Abstract
Recently zebrafish larvae have emerged as a high-throughput model for screening pharmacological activities. The present study was undertaken to investigate the effect of established anticonvulsants, such as valproic acid, carbamazepine, gabapentin, diazepam, lacosamide and pregabalin against pentylenetetrazole (6 mM) seizures in adult zebrafish. Different phases of seizures (increase swim activity, rapid whirlpool-like circling swim behaviour and brief clonus-like seizures leading to loss of posture) were elicited in zebrafish on exposure for 15 min to 6 mM pentylenetetrazole. The exposure of zebrafish to an increasing concentration of the anticonvulsants alongside 6 mM pentylenetetrazole showed concentration-dependent elevation of seizure latency against pentylenetetrazole-induced seizures except for pregabalin, which failed to produce any anticonvulsant activity in zebrafish. Moreover the proconvulsant activity of caffeine was also evaluated using suboptimal concentration (4 mM) of pentylenetetrazole in adult zebrafish. Decrease in seizure latency of different phases of seizures was observed with increasing concentration of caffeine compared with its respective control group. In view of the above findings, the results of the present study suggested that adult zebrafish produce the expected anticonvulsive and proconvulsive effects and could potentially be used as a screen in future epilepsy research.
Keywords: Anticonvulsant, caffeine, pentylenetetrazole, proconvulsant, seizures, zebrafish
Seizure liability is an undesirable property and is typically detected only in later stage preclinical safety studies using rodent assays[1]. To date, a vast majority of antiepileptic research has been performed in rodents, due to their homology to humans and ability to display key epilepsy-related phenotype[2,3,4]. Various animal models with chemically and electrically induced seizures as well as several genetic and reflex seizure models are used for initial screening. None are capable of identifying all the antiepileptics currently used and all are of relatively low throughput. Therefore there is a need for new approaches and experimental models to understand the pathogenesis of epilepsy and as tools for initial screening of new compounds.
Over the past decade, Zebrafish (Danio rerio), one of the unique vertebrate models, has risen to prominence in biomedical research because of its fully sequenced genome, genetic and physiological homology, convenient and ethical experimentation and many molecular biological methods are being developed and have been made available[5,6,7]. Lots of studies proved that the zebrafish has emerged as an experimental model for central nervous system (CNS) studies due to its similarities in overall organisation of the brain to other vertebrates[1,8,9,10]
Recently an epileptic model was described in zebrafish larvae[8,9]. Due to limitations of underdeveloped neural system and small size of larvae, assessment of certain behavioural parameters like different stages of seizures is difficult in zebrafish larvae; therefore, adult zebrafish may represent another promising approach in epileptic research. The present study describes the effects of selected antiepileptic agents in adult zebrafish exposed to a known chemoconvulsant, pentylenetetrazole (PTZ). The same screening approach was also used to investigate the effect of caffeine, a known proconvulsant compound in zebrafish.
Around 4- to 5-month-old stock of wild-type adult zebrafish (Danio rerio) of either sex was purchased from a local vendor (Big Fish, Gurgaon, India) and acclimatised for at least one month before starting the study. For all experiments zebrafish were housed in a 40 l tank, filled with deionised water maintained at 28-30° and a pH between 7.0 and 8.0 with constant filtration and aeration. Water conditioning and environmental quality was maintained according to the aquarium system use and care manual and the zebrafish book[11]. A dark-light cycle of 12 h light and 12 h dark (on: 8:00 am; off: 20:00 pm) was maintained. Utmost care was taken to ensure that all the animals were treated humanely. Zebrafish were fed twice daily with tetrabits adult zebrafish diet (Spectrum Brands Company, Germany), supplemented with live artemia. Behavioural testing of drug effects took place during the light phase between 10:00 am and 5:00 pm.
Caffeine, valproic acid, carbamazepine and PTZ were purchased from Sigma-Aldrich (Bangalore, India), diazepam injection (Calmpose) manufactured by Ranbaxy Laboratories Limited (Mumbai, India), gabapentin, lacosamide and pregabalin were procured from Ranbaxy Research Laboratories (Gurgaon, India). All drug solutions and PTZ solution were prepared daily. PTZ was dissolved in deionised water (1 l). Caffeine, valproic acid, gabapentin, pregabalin, diazepam and lacosamide dissolved in 6 mM PTZ solution and carbamazepine was dissolved in polyethylene glycol (PEG) 200+6 mM PTZ (10:990) solution. Table 1 shows the doses of different drugs used in this study. Selection of concentrations were based on the literature report[9] and the preliminary experiments conducted in our laboratory, such that the selected concentration of a drug would cause suppression of PTZ-induced seizures in a concentration-dependent manner.
TABLE 1.
SEIZURE LATENCY OF DIFFERENT ANTICONVULSANTS AT STAGE I, II AND III
The test was conducted in zebrafish placed individually in black boxes, which contained different concentrations (2, 4, 6 and 8 mM) of PTZ solution (1 l). Another set of black boxes contained the mixture of solution of PTZ 6 mM and the different concentrations of antiepileptic drugs (AEDs). Tests were conducted separately for each AED and PTZ combination in a different set of black boxes. During the test, fish were observed for the onset of different types of seizures by a trained blind observer. The time latencies from start of fish exploration in the bath medium to the appearance of stage I (characterised by increase swim activity), stage II (characterised by the rapid whirlpool-like circling swim behaviour) and stage III (characterised by a series of brief clonus-like seizures leading to loss of posture, for example fish falls on one side and remains immobile for 1-3 s) was recorded manually using stopwatch (Cole Parmer, US). Latency at different stages (stage I, II and III) of seizures of AEDs treatment group was compared with the latency of corresponding 6 mM PTZ group.
Proconvulsant activity was determined by keeping the zebrafish in a black box containing suboptimal concentration (4 mM) of PTZ or combination of PTZ (4 mM) with proconvulsive agent caffeine at 1 or 10 or 30 µM. The time latencies from start of fish exploration in the bath medium to the appearance of stage I, stage II and stage III was recorded manually using a stopwatch (Cole Parmer, USA). Latency at different stages of seizures induced by caffeine was compared with the latency of a corresponding PTZ 4 mM concentration group.
The mean time latency in seconds (Mean±SEM) to seizure response of AEDs and caffeine for all the three stages were calculated from the individual values of each fish and compared with the vehicle control group (6 mM PTZ for anticonvulsant activity, PEG 200+6 mM PTZ for carbamazepine and 4 mM PTZ for proconvulsant activity) by one-way analysis of variance (ANOVA) followed by Dunnett's post hoc test. PTZ (2, 4, 6 and 8 mM) and PEG 200+6 mM PTZ (vehicle for carbamazepine) groups were compared with control group (deionised water) by one-way ANOVA followed by Dunnett's post hoc test. EC50 value of each drug was calculated using log probit analysis[12]. P<0.05 was chosen as the criterion for statistical significance.
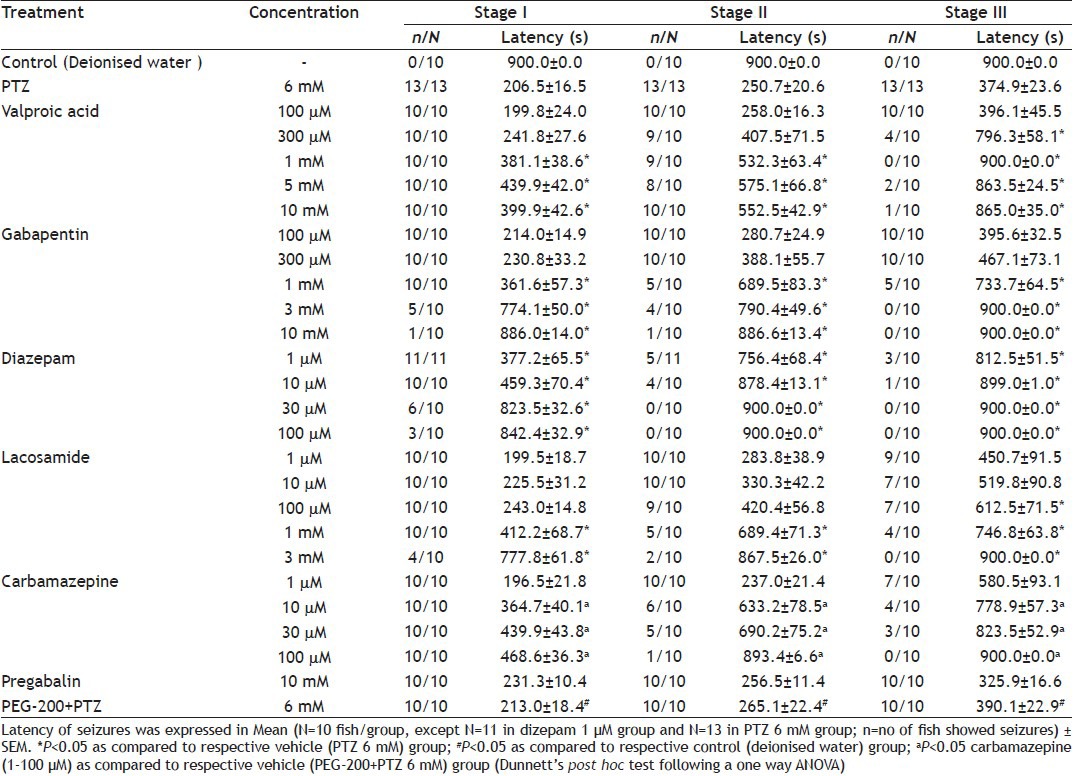
Fish exposed to normal bathing medium up to 15 min did not show any symptoms of seizures. The convulsive effect of PTZ in zebrafish was determined at different concentrations of PTZ (2-8 mM) during 15 min of exposure. Convulsive behaviour was confirmed by brief clonus-like seizures accompanied by rapid movement across the well followed by brief loss of posture. Exposure to PTZ showed a decrease in seizure latency (seconds) at all the stages of seizures (fig. 1) in a concentration-dependent manner as compared with the normal bathing medium group. Lower concentration of PTZ (2 mM) evoked only stage I (10/10) while only one (1/10) fish showed stage II. None of the fish showed stage III at lowest concentration. All the fish exposed to PTZ (4, 6 and 8 mM) solution showed stage I and II seizures, while only 3/10 fishes showed stage III at 4 mM. All fishes showed stage III seizures at 6 and 8 mM PTZ (Table 1). As 6 mM PTZ was the lowest achieved concentration, which showed significant seizures in all the zebrafish at all the stages without mortality compared with the deionised water group, it was selected as a dose for further evaluation of anticonvulsant compounds. The rationale for the selection of suboptimal concentration of PTZ 4 mM was as it failed to induce stage III seizures completely, while the latency of stage I and II was significantly decreased when compared with the deionised water group.
Fig. 1.
The anticonvulsant effect of standard AEDs with known effects in a rodent model of PTZ-evoked seizure was explored in adult zebrafish. All animals were exposed up to 15 min to monitor their convulsive activity in a solution of PTZ 6 mM (1 l) with standard AEDs valproic acid (100 µM to 10 mM), gabapentin (100 µM to 10 mM), diazepam (1-100 µM), carbamazepine (1-100 µM), lacosamide (1 µM to 3 mM) and pregabalin (10 mM). The latency at all the stages (I, II and III) of seizures were recorded manually as shown in Table 1.
Valproic acid, gabapentin, lacosamide and carbamazepine showed a concentration-dependent increase in latency at all the stages of seizures, which was significant for valproic acid at 300 µM to 10 mM, gabapentin at 1-10 mM, lacosamide at 100 µM to 3 mM and carbamazepine at 10-100 µM, while pregabalin failed to increase in seizure latency at all the stages compared with vehicle (PTZ 6 mM) group similar to the reported literature[3]. Gabapentin at 1 mM onwards and diazepam at 10 µM showed a saturated response, however, at higher concentration of diazepam (30 and 100 µM) group; complete inhibition of seizure was noticed at all the stages.
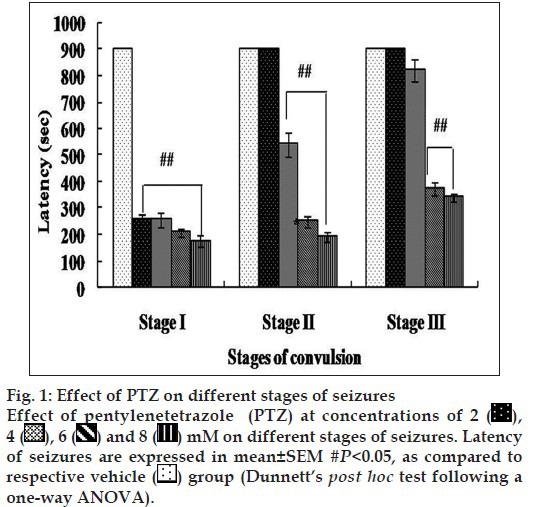
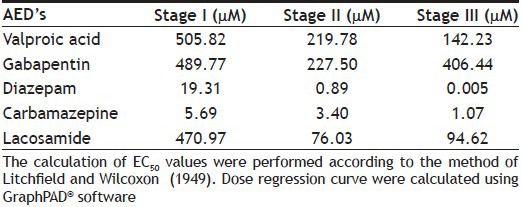
Carbamazepine at 100 µM showed a complete protection at stage II and stage III and noticeably showed almost equal potency to diazepam and more potent compared with other AEDs specifically valproic acid. EC50 was calculated using percent (%) change in seizure latency against the log dose of all the AEDs except pregabalin for all the stages (fig. 2 and Table 2). We considered stage II EC50 to evaluate the anticonvulsant potential of all the AEDs because the seizures were more prominent in stage II.
Fig. 2.
Dose-response curves illustrating % response in seizure latency at stage II
Dose-response curves of valproic acid (a), gabapentin (b), diazepam (c), carbamazepine (d) and lacosamide (e). EC50 values were calculated according to the method of Litchfield and Wilcoxon (1949). Dose regression curve were calculated using GraphPAD software
TABLE 2.
EC50 CALCULATION OF DIFFERENT AED'S AT STAGE I, II AND III
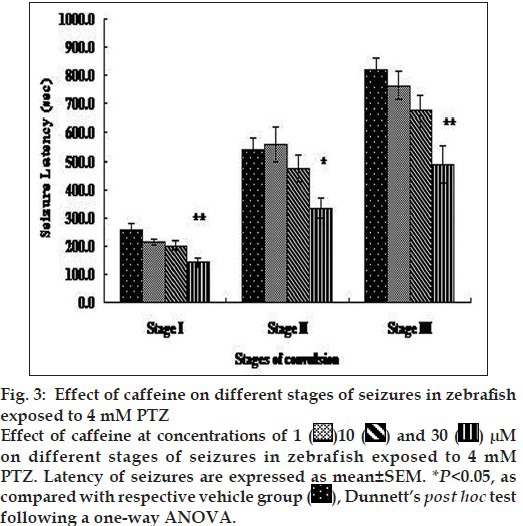
Proconvulsant effect of caffeine at different doses (1, 10 and 30 µM) was recorded using the sub threshold concentration of PTZ (4 mM) in adult zebrafish. Fish were observed for 15 min in a bath medium, containing a combination of PTZ 4 mM and caffeine (1, 10 and 30 µM). A concentration-dependent decrease in latency of seizures at different stages were observed, which was significant at caffeine (30 µM) compared with the latency of vehicle (PTZ 4 mM) group (fig. 3). All the fish (12/12) showed stage I seizure at 1, 10 and 30 µM, 10/12 fish showed stage II seizure at 1 µM while all the fish showed stage II seizure at the concentration of 10 and 30 µM of caffeine. Overall, 7/12, 9/12 and 12/12 fish showed concentration-dependent stage III seizures at 1, 10 and 30 µM of caffeine, respectively.
Fig. 3.
In this study, different AEDs were dissolved in PTZ solution and latencies of different stages of seizure were recorded up to 15 min and compared with the latencies of different stages of PTZ group. PTZ reliably elicited seizures in adult zebrafish similar to zebrafish larvae[8]. PTZ is a well known chemoconvulsant that competitively antagonizes the GABAA receptor, likely through an allosteric interaction in the Cl- channel and the site of PTZ is the benzodiazepine site of the GABAA receptor[13]. Many non-GABAergic drugs also prevent PTZ-induced seizure[14]. This makes the zebrafish PTZ screening relatively nondiscriminatory, in contrast to rat and mouse where PTZ seizures can be used to identify those anticonvulsants acting through GABA[9].
Inter animal variability in the onset of clonus in this model has been associated with brain concentration of PTZ. As described by Wayne et al. and Mandhane et al., the specific threshold concentration of PTZ in the brain is required to induce seizures in mice or rats[3,15]. Various investigators have reported that the onset time of myoclonus and clonus depends on the route of administration which determines how rapidly PTZ reaches optimum levels in the brain[15]. However, time of onset of stage I, II and III seizures depends upon the time required to attain the threshold brain concentration of PTZ in zebrafish, which might lead to inter animal variability in the onset of seizures.
Since, pharmacokinetic data of AED distribution in zebrafish are not available, all AEDs and other drugs concentration used in this study were chosen on the basis of reported literature[8,9] and the preliminary experiments conducted in our laboratory, such that the selected concentration of drug would cause suppression of PTZ-induced seizures in a concentration-dependent manner. A concentration-dependent decrease in latencies of stage I, II and III seizures were observed at PTZ 2, 4, 6 and 8 mM. In the present study, we used 6 mM concentration, as all the three stages of seizures were prominent and significant compared to vehicle (deionised) group at this concentration. Moreover, no mortality was observed at 6 mM in contrast to 8 mM in which few fish were dead. Further to this, chemically induced seizures at stage I, II and III was inhibited by concentration-dependent manner using conventional AEDs (valproic acid, carbamazepine, gabapentin, diazepam and lacosamide) as observed in their protection effect in zebrafish larvae[1,8,9]. Potency of all the AEDs used in this study was evaluated on the basis of EC50 calculated for stage II seizures and gradation was found as diazepam>carbamazepine>lacosamide>gabapentin>valproic acid>pregabalin.
It is well established from the available data in rodent model that much higher concentration of valproic acid is needed to demonstrate an anticonvulsant effect and both valproic acid and benzodiazepines (diazepam, nitrazepam and oxazepam) effectively inhibit PTZ-induced seizures[3]. Similarly data presented in this study also showed the high valproic acid concentration and very low concentration of diazepam required to nullify zebrafish seizures. It was also observed that carbamazepine and lacosamide were also equally effective compared with diazepam and more potent to valproic acid against PTZ-induced seizures. Though both lacosamide and carbamazepine were already reported highly potent against MES-induced seizures, but were relatively ineffective in the threshold PTZ test in rats and mice[16]. The high protective effect of lacosamide and carbamazepine against PTZ-induced seizures in zebrafish and very low EC50 might indicate the possibility of high systemic exposure of both the compounds in zebrafish.
It is interesting to note that the majority of AEDs were positively identified in this screen except pregabalin, which failed to protect seizures unexpectedly. The anticonvulsant activity of pregabalin in rodents was already reported in PTZ-induced seizures[3], however, we could not find any anticonvulsant activity even up to 10 mM. Pregabalin is completely ineffective for all types of GABA receptors and does not interfere with either GABA release; uptake or it does not get metabolised into a GABAergic compound[17]. Studies have shown that the anticonvulsant action of pregabalin and gabapentin is due to their binding affinity to the alpha-2-delta regulatory subunit of voltage-gated calcium channels (VGCC), inhibiting activity-dependent calcium influx in nerve terminals and consequently reducing the release of neurotransmitters such as glutamate, norepinephrine and substance P[18]. At the same time gabapentin showed the concentration-dependent decrease in PTZ-induced seizures in zebrafish. Though we could not find out the reason of different activity behaviour of gabapentin and pregabalin, but the possibility of low systemic exposure of pregabalin in zebrafish might be the probable reason of its failure to protect PTZ-induced seizures. While in case of gabapentin; its high systemic exposure in zebrafish and the reports of increase in cellular GABA within 2 h of oral dose of 20 mg/kg gabapentin in human subjects, increase GABA levels in the hippocampus in in vitro preparations and an increase in GABA synthesis in some brain regions[19] might be the probable reason for gabapentin showing the activity in zebrafish.
Proconvulsive effect of caffeine was also evaluated at different concentration range using the sub threshold concentration of PTZ (4 mM). The timing of induction of seizures of stage I, II and III was greatly decreased in concentration-dependent manner. Caffeine is known proconvulsant in rodent model[20] and administration of CNS stimulant caffeine along with PTZ lowers the PTZ-induced seizure threshold[4]. The worsening effect of caffeine on seizure are thought to be due to its antagonist effect on A1 receptor[21] or by interfering with GABA-BZD receptor complex and may decrease the cerebral blood flow in situation such as hypoxia, ischemia and seizures[22]. Findings of our study in caffeine group are also corroborating with the previous studies and provide the support for the utility of this model as screening tool. Overall, the data of present study concludes that adult zebrafish might be an option to develop different stages of convulsions and can be utilised for testing CNS effects (anticonvulsant and proconvulsant), that is efficacy and safety of the potential of new drugs as regular early stage screening.
ACKNOWLEDGEMENTS
The authors wish to thank the Ranbaxy Research Laboratories for providing facility to carry out the work. They are also grateful to Dr. Milind Deore, Dr. Venkatesha Udupa and Mr. Pravin J. Patil for the expert advice and helpful discussions while designing the experiments.
Footnotes
Gupta, et al.: Anticonvulsants and Zebrafish
REFERENCES
- 1.Winter MJ, Redfern WS, Hayfield AJ, Owen SF, Valentin JP, Hutchinson TH. Validation of larval zebrafish locomotor assay for assessing the seizure liability of early stage development drugs. J Pharmacol Toxicol Methods. 2008;57:176–87. doi: 10.1016/j.vascn.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Loscher W, Fiedler M. The role of technical, biological, and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. VII. Seasonal influences on anticonvulsant drug actions in mouse models of generalized seizures. Epilepsy Res. 2000;38:231–48. doi: 10.1016/s0920-1211(99)00095-9. [DOI] [PubMed] [Google Scholar]
- 3.Mandhane SN, Aavula K, Rajamannar T. Timed pentylenetetrazole infusion test: A comparative analysis with s.c. PTZ and MES models of anticonvulsant screening in mice. Seizure. 2007;16:636–44. doi: 10.1016/j.seizure.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Loscher W. Preclinical assessment of proconvulsant drug activity and its relevance for predicting adverse event in humans. Eur J Pharmacol. 2009;610:1–11. doi: 10.1016/j.ejphar.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Goldsmith P. Zebrafish as a pharmacological tool: The how, why and when. Curr Opin Pharmacol. 2004;4:504–12. doi: 10.1016/j.coph.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Rubinstein AL. Zebrafish assays for drug toxicity screening. Drug Metab Toxicol. 2006;2:231–40. doi: 10.1517/17425255.2.2.231. [DOI] [PubMed] [Google Scholar]
- 7.Barros TP, Alderton WK, Reynolds HM, Roach AG, Berghmans S. Zebrafish: An emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br J Pharmacol. 2008;154:1400–13. doi: 10.1038/bjp.2008.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole Induced Changes in Zebrafish behavior, neural activity and C-FOS expression. Neuroscience. 2005;131:759–68. doi: 10.1016/j.neuroscience.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Berghmans S, Hunt J, Roach A, Goldsmith P. Zebrafish offer the potential for a primary screen to identify a wide variety of potential anticonvulsants. Epilepsy Res. 2007;75:18–28. doi: 10.1016/j.eplepsyres.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Irons TD, MacPhail RC, Hunter DL, Padilla S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol Teratol. 2010;32:84–90. doi: 10.1016/j.ntt.2009.04.066. [DOI] [PubMed] [Google Scholar]
- 11.Westerfield M. 3rd ed. Eugene, USA: University of Oregon Press; 1995. The Zebrafish Book: A Guide for the Laboratory use of Zebrafish (Danio rerio) [Google Scholar]
- 12.Litchfield JT, Wilcoxan FA. A simplified method of evaluating dose- effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 13.Huang RQ, Cathy L, Horner B, Diba MI, Convey DF, Drewe JA, et al. Pentylenetetrazole-induced inhibition of recombinant ү-aminobutyric acid type A (GABA A ) receptors: Mechanism and site of action. J Pharmacol Exp Ther. 2001;298:986–95. [PubMed] [Google Scholar]
- 14.Olsen RW. The GABA postsynaptic membrane receptor-ionophore complex. Site of action of convulsant and anticonvulsant drugs. Mol Cell Biochem. 1981;39:261–79. doi: 10.1007/BF00232579. [DOI] [PubMed] [Google Scholar]
- 15.Wayne DY, Kupferberg HJ, Woodbury DM. Relationship between pentylenetetrazole-induced seizures and brain pentylenetetrazole levels in mice. J Pharmacol Exp Ther. 1980;214:584–93. [PubMed] [Google Scholar]
- 16.Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Loiseau P, Perucca E. Progress report on new antiepileptic drugs: a summary of the sixth eilat conference (EILAT VI) Epilepsy Res. 2002;51:31–71. doi: 10.1016/s0920-1211(02)00106-7. [DOI] [PubMed] [Google Scholar]
- 17.Czuczwar SJ, Ferenc R, Blaszczyk B, Borowicz KK. Neuroprotective effects of some newer and potential antiepileptic drugs. J Pre-Clin Clin Res. 2007;1:1–5. [Google Scholar]
- 18.Fink K, Dooley DJ, Meder WP, Chauhan NS, Duffy S, Clusmann H, et al. Inhibition of neuronal Ca 2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–36. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 19.Loscher W, Hoenack D, Taylor CP. Gabapentin increases aminooxyacetic acid-induced GABA accumulation in several regions of rat brain. Neurosci lett. 1991;128:150–4. doi: 10.1016/0304-3940(91)90249-s. [DOI] [PubMed] [Google Scholar]
- 20.Chu NS. Caffeine and aminophylline-induced seizures. Epilepsia. 1981;22:85–94. doi: 10.1111/j.1528-1157.1981.tb04335.x. [DOI] [PubMed] [Google Scholar]
- 21.Yacoubi EM, Ladent C, Menard JF, Parmentier M, Costentin M, Vaugeois JM. The stimulant effects of caffeine on locomotor behavior in mice are mediated through its blockade of adenosine A 2A receptor. Br J Pharmacol. 2000;129:1465–73. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dengiz GO, Halici Z, Bakirci A. Effect of omeprazole in caffeine and pentylenetetrazole-induced generalized seizures in mice. Erciyes Med J. 2007;29:184–8. [Google Scholar]


