |
Laboratory negative cases |
|||||
|---|---|---|---|---|---|---|
| n total | n deaths | (%) | n total | n deaths | (%) | |
| All | 36 | 22 | 61 | 48 | 21 | 44 |
| Ribavirin treatment | ||||||
| Yesa | 20 | 8 | 40 | NA | NA | NA |
| ≤6 days | 7 | 2 | 29 | NA | NA | NA |
| >6 days | 12 | 6 | 50 | NA | NA | NA |
| No | 16 | 14 | 87 | 48 | 21 | 44 |
| Gender | ||||||
| Female | 16 | 10 | 62 | 22 | 8 | 36 |
| Male | 20 | 12 | 60 | 26 | 13 | 50 |
| Pregnant women | 7 | 4 | 57 | 3 | 1 | 33 |
| Childrenb | ||||||
| <2 years | 13 | 8 | 62 | 21 | 12 | 57 |
| 2–5 years | 11 | 6 | 55 | 17 | 5 | 29 |
| >5–15 years | 5 | 4 | 80 | 5 | 2 | 40 |
| Diagnosis at admission | ||||||
| Malariac (all cases) | 20 | 12 | 60 | 22 | 14 | 64 |
| Malaria only | 9 | 5 | 56 | 14 | 9 | 64 |
| Malaria and pneumonia | 11 | 7 | 64 | 8 | 5 | 63 |
| Pneumonia only | 3 | 3 | 100 | 0 | 0 | ND |
| Obstetric related | 7 | 4 | 57 | 3 | 1 | 33 |
| Others | 6 | 3 | 50 | 4 | 3 | 75 |
| Missing | 0 | NA | NA | 19 | 3 | 16 |
| Symptoms at LF suspicion | ||||||
| Abnormal bleeding | 18 | 14 | 78 | 26 | 16 | 62 |
| Swollen neck or face | 12 | 9 | 75 | 15 | 7 | 47 |
| Conjunctivitis or sub-conjunctival hemorrhage | 5 | 4 | 80 | 3 | 1 | 33 |
| Proteinuria | 17 | 12 | 71 | 17 | 8 | 47 |
NA: not applicable; ND: not defined.
a One timing of admission missing.
b One missing data.
c Including 3 patients with unknown RDT results.
There were 48 patients with suspected LF for whom the laboratory results were negative and who thus did not receive ribavirin. Twenty one (44%; 21/48) deaths were recorded in this group.
Discussion
In a rural district hospital in an LF endemic area of Sierra Leone, patients with both suspected and confirmed LF have a very high case fatality despite the availability of ribavirin treatment. Six in 10 patients with confirmed LF died. The case fatality was lower when ribavirin was administered and the effect was even more marked when the administration occurred within six days after admission. To the best of our knowledge, this is one of the first studies reporting on treatment delays and associated mortality in children and pregnant women from a rural endemic area.
The strengths of this study are that it was conducted in an MSF hospital that is well resourced with clinicians (including paediatric specialists), data documentation and monitoring were rigorous, and the audit involved a careful review of patient files. In addition, the findings originated from a routine hospital setting and are thus likely to reflect operational reality. Despite a rather limited study population, this is still one of the largest cohorts reported from a programme setting since 1987.9
Limitations include the fact that Bo hospital is a referral facility and as such, more patients in the study cohort are likely to have had severe illness which in turn would have influenced case fatality. Due to the design of GRC our cohort involves only children and pregnant women, which might limit the generalizability of the findings. Patients presenting with obstetric haemorrhage may have had either a primary obstetric problem and/or LF. We were unable to distinguish between the two.
An important operational question is ‘what are the possible reasons for the high case fatality among confirmed LF cases?’ There are a number of possible reasons. First, health system delays are likely to be blamed. Patients with fever or malaria-like features present first at peripheral health centres, where they often receive antimalarial treatment and possibly an antibiotic. It is only when there is an unsatisfactory response to such treatment that they get referred to the hospital where at the time of presentation, systematic administration of anti-malarial treatment and an antibiotic is also likely to be the practice. This practice delays LF diagnosis which in turn worsens prognosis.
Second, current WHO recommendations stipulate administration of anti-malarial treatment and an antibiotic prior to raising suspicion of LF even at second level facilities. In our study, 20 (56%) of all confirmed LF cases also had a confirmed malaria test and 14 (39%) of them had clinical pneumonia (with or without malaria) at the time of admission. This clearly shows that the current WHO recommendation of ‘waiting for a response’ to anti-malarial and antibiotic treatment will result in unnecessary delays that worsen the prognosis. In addition, the WHO case definition is not well adapted for use in children. For instance, symptoms of tinnitus, headache, retrosternal pain and myalgia are difficult to determine in young children. Abnormal bleeding and swellings (features of advanced and late disease) were the main reasons that led to a suspicion of LF even in the presence of paediatric specialists. This highlights the lack of sensitivity of the current WHO LF case definition and calls for an urgent review of current recommendations.
Third, even when suspicion of LF is raised, there is still need for a confirmatory laboratory test, available only (like ribavirin treatment) at one centralized site. The transport related logistics are difficult and result in further loss of precious time. In our study, patients with confirmed LF who did not receive ribavirin had an 87% chance of death while among those treated more than six days after admission, case fatality was still 50%. However, if ribavirin was started relatively early, mortality was lower (29%). The clear message is that the whole process of diagnosis and treatment has to be hastened.
What can be done to change this paradigm?
First, a high index of suspicion and clinical vigilance is needed at a referral hospital in an endemic area. Any child who has already received a course of effective anti-malarial treatment and an antibiotic at peripheral health centres but still merits referral and inpatient admission should be tested for LF. A practical hurdle to such a strategy is the lack of a point-of-care rapid diagnostic test for acute LF. There is thus an urgent need for an affordable, easy-to-use, heat-stable and accurate LF RDT-like test similar to malaria RDT.19 Second, the limitations of the current WHO case definition need to be addressed to improve sensitivity of early diagnosis, in particular in children. Urgent expert deliberations on this issue are needed to provide guidance in this regard. Third, empiric ribavirin therapy for those with a high index of suspicion may be justified.
Cost of ribavirin and limited sources of procurement are important operational challenges and current treatment options are far from ideal. Advocacy efforts are needed to develop novel generic drugs that are affordable, less toxic and easy to administer at a decentralized level. Another unanswered operational question is why there was a high mortality among patients with Lassa-like clinical features who did not test positive for LF? Are we missing other fatal bacterial or viral diseases, are we dealing with other viral haemorrhagic fevers, and/or is it a problem with the sensitivity of the laboratory tests?
Finally, post-partum haemorrhage and bleeding were seen in over half of all obstetric cases (albeit relatively few). Further research into these different areas is merited.
In conclusion, we have highlighted several shortcomings in the current paradigm of LF management that contributes to high in-patient case fatality. Urgent steps are needed to address what has been so far a ‘neglected disease’. We call on the WHO and partners to improve diagnostics and effective treatment options.
Acknowledgments
Authors' contribution: AD, RZ and RVB conceived the study; AD, RZ, RVB designed the study protocol; AD and RZ carried out the file's audit; AD, RZ, RVB, MVH and JVG carried out the analysis and interpretation; AD, RZ and JVG drafted the manuscript; MVH, YN, JP, PA and AJ critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. RZ and JVG are guarantors of the paper.
Acknowledgements: This research was supported through an operational research course, which was jointly developed and run by the Centre for Operational Research, International Union Against Tuberculosis and Lung Disease, France, and the Operational Research unit, Médecins Sans Frontières, Brussels-Luxembourg. Additional support for running the course was provided by the Institute for Tropical Medicine, Antwerp, Belgium; the Center for International Health, University of Bergen; and the University of Nairobi, Kenya. We are particularly grateful to the laboratory services and management of the Kenema Government Hospital for supporting laboratory diagnosis and Lassa fever case management.
Funding: Funding for the course was from an anonymous donor and the Department for International Development, UK. The study implementation was funded by Médecins Sans Frontières, Operational Centre Brussels.
Competing interests: None declared
Ethical approval: This study met the MSF Ethics Review Board (Geneva, Switzerland) and the Union Ethics Advisory Group (International Union against Tuberculosis and Lung Disease, Paris, France) ethics criteria for studies using routinely-collected data. It also received approval from the Sierra Leone ethics review board.
References
- 1.Murphy FA. Arenavirus taxonomy: a review. Bull World Health Organ. 1975;52:389–91. [PMC free article] [PubMed] [Google Scholar]
- 2.Ogbu O, Ajuluchukwu E, Uneke CJ. Lassa fever in West African sub-region: an overview. J Vector Borne Dis. 2007;44:1–11. [PubMed] [Google Scholar]
- 3.Richmond JK, Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. 2003;327:1271–5. doi: 10.1136/bmj.327.7426.1271. BMJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asogun DA, Adomeh DI, Ehimuan J, et al. Molecular diagnostics for lassa fever at Irrua specialist teaching hospital, Nigeria: lessons learnt from two years of laboratory operation. PLoS Negl Trop Dis. 2012;6 doi: 10.1371/journal.pntd.0001839. e1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. doi: 10.1007/978-3-642-56029-3_4. [DOI] [PubMed] [Google Scholar]
- 6.Fisher-Hoch SP. Lessons from nosocomial viral haemorrhagic fever outbreaks. Br Med Bull. 2005:73–74. doi: 10.1093/bmb/ldh054. 123–37. [DOI] [PubMed] [Google Scholar]
- 7.McCormick JB, Webb PA, Krebs JW, et al. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987;155:437–44. doi: 10.1093/infdis/155.3.437. [DOI] [PubMed] [Google Scholar]
- 8.Fichet-Calvet E, Rogers DJ. Risk maps of Lassa fever in West Africa. PLoS Negl Trop Dis. 2009;3 doi: 10.1371/journal.pntd.0000388. e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amorosa V, MacNeil A, McConnell R, et al. Imported Lassa fever, Pennsylvania, USA, 2010. Emerg Infect Dis. 2010;16:1598–600. doi: 10.3201/eid1610.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunther S, Emmerich P, Laue T, et al. Imported Lassa fever in Germany: molecular characterization of a new Lassa virus strain. Emerg Infect Dis. 2000;6:466–76. doi: 10.3201/eid0605.000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkin S, Anaraki S, Gothard P, et al. The first case of Lassa fever imported from Mali to the United Kingdom, February 2009. Euro Surveill. 2009 14(6). pii19117. [PubMed] [Google Scholar]
- 12.Kitching A, Addiman S, Cathcart S, et al. A fatal case of Lassa fever in London, January 2009. Euro Surveill. 2009 14(10). pii19145. [PubMed] [Google Scholar]
- 13.Macher AM, Wolfe MS. Historical Lassa fever reports and 30-year clinical update. Emerg Infect Dis. 2006;12:835–7. doi: 10.3201/eid1205.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormick JB. Lassa fever. In: Saluzzo JF, Dodet B, editors. 1999. pp. 177–95. Emergence and Control of Rodent-borne Viral Diseases. Paris: Elsevier. [Google Scholar]
- 15.WHO. Geneva: World Health Organization; 2000. Lassa Fever. Fact Sheet No 179. http://www.who.int/inf-fs/en/fact179.html. [accessed 16 December 2011] [Google Scholar]
- 16.Grove JN, Branco LM, Boisen ML, et al. Capacity building permitting comprehensive monitoring of a severe case of Lassa hemorrhagic fever in Sierra Leone with a positive outcome: case report. Virol J. 2011;8:314. doi: 10.1186/1743-422X-8-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vieth S, Drosten C, Lenz O, et al. RT-PCR assay for detection of Lassa virus and related Old World arenaviruses targeting the L gene. Trans R Soc Trop Med Hyg. 2007;101:1253–64. doi: 10.1016/j.trstmh.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Bausch DG, Rollin PE, Demby AH, et al. Diagnosis and clinical virology of Lassa fever as evaluated by enzyme-linked immunosorbent assay, indirect fluorescent-antibody test, and virus isolation. J Clin Microbiol. 2000;38:2670–7. doi: 10.1128/jcm.38.7.2670-2677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Branco LM, Boisen ML, Andersen KG, et al. Lassa hemorrhagic fever in a late term pregnancy from northern Sierra Leone with a positive maternal outcome: case report. Virol J. 2011;8:404. doi: 10.1186/1743-422X-8-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MSF. Lassa Fever Guidelines. Brussels: Médecins Sans Frontières. 2005 [Google Scholar]