Abstract
Association of urinary arsenic concentration with incident diabetes was examined in American Indians from Arizona who have a high prevalence of type 2 diabetes and were screened for diabetes between 1982 and 2007. The population resides where drinking water contains arsenic at concentrations above federally recommended limits. A total of 150 nondiabetic subjects aged ≥25 years who subsequently developed type 2 diabetes were matched by year of examination and sex to 150 controls who remained nondiabetic for ≥10 years. Total urinary arsenic concentration, adjusted for urinary creatinine level, ranged from 6.6 µg/L to 123.1 µg/L, and inorganic arsenic concentration ranged from 0.1 µg/L to 36.0 µg/L. In logistic regression models adjusted for age, sex, body mass index, and urinary creatinine level, the odds ratios for incident diabetes were 1.11 (95% confidence interval (CI): 0.79, 1.57) and 1.16 (95% CI: 0.89, 1.53) for a 2-fold increase in total arsenic and inorganic arsenic, respectively. Categorical analyses suggested a positive relationship between quartiles of inorganic arsenic and incident diabetes (P = 0.056); post-hoc comparison of quartiles 2–4 with quartile 1 revealed 2-fold higher odds of diabetes in the upper quartiles (OR = 2.14, 95% CI: 1.19, 3.85). Modestly elevated exposure to inorganic arsenic may predict type 2 diabetes in American Indians. Larger studies that include measures of speciated arsenic are required for confirmation.
Keywords: arsenic; diabetes mellitus, type 2; incidence; Indians, North American; nested case-control studies
Environmental exposure to arsenic is associated with adverse health outcomes, including reproductive abnormalities, cardiovascular disease, and cancer. Inorganic forms of arsenic (arsenite and arsenate), derived principally from ingestion of contaminated drinking water or food, are responsible for these effects, whereas arsenobetaine, arsenosugars, and arsenolipids, which are organic forms derived principally from ingestion of seafood, are considered nontoxic (1). Very high levels of inorganic arsenic in drinking water, typically above 500 μg/L, are reportedly associated with an increased risk of type 2 diabetes in Taiwan and Bangladesh (2–4), and even moderate concentrations are associated with increased risk in Mexico (5). Analysis of the 2003–2004 National Health and Nutrition Examination Survey (NHANES) suggests that exposure to low or moderate concentrations of arsenic may substantially increase the risk of type 2 diabetes (6).
The relationship between arsenic exposure and type 2 diabetes, however, is controversial. Most evidence supporting the relationship in populations with high arsenic exposure comes from ecological studies, which have no data on exposure within individuals. A recent study of 11,319 participants in Bangladesh, in which each individual's concentration of urinary arsenic was measured and reflected high levels of exposure, found no relationship between urinary arsenic concentration and the prevalence of diabetes (7). On the other hand, total urinary arsenic concentrations in a representative sample of the South Korean population, in which levels of exposure were lower than in Bangladesh, were associated significantly with the prevalence of diabetes, after adjustment for other diabetes risk factors and seafood intake (8). Reanalysis of the NHANES data described above found no relationship between urinary arsenic concentration and diabetes, prompting some to suggest that the association reported previously did not properly estimate the exposure to inorganic arsenic (9). However, the assumptions on which this reanalysis was based were subsequently questioned (10, 11). Given that millions of people could be at risk of type 2 diabetes if modest exposure to inorganic arsenic were associated with diabetes risk (6), further examination of this relationship in informative populations is warranted (11).
We conducted a case-control study nested within a longitudinal study to investigate the impact of urinary arsenic species on development of diabetes in a southwestern American Indian population with a high prevalence of type 2 diabetes. This population resides in a region of the United States where arsenic exposure in drinking water above the federally recommended limit of 10 μg/L disproportionately affects small communities (12). The population has limited exposure to nontoxic organic forms of arsenic because of low seafood consumption, so the relationship between toxic forms of arsenic and diabetes can more easily be assessed.
MATERIALS AND METHODS
Participants
Between 1965 and 2007, Pima Indians from the Gila River Indian Community participated in a longitudinal study that examined diabetes and its complications. Each member of the community aged 5 years or more was invited to undergo a research examination every 2 years, regardless of health. These biennial examinations included an oral glucose tolerance test. Diabetes was defined by the 1985 World Health Organization criteria (13) when the 2-hour postload plasma glucose concentration following a 75-g oral glucose tolerance test was ≥11.1 mmol/L (≥200 mg/dL). The date of diabetes diagnosis was determined from study examinations or from review of clinical records if diabetes was diagnosed during routine care. Urinary albumin concentration was measured at all examinations on or after July 1, 1982, by nephelometric immunoassay, and urinary creatinine concentration was measured by means of a modification of the Jaffé reaction (14). Remaining urine from these specimens was stored at −20°C. Urinary albumin excretion was quantified by means of the urinary albumin:creatinine ratio and reported in milligrams per gram of creatinine. Subjects with an undetectable urinary albumin concentration (n = 62) were assigned a level of 6.8 mg/L, the threshold below which albuminuria cannot be detected by the assay.
Subjects aged ≥25 years with no history of diabetes and confirmed to be nondiabetic at a research examination between July 1, 1982, and December 31, 1989, were eligible for this analysis. Potential cases developed diabetes before follow-up ended in 2007. Potential controls remained nondiabetic through the end of follow-up and had at least 1 follow-up examination after January 1, 2000, that confirmed the absence of diabetes for at least 10 years following the baseline examination. From all qualifying participants, 150 cases that developed diabetes during follow-up were randomly selected and frequency-matched by year of baseline visit and sex to 150 controls. Concentrations of arsenic and its metabolites were measured in stored urine samples obtained at the baseline examination and related to the development of type 2 diabetes. We did not measure arsenic concentration in well water used as the source of drinking water. Place of residence since 1965 was recorded for each community member; the proportion of time spent within the community from 1965 to the sample collection date averaged 86% (median, 100%), indicating that virtually all participants were long-term residents. The study was approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases. Each participant gave informed consent.
Measurement of arsenic and its metabolites
Concentration of total arsenic was determined using an inductively coupled plasma mass spectrometer (XSERIES 2; Thermo Fisher Scientific, Franklin, Massachusetts). The instrument's collision cell was used to mitigate the polyatomic argon chloride-75 interference when monitoring arsenic-75. Prior to assay, urine samples were allowed to reach room temperature and 0.25 mL of the urine sample was combined with 0.5 mL of high-purity, Ultrex-grade nitric acid (J. T. Baker, Phillipsburg, New Jersey). Samples were then heated to 100°C. To monitor data quality, a Standard Reference Material (SRM) from the National Institute of Standards and Technology (NIST), NIST SRM 2699 (Arsenic Species in Human Urine, Level 1 and Level 2), was analyzed. Average total arsenic recoveries for levels 1 and 2, calculated from data generated on 9 different days, were 112% and 104%, respectively.
Speciation was performed using a PRP-X100 anion exchange chromatography column (Hamilton Company, Reno, Nevada) and an ultraperformance liquid chromatographic system (AQUITY; Waters Corporation, Milford, Massachusetts) interfaced with the inductively coupled plasma mass spectrometer (15). A 30 mm tris(hydroxymethyl)aminomethane (Tris) (Sigma Aldrich Corporation, St. Louis, Missouri) solution buffered to a pH of 8.8 with nitric acid (J. T. Baker) was used as eluent A, and a 30 mm Tris and 20 mm ammonium sulfate (J. T. Baker) solution buffered to a pH of 7.8 with nitric acid was used as eluent B. The column was operated at ambient temperature, the injection volume was 20 µL, and the gradient was: time 0 minutes (99% A, 1% B), time 0.5 minutes (99% A, 1% B), time 1.5 minutes (1% A, 99% B), time 5.25 minutes (99% A, 1% B), and time 6.0 minutes (99% A, 1% B). Speciation analysis was not performed in the 19 samples with total arsenic concentrations less than 5 µg/L. Concentrations of several arsenic species (arsenobetaine, arsenocholine, monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA)) were determined. Data on arsenite and arsenate were not available from the speciation analysis. The calibration range used in the assays was 0.25–50 µg/L. Prior to assay, urine samples were allowed to reach room temperature and were subsequently diluted 5× with 20 mm ammonium acetate (Sigma Aldrich Corporation) buffered to a pH of 5.25 with acetic acid (J. T. Baker). Instrument detection limits, taken from data generated on 7 different days, were calculated as 3 times the standard deviation of the lowest calibration standard concentration (0.25 µg/L) and were 0.034 µg/L for arsenocholine, 0.028 µg/L for arsenobetaine, 0.043 µg/L for DMA, and 0.022 µg/L for MMA. Taking into account a 5× dilution during sample preparation, the method detection limits were 0.17 µg/L for arsenocholine, 0.14 µg/L for arsenobetaine, 0.21 µg/L for DMA, and 0.11 µg/L for MMA. Among the 281 subjects who had speciation data, the majority had concentrations greater than the method detection limits, with the exception of arsenocholine. To monitor data quality, NIST SRM 2699 was analyzed. Average arsenic species recoveries, calculated from data generated on 7 different days, for level 1 were 104%, 92.9%, and 103% for arsenobetaine, DMA, and MMA, respectively, with certified values of 12.4 µg/L, 3.47 µg/L, and 1.87 µg/L. Average species recoveries for level 2 were 82.4%, 91.9%, 94.4%, and 99.8% for arsenocholine, arsenobetaine, DMA, and MMA, respectively, with certified values of 3.74 µg/L, 1.43 µg/L, 25.2 µg/L, and 7.18 µg/L.
Reproducibility of total and speciated arsenic concentration was assessed by intraclass correlation of measurements from 50 duplicate samples blinded to the arsenic laboratory. Correlations were computed on the arithmetic scale for percent methylation capacity and on the logarithmic scale for total arsenic, arsenobetaine, inorganic arsenic, DMA, and MMA because of their skewed distributions on the arithmetic scale. Intraclass correlations were excellent, exceeding 0.98 for all measures except inorganic arsenic, which was 0.56. When 1 extremely low outlier for inorganic arsenic was removed, the correlation was 0.78. None of the other results changed appreciably with exclusion of the single outlier, so the results reported include the outlier.
Calculation of organic arsenic, inorganic arsenic, and methylation indices
The concentration of inorganic arsenic was estimated by subtracting the concentrations of arsenobetaine, MMA, and DMA from the concentration of total arsenic. Arsenocholine was not included in this computation, because nearly all subjects had arsenocholine concentrations below the method detection limits. For subjects with arsenobetaine, MMA, or DMA concentrations below the method detection limits, an assigned value equivalent to the method detection limits divided by the square root of 2 was used for statistical analysis. Percentages of MMA and DMA metabolites were calculated from the percentages of each species relative to total arsenic minus arsenobetaine. The methylation capacity of arsenic was computed by dividing the sum of MMA and DMA by total arsenic minus arsenobetaine.
Statistical analysis
Clinical features at baseline were compared between cases and controls. Normally distributed variables were expressed as mean values, and differences by group were assessed by Student's t test. Non–normally distributed variables were expressed as median values (with interquartile ranges), and differences by group were assessed using the Kruskal-Wallis test. Distributions and differences of total arsenic, arsenobetaine, inorganic arsenic, MMA, and DMA were reported by group after adjustment for urinary creatinine concentration by linear regression to account for differences in urinary flow. Frequency distributions of arsenic species and methylation capacity were examined. Spearman correlation coefficients were calculated between variables of interest, since most were not normally distributed. Partial correlations involving total arsenic, arsenobetaine, inorganic arsenic, MMA, DMA, and urinary albumin were adjusted for urinary creatinine.
Odds ratios for diabetes were computed by logistic regression. Data for variables representing total and speciated arsenic concentrations were highly skewed and were therefore logarithmically transformed. A logarithm base 2 transformation was used to reflect the association with diabetes corresponding to a doubling in the arsenic concentration. For %DMA (DMA/(total arsenic – arsenobetaine) × 100), %MMA (MMA/(total arsenic – arsenobetaine) × 100), and percent methylation capacity ([(MMA + DMA)/(total arsenic – arsenobetaine)] × 100), the odds ratios were expressed for a difference of 10 in these ratios (e.g., the difference between 40% and 50%). Three logistic regression models were presented for each arsenic species and for percent methylation capacity. Model 1 was adjusted for the logarithm of urinary creatinine concentration, except for percent methylation capacity, for which there was no adjustment. Model 2 was additionally adjusted for age and sex, and model 3 for body mass index (weight (kg)/height (m)2). A quadratic term for each arsenic species did not enhance the fit of any model, so it was not included in the final models. Models were also computed for quartiles of each arsenic species, adjusted for urinary creatinine concentration, to further assess the shape of the dose-response relationship.
The association of inorganic arsenic with development of diabetes was also examined using an approach advocated by Navas-Acien et al. (6), in which covariates for both total arsenic and arsenobetaine were included in the logistic regression model. By adjusting for arsenobetaine in a model that included total arsenic, the association of inorganic arsenic with diabetes could be assessed indirectly (6).
RESULTS
Clinical characteristics of the cases and controls are shown in Table 1. Mean age, systolic blood pressure, and median albumin:creatinine ratio were similar in the 2 groups. Mean body mass index, fasting plasma glucose, and 2-hour postload plasma glucose were higher in subjects who developed diabetes. The median storage time of the urine samples was 24.0 years in cases and 23.2 years in controls. Total and speciated arsenic levels, with the exception of arsenobetaine, were correlated positively with storage time; no concentrations were significantly different between cases and controls, except for MMA and %MMA, levels of which were lower in cases.
Table 1.
Baseline Characteristics of Case and Control Groups of Southwestern American Indians in Arizona Who Were Screened for Diabetes Between 1982 and 2007
| Variable | Controls (n = 150) |
Cases (n = 150) |
P Valuea | ||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Age, years | 31.6 (8.0) | 33.0 (6.9) | 0.10 | ||
| Body mass indexb | 30.2 (5.7) | 35.1 (6.9) | <0.0001 | ||
| Systolic blood pressure, mm Hgc | 115 (15) | 116 (14) | 0.66 | ||
| Fasting plasma glucose, mmol/L | 5.2 (0.4) | 5.5 (0.6) | <0.0001 | ||
| 2-hour postload plasma glucose, mmol/Lc | 5.6 (1.2) | 6.8 (1.6) | <0.0001 | ||
| Albumin:creatinine ratio, mg/g | 7.8 (4.8, 13.6) | 7.6 (5.0, 13.1) | 0.93 | ||
| Total arsenic, µg/Ld | 20.7 (14.9, 29.9) | 22.1 (15.8, 29.1) | 0.97 | ||
| Arsenobetaine, µg/Ld | 0.3 (0.2, 1.1) | 0.3 (0.1, 1.1) | 0.73 | ||
| Inorganic arsenic, µg/Ld | 6.9 (4.2, 9.7) | 7.0 (5.0, 9.3) | 0.44 | ||
| DMA, µg/Ld | 9.8 (6.3, 15.2) | 10.2 (6.5, 14.4) | 0.84 | ||
| MMA, µg/Ld | 2.4 (1.6, 3.6) | 2.0 (1.2, 3.0) | 0.04 | ||
| %DMAe | 53.8 (46.3, 61.4) | 53.9 (45.2, 61.0) | 0.88 | ||
| %MMAe | 13.0 (9.5, 17.0) | 10.5 (7.5, 15.0) | 0.01 | ||
| % methylation capacitye | 67.5 (59.7, 74.5) | 66.4 (58.5, 72.9) | 0.33 | ||
| Storage time, years | 23.2 (21.2, 25.1) | 24.0 (21.7, 25.4) | 0.14 | ||
| Follow-up time, years | 18.3 (16.5,20.8) | 11.4 (6.7,15.6) | <0.0001 | ||
Abbreviations: DMA, dimethylarsinic acid; IQR, interquartile range; MMA, monomethylarsonic acid; SD, standard deviation.
a P values (2-sided) were based on Student's t test for normally distributed variables and on the Kruskal-Wallis test for non–normally distributed variables.
b Weight (kg)/height (m)2.
c Information on systolic blood pressure was missing for 3 subjects, and information on 2-hour postload plasma glucose was missing for 5 subjects.
d Values were adjusted for urinary creatinine concentration.
e %DMA = DMA/(total arsenic – arsenobetaine) × 100; %MMA = MMA/(total arsenic – arsenobetaine) × 100; % methylation capacity = [(MMA + DMA)/(total arsenic – arsenobetaine)] × 100.
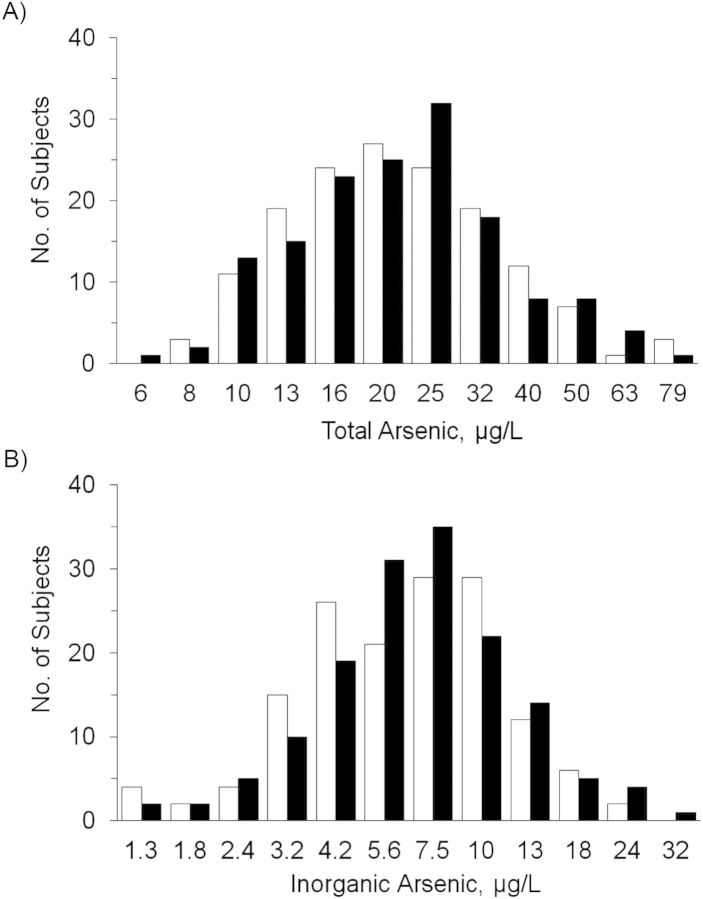
Distributions of total and speciated arsenic are shown in Table 2. The median total arsenic concentration was 21.1 µg/L (interquartile range, 15.3–29.4), and for inorganic arsenic it was 7.0 µg/L (interquartile range, 4.6–9.5). The frequency distributions of total arsenic concentration, adjusted for urinary creatinine concentration and sample date, were nearly identical between cases and controls, whereas the frequency distribution of inorganic arsenic was somewhat higher in cases than in controls (Figure 1).
Table 2.
Distributions of Total and Speciated Arsenic Concentrations at Baseline Among 300 Southwestern American Indians in Arizona Who Were Screened for Diabetes Between 1982 and 2007
| Variable | Minimum | 25% Percentile | Median | 75% Percentile | Maximum |
|---|---|---|---|---|---|
| Total arsenic, µg/La | 6.6 | 15.3 | 21.1 | 29.4 | 123.1 |
| Arsenobetaine, µg/La | 0.04 | 0.1 | 0.3 | 1.1 | 74.7 |
| Inorganic arsenic, µg/La | 0.1 | 4.6 | 7.0 | 9.5 | 36.0 |
| DMA, µg/La | 0.4 | 6.4 | 10.1 | 14.5 | 57.6 |
| MMA, µg/La | 0.1 | 1.4 | 2.3 | 3.4 | 12.1 |
| %DMAb | 3.1 | 45.9 | 53.9 | 61.1 | 82.5 |
| %MMAb | 0.6 | 8.3 | 12.1 | 16.0 | 30.0 |
| % methylation capacityb | 4.6 | 59.1 | 66.9 | 73.7 | 99.3 |
Abbreviations: DMA, dimethylarsinic acid; MMA, monomethylarsonic acid.
a Values were adjusted for urinary creatinine concentration.
b %DMA = DMA/(total arsenic – arsenobetaine) × 100; %MMA = MMA/(total arsenic – arsenobetaine) × 100; % methylation capacity = [(MMA + DMA)/(total arsenic – arsenobetaine)] × 100.
Figure 1.
Distributions of total and inorganic urinary arsenic concentrations on a logarithmic scale, adjusted for urinary creatinine concentration and sample date, among southwestern American Indians in Arizona who were screened for diabetes between 1982 and 2007. Black bars, type 2 diabetes cases; white bars, controls.
Correlations between arsenic compounds and baseline clinical variables are shown in Table 3. Body mass index was correlated negatively with MMA and %MMA and positively with %DMA; fasting plasma glucose was correlated negatively with %MMA and positively with total arsenic, arsenobetaine, inorganic arsenic, and DMA; and 2-hour postload plasma glucose was correlated negatively with MMA and %MMA. Correlations between %MMA or DMA and fasting plasma glucose were attenuated slightly following additional adjustment for body mass index (r = −0.06 (P = 0.35) vs. r = −0.17 (P = 0.01) for %MMA; r = 0.11 (P = 0.06) vs. r = 0.14 (P = 0.02) for DMA), as were correlations between MMA or %MMA and 2-hour postload plasma glucose (r = −0.18 (P = 0.002) vs. r = −0.25 (P < 0.001) for MMA; r = −0.25 (P < 0.001) vs. r = −0.32 (P < 0.001) for %MMA).
Table 3.
Spearman Correlations Between Total and Speciated Arsenic and Other Baseline Covariates Among Southwestern American Indians in Arizona Who Were Screened for Diabetes Between 1982 and 2007
| Characteristic | Age (n = 300) |
Body Mass Indexa (n = 300) |
Systolic Blood Pressure (n = 297) |
Fasting Plasma Glucose (n = 272) |
2-Hour Postload Plasma Glucose (n = 295) |
Urinary Albumin (n = 300) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P Valueb | Spearman's r | P Value | Spearman's r | P Value | Spearman's r | P Value | Spearman's r | P Value | Spearman's r | P Value | Spearman's r | |
| Total arsenicc | 0.21 | 0.07 | 0.45 | −0.04 | 0.11 | 0.09 | 0.03 | 0.13 | 0.63 | −0.03 | 0.79 | 0.02 |
| Arsenobetainec | 0.88 | 0.01 | 0.83 | −0.01 | 0.14 | 0.09 | 0.03 | 0.13 | 0.47 | 0.04 | 0.23 | −0.07 |
| Inorganic arsenicc | 0.13 | 0.09 | 0.32 | −0.06 | 0.12 | 0.09 | 0.03 | 0.13 | 0.93 | 0.00 | 0.50 | −0.04 |
| DMAc | 0.22 | 0.07 | 0.38 | 0.05 | 0.09 | 0.10 | 0.02 | 0.14 | 0.89 | −0.01 | 0.18 | 0.08 |
| MMAc | 0.45 | 0.04 | <0.001 | −0.29 | 0.15 | 0.08 | 0.57 | −0.03 | <0.001 | −0.25 | 0.75 | −0.02 |
| %DMAd | 0.90 | 0.01 | <0.001 | 0.24 | 0.71 | 0.02 | 0.18 | 0.08 | 0.12 | 0.09 | 0.04 | 0.12 |
| %MMAd | 0.49 | −0.04 | <0.001 | −0.31 | 0.97 | 0.00 | 0.01 | −0.17 | <0.001 | −0.13 | 0.83 | −0.01 |
| % methylation capacityd | 0.96 | 0.00 | 0.10 | 0.09 | 0.65 | 0.03 | 0.82 | −0.01 | 0.41 | −0.05 | 0.08 | 0.10 |
Abbreviations: DMA, dimethylarsinic acid; MMA, monomethylarsonic acid.
a Weight (kg)/height (m)2.
b P values (2-sided) were based on a comparison of the correlation with its standard error.
c Partial correlations involving this variable were adjusted for urinary creatinine concentration.
d %DMA = DMA/(total arsenic – arsenobetaine) × 100; %MMA = MMA/(total arsenic – arsenobetaine) × 100; % methylation capacity = [(MMA + DMA)/(total arsenic – arsenobetaine)] × 100.
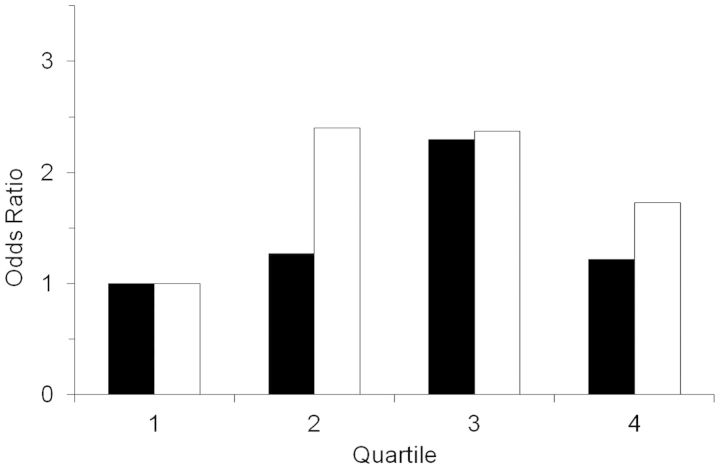
Neither total arsenic nor inorganic arsenic was associated with development of type 2 diabetes when assessed as a continuous variable (Table 4). Further adjustment of the total arsenic model by adding arsenobetaine as a covariate did not change the results. The odds ratio for development of diabetes associated with a doubling of total arsenic concentration was 1.12 (95% confidence interval: 0.77, 1.62) after adjustment for arsenobetaine in addition to age, sex, body mass index, and urinary creatinine concentration. Categorical analyses, however, suggested positive relationships between quartiles of total and inorganic arsenic and incident type 2 diabetes (Figure 2); the stronger relationship was with inorganic arsenic (P = 0.12 for total arsenic, P = 0.056 for inorganic arsenic). Post-hoc analyses comparing quartiles 2–4 with quartile 1 of inorganic arsenic concentration revealed 2-fold higher odds of diabetes in the upper 3 quartiles (odds ratio = 2.14, 95% confidence interval: 1.19, 3.85).
Table 4.
Odds Ratios for Development of Diabetes According to Total Arsenic Concentration and Species of Arsenic Concentration Among Southwestern American Indians in Arizona Who Were Screened for Diabetes Between 1982 and 2007
| Variable | Model 1a |
Model 2b |
Model 3c |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ORd | 95% CI | P Valuee | OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Total arsenic | 1.05 | 0.77, 1.44 | 0.74 | 1.06 | 0.77, 1.45 | 0.74 | 1.11 | 0.79, 1.57 | 0.55 |
| Arsenobetaine | 1.00 | 0.91, 1.11 | 0.98 | 1.00 | 0.91, 1.11 | 0.97 | 1.01 | 0.91, 1.12 | 0.87 |
| Inorganic arsenic | 1.10 | 0.86, 1.42 | 0.46 | 1.10 | 0.85, 1.42 | 0.46 | 1.16 | 0.89, 1.53 | 0.28 |
| DMA | 1.00 | 0.84, 1.19 | 0.99 | 1.01 | 0.84, 1.20 | 0.96 | 0.99 | 0.82, 1.19 | 0.89 |
| MMA | 0.89 | 0.74, 1.07 | 0.21 | 0.89 | 0.74, 1.08 | 0.24 | 1.00 | 0.82, 1.22 | 0.99 |
| %DMAf | 0.99 | 0.85, 1.15 | 0.86 | 0.99 | 0.85, 1.15 | 0.87 | 0.91 | 0.77, 1.07 | 0.25 |
| %MMAf | 0.58 | 0.38, 0.88 | 0.01 | 0.57 | 0.37, 0.89 | 0.01 | 0.83 | 0.51, 1.33 | 0.43 |
| % methylation capacitya,f | 0.93 | 0.81, 1.07 | 0.31 | 0.94 | 0.82, 1.07 | 0.35 | 0.91 | 0.78, 1.05 | 0.21 |
Abbreviations: CI, confidence interval; DMA, dimethylarsinic acid; MMA, monomethylarsonic acid; OR, odds ratio.
a Model 1 adjusted for urinary creatinine concentration, except for percent methylation capacity, which was unadjusted.
b Model 2 included model 1 adjustments plus age and sex.
c Model 3 included model 2 adjustments plus body mass index.
d OR for a 2-fold difference in total and speciated arsenic concentrations, which were analyzed on a logarithmic scale (e.g., the difference between 10 µg/L and 20 µg/L). For %DMA, %MMA, and percent methylation capacity, the ORs are expressed for a difference of 10 in these ratios (e.g., the difference between 40% and 50%).
e P values (2-sided) were based on the Wald χ2 test.
f %DMA = DMA/(total arsenic – arsenobetaine) × 100; %MMA = MMA/(total arsenic – arsenobetaine) × 100; % methylation capacity = [(MMA + DMA)/(total arsenic – arsenobetaine)] × 100.
Figure 2.
Odds ratios for incident type 2 diabetes among southwestern American Indians in Arizona who were screened for diabetes between 1982 and 2007, by quartiles of total arsenic (black bars) and inorganic arsenic (white bars) concentration, relative to the lowest quartile. Results were obtained from logistic regression models that controlled for age, sex, body mass index, and urinary creatinine concentration. Neither relationship was statistically significant, but there was a suggestion of higher incidence of diabetes in persons with higher arsenic concentrations, particularly for inorganic arsenic (P = 0.12 for total arsenic; P = 0.056 for inorganic arsenic). The P values (2-sided) were based on the Wald χ2 test.
The %MMA was associated with development of type 2 diabetes when adjusted for urinary creatinine concentration alone and when also adjusted for age and sex. With additional adjustment for body mass index, the relationship with %MMA was attenuated (Table 4).
DISCUSSION
Modestly elevated exposure to inorganic arsenic in drinking water, as estimated by urinary arsenic concentration, may predict type 2 diabetes in southwestern American Indians. The putative relationship was most apparent in the categorical analysis. Median total urinary arsenic concentration in study participants was 2- to 3-fold higher than that reported in NHANES (6). Low levels of arsenobetaine in the urine samples confirmed the extremely low seafood consumption in the local diet and the assumption that urinary arsenic levels were almost entirely attributable to inorganic arsenic and its methylated metabolites. Accordingly, these analyses were less confounded by organic arsenic than may be the case in other populations with greater seafood intake. Associations of methylated metabolites with fasting plasma glucose and 2-hour postload plasma glucose were attenuated slightly by adjustment for body mass index, suggesting that these relationships may partly reflect a shared correlation with body mass index.
Although the present findings only suggest an association between arsenic exposure and the risk of diabetes, there is other evidence for a relationship. First, concentrations of inorganic arsenic were slightly higher in cases than in controls. Moreover, concentrations of inorganic arsenic increase glucose and insulin levels in animal studies (16), disrupt glucose uptake and transport mechanisms (17–19), interfere with transcription factors involved in insulin-related gene expression (18, 20), and adversely affect β-cell function (21, 22). Second, studies from countries with very high inorganic arsenic concentrations in the drinking water associated these high concentrations with diabetes (2–4), although these studies assessed the relationship ecologically and therefore did not examine data within individuals. Third, some evidence from epidemiologic studies within individuals supports this relationship. In central Taiwan, for example, with modestly elevated arsenic concentrations in drinking water, total arsenic concentrations in hair were positively associated with elevated plasma glucose concentrations and with the prevalence of metabolic syndrome (23). In a case-control study conducted in an arsenic-endemic region in Mexico, urinary total arsenic levels had a dose-response relationship with the presence of diabetes (5). However, these studies were cross-sectional and did not speciate arsenic concentrations to distinguish organic forms from inorganic forms. In a cross-sectional analysis of data from the 2003–2004 NHANES (6), a nearly 4-fold increased risk of diabetes was reported when comparing the 80th percentile of total arsenic concentration with the 20th percentile, after adjustment for arsenobetaine as a means of examining the association with inorganic arsenic. Our results were nearly identical with and without arsenobetaine in the models due to low levels of arsenobetaine overall.
High detection limits for measurement of inorganic arsenic species generally preclude their direct measurement. Accordingly, subtracting arsenobetaine concentration from total arsenic concentration has been used as an indirect measure of inorganic arsenic exposure, since it removes the most common form of organic arsenic (9). In populations with low seafood consumption, this approach may provide valid estimates of inorganic arsenic concentrations. Conversely, in populations with high seafood consumption, subtracting arsenobetaine concentration may not be sufficient to remove the contribution of other organic arsenic species, such as arsenosugars and arsenolipids, and their metabolites, such as DMA, from the urine. Navas-Acien et al. (24) found that seafood consumption was a major determinant of increased urinary arsenic concentrations in NHANES. In the present study, conducted in a population with a very low seafood intake, we calculated inorganic arsenic concentration by subtracting arsenobetaine, MMA, and DMA from total arsenic concentration to eliminate the modest contribution of seafood-originated DMA and because MMA and DMA are considered nontoxic metabolites of inorganic arsenic (25). By means of this approach, we found no relationship between inorganic arsenic exposure and diabetes when inorganic arsenic was modeled as a continuous variable, but we found a suggestive positive relationship when it was modeled categorically.
Urinary arsenic levels are higher in American Indians in Arizona than among American Indians in the Dakotas or in Oklahoma (12). These differences may relate to different arsenic concentrations in drinking water, since previous epidemiologic studies consistently found that arsenic concentration in drinking water correlates positively with urinary inorganic and methylated arsenic concentrations (26, 27). The present results suggest that higher arsenic exposure in the Southwest may, in part, contribute to the higher prevalence of diabetes among American Indians in Arizona.
Reduced methylation capacity may enhance the toxicity of inorganic arsenic by inhibiting conversion to less toxic metabolites (25). In addition to examining the association of inorganic arsenic with the risk of developing diabetes, we also examined whether less efficient methylation of arsenic was associated with increased diabetes risk. Examining the role of methylation was possible in the present study, since MMA and DMA concentrations in most subjects were measurable; in a past study, it could not be done because MMA and DMA levels were undetectable (6). Consistent with previous observations (28, 29), we found associations between arsenic metabolites and body mass index, suggesting that body fat may affect the storage and metabolism of arsenic. However, methylation capacity did not predict development of diabetes.
The present study was based on urinary arsenic concentration measured in a single spot urine specimen and therefore reflected arsenic exposure at only 1 point in time. The very low concentration of arsenobetaine in the urine, however, suggests that much of the arsenic exposure in the population was due to inorganic arsenic in groundwater, which would not be expected to fluctuate substantially over time. In addition, samples were stored frozen for many years prior to assay, which may affect the concentration of some substances. A previous study in American Indians demonstrated long-term constancy of urinary arsenic levels and excretion patterns (12), but we found significant positive correlations between sample storage time and total and speciated arsenic concentrations, except for arsenobetaine. Finally, several factors, including the limited sample size, insufficient variability in exposure levels within this homogenous population, and use of the single arsenic measure, could have reduced the level of association between urinary arsenic concentration and incident diabetes in the present study. The small sample size is particularly relevant, given the suggestive association (P = 0.056) with incident diabetes observed in the categorical analysis, which implies an increased risk of diabetes with higher levels of inorganic arsenic.
In conclusion, the association of arsenic exposure with development of type 2 diabetes was examined in a population of American Indians at high risk for type 2 diabetes. This population consumes little seafood and lives where drinking water has moderately high inorganic arsenic concentrations—sufficiently high that methylation capacity and arsenic species could be measured in the urine. Using several accepted approaches to eliminate potential confounding by organic arsenic in the analysis, we found that moderately elevated exposure to inorganic arsenic may predict type 2 diabetes in this population. Larger studies that include measures of these arsenic species are required to confirm this finding. Regardless of the outcome of these studies, arsenic exposure should be reduced to protect community members from its other well-established health effects.
ACKNOWLEDGMENTS
Author affiliations: Diabetes Epidemiology and Clinical Research Section, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, Arizona (Nan Hee Kim, Clinton C. Mason, Robert G. Nelson, William C. Knowler); Research Triangle Institute, Research Triangle Park, North Carolina (Scott E. Afton, Amal S. Essader, James E. Medlin, Keith E. Levine); and National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Jane A. Hoppin, Cynthia Lin, Dale P. Sandler).
This research was supported by the Intramural Research Programs of the National Institute of Environmental Health Sciences and the National Institute of Diabetes and Digestive and Kidney Diseases.
Conflict of interest: none declared.
REFERENCES
- 1.Ng JC. Environmental contamination of arsenic and its toxicological impact on humans. Environ Chem. 2005;2(3):146–160. [Google Scholar]
- 2.Wang SL, Chiou JM, Chen CJ, et al. Prevalence of non-insulin-dependent diabetes mellitus and related vascular diseases in southwestern arseniasis-endemic and nonendemic areas in Taiwan. Environ Health Perspect. 2003;111(2):155–159. doi: 10.1289/ehp.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai MS, Hsueh YM, Chen CJ, et al. Ingested inorganic arsenic and prevalence of diabetes mellitus. Am J Epidemiol. 1994;139(5):484–492. doi: 10.1093/oxfordjournals.aje.a117031. [DOI] [PubMed] [Google Scholar]
- 4.Rahman M, Tondel M, Ahmad SA, et al. Diabetes mellitus associated with arsenic exposure in Bangladesh. Am J Epidemiol. 1998;148(2):198–203. doi: 10.1093/oxfordjournals.aje.a009624. [DOI] [PubMed] [Google Scholar]
- 5.Coronado-González JA, Del Razo LM, Garcia-Vargas G, et al. Inorganic arsenic exposure and type 2 diabetes mellitus in Mexico. Environ Res. 2007;104(3):383–389. doi: 10.1016/j.envres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, et al. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300(7):814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Ahsan H, Slavkovich V, et al. No association between arsenic exposure from drinking water and diabetes mellitus: a cross-sectional study in Bangladesh. Environ Health Perspect. 2010;118(9):1299–1305. doi: 10.1289/ehp.0901559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim Y, Lee B-K. Association between urinary arsenic and diabetes mellitus in the Korean general population according to KNHANES 2008. Sci Total Environ. 2011;409(19):4054–4062. doi: 10.1016/j.scitotenv.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Steinmaus C, Yuan Y, Liaw J, et al. Low-level population exposure to inorganic arsenic in the United States and diabetes mellitus: a reanalysis. Epidemiology. 2009;20(6):807–815. doi: 10.1097/EDE.0b013e3181b0fd29. [DOI] [PubMed] [Google Scholar]
- 10.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, et al. Rejoinder: arsenic exposure and prevalence of type 2 diabetes. Updated findings from the National Health and Nutrition Examination Survey, 2003–2006. Epidemiology. 2009;20(6):816–820. doi: 10.1097/EDE.0b013e3181afef88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longnecker MP. On confounded fishy results regarding arsenic and diabetes. Epidemiology. 2009;20(6):821–823. doi: 10.1097/EDE.0b013e3181b26bce. [DOI] [PubMed] [Google Scholar]
- 12.Navas-Acien A, Umans JG, Howard BV, et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environ Health Perspect. 2009;117(9):1428–1433. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Diabetes Mellitus:Report of a WHO Study Group. Geneva, Switzerland: World Health Organization; 1985. (WHO Technical Report Series no. 727). [Google Scholar]
- 14.Chasson AL, Grady HJ, Stanley MA. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol. 1960;30(12):207–212. [PubMed] [Google Scholar]
- 15.Afton S, Kubachka K, Catron B, et al. Simultaneous characterization of selenium and arsenic analytes via ion-pairing reversed phase chromatography with inductively coupled plasma and electrospray ionization ion trap mass spectrometry for detection. Applications to river water, plant extract and urine matrices. J Chromatogr A. 2008;1208(1-2):156–163. doi: 10.1016/j.chroma.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 16.Izquierdo-Vega JA, Soto CA, Sanchez-Pena LC, et al. Diabetogenic effects and pancreatic oxidative damage in rats subchronically exposed to arsenite. Toxicol Lett. 2006;160(2):135–142. doi: 10.1016/j.toxlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Walton FS, Harmon AW, Paul DS, et al. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicol Appl Pharmacol. 2004;198(3):424–433. doi: 10.1016/j.taap.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Paul DS, Harmon AW, Devesa V, et al. Molecular mechanisms of the diabetogenic effects of arsenic: inhibition of insulin signaling by arsenite and methylarsonous acid. Environ Health Perspect. 2007;115(5):734–742. doi: 10.1289/ehp.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Druwe IL, Vaillancourt RR. Influence of arsenate and arsenite on signal transduction pathways: an update. Arch Toxicol. 2010;84(8):585–596. doi: 10.1007/s00204-010-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salazard B, Bellon L, Jean S, et al. Low-level arsenite activates the transcription of genes involved in adipose differentiation. Cell Biol Toxicol. 2004;20(6):375–385. doi: 10.1007/s10565-004-1471-1. [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Villasenor A, Burns AL, Salazar AM, et al. Arsenite reduces insulin secretion in rat pancreatic beta cells by decreasing the calcium-dependent calpain-10 proteolysis of SNAP-25. Toxicol Appl Pharmacol. 2008;231(3):291–299. doi: 10.1016/j.taap.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Fu J, Woods CG, Yehuda-Shnaidman E, et al. Low level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta-cells: involvement of cellular adaptive response to oxidative stress. Environ Health Perspect. 2010;118(6):864–870. doi: 10.1289/ehp.0901608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang SL, Chang FH, Liou SH, et al. Inorganic arsenic exposure and its relation to metabolic syndrome in an industrial area of Taiwan. Environ Int. 2007;33(6):805–811. doi: 10.1016/j.envint.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Navas-Acien A, Francesconi KA, Silbergeld EK, et al. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ Res. 2011;111(1):110–118. doi: 10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng CH. Arsenic methylation, urinary arsenic metabolites and human diseases: current perspective. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25(1):1–22. doi: 10.1080/10590500701201695. [DOI] [PubMed] [Google Scholar]
- 26.Karagas M, Le X, Morris S, et al. Markers of low level arsenic exposure for evaluating human cancer risks in a US population. Int J Occup Med Environ Health. 2001;14(2):171–175. [PubMed] [Google Scholar]
- 27.Rivera-Núñez Z, Meliker JR, Meeker JD, et al. Urinary arsenic species, toenail arsenic, and arsenic intake estimates in a Michigan population with low levels of arsenic in drinking water. J Expo Sci Environ Epidemiol. 2012;22(2):182–190. doi: 10.1038/jes.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng CH, Huang YK, Huang YL, et al. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol Appl Pharmacol. 2005;206(3):299–308. doi: 10.1016/j.taap.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Rubio P, Roberge J, Arendell L, et al. Associations between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol Appl Pharmacol. 2011;252(2):176–182. doi: 10.1016/j.taap.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]