Abstract
Because neutropenia may aggravate infections in alcoholics, effects of ethanol on the generation of myeloid growth factors by human umbilical vein endothelial cells (HUVECs) and on interactions with neutrophils were examined in vitro. Exposure of HUVECs to ethanol (0.01%–1%) dose-dependently inhibited (by 12%–27%) the release of stem cell factor, granulocyte-macrophage and granulocyte colony-stimulating factors (CSFs), or interleukin (IL)–8, but not of macrophage CSF triggered by lipopolysaccharide (LPS) or IL-1. Ethanol also inhibited the LPS-induced increase in HUVECs to bind neutrophils by 28% (without affecting the expression of intracellular adhesion molecule–1 and E-selectin) and inhibited the translocation of the p65 subunit of NF-κB from the cytoplasm to the nucleus by 46%. Thus, exposure of HUVECs to ethanol inhibited the generation of cytokines important for myeloid cell development and reduced the adhesiveness of HUVECs for neutrophils: effects that are possibly linked to the reduced activation of NF-κB
Ethanol abuse impairs host defense against microorganisms [1]. Many studies have demonstrated an association between alcoholism and pulmonary infections, such as those caused by pneumococci and Klebsiella organisms. Alcohol-related infections also may become more prevalent and severe in trauma victims [2]. The influence of alcohol on infection has been attributed to effects of ethanol (or its metabolites) on phagocytic cells. These effects include the inhibition of polymorphonuclear leukocyte adherence to various substrates and of the generation of superoxide ions by chemotaxis, as well as other bactericidal functions of these cells [1, 3–7]. Such actions are linked to the ability of ethanol to block the production of both intracellular messengers by phospholipase D [8, 9] and cytokines and by its inhibition of lipopolysaccharide (LPS)–induced signaling [10–12]
In addition to its impairment of phagocytic cell function, effects of ethanol on the number of such cells may contribute to the severity of alcohol-related infections. Thus, neutropenia (and thrombocytopenia), rather than the anticipated neutrophilia, may occur in alcoholics during infections [13–17]. Although the mechanisms of such actions remain unclear, a direct toxic effect of ethanol or its metabolites on myeloid precursor cells may be a contributing factor [15]. Thus, administration of the myeloid lineage-specific growth factor, granulocyte colony-stimulating factor (G-CSF), can ameliorate alcohol-associated infections [11, 18]. This effect of G-CSF has been attributed to its ability to increase the production of mature neutrophils and to enhance the bactericidal functions of these cells
Endothelial cells play an important role in host defense. The expression of adhesion molecules (e.g., E-selectin, intercellular adhesion molecule–1 [ICAM-1], and vascular cell adhesion molecule–1 [VCAM-1]) and of activation molecules (e.g., platelet-activating factor, interleukin [IL]–1, tumor necrosis factor [TNF], and IL-8) by these cells thus contributes to the extravasation of leukocytes into infected tissues. Endothelial cells also are an important source of myeloid growth factors, such as G-CSF, granulocyte-macrophage CSF (GM-CSF), macrophage CSF (M-CSF), thrombopoietin (TPO), and stem cell factor (SCF), which are released in response to stimulation with LPS, IL-1, or other agents [19–22]. The proximity of blood cell progenitors to the endothelium in highly vascularized bone marrow suggests that impairment of endothelial secretion of growth factors might affect myeloid development
We investigated the effects of ethanol in clinically relevant and pharmacologic concentrations on the production of myeloid growth factors by human endothelial cells and on the interaction of these cells with neutrophils in vitro. Because human bone marrow endothelial cells are notoriously difficult to grow in vitro, we used human umbilical vein endothelial cells (HUVECs)
Materials and Methods
MaterialsChemicals and antibodies were obtained as follows: LPS from Escherichia coli serotype O55:B5 (Sigma Chemical); IL-1β (Boehringer Mannheim); IL-1α (R&D Systems); endothelial cell growth factor (Collaborative Research); fetal bovine serum, HEPES, penicillin, streptomycin, RPMI 1640, PBS, and Hanks’s balanced salt solution (HBSS; Life Technologies); collagenase (type 3; Worthington); ethanol (Kemetyl), acetone, and methanol (Apoteksbolaget); and polystyrene plates (96 and 24 well) and other tissue culture plastic materials (TPP). All other chemicals were obtained from Sigma
We used murine monoclonal antibodies to E-selectin (33361A) or to VCAM-1 (33351A; PharMingen) and to ICAM-1 (84H10; Serotec). Rabbit polyclonal antibodies to human NF-κB subunits p50 and p65 were from Calbiochem, horseradish peroxidase (HRP)–conjugated goat antibodies to mouse immunoglobulin (IgG) were from Bio-Rad, biotinylated goat antibodies to rabbit IgG were from Vector, and HRP-conjugated porcine antibodies to rabbit IgG were from Dako
Alcohol concentrations are expressed as vol/vol percentages. Thus, 1% ethanol corresponds to a 7.9 mg/mL solution, or 0.17 M. This pharmacologic concentration was used in most experiments to detect an effect of ethanol. If such an effect was apparent, we also examined lower ethanol concentrations (0.01%–0.5%) that are relevant for mild-to-severe intoxication or abuse
Endothelial cell culture and neutrophil isolationHUVECs were obtained, as described elsewhere [23], and were used for experiments as confluent monolayers in the second or third passage. Neutrophils were isolated from healthy donors by 1-step discontinuous gradient centrifugation on Percoll (Amersham Pharmacia Biotech), as described elsewhere [24]. Purified neutrophils (>95% purity and viability) were loaded with 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM; Molecular Probes) for assay of adherence, as described elsewhere [25]. We treated HUVECs for 10 min with ethanol dissolved in HBSS containing 1% fetal bovine serum, since previous studies with neutrophils indicated that all effects of ethanol were apparent within a few minutes and were reversible by washing the cells [4, 5, 9]. Thus, ethanol was present continuously during incubation with agonists
Measurement of cytokine production by HUVECsAfter incubation of HUVECs with various agonists for 4 h, culture supernatants and cells were harvested. The concentrations of cytokines were determined by use of Quantikine assays (R&D Systems). To compensate for differences in cytokine production among HUVEC donors, we expressed cytokine concentrations relative to those determined for LPS-stimulated cells. At the concentrations used in the present study, ethanol had no effect on the cytokine assays
Expression of endothelial cell adhesion moleculesThe expression of E-selectin, VCAM-1, and ICAM-1 on the surface of HUVECs was examined by a modified cellular ELISA [26]. After treatment with ethanol and stimulation with LPS (10 ng/mL) for 4 h, HUVECs were fixed with 0.1% paraformaldehyde and were incubated for 1 h at room temperature with 5% nonfat dried milk in PBS. Adhesion molecules were labeled with specific monoclonal antibodies (1 μg/mL) for 2 h and then for 1 h with HRP-conjugated secondary antibodies. The cells were washed, and immune complexes then were detected by incubation with TMB peroxidase substrate (Bio-Rad) for 30 min, after which the reaction was terminated with sulfuric acid, and absorbance was measured at 450 nm. Data are expressed in arbitrary units and as a percentage of the level of adhesion molecule expression apparent in LPS-stimulated cells
In vitro assay of neutrophil adherenceAdherence of neutrophils to LPS-stimulated HUVEC monolayers was assessed, as described elsewhere [25, 27]. After stimulation and washing, HUVECs were exposed to BCECF-loaded neutrophils for 10 min. Nonadherent neutrophils then were removed, and fluorescence of the adherent cells was measured. We expressed the number of adherent cells as a percentage of the total number of added neutrophils. The contribution of possible interfering factors, such as neutrophil aggregation, to the adherence assay was evaluated elsewhere [25, 27]. Thus, neutrophils were not exposed to ethanol
Immunostaining of NF-κBThe staining of NF-κB was based on a method described elsewhere [28]. HUVECs grown on glass cover slips to confluence were incubated with ethanol and then were stimulated with LPS, fixed in methanol, permeabilized with acetone, and exposed to 3% H2O2. The cells were incubated with 1.5% normal goat serum for 20 min and then with antibodies to NF-κB subunits p50 and p65. Immune complexes were detected with a Vectastain ABC kit and DAB substrate for peroxidase (Vector). The cells were examined with an Olympus microscope, and micrographs were scanned with a Jandel SigmaScan Pro instrument for densitometric assessment of the ratio of staining between the nucleus and cytoplasm. We analyzed ⩾5 cells on each micrograph
Immunoblot analysis of NF-κB in cytoplasmic and nuclear extractsExtracts were prepared from HUVECs by a modified miniextraction protocol [29]. Cells were harvested by centrifugation and then were resuspended and incubated for 15 min in hypertonic buffer A [29]. After the addition of 10% of the detergent Igepal CA-630 to the suspension, nuclei were isolated by centrifugation at 13,000 g and the resulting supernatant was saved and frozen as the cytoplasmic extract. The nuclear pellet was extracted in buffer C [29]. After sonication, mixing, and centrifugation of the mixture at 13,000 g the resulting supernatant was saved and frozen as the nuclear extract. Protein concentration of the extracts was measured using the Bio-Rad protein assay
Portions of cytoplasmic and nuclear extracts were fractionated by SDS-PAGE on a 12% gel. The separated proteins were transferred electrophoretically to a nitrocellulose membrane (Bio-Rad) and were stained consecutively with rabbit antibodies to the p65 subunit of NF-κB (1 μg/mL) and HRP-conjugated secondary antibodies. Immune complexes were detected with an ECL kit (Amersham Pharmacia Biotech), according to the protocol provided by the manufacturer
Detection of apoptotic cellsAfter stimulation, HUVECs were labeled with Annexin-V-Biotin (Oncogene Research Products), and ⩾200 cells were assessed for fluorescence by UV microscopy. Apoptosis also was evaluated using the TUNEL assay (Boehringer Mannheim). Stimulated cells were fixed with 2% formaldehyde, were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate, and were incubated with fluorescein-dUTP for the direct detection of DNA fragmentation by flow cytometry
Assessment of cell viabilityHUVEC viability was assessed before experiments or after incubation with ethanol. Less than 5% of cells incubated with 1% ethanol for 4 h exhibited an altered morphology or uptake of trypan blue, indicating that membrane integrity was not disrupted under the experimental conditions of the present study. Consistent with this conclusion, the amount of lactate dehydrogenase released into culture supernatants was not increased by experimental treatments
Statistical analysisData are presented as mean±SE for the indicated number of separate experiments. Each experiment was done at least in duplicate and each assay at least in triplicate. Data were analyzed by 1-way analysis of variance, followed by the Newman-Keuls test for multiple comparisons with use of the Statistica software package (Stat Soft). All analyses are based on ⩾3 separate experiments performed with HUVECs or neutrophils from different donors
Results
Cytokine productionQuiescent HUVECs released only relatively small amounts of G-CSF, GM-CSF, and M-CSF (58±29, 20±6, and 263±30 pg/mL, respectively; n=4–12) and of TPO, SCF, and IL-8 (8±3, 9±4, and 2638±1262 pg/mL, respectively; n=3–6) into the culture supernatant. Exposure of cells to 0.01%, 0.1%, or 1% ethanol alone did not affect the release of these cytokines (figure 1; data not shown). Treatment of cells for 4 h with various agents revealed that LPS and IL-1β, but not TNF-α, leukotriene B4, lipoxin A4, histamine, or H2O2 (at concentrations of 100 ng/mL, 100 nM 100 nM 100 nM and 10 μM respectively), induced a substantial increase in G-CSF release into the culture supernatant (to 1130±140 pg/mL with LPS; n=12; P<.01 vs. quiescent cells; data not shown). An incubation time of 4 h was chosen after data from preliminary experiments and of previous studies [19, 30] suggested it would be appropriate for the detection of both stimulation and inhibition of G-CSF, GM-CSF, and IL-8 release
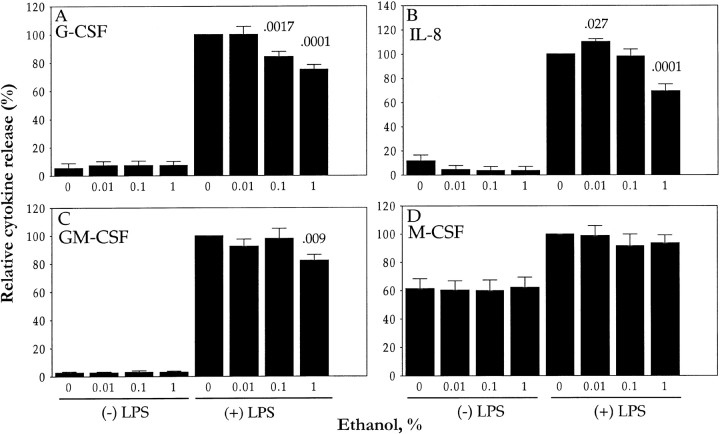
Figure 1.
Effects of ethanol on lipopolysaccharide (LPS)–induced release of granulocyte colony-stimulating factor (G-CSF; A), interleukin (IL)–8 (B) granulocyte-macrophage CSF (GM-CSF; C), and macrophage CSF (M-CSF; D) by human umbilical vein endothelial cells (HUVECs). HUVECs were exposed for 10 min to indicated ethanol concentrations and then were incubated (in continued presence of ethanol) for 4 h, with (+) or without (−) LPS (10 ng/mL), before cytokine concentrations were measured in culture supernatants. Data are mean±SE (n=3–12), expressed as a percentage of corresponding values for LPS-stimulated cells not exposed to ethanol (see Results). P values (above columns) vs. 100% value
The G-CSF response to LPS was dose dependent; it was apparent at an LPS concentration of 1 ng/mL and maximal (∼100-fold increase) at 40 ng/mL (figure 2). Higher concentrations of LPS tended to exhibit a less than maximal effect on G-CSF release and induced apoptosis, as determined by staining of cells with annexin-V (data not shown). Because the median effective dose of LPS was ∼10 ng/mL, we chose this concentration for most of the following studies. Similar experiments suggested that an IL-1β concentration of 5 U/mL was optimal for our experiments
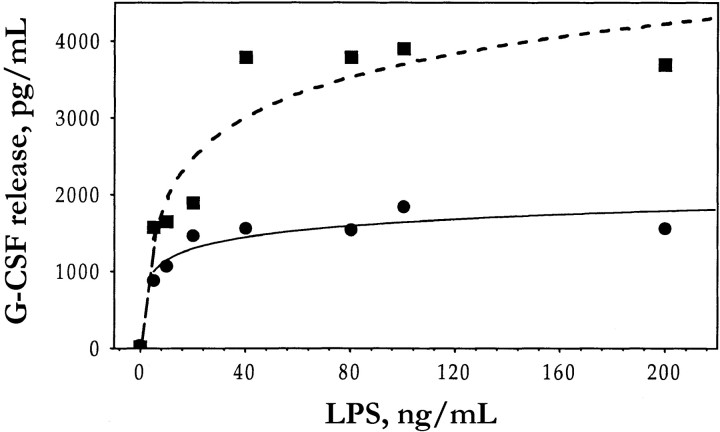
Figure 2.
Effect of lipopolysaccharide (LPS) concentration on granulocyte colony-stimulating factor (G-CSF) release by human umbilical vein endothelial cells (HUVECs) in absence (▪) or presence (•) of ethanol. HUVECs were incubated for 10 min in absence or presence of 1% ethanol and then for 4 h (in continued absence or presence of ethanol), with indicated concentrations of LPS. Culture supernatants then were assayed for G-CSF. Data are means (n=2)
Exposure of HUVECs to ethanol for 10 min before incubation with LPS (figure 1A) or IL-1β (data not shown) resulted in a dose-dependent inhibition of stimulated G-CSF release. Maximal inhibition (25%) of LPS-induced G-CSF release was apparent at an ethanol concentration of 1%; a significant inhibitory effect (16%) also was apparent at 0.1% ethanol. The percentage of inhibition of G-CSF release by 1% ethanol was similar over a wide range of LPS concentrations (figure 2), which suggests that the effect of ethanol is noncompetitive
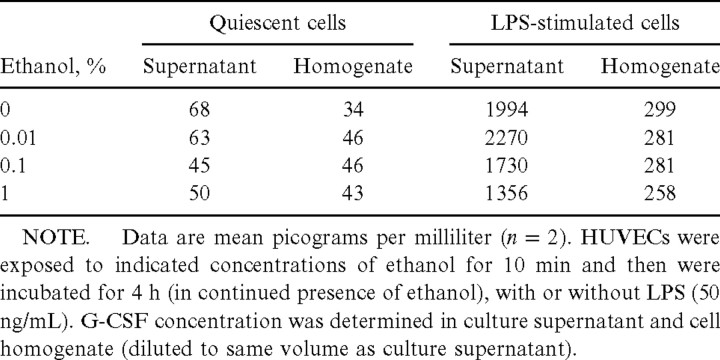
It is possible that ethanol impaired the actual secretion rather than the synthesis of G-CSF by HUVECs, which would be similar to the inhibition by ethanol of degranulation in polymorphonuclear leukocytes [5]. To investigate this possibility, we also measured the amount of this cytokine in cell homogenates. The amount of intracellular G-CSF in quiescent HUVECs was only ∼50% of that released into the culture supernatant (table 1). Although LPS induced an ∼9-fold increase in the amount of intracellular G-CSF, it increased the amount of extracellular G-CSF by a factor of 29 in these experiments. However, although it reduced the amount of G-CSF released into the culture supernatant of LPS-stimulated HUVECs in a dose-dependent manner, ethanol did not markedly affect either the release of G-CSF by quiescent cells or the amount of intracellular G-CSF in quiescent or LPS-stimulated cells. Thus, most of the G-CSF produced by HUVECs was released into the culture medium, and ethanol affected only the LPS-induced increase in this portion of total cellular G-CSF; thus, ethanol appeared to inhibit the synthesis of this cytokine by HUVECs
Table 1.
Effects of ethanol on the intracellular (cell homogenate) and extracellular (culture supernatant) concentrations of granulocyte colony-stimulating factor (G-CSF) for quiescent and lipopolysaccharide (LPS)–stimulated human umbilical vein endothelial cells (HUVECs) in vitro
IL-1β induced a dose-dependent increase in total G-CSF production (from 24.3±6.2 for quiescent HUVECs to 1215±173 pg/mL for cells stimulated with IL-1β at 5 U/mL). Ethanol (1%) reduced total G-CSF production (released plus intracellular cytokine), in the presence of 5 U of IL-1β/mL, to 696±72 pg/mL (i.e., mean±SE, 65%±6% of the value for cells exposed to IL-1β alone; n=4; P=.008). Thus, ethanol similarly affected LPS and IL-1β signaling pathways
LPS induced an 8-fold increase in the release of IL-8 by HUVECs (to 21.2±3.5 ng/mL; n=6; P<.01, compared with quiescent cells). However, although 1% ethanol inhibited LPS-induced IL-8 release by 27%, 0.01% ethanol induced a small (12%) but significant increase in LPS-stimulated IL-8 release (figure 1B). As with G-CSF, most IL-8 produced by HUVECs is secreted [30]. LPS also induced a 33-fold increase in the release of GM-CSF by HUVECs (to 668±65 pg/mL; n=9; P=.01). This effect of LPS was inhibited by 18% in the presence of 1% ethanol (figure 1C). LPS about doubled the release of M-CSF by HUVECs (to 488±80 pg/mL; n=9; P<.01). However, in contrast to its effects on G-CSF, GM-CSF, and IL-8 release, ethanol (1%) had no effect on LPS-induced M-CSF release (figure 1D)
Incubation of HUVECs with LPS or IL-1β for 4 or 24 h had no effect on the released or intracellular concentrations of TPO or SCF (14±5 and 8±5 pg/mL, respectively, after 4 h; n=3 and n=5). In contrast, incubation of cells with IL-1α (2 ng/mL) for 24 h increased the concentration of SCF in the culture supernatant to 59±13 pg/mL, compared with 31±8 pg/mL for quiescent cells (mean±SE; n=5; P<.05). IL-1α had no effect on TPO release. Ethanol at concentrations of 0.5% and 1% inhibited the IL-1α–induced release of SCF by 18%±1.5% and 11.8%±2.3%, respectively (P<.05; n=5 for each)
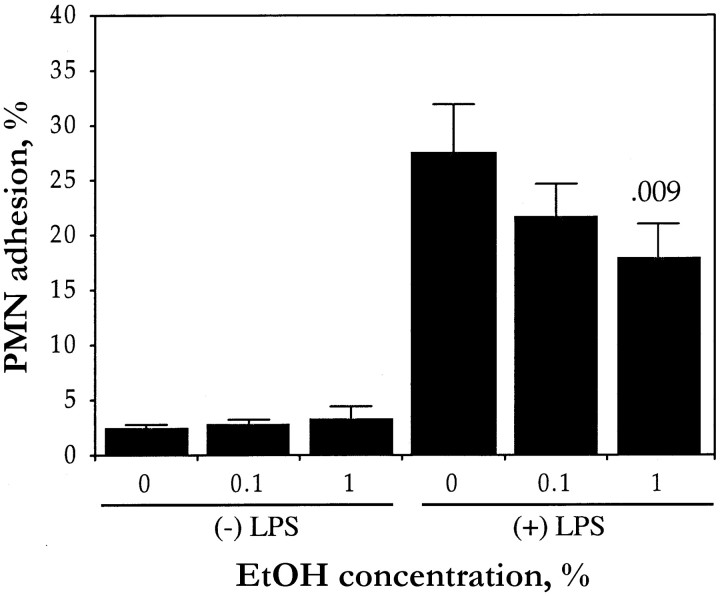
Neutrophil adhesion to HUVECsWe next investigated whether the ethanol-induced inhibition of cytokine production, particularly that of IL-8 (since IL-8 is an important activation molecule that is coexpressed with E-selectin), by stimulated HUVECs was associated with a reduction in the adhesion of neutrophils to these cells. In our assay, the number of neutrophils that adhered to quiescent HUVECs was 1%–5% of the total number of added cells and was not affected by exposure of the HUVECs to ethanol (figure 3). Stimulation of HUVECs with LPS for 4 h induced an ∼10-fold increase in the adhesion of neutrophils. However, exposure of HUVEC monolayers to ethanol resulted in a dose-dependent inhibition of LPS-induced neutrophil adhesion: 18% and 28% at concentrations of 0.1% and 1%, respectively
Figure 3.
Effect of ethanol (EtOH) on neutrophil (PMN) adhesion to human umbilical vein endothelial cells (HUVECs). HUVECs were incubated for 10 min with indicated EtOH concentrations and then for 4 h (in continued presence of EtOH), with (+) or without (−) lipopolysaccharide (LPS; 10 ng/mL). Cells were washed and were incubated for 10 min with 2×105 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester–loaded neutrophils, before the determination of percentage of adherent neutrophils. Data are mean±SE (n=8). P value (above column) compares LPS-stimulated HUVECs not exposed to EtOH
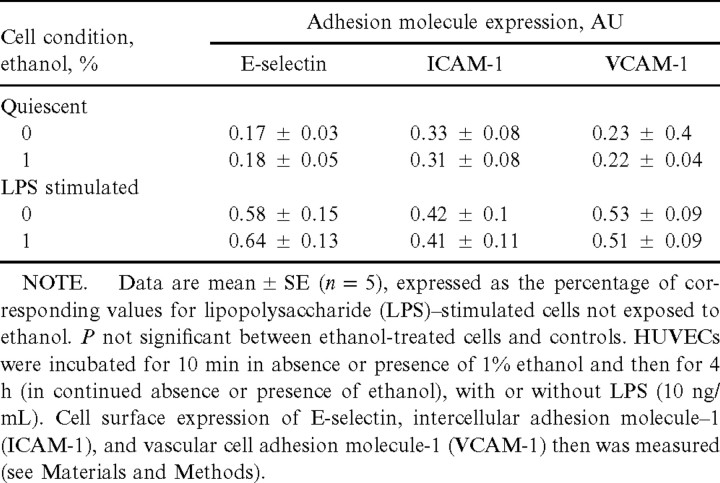
Expression of adhesion molecules by HUVECsTo determine whether the ethanol-induced inhibition of HUVEC adhesiveness was accompanied by a reduction in the extent of adhesion molecule expression by these cells, we analyzed the surface abundance of E-selectin, ICAM-1, and VCAM-1. Both E-selectin and ICAM-1 are coexpressed with IL-8 in LPS-stimulated HUVECs and are essential for the binding of neutrophils, whereas VCAM-1 mediates the binding by HUVECs of other leukocytes. Quiescent HUVECs exhibited only a low level of expression of E-selectin, ICAM-1, and VCAM-1 (0.17±0.03, 0.35±0.08, and 0.23±0.04 arbitrary units, respectively), and ethanol did not affect the expression of these molecules. Stimulation of cells with LPS for 4 h induced 3.4-, 1.3-, and 2.3-fold increases in the abundance of E-selectin, ICAM-1, and VCAM-1, respectively. However, unlike its effects on IL-8 production and neutrophil adhesion, ethanol did not markedly affect the LPS-induced expression of any of these 3 adhesion molecules (table 2). Thus, the inhibitory effect of ethanol on the ability of LPS-stimulated HUVECs to bind neutrophils does not appear to be attributable to a reduced expression of adhesion molecules. However, we cannot rule out the possibility that ethanol affects the biologic activity of these adhesion molecules
Table 2.
Effects of ethanol on surface expression of adhesion molecules by human umbilical vein endothelial cells (HUVECs) in vitro
NF-κB translocation in HUVECsGiven that NF-κB mediates the transcriptional activation of the genes that encode G-CSF, GM-CSF, and IL-8 [31], we investigated the possible role of this transcription factor in the ethanol-induced inhibition of the production of these cytokines by HUVECs. Therefore, we examined the effect of ethanol on the translocation of NF-κB from the cytoplasm to the nucleus
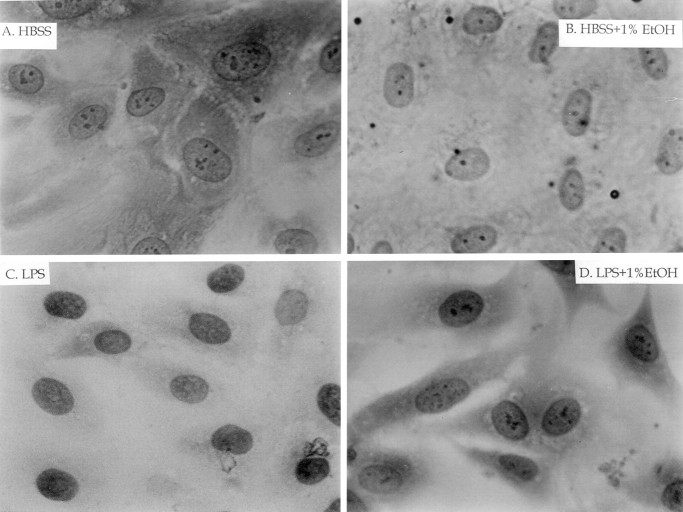
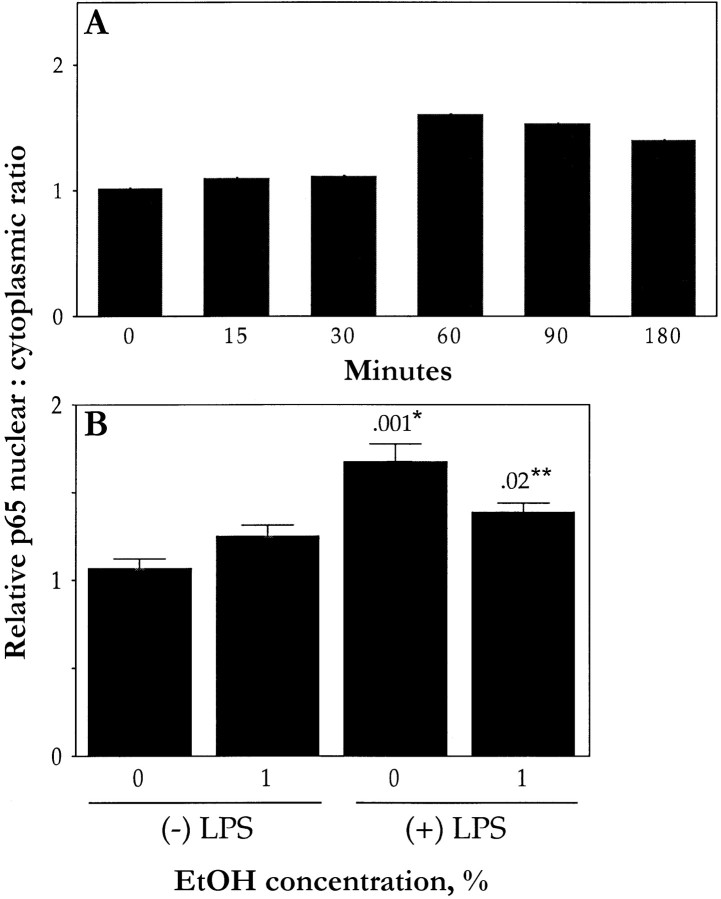
We first examined the translocation of the p65 subunit of NF-κB by immunostaining. In quiescent HUVECs, p65 was detected in the cytoplasm but not in the nucleus (figure 4A). Conversely, LPS-stimulated cells exhibited pronounced staining for p65 in the nucleus, with minimal staining in the cytoplasm (figure 4C), presumably reflecting the translocation of p65 from the cytoplasm to the nucleus. Densitometric analysis showed that the LPS-induced translocation of p65 to the nucleus was time dependent; the effect of LPS (10 ng/mL) was apparent after 30 min and maximal after 60 min (figure 5A). Similar analysis revealed that the median effective dose for this effect of LPS was 10 ng/mL (data not shown). Exposure of cells to ethanol inhibited the LPS-induced translocation of p65 to the nucleus by 46% (figure 4D and figure 5B); ethanol had no effect on the subcellular distribution of p65 in quiescent cells (figure 4B and figure 5B). Immunostaining and densitometric analysis with antibodies to the p50 subunit of NF-κB showed an increased nuclear accumulation after LPS treatment (data not shown). Ethanol (1%) reduced the LPS-induced translocation of p50 to the nucleus by only 13% (P>.05)
Figure 4.
Immunostaining analysis of effects of lipopolysaccharide (LPS) and ethanol (EtOH) on subcellular distribution of p65 subunit of NF-κB in human umbilical vein endothelial cells (HUVECs). HUVECs were incubated for 10 min in absence (A and C) or presence (B and D) of 1% EtOH and then for 1 h (in continued absence or presence of EtOH) with (C and D) or without (A and B) LPS (10 ng/mL). HUVECs were examined by interference microscopy. Original magnification, ×1000. HBSS, Hanks’s balanced salt solution
Figure 5.
Densitometric analysis of p65 translocation in human umbilical vein endothelial cells (HUVECs). A HUVECs incubated for indicated times in presence of lipopolysaccharide (LPS; 10 ng/mL) and then immunostained with antibodies to p65 and densitometric analysis. Nuclear:cytoplasmic ratio of p65 staining was determined (results are expressed relative to value for zero time point). Data are means (n=2). B HUVECs incubated for 10 min in absence or presence of 1% ethanol (EtOH) and then for 1 h (in continued absence or presence of ethanol), with (+) or without (−) LPS (10 ng/mL), and analyzed as in panel A. Data are mean±SE (n=3). P values (above bars) are for comparisons with cells not exposed to either ethanol or LPS (*) or with LPS-stimulated cells not exposed to ethanol (**)
Immunoblot analysis also revealed that most p65 in quiescent HUVECs was present in the cytoplasmic fraction (figure 6). Stimulation of cells with LPS resulted in a decrease of p65 in the cytoplasmic fraction and a corresponding increase in the amount of this subunit in the nuclear fraction. Exposure of cells to 1% ethanol reduced the extent of these effects of LPS on the abundance of p65 in the cytoplasmic and nuclear fractions
Figure 6.
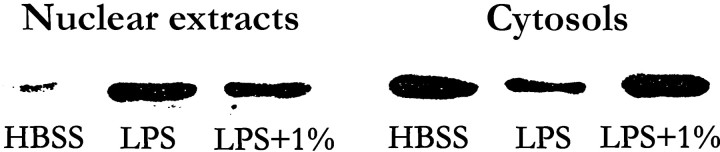
Immunoblot analysis of nuclear translocation of p65. Human umbilical vein endothelial cells were incubated for 10 min in the absence or presence of 1% ethanol and then for 1 h (in continued absence or presence of ethanol), with or without lipopolysaccharide (LPS; 10 ng/mL), as indicated. Nuclear and cytoplasmic extracts (cytosols) then were prepared and were subjected (10 μg of protein) to immunoblot analysis with antibodies to p65. Data are for 1 experiment (repeated 3 times with similar results). HBSS, Hanks’s balanced salt solution
Because these experiments suggested a relationship between inhibition of NF-κB translocation and cytokine generation, we assessed the effect of an NF-κB antagonist, pyrrolidine dithiocarbamate (PDTC; 100 μM added 30 min before LPS stimulation of HUVECs) on G-CSF release. PDTC caused a 54.6% reduction (n=3), which suggests that NF-κB was essential for the reaction
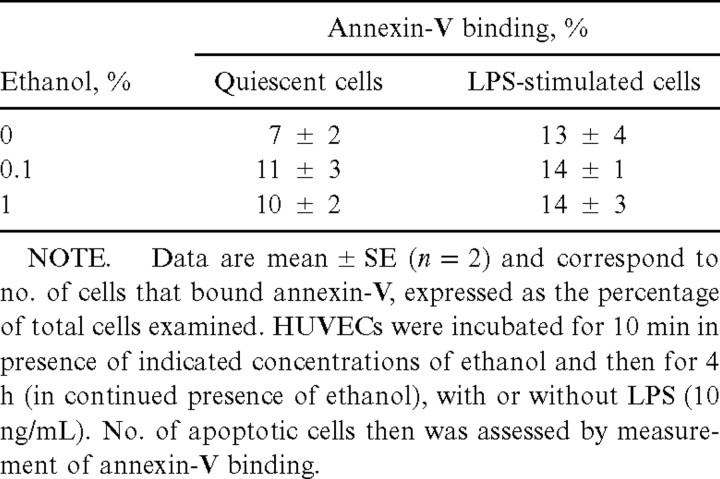
ApoptosisTo demonstrate that the apparent inhibition by ethanol of cytokine release and NF-κB translocation in LPS-stimulated HUVECs was not actually due to an ongoing apoptotic process (which would not result in cytolysis detectable with trypan blue or lactate dehydrogenase release), we assessed the extent of apoptosis in the cell cultures. Incubation of HUVECs for 4 h with 1% ethanol in the absence or presence of LPS did not increase the surface binding of annexin-V (table 3), which suggests that ethanol does not induce apoptosis in these cells. This conclusion was verified by TUNEL assay, to ensure that the effects of ethanol on membrane fluidity and phospholipid expression [32] did not interfere with annexin-V binding. The TUNEL assay failed to detect apoptosis in ethanol-treated cells (data not shown)
Table 3.
Effect of ethanol on apoptosis in quiescent or lipopolysaccharide (LPS)–stimulated human umbilical vein endothelial cells (HUVECs) in vitro
Ethanol specificityWe also investigated the specificity of the observed effects of ethanol by examining the effects of methanol, propanol, and butanol on cell viability and G-CSF release. These alcohols were chosen because they affect cell membrane fluidity and exert osmotic effects [32]. At a concentration of 0.1%, butanol and propanol each induced the detachment of ∼25%–50% of HUVECs from the substratum. At a concentration of 1%, methanol did not appear to affect the morphology of HUVECs, and it inhibited the G-CSF release induced by LPS (10 ng/mL) by ∼50%; the inhibitory effect of ethanol also was ∼50% in this particular experiment (data not shown). Thus, the short-chain alcohols methanol and ethanol similarly impaired G-CSF release, whereas the cytotoxic action of longer-chain alcohols prevented their further evaluation
Discussion
Our results show that ethanol inhibits the release of G-CSF, GM-CSF, and IL-8 induced by LPS in HUVECs. It also inhibits the IL-1β–induced release of G-CSF and the IL-1α–induced release of SCF by these cells. The inhibition of LPS-induced cytokine release by ethanol was accompanied by inhibitory effects on both the nuclear translocation of NF-κB and the ability of HUVECs to bind neutrophils. In contrast, the LPS-induced release of M-CSF and the surface expression of ICAM-1, VCAM-1, and E-selectin in HUVECs were not affected by ethanol. Because endothelial cells do not express alcohol dehydrogenase, it is very unlikely that acetaldehyde, the major metabolite of ethanol in vivo, mediates the in vitro effects observed here. Also, because ethanol did not affect M-CSF production elicited by LPS, and since LPS- and IL-1–induced G-CSF release were similarly inhibited, it is unlikely that ethanol altered the physical state of LPS in the incubation medium
Given that G-CSF promotes both neutrophil proliferation and maturation and facilitates various functional responses of mature neutrophils, the inhibition of G-CSF release by endothelial cells may contribute to the increased incidence and poorer outcome of bacterial diseases and to the neutropenia associated with such infections in persons who abuse alcohol [1, 2, 11, 13, 14, 16, 17, 33]. The inhibition by ethanol of GM-CSF and SCF release may similarly contribute to the increased susceptibility to bacterial infection that is associated with alcohol abuse. Although the inhibitory effects of ethanol on the release of these myeloid growth factors were each relatively modest and apparent only at the higher concentrations of ethanol examined, the combination of these actions might be of clinical relevance. This conclusion is supported by the observation that ethanol intake markedly reduces the plasma concentration of G-CSF in rodents when the blood ethanol concentration reaches ∼300–350 mg/dL (∼0.4%) [34]
The inhibitory effect of ethanol on IL-8 production also might contribute to the impairment of host defenses by alcohol, given that this chemokine induces the release of mature neutrophils from the bone marrow and promotes neutrophil adhesion to endothelial cells and the subsequent migration and chemotaxis of these leukocytes to foci of infection [35]. In particular, the coexpression of E-selectin and IL-8 by endothelial cells constitutes a powerful signal for neutrophils to attach to the endothelial surface and activates them through specific IL-8 receptors [30]. Our observation that ethanol inhibited neutrophil adhesion to LPS-stimulated HUVECs is consistent with previous findings, that ethanol reduces the adherence of neutrophils to various surfaces [4]. The neutrophils were not exposed to ethanol in our experiments, which suggests that the inhibitory effect of ethanol was mediated only at the level of the endothelial cells. Ethanol did not affect the surface expression by HUVECs of adhesion molecules that are thought to play important roles in neutrophil adherence to LPS- or IL-1β–stimulated endothelial cells. This lack of effect suggests that ethanol exerts a selective action on cell signaling in HUVECs that may or may not be related to the inhibition of phospholipase D–dependent Ca2+ transients [36]
In addition to adhesion molecule expression, the LPS-induced release of M-CSF by HUVECs was resistant to ethanol treatment. This observation suggests that different signaling pathways underlie the effects of LPS on G-CSF, GM-CSF, SCF, and IL-8 generation on the one hand and those on M-CSF production and adhesion molecule expression on the other. Since NF-κB mediates the transcriptional activation of G-CSF, GM-CSF, and IL-8 genes [31] and previous data suggest that TNF-α–induced M-CSF release is only partly, if at all, dependent on NF-κΒ activation [37], we examined the effect of ethanol on the NF-κB signaling pathway. We showed that LPS induces the translocation of both the p50 and p65 subunits of NF-κB from the cytoplasm to the nucleus of HUVECs and that ethanol inhibits this effect of LPS. Ethanol previously was shown to inhibit the nuclear translocation of NF-κB and subsequent cytokine production in rat alveolar macrophages, rat Kupffer cells, and human monocytes [38–40]. In experiments on A549 cells, we found that ethanol does not influence the binding of NF-κB subunits to a transfected reporter gene or the activity of a constitutively active CMV construct, which suggests that the effects of ethanol occurred upstream of the gene transcription steps (authors’ unpublished data)
The lack of effect of ethanol on LPS-induced expression of adhesion molecules appears to be inconsistent with the ethanol-induced inhibition of the nuclear translocation of NF-κB observed in LPS-stimulated cells, since transcription of E-selectin, ICAM-1, and VCAM-1 genes is thought to be activated by NF-κB [31]. However, the specific signaling and transcriptional activation mechanisms that underlie E-selectin, ICAM-1, and V-CAM-1 expression probably differ from those responsible for G-CSF, GM-CSF, and IL-8 expression. Thus, NF-κB appears to interact with various other signaling and transcription factors, including components of the p38 pathway [12], NF-IL-6 [41], and AP-1 [42]. Moreover, the coactivators p300 and CBP (CREB-binding protein) potentiate the ability of p65 to regulate gene expression [43]
The inhibition by ethanol of G-CSF release from endothelial cells, a major producer of myeloid growth factors in the bone marrow stroma, also might be relevant to the beneficial effects of exogenous G-CSF in ethanol-treated animals. Administration of G-CSF to such animals reduces mortality associated with LPS-induced inflammation or bacterial infection [11, 18]. Thus, the immunosuppressive and anti-inflammatory effects of ethanol might be of interest in relation to prevention of cardiovascular disease [44]
Acknowledgments
We thank A. Landström, K. Rosendahl, and H. Farzin, for technical assistance, and J. Lidén, for advice on Western blot analyses of p65
Footnotes
Financial support: Swedish Medical Research Council (grants 19X-05991 and 71XS-13135); Swedish Association against Rheumatism; Karolinska Institute; Huddinge University Hospital; Funds of Å. Wiberg, I.-B. and A. Lundberg; King Gustaf V’s 80-Year, Uggla, N. Svartz, Vårdal; and Cancer Society of Stockholm
References
- 1.MacGregor RR. Alcohol and immune defense. JAMA. 1986;256:1474–8. [PubMed] [Google Scholar]
- 2.Jurkovich GJ, Rivara FP, Gurney JG, et al. The effect of acute alcohol intoxication and chronic alcohol abuse on outcome from trauma. JAMA. 1993;270:51–6. [PubMed] [Google Scholar]
- 3.MacGregor RR, Safford M, Shalit M. Effect of ethanol on functions required for the delivery of neutrophils to sites of inflammation. J Infect Dis. 1988;157:682–9. doi: 10.1093/infdis/157.4.682. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson E, Lindström P, Patarroyo M, et al. Ethanol impairs certain aspects of neutrophil adhesion in vitro: comparisons with inhibition of expression of the CD18 antigen. J Infect Dis. 1991;163:591–7. doi: 10.1093/infdis/163.3.591. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson E, Halldén G, Magnusson KE, Hed J, Palmblad J. In vitro effects of ethanol on polymorphonuclear leukocyte membrane receptor expression and mobility. Biochem Pharmacol. 1996;51:225–31. doi: 10.1016/0006-2952(95)02120-5. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson E, Thomsen P, Ericsson L, Palmblad J. Rabbit polymorphonuclear granulocyte function during ethanol administration: migration and oxidative responses in a joint with immune complex synovitis. Clin Exp Immunol. 1995;102:137–43. doi: 10.1111/j.1365-2249.1995.tb06647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libon C, Forestier F, Cotte-Lafitte J, Labarre C, Quero AM. Effect of acute oral administration of alcohol on superoxide anion production from mouse alveolar macrophages. J Leukoc Biol. 1993;53:93–8. doi: 10.1002/jlb.53.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Bosner RW, Thompson NT, Randall RW, Garland LG. Phospholipase D activation is functionally linked to superoxide generation in the human neutrophil. Biochem J. 1989;264:617–20. doi: 10.1042/bj2640617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilsson E, Andersson T, Fällman M, Rosendahl K, Palmblad J. Effects of ethanol on the chemotactic peptide-induced second messenger generation and superoxide production in polymorphonuclear leukocytes. J Infect Dis. 1992;166:854–60. doi: 10.1093/infdis/166.4.854. [DOI] [PubMed] [Google Scholar]
- 10.Verma BK, Fogarasi M, Szabo G. Down-regulation of tumor necrosis factor alpha activity by acute ethanol treatment in human peripheral blood monocytes. J Clin Immunol. 1993;13:8–22. doi: 10.1007/BF00920631. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P, Bagby GJ, Stoltz DA, Summer WB, Nelson S. Granulocyte colony-stimulating factor modulates the pulmonary host response to endotoxin in the absence and presence of acute ethanol intoxication. J Infect Dis. 1999;179:1441–8. doi: 10.1086/314763. [DOI] [PubMed] [Google Scholar]
- 12.Arbabi S, Garcia I, Bauer GJ, Maier RV. Alcohol (ethanol) inhibits IL-8 and TNF: role of the p38 pathway. J Immunol. 1999;162:7441–5. [PubMed] [Google Scholar]
- 13.Liu YK. Effects of alcohol on granulocytes and lymphocytes. Semin Hematol. 1980;17:130–6. [PubMed] [Google Scholar]
- 14.Perlino CA, Rimland D. Alcoholism, leukopenia, and pneumococcal sepsis. Am Rev Respir Dis. 1985;132:757–60. doi: 10.1164/arrd.1985.132.4.757. [DOI] [PubMed] [Google Scholar]
- 15.Meagher RC, Sieber F, Spivak JL. Suppression of hematopoietic-progenitor-cell proliferation by ethanol and acetaldehyde. N Engl J Med. 1982;307:845–9. doi: 10.1056/NEJM198209303071402. [DOI] [PubMed] [Google Scholar]
- 16.Imperia PS, Chikkappa G, Philips PG. Mechanism of inhibition of granulopoiesis by ethanol. Proc Soc Exp Biol Med. 1984;175:219–25. doi: 10.3181/00379727-175-41792. [DOI] [PubMed] [Google Scholar]
- 17.Malik F, Wickramasinghe SN. Haematological abnormalities in mice continuously exposed to ethanol vapour. Br J Exp Pathol. 1986;67:831–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson S, Summer W, Bagby G, et al. Granulocyte colony-stimulating factor enhances pulmonary host defenses in normal and ethanol-treated rats. J Infect Dis. 1991;164:901–6. doi: 10.1093/infdis/164.5.901. [DOI] [PubMed] [Google Scholar]
- 19.Lenhoff S, Olofsson T. Cytokine regulation of GM-CSF and G-CSF secretion by human umbilical cord vein endothelial cells (HUVEC) Cytokine. 1996;8:702–9. doi: 10.1006/cyto.1996.0093. [DOI] [PubMed] [Google Scholar]
- 20.Fixe P, Praloran V. M-CSF: hematopoietic growth factor or inflammatory cytokine. Cytokine. 1998;10:32–7. doi: 10.1006/cyto.1997.0249. [DOI] [PubMed] [Google Scholar]
- 21.Cardier JE, Dempsey J. Thrombopoietin and its receptor, c-mpl, are constitutively expressed by mouse liver endothelial cells. Blood. 1998;91:923–9. [PubMed] [Google Scholar]
- 22.Koenig A, Yakisan E, Reuter M, et al. Differential regulation of stem cell factor mRNA expression in human endothelial cells by bacterial pathogens: an in vitro model of inflammation. Blood. 1994;83:2836–43. [PubMed] [Google Scholar]
- 23.Palmblad J, Lerner R, Larsson SH. Signal transduction mechanism for leukotriene B4 induced hyperadhesiveness of endothelial cells for neutrophils. J Immunol. 1994;152:262–9. [PubMed] [Google Scholar]
- 24.Ringertz B, Palmblad J, Lindgren JÅ Stimulus-specific neutrophil aggregation: evaluation of possible mechanisms for the stimulus-response apparatus. J Lab Clin Med. 1985;106:132–40. [PubMed] [Google Scholar]
- 25.Lerner R, Heimburger M, Palmblad J. Lipoxin A4 induces hyperadhesiveness in human endothelial cells for neutrophils. Blood. 1993;82:948–53. [PubMed] [Google Scholar]
- 26.Hashemi S, Smith CD, Izaguirre CA. Anti-endothelial cell antibodies: detection and characterization using a cellular enzyme-linked immunosorbent assay. J Lab Clin Med. 1987;109:434–40. [PubMed] [Google Scholar]
- 27.Heimburger M, Palmblad JE. Effects of leukotriene C4 and D4, histamine and bradykinin on cytosolic calcium concentrations and adhesiveness of endothelial cells and neutrophils. Clin Exp Immunol. 1996;103:454–60. doi: 10.1111/j.1365-2249.1996.tb08302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weyrich AS, McIntyre TM, McEver RP, Prescott SM, Zimmerman G. Monocyte tethering by P-selectin regulates monocyte chemotactic protein–1 and tumor necrosis factor–α secretion. J Clin Invest. 1995;95:2297–303. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with “mini-extracts” prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bratt J, Palmblad J. Cytokine-induced neutrophil-mediated injury of human endothelial cells. J Immunol. 1997;159:912–8. [PubMed] [Google Scholar]
- 31.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 32.Yuli I, Tomonaga A, Snyderman R. Chemoattractant receptor functions in human polymorphonuclear leukocytes are divergently altered by membrane fluidizers. Proc Natl Acad Sci USA. 1982;79:5906–10. doi: 10.1073/pnas.79.19.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandes-Sola J, Junque A, Estruch R, Monforte R, Torres A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155:1649–54. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- 34.Bragby GJ, Zhang P, Stoltz DA, Nelson S. Suppression of the granulocyte colony-stimulating factor response to E. coli challenge by alcohol intoxication. Alcohol Clin Exp Res. 1998;22:1740–5. [PubMed] [Google Scholar]
- 35.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 36.Lerner R. Changes of cytosolic calcium ion concentrations in human endothelial cells in response to thrombin, platelet-activating factor, and leukotriene B4. J Lab Clin Med. 1994;124:723–9. [PubMed] [Google Scholar]
- 37.Isaacs SD, Fan X, Fan D, et al. Role of NFκB in the regulation of macrophage colony stimulating factor by tumor necrosis factor–α in ST2 bone stromal cells. J Cell Physiol. 1999;179:193–200. doi: 10.1002/(SICI)1097-4652(199905)179:2<193::AID-JCP9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Greenberg SS, Ouyang J, Zhao X, Xie J, Wang JF. Interaction of ethanol with inducible nitric oxide synthase messenger RNA and protein: direct effects on autocoids and endotoxin in vivo. J Pharmacol Exp Ther. 1997;282:1044–54. [PubMed] [Google Scholar]
- 39.Fox ES, Cantrell CH, Leingang KA. Inhibition of the Kupffer cell inflammatory response by acute ethanol: NFκB activation and subsequent cytokine production. Biochem Biophys Res Commun. 1996;225:134–40. doi: 10.1006/bbrc.1996.1142. [DOI] [PubMed] [Google Scholar]
- 40.Mandrekar P, Catalano D, Szabo G. Inhibition of polysaccharide-mediated NFκB activation by ethanol in human monocytes. Int Immunol. 1999;11:1781–90. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- 41.Dunn SM, Coles LS, Lang RK, Gerondikes S, Vadas MA. Requirement for nuclear factor (NF)–κB p65 and NF-interleukin–binding elements in the tumor necrosis factor response region of the granulocyte colony-stimulating factor promoter. Blood. 1994;83:2469–79. [PubMed] [Google Scholar]
- 42.Ahmad M, Theofanidis P, Medford RM. Role of activating protein-1 in the regulation of the vascular cell adhesion molecule–1 gene expression by tumor necrosis factor–α. J Biol Chem. 1998;273:4616–21. doi: 10.1074/jbc.273.8.4616. [DOI] [PubMed] [Google Scholar]
- 43.Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927–32. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB. Effect of alcohol on consumption on systemic markers of inflammation. Lancet. 2001;357:763–7. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]