Abstract
The natriuretic peptides (NPs) family, including atrial, B-type, and C-type NPs, is a group of hormones possessing relevant haemodynamic and anti-remodelling actions in the cardiovascular (CV) system. Due to their diuretic, natriuretic, vasorelaxant, anti-proliferative, and anti-hypertrophic effects, they are involved in the pathogenic mechanisms leading to major CV diseases, such as heart failure (HF), coronary artery disease, hypertension and left ventricular hypertrophy, and cerebrovascular accidents. Blood levels of NPs have established predictive value in the diagnosis of HF, as well as for its prognostic stratification. In addition, they provide useful clinical information in hypertension and in both stable and unstable coronary artery disease. Structural abnormalities of atrial natriuretic peptide gene (NPPA), as well as genetically induced changes in circulating levels of NPs, have a pathogenic causal link with CV diseases and represent emerging markers of CV risk. Novel NP-based therapeutic strategies are currently under advanced clinical development, as they are expected to contribute to the future management of hypertension and HF.
The present review provides a current appraisal of NPs’ clinical implications and a critical perspective of the potential therapeutic impact of pharmacological manipulation of this class of CV hormones.
Keywords: Natriuretic peptides, Cardiovascular diseases, Genetics, Natriuretic peptides analogues, NEP inhibitors, ARNi
Biology of the natriuretic peptides system
The natriuretic peptides (NPs) family includes three well-characterized hormones: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP) that are intimately involved in the maintenance of cardio-renal homeostasis. Atrial natriuretic peptide and BNP are synthetized mainly in the heart, and to a lesser extent in other organs, whereas CNP is mainly produced by endothelial cells.1,2 They are synthetized as pre-prohormones and subsequently cleaved to obtain a biological active α-carboxy terminal peptide along with the amino-terminal end3–5 (Figure 1).
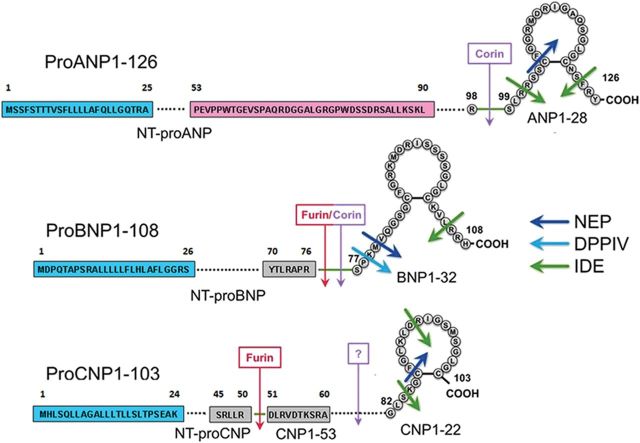
Figure 1.
Schematic representation of natriuretic peptides structure, cleavage processing, and degradation. ProANP1-126 and signal peptide, cleaved to NT-proANP1-98 and the active hormone ANP1-28. Recognition of the mid-regional portion (amino acids 53–90, pink colour) within NT-proANP allows measurement of MR-proANP; ProBNP1-108 and signal peptide, cleaved to NT-proBNP1-76 and the active hormone BNP1-32; ProCNP1-103 and signal peptide, cleaved to NT-proCNP1-80(50) and the active hormones CNP1-53 and CNP1-22. Red and purple arrows indicate processing sites by furin and corin. Blue and green arrows indicate NPs degrading enzymes: neutral endopeptidase (NEP), dipeptydil peptidase IV (DPPIV), and insulin degrading enzyme (IDE).
Soluble guanylyl cyclase (GC) receptors mediate NPs effects in target tissues. GC-A receptor is the main effector of both ANP and BNP actions, whereas GC-B receptor mediates CNP effects. An increase in cyclic guanylate monophosphate (cGMP) levels follows the activation of both GC-A and B receptors6 (Figure 2). An additional natriuretic peptide receptor (NPR-C) plays a fundamental role in NPs clearance. Moreover, NPR-C mediates vasoprotective properties of CNP and it has been also involved in cellular signalling pathways leading to antiproliferative and pro-apoptotic effects in specific circumstances.7 Different from GC-A and B receptors, NPR-C contains a 37-amino acid cytoplasmic domain with a Gα inhibitory protein-activating sequence and it is devoid of kinase and GC activities. A decrease in cyclic adenylate monophosphate levels follows its activation (Figure 2).7
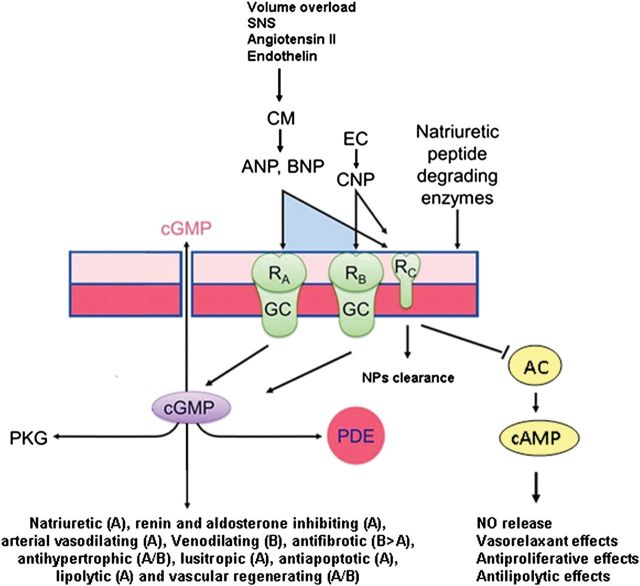
Figure 2.
Natriuretic peptides produced by cardiomyocytes (CM) upon haemodynamic and neuroendocrine regulation act on target cells through specific receptors, soluble guanylyl cyclase (sGC), type A and B (GC-A and GC-B). The type of biological effect mediated by each receptor through the cGMP pathway, involving protein kinase G (PKG) and phosphodiesterase (PDE), is described. C-type natriuretic peptide produced by endothelial cells (EC) acts through GC-B and also through natriuretic peptide receptor (NPR-C), a non-GC receptor able to inhibit adenylate cyclase (AC) and to reduce cAMP levels. The biological effects of NPR-C within the cardiovascular system are shown. NPR-C plays also a fundamental role in NPs clearance.
Old and novel functional properties of natriuretic peptides
Natriuretic peptides have long been viewed as important cardio-renal hormones mostly because of their key role in the regulation of electrolytes and water balance homeostasis as well as of blood pressure (BP) levels through diuretic, natriuretic, and vasorelaxant effects, along with the ability to inhibit the renin–angiotensin–aldosterone system (RAAS) and the sympathetic nervous system (SNS).1,2 They also contribute to modulate systemic vascular resistance mainly by inhibiting the contraction of vascular smooth muscle cells through cGMP-dependent kinases.2 Of note, it was shown that supraphysiologic concentrations of ANP did not produce vasodilation in the intestinal and skeletal muscle microcirculation, and that ANP did not counteract the vasoconstriction dependent from norepinephrine and arginine vasopressin in these vascular districts,8 supporting the existence of regional differences in vascular sensitivity to ANP.9
A part from mechanical factors, i.e. increased volume overload and myocytes stress, hormonal stimuli are involved in the control of NPs release.1–3,10,11 Brain natriuretic peptide increases in a broad range of disease conditions, i.e. myocardial fibrosis and ischaemia, ventricular remodelling, and overload, all underlying chronic left ventricular (LV) dysfunction.
Besides the well-described haemodynamic functions, new properties of NPs have been discovered over the past two decades that relate to the interaction with cellular growth and proliferation at the vascular level.12 Natriuretic peptides are known to preserve vascular health in both endothelial and vascular smooth muscle cells by interfering with the key mechanisms of atherosclerosis, i.e. proliferation, angiogenesis, apoptosis, and inflammation.12 Of interest, ANP appears to be a powerful contributing factor to endothelial function in the general population.13 In addition, NPs are known to exert anti-hypertrophic and anti-fibrotic roles within the heart,14 through mechanisms that have been extensively reviewed elsewhere.15 A negative inotropic effect of ANP has been described in normal LV myocytes.16
Finally, a role of NPs in the control of lipid metabolism, in the promotion of mitochondria biogenesis in adipocytes, and in the process of ‘browning’ of white adipocytes to increase energy expenditure has been recently identified.17
As a result of their complex cardiovascular (CV) properties, NPs are currently viewed as active players in the process of CV remodelling and in the natural history of heart failure (HF).
Predictive role of cardiovascular risk in the general population
The issue of a potential usefulness of NPs levels for stratifying cardiovascular risk (CVR) in asymptomatic individuals from the general population currently represents an active subject of investigation.18
In the offspring study from the Framingham population, NPs levels significantly predicted mortality from CV causes in apparently healthy subjects.19 NT-proBNP predicted major adverse CV events (MACE) and death in a population-based study performed in the city of Copenhaghen.20 Cardiovascular risk, as estimated by two validated algorithms for risk stratification, i.e. the SCORE project chart and the Framingham algorithm, increased gradually across tertiles of NT-proANP in a cohort of adult males from Southern Italy.21 In a community-based cohort from the USA, free from HF, NT-proBNP levels emerged as a more robust cardiac biomarker able to predict CV mortality and morbidity, independently from conventional clinical risk factors and cardiac structural abnormalities.22
Assessment of either BNP or NT-proBNP levels, in addition to measurement of conventional cardiovascular disease (CVD) risk factors, for improvement in risk discrimination for subsequent CVD has led to variable results, from only modest incremental improvement for CVD23 to a net reclassification improvement for major CV events.24 This controversial issue was recently addressed by a primary prevention study considering a cohort of hypercholesterolaemic middle-aged men at moderate CVR. In this study, NT-proBNP, once added to conventional risk factors, almost doubled the incremental gain for prediction of CVD compared with C-reactive protein, even when excluding subjects with minor as well as with major cardiac abnormalities.25
The predictive role of NPs for CVR in apparently healthy individuals may serve as a useful tool for early identification of ‘at risk subjects’. A major issue that remains to be verified in future studies is whether reduction of NPs levels in this subjects category may truly contribute to decrease their CVR and therefore to effectively prevent CVDs.
Of note, studies supporting the suitability of NPs levels as markers of CVR in asymptomatic individuals parallel those showing the ability of NPs to detect subclinical damage, as it was shown in the context of hypertension26 and of chronic kidney disease.27
Thus, based on the whole CV actions of NPs and on their tight relationships with the neuroendocrine regulation, NPs can be viewed as sensitive markers of preclinical haemodynamic and structural CV changes.
Diagnostic and prognostic role of natriuretic peptides circulating levels in cardiovascular diseases
Heart failure
The diagnostic role of NPs in both acute and chronic HF is well established. Both ANP and BNP levels increase in parallel with the degree of LV dysfunction and haemodynamic stress,28 although they are not useful to discriminate between systolic and diastolic HF. Furthermore, the value of NPs as reliable markers for long-term prognostic stratification both in acute and chronic HF conditions is well documented. In a study of patients presenting with acute HF, measurement of BNP, NT-proBNP, and mid regional (MR)-proANP levels showed significant diagnostic performance, although only MR-proANP had long-term prognostic value.29 In a large collection of patients hospitalized for acutely destabilized HF (ADHF), the prognostic performance of both NT-proBNP and MR-proANP levels was confirmed by evidence of incremental prognostic value to clinical risk factors for predicting mortality at 1 year.30 In chronic HF, subsequent measurements of either BNP or NT-proBNP levels provide independent information regarding the risk for disease progression across a wide spectrum of adverse outcomes: ventricular remodelling, malignant ventricular arrhythmias, hospitalization for HF, need for transplantation, and death.31 In one of the longest available follow-up study of patients with chronic HF, evaluating the prognostic power of multiple biomarkers, plasma ANP levels turned out to be the strongest long-term predictor of death in all disease stages.32 Prognostic value of NPs has been shown both in HF with reduced and with preserved EF.33
Even a preventive role of BNP-based screening towards HF development has been recently documented. Among patients with CV risk factors, at risk of HF, BNP assessment, associated with collaborative care, reduced the combined rates of LV systolic dysfunction, diastolic dysfunction, and HF.34
Natriuretic peptides guidance in heart failure therapy
The important clinical implications of NPs in HF indicate that NPs level measurement is one of the most effective tool for a HF hormonal-guided therapy. In ADHF, BNP measurement led to better accuracy in diagnosis, reduced rate of hospitalizations and of admissions in intensive care units, and had favourable effects on treatment costs and mortality rates.35 In fact, a useful algorithm for BNP-guided treatment of ADHF has been developed.35 Also in chronic HF, NPs level represents a useful marker to monitor the course of the disease in relation to the benefits of therapeutic strategies.36 However, due to uncertainties produced by different studies, a low level of recommendation for NP-guided therapy in chronic HF has been assigned by AHA-ACC-HF guidelines.37 A recent meta-analysis, performed in the attempt to overcome existing controversies and including 12 randomized clinical trials, has shown that in patients with chronic HF due to systolic dysfunction NP-guided therapy reduced all-cause mortality and HF-related hospitalizations.38 It was confirmed that individuals older than 75 years are those who benefit less, likely as a result of increased rate of co-morbidities in this age range.38
Hypertension
A diagnostic independent role of NPs levels has not been assessed in hypertension. On the other hand, few studies examined the significance of NPs in terms of prediction of CVR in hypertensive patients. Higher NT-proBNP levels predicted mortality in hypertensive patients.39,40 MR-proANP levels were significantly associated with lower ankle-brachial index, higher urinary albumin-creatinine ratio, greater LV mass index, independently of age, gender, body mass index, systolic BP, glomerular filtration rate, smoking, diabetes, cholesterol levels, medication use, previous acute myocardial infarction (AMI), or stroke, particularly in African-Americans.41
Stable and unstable atherosclerotic disease
Natriuretic peptides are sensitive markers of both clinically evident and subclinical myocardial ischaemia,42 as a consequence of the ischaemia-dependent LV dysfunction. In addition, a growing body of evidence indicates that NPs may play a direct contributory role into atherosclerotic disease pathogenesis. It is known that NPs affect vascular remodelling by exerting a significant impact on vitality, proliferation, apoptosis, and necrosis of both endothelial and vascular smooth muscle cells.12 Natriuretic peptides are significantly more expressed in human coronary explants of advanced atherosclerotic lesions in comparison with early atherosclerotic lesions.43 Furthermore, well-defined stages of atherosclerosis are characterized by progressively more pronounced increases in NT-proANP and NT-proBNP levels, from endothelial dysfunction to moderate and severe coronary atherosclerosis, irrespective of the underlying myocardial disease.44
As a consequence, clinical usefulness of NT-proNPs appears well established in both stable and unstable atherosclerotic disease, with particular regard to prognostic implications. In subjects experiencing AMI, a Kaplan–Meyer curve, based on either NT-proANP or NT-proBNP or both being above the respective median value, led to a significant prediction of future MACE.45 Similar data were obtained in patients experiencing either AMI or stroke.46
In a 10-year follow-up study performed in patients with stable CAD, NT-proBNP levels within the highest quartile were associated to 50% survival rate.47 Moreover, NT-proBNP levels in the 75–100 percentile were significantly associated with AMI, CV death, and all-cause death in the Claricor trial.48 Similar prognostic value has been reported for NT-proANP levels in patients with stable angina. In a multimarker model, involving MR-proANP, midregional pro-adrenomedullin (MR-proADM), C-terminal pro-endothelin-1 (CT proET1) and copeptin, adjusted for clinical factors, MR-proANP, and MR-proADM proved to be the strongest biomarkers in identifying patients with stable ischaemic heart disease at higher risk of CV death and HF.49 In the latter study, elevated levels of MR-proANP and MR-proADM identified patients in whom, despite apparent low clinical risk, therapy with ACEI resulted in a reduction in the risk of CV death or HF. This evidence represents an excellent example of the potential additive prognostic role of NPs in CAD patients. Future studies are required to confirm and expand this observation.
As mentioned above, MR-proANP is emerging in CVD diagnosis and prognosis. Identification of the mid-regional portion of the NT prosegment of proANP derives from a technical improvement in the hormonal assay detection50 (Figure 1). Due to its higher resistance to degradation, MR-proANP appears to behave as a more reliable marker for diagnostic and prognostic purposes in CVD patients, being often superior to BNP and to other biomarkers.29,41,49 Further confirmation of this role may reveal useful to improve CVD management.
Valve disease
Both diagnostic and prognostic power of NPs levels have been investigated in valve heart disease. In aortic stenosis, NPs correlate extensively with the severity of stenosis, LV chamber size, wall thickness and stress, EF, left atrial size, and right ventricular pressure. Since NT-proBNP correlates better to severity of aortic stenosis and LV remodelling, whereas ANP correlates more closely with atrial pressure, measurement of both peptides may reveal more useful for diagnostic purposes.51 Although BNP cannot discriminate between asymptomatic and mildly symptomatic patients with aortic stenosis, it may help reveal the transition from compensated hypertrophy to early decompensation in patients with preserved EF.52 Both peptides are related to the severity of mitral regurgitation. However, BNP is superior to ANP in reflecting the cardiac consequences of mitral regurgitation including adverse clinical outcomes.53
Although evidence is still insufficient, it is reasonable to expect that increased NPs levels may integrate the standard indications for surgery in order to identify patients who need a prompt operation. In addition, NPs level may reveal helpful to monitor the post-operative outcome.
Natriuretic peptides genetic variants and cardiovascular risk
Common genetic variants of NPPA (ANP) and NPPB (BNP), able to modulate NPs levels, contribute significantly to hypertension risk. In fact, in genome-wide association studies performed in both Caucasian and Asian populations54–56 genetic variants at the MTHFR-NPPB locus (which contains both NPPA and NPPB) act through increased ANP/BNP production to lower BP and, consequently, to influence susceptibility to hypertension development. Among others, the minor allele at rs5068 NPPA variant is associated to higher ANP levels and plays a fundamental role in hypertension risk.55
Notably, CVR can also be influenced by genetic variations of NPs in a BP-independent manner. Available evidence indicates that NPPA promoter variants associate directly to higher degree of LV hypertrophy in hypertension.57,58 Moreover, the common T2238C ANP coding variant induced endothelial-dependent impaired vasorelaxation in young healthy subjects without affecting BP59; it increased the risk of acute CV events (stroke and AMI) in different cohorts60,61; it exerted a significant prognostic role on CV outcomes in patients with stable ischaemic heart disease,62 and finally, it showed a significant gene-by-treatment interaction in a large cohort of hypertensive patients.63 This variant, resulting from a T-to-C nucleotide substitution at position 2238 of the gene, leads to a change from a stop codon to an arginine amino acid. The mechanisms of vascular disease promotion, resulting from the structurally altered ANP molecule, have been dissected out providing a good basis for a molecular to clinical level translation.59 In fact, it was discovered that C2238/ANP does not differently activate NPR-A, compared with wild-type ANP. In contrast, it activates NPR-C with a significantly higher affinity than wild-type ANP, and, by doing so, it promotes endothelial cell damage in vitro and endothelial dysfunction in vivo, ultimately leading to increased CVR.59 Selective blockade of NPR-C in C2238/ANP carriers may represent a suitable strategy to reduce their CVR.
Other NPPA variants, including G664A within exon 1, have been associated to CVD phenotypes with controversial results.61 The minor allele at NPPA rs5068 was reported to lower cardiometabolic risk,64 thus supporting the protective role of higher NP levels.
Natriuretic peptides as an emerging target for cardiovascular diseases
Renin–angiotensin–aldosterone system and SNS represent established targets in the therapeutic management of major CVDs. In fact, the well-defined involvement of both systems in the control of cardiac output, systemic vascular resistance, blood volume, and BP, as well as of CV remodelling, has provided solid pathophysiological basis for the significant benefits obtained with both RAAS-blocking agents and beta-blockers in the treatment of hypertension, CAD, and HF (Figure 3).
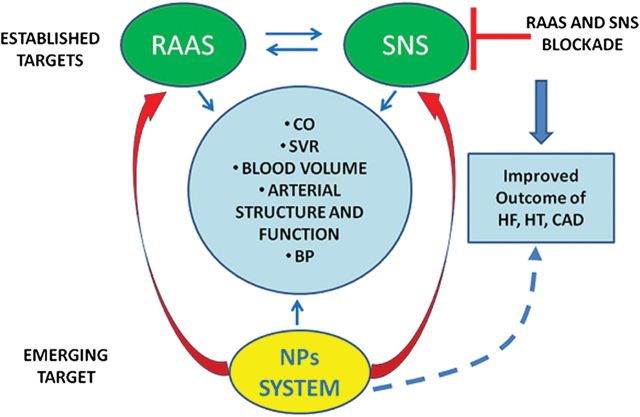
Figure 3.
Both renin–angiotensin–aldosterone system (RAAS) and sympathetic nervous system (SNS) blockade represent established therapeutic tools for controlling cardiovascular function and structure, and they both lead to improved outcome of heart failure (HF), hypertension (HT), and coronary artery disease (CAD). The pharmacological modulation of NPs family represents an emerging target to combat CVDs and their outcomes. It is expected to integrate the effects obtained by both RAAS and SNS blockade and to contribute to optimize control of CVDs and of their outcomes (dashed line). CO, cardiac output; SVR, systemic vascular resistance; BP, blood pressure.
However, in spite of the optimal currently available treatment strategies, hypertension and CAD are still far from being considered under control and CVDs are still the leading cause of death and morbidity in Western countries.65 For these reasons, novel therapeutic tools for the care of these conditions are actively searched. Among the potential targets, the NPs family has long represented an attractive candidate. In fact, based on its wide range of CV functions, NPs-derived therapies are expected to improve CVD treatment. Evidence of the protective effects of NP-based therapeutic approaches is already available with regard to major CVDs, as it will be summarized below. However, on the basis of previous negative findings, uncertainties necessarily exist about the theoretical benefits of the developing NPs-based therapies.
Hypertension
The use of ANP as a therapeutic anti-hypertensive agent has been explored extensively. Both short- and long-term infusions of ANP have been tested in essential hypertension as well as in secondary forms of hypertension, producing reduction in BP, along with natriuresis and diuresis.12,66 Due to the lack of peptide stability following oral administration, an alternative approach was attempted by a strategy based on pharmacologically increased circulating ANP levels. For this purpose, a new class of drugs was introduced: selective inhibitors of neutral endopeptidase (NEP). However, candoxatril revealed no substantial benefits in hypertension partly due to simultaneous increases of both vasodilators (NPs, bradikinin) and vasoconstrictors (endothelin-1, angiotensin II).67,68 To circumvent the limitations of selective NEP inhibition, vasopeptidase inhibitors (VPI) were developed.69 By simultaneously blocking NEP and ACE, VPI led to reduced degradation of NPs and bradykinin with a concomitant inhibition of angiotensin II generation. In fact, use of omapatrilat resulted in a BP lowering effect greater than that achieved by ACEI alone.70 However, a three-fold greater risk of angioedema, particularly in black individuals, prevented a safe introduction of NEP/ACEI inhibitors into clinical practice.71
Recently, a novel class of drugs (ARNi), which combine the action of both Angiotensin II receptor blocker (ARB) and NEP inhibition, was introduced. Among them, the LCZ696 compound provides a 1:1 ratio blockade of Angiotensin type 1 receptor with a valsartan moiety and NEP inhibition with a prodrug moiety (AHU377) metabolized in the final LBQ657 molecule.72 Its greater efficacy in controlling both sitting systolic and diastolic BP, when compared with valsartan, was reported in a randomized double-blind Phase II trial.73,74 LCZ696 was well tolerated and no cases of angioedema were reported, not even in black subjects. These findings need to be confirmed in larger studies. Moreover, the efficacy of these drugs on target organ protection and on major CV endpoints prevention needs to be verified.
Heart failure
Efficacy of VPI was investigated in chronic HF. Two major trials75,76 provided no definitive information on the effects of dual ACE/NEP inhibition. In fact, omapatrilat was not superior to enalapril with respect to the primary endpoint in HF.75 Furthermore, occurrence of hypotension and dizziness were more frequently observed with the use of omapatrilat rather than with that of ACEI in HF patients.76 No symptomatic benefits were observed in chronic HF with the use of sinorphan.77
Currently, NPs analogues may offer a valid alternative for the treatment of ADHF compared with the original molecules. Carperitide is a 28-amino acid molecule synthesized by genetic recombination.78 Its main effects are vasodilation, natriuresis, and RAAS inhibition, thus reducing both preload and afterload, causing hypotension and increasing cardiac output. Carperitide was approved for the treatment of ADHF in Japan in 1995. It is currently used in patients with either normotensive or hypertensive ADHF leading to systolic BP improvement, filling pressures, and enhanced diuresis.79 Brain natriuretic peptide itself can be administered as a treatment for HF with beneficial actions on myocardial structure and function.80 In 2001, the FDA approved the use of nesiritide, which is a recombinant form of human BNP, for the treatment of ADHF. However, it was observed that nesiritide may worsen renal function and increase the risk of short-term mortality when given to patients with ADHF.81 The latter evidence was not confirmed in patients with decompensated HF and preserved EF.82 It is noteworthy that no studies, including the ATTEND Investigators trial,79 have shown a significant impact on major CV endpoints in HF under administration of either ANP or BNP recombinant, thus leaving still open the question of the true beneficial effects of NPs recombinants on CVD outcomes.
The new dual Angiotensin receptor and neprilysin inhibitor LCZ696 have been tested in HF patients with preserved EF.83 It reduced NT-proBNP levels, as a consequence of improvement in LV wall stress, more than valsartan alone. Its impact on chronic systolic HF, as well as on morbidity and mortality in HF, is currently being tested in a large prospective trial in comparison with ACEI.84
Acute myocardial infarction
Atrial natriuretic peptide can inhibit early activation of neurohormonal factors and inflammation after reperfusion therapy. It can also reverse arrhythmias, apoptosis of cardiac myocytes and endothelial cells, and limit infarct size and LV remodelling, thereby improving LV function in animal models with ischaemia/reperfusion injury.85
The ability of ANP to prevent LV dilation and remodelling after primary coronary angioplasty in patients with AMI has been proven.86 Patients received either ANP infusion or isosorbide dinitrate administration immediately after primary coronary angioplasty. The outcome after ANP infusion was superior to that obtained with isosorbide dinitrate. Findings from a multicentre study also suggest that ANP can reduce subsequent re-hospitalization or death due to HF. These cardioprotective effects of ANP were observed at a low dose of ANP without a large effect on systemic BP in patients with AMI.87
Nesiritide, given soon after AMI, induced increments in plasma cGMP and CNP and decrements in other endogenous cardiac peptides with a neutral effect on renal function and a trend towards favourable LV remodelling.88
Of note, few studies have shown that CNP also acts as an efficient anti-remodelling agent after AMI. In fact, serial infusions of CNP in rats after AMI were associated with reduction in LV dilatation, increase in systolic and diastolic performance, and reduction in ventricular fibrosis.12
All mentioned studies reinforce the therapeutic potential of NPs in the treatment of AMI, and their beneficial effects on morbidity and mortality in post-MI patients. The contributory role of the dual angiotensin receptor and neprilysin inhibitor in ischaemic heart disease remains a matter of future investigation.
Cardiac surgery
A promising field of therapeutic application for NPs is represented by cardiac surgery. In this regard, continuous intravenous administration of either ANP or BNP has been performed in cardiac surgery resulting in cardiac and reno-protective effects.89 In the NAPA trial, use of BNP led to lower creatinine values, higher glomerular filtration rate, and higher survival rate.90 A trial of BNP in patients with preoperative renal dysfunction showed better postoperative results in the BNP patients.91 A recent pilot study evaluated the cardioprotective effects of ANP shot, simultaneously to the induction of cardioplegia during CABG surgery, and reported evidence of lower operative mortality and complications, lower incidence of arrhythmias and of HF.92
Conclusions
Three decades of research have outlined an important contribution of NPs in CV physiology and pathology. Following assessment of their diagnostic role, particularly in HF, a significant prognostic implication has emerged towards CV outcome in HF, hypertension, and CAD. Furthermore, NPs have shown predictive power towards CVD occurrence in the general population. Based on the wide range of CV implications of these hormones, NP-derived therapeutic approaches are currently viewed as a reasonable tool to fight CVDs. Available evidence obtained with NPs-based therapeutic approaches is generating active clinical research. The next years will assess whether these compounds will contribute to improve CVD therapeutic management.
Funding
This work was supported by a grant (Ricerca Corrente) from the Italian Ministry of Health to M.V. and S.R.; by the 5‰ grant to M.V. and S.R.; by PRIN 2009 to S.R.; by InGenious Hypercare European project to M.V.
Conflict of interest: none declared.
References
- 1.Levis ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 2.Lee CY, Burnett JC., Jr Natriuretic peptides and therapeutic applications. Heart Fail Rev. 2007;12:131–142. doi: 10.1007/s10741-007-9016-3. [DOI] [PubMed] [Google Scholar]
- 3.Rubattu S, Volpe M. The atrial natriuretic peptide: a changing view. J Hypertens. 2001;19:1923–1931. doi: 10.1097/00004872-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Tonne JM, Campbell JM, Cataliotti A, Ohmine S, Thatava T, Sakuma T, Macheret F, Huntley BK, Burnett JC, Jr, Ikeda Y. Secretion of glycosylated pro-B-type natriuretic peptide from normal cardiomyocytes. Clin Chem. 2011;57:864–873. doi: 10.1373/clinchem.2010.157438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan SD, Hobbs AJ, Ahluwalia A. C-type natriuretic peptide: new candidate for endothelium-derived hyperpolarizing factor. Int J Biochem Cell Biol. 2004;36:1878–1881. doi: 10.1016/j.biocel.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn M. Molecular physiology of natriuretic peptide signaling. Basic Res Cardiol. 2004;99:76–82. doi: 10.1007/s00395-004-0460-0. [DOI] [PubMed] [Google Scholar]
- 7.Rubattu S, Sciarretta S, Morriello A, Calvieri C, Battistoni A, Volpe M. NPR-C: a component of the natriuretic peptide family with implications in human diseases. J Mol Med. 2010;88:889–897. doi: 10.1007/s00109-010-0641-2. [DOI] [PubMed] [Google Scholar]
- 8.Proctor KG, Bealer SL. Selective antagonism of hormone-induced vasoconstriction by synthetic atrial natriuretic factor in the rat microcirculation. Circ Res. 1987;61:42–49. doi: 10.1161/01.res.61.1.42. [DOI] [PubMed] [Google Scholar]
- 9.Woods RL, Smolich JJ. Regional blood flow effects of ANP in conscious dogs: preferential gastrointestinal vasoconstriction. Am J Physiol. 1991;261(6 Pt 2):H1961–H1969. doi: 10.1152/ajpheart.1991.261.6.H1961. [DOI] [PubMed] [Google Scholar]
- 10.Focaccio A, Volpe M, Ambrosio G, Lembo G, Pannain S, Rubattu S, Enea I, Pignalosa S, Chiariello M. Angiotensin II directly stimulates release of atrial natriuretic factor in isolated rabbit hearts. Circulation. 1993;87:192–198. doi: 10.1161/01.cir.87.1.192. [DOI] [PubMed] [Google Scholar]
- 11.Bruneau BG, de Bold AJ. Selective changes in natriuretic peptide and early response gene expression in isolated rat atria following stimulation by stretch or endothelin-1. Cardiovasc Res. 1994;28:1519–1525. doi: 10.1093/cvr/28.10.1519. [DOI] [PubMed] [Google Scholar]
- 12.Rubattu S, Sciarretta S, Valenti V, Stanzione R, Volpe M. Natriuretic peptides: an update on bioactivity, potential therapeutic use and implication in cardiovascular diseases. Am J Hypertens. 2008;21:733–741. doi: 10.1038/ajh.2008.174. [DOI] [PubMed] [Google Scholar]
- 13.Kathiresan S, Gona P, Larson MG, Vita JA, Mitchell GF, Tofler GH, Levy D, Newton-Cheh C, Wang TJ, Benjamin EJ, Vasan RS. Cross-sectional relations of multiple biomarkers from distinct biological pathways to brachial artery endothelial function. Circulation. 2006;113:938–945. doi: 10.1161/CIRCULATIONAHA.105.580233. [DOI] [PubMed] [Google Scholar]
- 14.Molkentin JD. A friend within the heart: natriuretic peptide receptor signaling. J Clin Invest. 2003;111:1275–1277. doi: 10.1172/JCI18389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvieri C, Rubattu S, Volpe M. Molecular mechanisms underlying cardiac antihypertrophic and antifibrotic effects of natriuretic peptides. J Mol Med. 2012;90:5–13. doi: 10.1007/s00109-011-0801-z. [DOI] [PubMed] [Google Scholar]
- 16.Tajima M, Bartunek J, Weinberg EO, Ito N, Lorell BH. Atrial natriuretic peptide has different effects on contractility and intracellular pH in normal and hypertrophied myocytes from pressure-overloaded hearts. Circulation. 1998;98:2760–2764. doi: 10.1161/01.cir.98.24.2760. [DOI] [PubMed] [Google Scholar]
- 17.Bordicchia M, Liu D, Amri E-Z, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniels LB. Natriuretic peptides and assessment of cardiovascular disease risk in asymptomatic persons. Curr Cardio Risk Rep. 2010;4:120–127. doi: 10.1007/s12170-010-0078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 20.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 21.Barbato A, Sciarretta S, Marchitti S, Iacone R, Di Castro S, Stanzione R, Cotugno M, Ippolito R, Palmieri L, Calvieri C, Battistoni A, Volpe M, Strazzullo P, Rubattu S. Aminoterminal natriuretic peptides and cardiovascular risk in an Italian male adult cohort. Int J Cardiol. 2011;152:245–246. doi: 10.1016/j.ijcard.2011.07.077. [DOI] [PubMed] [Google Scholar]
- 22.McKie PM, Cataliotti A, Sangaralingham SJ, Ichiki T, Cannone V, Bailey KR, Redfield MM, Rodeheffer RJ, Burnett JC. Predictive utility of atrial, N-terminal pro-atrial, and N-terminal pro-B-type natriuretic peptides for mortality and cardiovascular events in the general community: a 9-year follow-up study. Mayo Clin Proc. 2011;86:1154–1160. doi: 10.4065/mcp.2011.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Angelantonio E, Chowdhury R, Sarwar N, Ray KK, Gobin R, Saleheen D, Thompson A, Gudnason V, Sattar N, Danesh J. B-type natriuretic peptides and cardiovascular risk. Systematic Review and Meta-Analysis of 40 Prospective Studies. Circulation. 2009;120:2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 24.Wannamethee G, Welsh P, Lowe GD, Gudnason V, Di Angelantonio E, Lennon L, Rumley A, Whincup PH, Sattar N. N-terminal pro-brain natriuretic peptide is a more useful predictor of cardiovascular disease risk than C-reactive protein in older men with and without pre-existing cardiovascular disease. J Am Coll Cardiol. 2011;58:56–64. doi: 10.1016/j.jacc.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 25.Welsh P, Doolin O, Willeit P, Packard C, Macfarlane P, Cobbe S, Gudnason V, Di Angelantonio E, Ford I, Sattar N. N-terminal pro-B-type natriuretic peptide and the prediction of primary cardiovascular events: results from 15-year follow-up of WOSCOPS. Eur Heart J. 2013;34:443–450. doi: 10.1093/eurheartj/ehs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishikimi T, Yoshihara F, Morimoto A, Ishikawa K, Ishimitsu T, Saito Y, Kangawa K, Matsuo H, Omae T, Matsuoka H. Relationship between left ventricular geometry and natriuretic peptide levels in essential hypertension. Hypertension. 1996;28:22–30. doi: 10.1161/01.hyp.28.1.22. [DOI] [PubMed] [Google Scholar]
- 27.Zhou W, Ni Z, Yu Z, Shi B, Wang Q. Brain natriuretic peptide is related to carotid plaques and predicts atherosclerosis in pre-dialysis patients with chronic kidney disease. Eur J Int Med. 2012;23:539–544. doi: 10.1016/j.ejim.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 29.Seronde MF, Gayat E, Logeart D, Lassus J, Laribi S, Boukef R, Sibellas F, Launay JM, Manivet P, Sadoune M, Nouira S, Cohen Solal A, Mebazaa A. Great Network. Comparison of the diagnostic and prognostic values of B-type and atrial-type natriuretic peptides in acute heart failure. Intern J Cardiol. 2013;168:3404–3411. doi: 10.1016/j.ijcard.2013.04.164. [DOI] [PubMed] [Google Scholar]
- 30.Lassus J, Gayat E, Mueller C, Peacock WF, Spinar J, Harjola V-P, van Kimmenade R, Pathak A, Mueller T, Di Somma S, Metra M, Pascual-Figal D, Laribi S, Logeart D, Nouira S, Sato N, Potocki M, Parenica J, Collet C, Cohen-Solal A, Januzzi JL, Jr, Mebaza A for the Great-Network. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: The Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol. 2013;168:2186–2194. doi: 10.1016/j.ijcard.2013.01.228. [DOI] [PubMed] [Google Scholar]
- 31.Boerrigter G, Costello-Boerrigter LC, Burnett JC., Jr Natriuretic peptides in the diagnosis and management of chronic heart failure. Heart Fail Clin. 2009;5:501–514. doi: 10.1016/j.hfc.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpe M, Francia P, Tocci G, Rubattu S, Cangianiello S, Rao MAE, Trimarco B, Condorelli M. Prediction of long-term survival in chronic heart failure by multiple biomarker assessment: A 15-year prospective follow-up study. Clin Cardiol. 2010;33:700–707. doi: 10.1002/clc.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA, Hillege HL. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–1506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 34.Ledwidge M, Gallagher J, Conlon C, Tallon E, O'Connell E, Dawkins I, Watson C, O'Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Voon V, Tilson L, Barry M, McDonald L, Maurer B, McDonald K. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310:66–74. doi: 10.1001/jama.2013.7588. [DOI] [PubMed] [Google Scholar]
- 35.Bhardwaj A, Januzzi JL. Natriuretic peptide-guided management of acutely destabilized heart failure. Rationale and treatment algorithm. Critical Pathways Cardiol. 2009;8:146–150. doi: 10.1097/HPC.0b013e3181c4a0c6. [DOI] [PubMed] [Google Scholar]
- 36.Motiwala SR, Januzzi JL. The role of natriuretic peptides as biomarkers for guiding the management of chronic heart failure. Clin Pharm Therap. 2013;93:57–67. doi: 10.1038/clpt.2012.187. [DOI] [PubMed] [Google Scholar]
- 37.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 38.Savarese G, Trimarco B, Dellegrottaglie S, Prastaro M, Gambardella F, Rengo G, Leosco D, Perrone-Filardi P. Natriuretic peptide-guided therapy in chronic heart failure: a meta-analysis of 2,686 patients in 12 randomized trials. PLoS ONE. 2013;8:e58287. doi: 10.1371/journal.pone.0058287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsen MH, Wachtell K, Nielsen OW, Hall C, Wergeland R, Ibsen H, Kjeldsen SE, Devereux RB, Dahlöf B, Hildebrandt PR. N-terminal brain natriuretic peptide predicted cardiovascular events stronger than high-sensitivity C-reactive protein in hypertension: a LIFE substudy. J Hypertens. 2006;24:1531–1539. doi: 10.1097/01.hjh.0000239288.10013.04. [DOI] [PubMed] [Google Scholar]
- 40.Paget V, Legedz L, Gaudebout N, Girerd N, Bricca G, Milon H, Vincent M, Lantelme P. N-terminal pro-brain natriuretic peptide. A powerful predictor of mortality in hypertension. Hypertension. 2011;57:702–709. doi: 10.1161/HYPERTENSIONAHA.110.163550. [DOI] [PubMed] [Google Scholar]
- 41.Kaleghi M, Al-Omari MA, Kondragunta V, Morgenthaler NG, Struck J, Bergmann A, Mosley TH, Kuoo IJ. Relation of plasma midregional natriuretic peptide to target organ damage in adults with systemic hypertension. Am J Cardiol. 2009;103:1255–1260. doi: 10.1016/j.amjcard.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noman A, George J, Struthers A. A new use for B-type natriuretic peptide: to detect myocardial ischemia in non-heart failure patients. Br J Diabetes Vasc Dis. 2010;10:78–82. [Google Scholar]
- 43.Casco VH, Veinot JP, Kuroski de Bold MLK, Masters RG, Stevenson MM, de Bold AJ. Natriuretic peptide system gene expression in human coronary arteries. J Histochem Cytochem. 2002;50:799–809. doi: 10.1177/002215540205000606. [DOI] [PubMed] [Google Scholar]
- 44.Barbato E, Rubattu S, Bartunek J, Berni A, Sarno G, Vanderheyden M, Delrue L, Zardi D, Pace B, De Bruyne B, Coluccia R, Wijns W, Volpe M. Role of cardiac natriuretic peptides in human coronary atherosclerosis. Atherosclerosis. 2009;206:258–264. doi: 10.1016/j.atherosclerosis.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 45.Squire IB, O'Brien RJ, Demme B, Davies JE, Ng LL. N-terminal pro-atrial natriuretic peptide (N-ANP) and N-terminal pro-B-type natriuretic peptide (N-BNP) in the prediction of death and heart failure in unselected patients following acute myocardial infarction. Clin Sci. 2004;107:309–316. doi: 10.1042/CS20040087. [DOI] [PubMed] [Google Scholar]
- 46.Makikallio AM, Makikallio TH, Korpelainen JT, Vuolteenaho O, Tapanainen JM, Ylitalo K, Sotaniemi KA, Huikuri HV, Myllyla VV. Natriuretic peptides and mortality after stroke. Stroke. 2005;36:1016–1020. doi: 10.1161/01.STR.0000162751.54349.ae. [DOI] [PubMed] [Google Scholar]
- 47.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal proB-type natriuretic peptide and long-term mortality in stable coronary heart disease. New Engl J Med. 2005;352:666–675. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 48.Harutyunyan MJ, Mathiasen AB, Winkel P, Gotze JP, Hansen JF, Hildebrandt P, Jensen JB, Hilden J, Jespersen CM, Kjoller E, Kolmos HJ, Gluud C, Kastrup J CLARICOR Trial Group. High-sensitivity C-reactive protein and N-terminal pro-B-type natriuretic peptide in patients with stable coronary heart disease: a prognostic study within the CLARICOR trial. Scand J Clin Lab Invest. 2011;71:52–62. doi: 10.3109/00365513.2010.538081. [DOI] [PubMed] [Google Scholar]
- 49.Sabatine MS, Morrow DA, de Lemos JA, Omland T, Sloan S, Jarolim P, Solomon SD, Pfeffer MA, Braunwald E. Evaluation of multiple biomarkers of cardiovascular stress for risk prediction and guiding medical therapy in patients with stable coronary disease. Circulation. 2012;125:233–240. doi: 10.1161/CIRCULATIONAHA.111.063842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgenthaler NG, Struck J, Thomas B, Bergmann A. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin Chem. 2004;50:234–236. doi: 10.1373/clinchem.2003.021204. [DOI] [PubMed] [Google Scholar]
- 51.Ray SG. Natriuretic peptides in heart valve disease. Heart. 2006;92:1194–1197. doi: 10.1136/hrt.2005.074161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanderheyden M, Goethals M, Verstreken S, De Bruyne B, Muller K, Van Schuerbeeck E, Bartunek J. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol. 2004;44:2349–2354. doi: 10.1016/j.jacc.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 53.Detaint D, Messika-Zeitoun D, Avierinos J-F, Avierinos JF, Scott C, Chen H, Burnett JC, Jr, Enriquez-Sarano M. B-type natriuretic peptide in organic mitral regurgitation. Circulation. 2005;11:2391–2397. doi: 10.1161/01.CIR.0000164269.80908.9D. [DOI] [PubMed] [Google Scholar]
- 54.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O'Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sõber S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjögren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimäki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, Aspelund T, Garcia M, Chang YP, O'Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kähönen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Köttgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grässler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Soler Artigas M, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stančáková A, Raffel LJ, Yao J, Kathiresan S, O'Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT, Jr, Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikäinen LP, Soininen P, Tukiainen T, Würtz P, Ong RT, Dörr M, Kroemer HK, Völker U, Völzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MV, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FG, Charchar FJ, Schwarz PE, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han BG, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJ, Altshuler D, Loos RJ, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JC, Hartikainen AL, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Järvelin MR, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T The International Consortium for Blood Pressure Genome-Wide association studies CARDIoGRAMConsortium; CKDGen Consortium; KidneyGenConsortium; EchoGen Consortium; CHARGE-HF Consortium. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–353. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, Tay WT, Chen C-H, Zhang Y, Yamamoto K, Katsuya T, Yokota M, Kim YJ, Ong RT, Nabika T, Gu D, Chang LC, Kokubo Y, Huang W, Ohnaka K, Yamori Y, Nakashima E, Jaquish CE, Lee JY, Seielstad M, Isono M, Hixson JE, Chen YT, Miki T, Zhou X, Sugiyama T, Jeon JP, Liu JJ, Takayanagi R, Kim SS, Aung T, Sung YJ, Zhang X, Wong TY, Han BG, Kobayashi S, Ogihara T, Zhu D, Iwai N, Wu JY, Teo YY, Tai ES, Cho YS, He J. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet. 2011;43:531–538. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubattu S, Bigatti G, Evangelista A, Lanzani C, Stanzione R, Zagato L, Manunta P, Marchitti S, Venturelli V, Bianchi G, Volpe M, Stella P. Association of atrial natriuretic and type-A natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J Am Coll Cardiol. 2006;48:499–505. doi: 10.1016/j.jacc.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 58.Xue H, Wang S, Wang H, Sun K, Song X, Zhang W, Fu C, Han Y, Hui R. Atrial natriuretic peptide gene promoter polymorphism is associated with left ventricular hypertrophy in hypertension. Clin Sci (Lond) 2007;114:131–137. doi: 10.1042/CS20070109. [DOI] [PubMed] [Google Scholar]
- 59.Sciarretta S, Marchitti S, Bianchi f, Moyes A, Barbato E, Di Castro S, Stanzione R, Cotugno M, Castello L, Calvieri C, Iberini I, Sadoshima J, Hobbs AJ, Volpe M, Rubattu S. The C2238 atrial natriuretic peptide molecular variant is associated with endothelial damage and dysfunction through natriuretic peptide receptor C signaling. Circ Res. 2013;112:1355–1364. doi: 10.1161/CIRCRESAHA.113.301325. [DOI] [PubMed] [Google Scholar]
- 60.Rubattu S, Stanzione R, Di Angelantonio E, Zanda B, Evangelista A, Tarasi D, Gigante B, Pirisi A, Brunetti E, Volpe M. Atrial natriuretic peptide gene polymorphisms and the risk of ischemic stroke in humans. Stroke. 2004;35:814–818. doi: 10.1161/01.STR.0000119381.52589.AB. [DOI] [PubMed] [Google Scholar]
- 61.Lynch AI, Claas SA, Arnett DK. A review of the role of atrial natriuretic peptide gene polymorphisms in hypertension and its sequelae. Curr Hypertens Rep. 2009;11:35–42. doi: 10.1007/s11906-009-0008-7. [DOI] [PubMed] [Google Scholar]
- 62.Barbato E, Bartunek J, Mangiacapra F, Sciarretta S, Stanzione R, Delrue L, Cotugno M, Marchitti S, Iaccarino G, Sirico G, Di Castro S, Evangelista A, Lambrechts D, Sinnaeve P, De Bruyne B, Van De Werf F, Janssens S, Fox K-A-A, Wijns W, Volpe M, Rubattu S. Influence of rs5065 atrial natriuretic peptide gene variant on coronary artery disease. J Am Coll Cardiol. 2012;59:1763–1770. doi: 10.1016/j.jacc.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 63.Lynch AI, Boerwinkle E, Davis BR, Ford CE, Eckfeldt JH, Leiendecker-Foster C, Arnett DK. Pharmacogenetic association of the NPPA T2238C genetic variant with cardiovascular disease outcomes in patients with hypertension. JAMA. 2008;299:296–307. doi: 10.1001/jama.299.3.296. [DOI] [PubMed] [Google Scholar]
- 64.Cannone V, Boerrigter G, Cataliotti A, Costello-Boerrigter LC, Olson TM, McKie PM, Heublein DM, Lahr BD, Bailey KR, Averna M, Redfield MM, Rodeheffer RJ, Burnett JC., Jr A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J Am Coll Cardiol. 2011;58:629–636. doi: 10.1016/j.jacc.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravat DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: Heart disease and stroke statistics-2013 update. A report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 66.Volpe M, Mele AF, Indolfi C, De Luca N, Lembo G, Focaccio A, Condorelli M, Trimarco B. Haemodynamic and hormonal effects of atrial natriuretic factor in patients with essential hypertension. J Am Coll Cardiol. 1987;10:787–793. doi: 10.1016/s0735-1097(87)80271-1. [DOI] [PubMed] [Google Scholar]
- 67.Ando S, Ramhan MA, Butler GC, Senn BL, Floras JS. Comparison of candoxatril and atrial natriuretic factor in healthy men. Effects on hemodynamics, sympathetic activity, heart rate variability, and endothelin. Hypertension. 1995;26(6 Pt 2):1160–1166. doi: 10.1161/01.hyp.26.6.1160. [DOI] [PubMed] [Google Scholar]
- 68.Bevan EG, Connell JM, Doyle J, Carmichael HA, Davies DL, Lorimer AR, McInnes GT. Candoxatril, a neutral endopeptidase inhibitor: efficacy and tolerability in essential hypertension. J Hypertens. 1992;10:607–613. [PubMed] [Google Scholar]
- 69.Corti R, Burnett JC, Jr, Rouleau JL, Ruschitzka F, Luscher TF. Vasopeptidase inhibitors: a new therapeutic concept in cardiovascular disease? Circulation. 2001;104:1856–1862. doi: 10.1161/hc4001.097191. [DOI] [PubMed] [Google Scholar]
- 70.Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 71.Campbell DJ. Vasopeptidase inhibition: a double-edged sword? Hypertension. 2003;41:383–389. doi: 10.1161/01.HYP.0000054215.71691.16. [DOI] [PubMed] [Google Scholar]
- 72.Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Kin TH, Zheng W, Dole WP. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi) J Clin Pharmacol. 2010;50:401–414. doi: 10.1177/0091270009343932. [DOI] [PubMed] [Google Scholar]
- 73.Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375:1255–1266. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- 74.Mangiafico S, Costello-Boerrigter LC, Andersen IA, Cataliotti A, Burnett JC., Jr Neutral endopetidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2013;34:886–893. doi: 10.1093/eurheartj/ehs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) Circulation. 2002;106:920–926. doi: 10.1161/01.cir.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- 76.Rouleau JL, Pfeffer MA, Stewart DJ, Isaac D, Sestier F, Kerut EK, Porter CB, Proulx G, Qian C, Block AJ. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356:615–620. doi: 10.1016/s0140-6736(00)02602-7. [DOI] [PubMed] [Google Scholar]
- 77.Cleland JG, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet. 1998;351:1657–1658. doi: 10.1016/s0140-6736(05)77712-6. [DOI] [PubMed] [Google Scholar]
- 78.Kangawa K, Tawaragi Y, Oikawa S, Mizuno A, Sakuragawa Y, Nakazato H, Fukuda A, Minamino N, Matsuo H. Identification of rat gamma atrial natriuretic polypeptide and characterization of the cDNA encoding its precursor. Nature. 1984;312:152–155. doi: 10.1038/312152a0. [DOI] [PubMed] [Google Scholar]
- 79.Sato N, Kajimoto K, Asai K, Mizuno M, Minami Y, Nagashima M, Murai K, Muanakata R, Yumino D, Meguro T, Kawana M, Nejima J, Satoh T, Mizuno K, Tanaka K, Kasanuki H, Takano T ATTEND Investigators. Acute decompensated heart failure syndromes (ATTEND) registry. A prospective observational multicenter cohort study: rationale, design, and preliminary data. Am Heart J. 1010;159:949–955. doi: 10.1016/j.ahj.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 80.Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC., Jr Novel protein therapeutics for systolic heart failure. J Am Coll Cardiol. 2012;60:2305–2312. doi: 10.1016/j.jacc.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sackner-Berstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 82.Kelesidis I, Mazurek J, Khullar P, Saeed W, Vittorio T, Zolty R. The effect of nesiritide on renal function and other clinical parameters in patients with decompensated heart failure and preserved ejection fraction. Congest Heart Fail. 2012;18:158–164. doi: 10.1111/j.1751-7133.2011.00272.x. [DOI] [PubMed] [Google Scholar]
- 83.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJV. For the prospective comparison of ARNi with ARB on Management of Heart Failure with preserved ejection fraction (PARAMOUNT) Investigators. Lancet. 2012;380:1387–1395. [Google Scholar]
- 84.McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR on behalf of the PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) Eur J Heart Failure. 2013;15:1062–1073. doi: 10.1093/eurjhf/hft052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kasama S, Furuya M, Toyama T, Ichikawa S, Kurabayashi M. Effect of atrial natriuretic peptide on left ventricular remodelling in patients with acute myocardial infarction. Eur Heart J. 2008;29:1485–1494. doi: 10.1093/eurheartj/ehn206. [DOI] [PubMed] [Google Scholar]
- 86.Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M. Effects of intravenous atrial natriuretic peptide on cardiac sympathetic nerve activity and left ventricular remodeling in patients with first anterior acute myocardial infarction. J Am Coll Cardiol. 2007;49:667–674. doi: 10.1016/j.jacc.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 87.Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H, Kitamura S J-WIND investigators. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 88.Chen HH, Martin FL, Gibbons RJ, Schirger JA, Wright RS, Schears RM, Redfield MM, Simari RD, Lerman A, Cataliotti A, Burnett JC., Jr Low-dose nesiritide in human anterior myocardial infarction suppresses aldosterone and preserves ventricular function and structure: a proof of concept study. Heart. 2009;95:1315–1319. doi: 10.1136/hrt.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sezai A, Hata M, Niino T, Yoshitake I, Unosawa S, Wakui S, Kimura H, Shiono M, Takayama T, Hirayama A. Continuos low-dose infusion of human atrial natriuretic peptide in patients with left ventricular dysfunction undergoing coronary artery bypass grafting: The NU-HIT (Nihon University working group study of low-dose Human ANP Infusion therapy during cardiac surgery) for left ventricular dysfunction. J Am Coll Cardiol. 2010;55:1844–1851. doi: 10.1016/j.jacc.2009.11.085. [DOI] [PubMed] [Google Scholar]
- 90.Mentzer RM, Oz MC, Sladen RN, Graeve AH, Hebeler RF, Luber JM, Jr, Smedira NG NAPA Investigators. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery. J Am Coll Cardiol. 2007;49:716–726. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 91.Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC. Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double blind placebo-controlled pilot study. Circulation. 2007;116:I134–I138. doi: 10.1161/CIRCULATIONAHA.106.697250. [DOI] [PubMed] [Google Scholar]
- 92.Sezai A, Wakui S, Akiyama K, Hata M, Yoshitake I, Unosawa S, Shiono M, Hirayama A. Myocardial protective effect of human atrial natriuretic peptide in cardiac surgery. Circulation J. 2011;75:2144–2150. doi: 10.1253/circj.cj-11-0185. [DOI] [PubMed] [Google Scholar]