Abstract
Background
There are limited data on the epidemiology of paediatric healthcare-associated infection (HCAI) and infection control in low-income countries. We describe the value of intermittent point-prevalence surveys for monitoring HCAI and evaluating infection control interventions in a Cambodian paediatric hospital.
Methods
Hospital-wide, point-prevalence surveys were performed monthly in 2011. Infection control interventions introduced during this period included a hand hygiene programme and a ventilator-associated pneumonia (VAP) care bundle.
Results
Overall HCAI prevalence was 13.8/100 patients at-risk, with a significant decline over time. The highest HCAI rates (50%) were observed in critical care; the majority of HCAIs were respiratory (61%). Klebsiella pneumoniae was most commonly isolated and antimicrobial resistance was widespread. Hand hygiene compliance doubled to 51.6%, and total VAP cases/1000 patient-ventilator days fell from 30 to 10.
Conclusion
Rates of HCAI were substantial in our institution, and antimicrobial resistance a major concern. Point-prevalence surveys are effective for HCAI surveillance, and in monitoring trends in response to infection control interventions.
Keywords: Cambodia, Paediatric, Hospital-associated infection, Healthcare-associated infection, HCAI, Nosocomial infection
Introduction
Healthcare-associated infection (HCAI) is a major threat to patient safety, particularly in low-income countries.1 Here, the capacity to implement infection control (IC) programmes is hampered by a lack of resources, infrastructure, guidelines, surveillance, and training.1,2 Current data suggests the prevalence of HCAI in resource-limited settings is 15.5/100 patients, with the highest infection densities in intensive care (47.9/1000 patient/days); these are 3–4 times the figures in the USA.1 Data on paediatric HCAIs from resource-limited countries are under-represented in the literature,1 but associated mortality, morbidity and healthcare costs are substantial.3
Cambodia is a resource-limited country in South-East Asia, with some of the poorest regional health indicators.4 Recent IC initiatives have been undertaken by the Ministry of Health in conjunction with the WHO and private partners, and include a National Infection Control Policy (2010) and Hand Hygiene Programme (2011). However, HCAI surveillance and outcome data are limited. Here we report our experience of using intermittent point-prevalence surveys to monitor HCAI at a regional paediatric hospital in Cambodia and their value in evaluating our efforts at implementing IC.
Methods
Setting
Angkor Hospital for Children (AHC) is a charitably-funded, 50-bedded paediatric hospital in Siem Reap, Cambodia. It is the provincial paediatric referral centre, and also manages patients from surrounding provinces (120 000 attendances/year; 2000–2500 admissions/year). Healthcare is free to children <16 years of age. There is an intensive care unit (ICU; 4 isolation rooms, 700–750 admissions/year), and ventilated/high-dependency patients are also sometimes managed in the adjacent emergency room (ER). Other facilities include surgical (SU), medical (in-patient department/IPD) and ‘step-down’ care units (low acuity/LAU).
Healthcare-associated infection surveillance
An IC programme was implemented in late 2010, with regular HCAI surveillance from January 2011. During single-day, monthly cross-sectional point-prevalence surveys, standardised data is collected on all patients, detailing: location, age at admission, admission duration, diagnosis, comorbidities, current antimicrobials, presence/site of HCAI and of invasive devices. HCAI diagnoses are based on locally adapted, simplified versions of the CDC/NHSN definitions.5 An individual considered at-risk of HCAI was defined as a patient with an admission duration of >48 hours.
Infection control interventions
Hand hygiene programme
Development of a hand hygiene (HH) programme commenced late 2010 to early 2011, including an HH policy, perception surveys of HH, and the introduction of an alcohol-based hand-rub. Staff HH training was formalised from July 2011 and incorporated into the induction process for new recruits. Baseline HH compliance was audited in June 2011 (adapted from the WHO ‘Save Lives: Clean Your Hands’ tools available at http://www.who.int/gpsc/5may/tools/en) and two-monthly thereafter. Compliance targets were set at 50% by January 2012, 75% by July 2012, and >90% by July 2013.
Ventilator-associated pneumonia monitoring and care bundle implementation
Ventilator-associated pneumonia (VAP) monitoring commenced June 2011, with a care bundle phased in from July. This included: intubation and suctioning protocols; elevation of bedheads; circuitry care; chlorhexidine mouthcare; and aggressive weaning as tolerated.
Screening for carriage of Staphylococcus aureus and multi-drug resistant-Gram negative bacilli
In December 2011, cross-sectional screening on a single day was undertaken on all inpatients, using a nasal swab for S. aureus, and a faecal sample for third-generation cephalosporin and/or carbapenem resistance in Gram negative bacilli (GNB).
Nasal samples were cultured on mannitol salt agar; S. aureus isolation was confirmed by Gram-stain and microscopy, catalase testing and antigen detection (Staphaurex, Thermo Scientific, Lenexa, KS, USA). Faecal specimens were diluted 1:10 with sterile 0.9% saline and 10 µl cultured aerobically on MacConkey agar with cefpodoxime (10 µg) and imipenem (10 µg) discs. Speciation of resistant organisms was undertaken using phenotypic tests. Confirmation of extended-spectrum beta-lactamase (ESBL)-producing status for GNB was performed with combination disc testing and susceptibility testing for all isolates was undertaken in accordance with published guidelines.6
Statistical analysis
Statistical analysis was performed using Stata 11.1 (StataCorp, College Station, TX, USA). Fisher's exact and Kruskal-Wallis tests were used for between-group comparisons of categorical and continuous variables respectively. The Mantel–Haenszel χ2-test was used to analyse trends in HH compliance. Linear regression was used to characterise crude trends in HCAI prevalence/antibiotic consumption. Multivariable logistic regression was used to characterise risk factors for binary outcomes. A p-value of <0.05 was considered statistically significant.
Results
Healthcare-associated infection surveillance data
During 2011 613 (median; range/month [53; 46–55]) patients were surveyed: 47 on ICU (7.7%), 44 (7.2%) in the ER, 312 in the IPD (50.9%), 98 (16.0%) in the SU and 112 (18.2%) on LAU. Median (IQR; range) age at survey was 2.6 (0.5–8.7; 0–15.9) years; this differed between units, with admissions to ICU/ER being younger. Overall, 428/613 (69.8%) patients were at-risk of HCAI; again this was significantly different between units (Table 1).
Table 1.
Demographic and clinical details of patients surveyed by ward location
| Ward Location (number of patients surveyed) |
||||||
|---|---|---|---|---|---|---|
| ICU (47) | ER (44) | IPD (312) | SU (98) | LAU (112) | p value | |
| Median age at survey in years (IQR) | 0.2 (0.1–2.3) | 0.2 (0–1.2) | 1.5 (0.4–7.4) | 4.5 (1.2–11.1) | 6.2 (3.3–11.3) | 0.0001 |
| Number at riska of HCAI (% of patients surveyed on ward) | 45 (96) | 25 (57) | 192 (61.5) | 87 (89) | 79 (70.5) | <0.001 |
| Median duration of admission in days (IQR) | 17 (8–29) | 4 (1–8.5) | 3 (1.5–8) | 8 (4–16) | 4 (2–7) | 0.0001 |
| Number of patients surveyed with a comorbidity (% of patients surveyed on ward) | 27 (57) | 25 (57) | 140 (44.9) | 50 (51) | 14 (12.5) | <0.001 |
| Number of patients surveyed with a deviceb in situ (% of patients surveyed on ward) | 46 (98) | 44 (100) | 295 (94.5) | 63 (64) | 100 (89.3) | <0.001 |
| Number of patients on antibiotics (% of patients surveyed on ward) | 38 (81) | 41 (93) | 248 (79.5) | 64 (65) | 92 (82.1) | 0.002 |
ER: Emergency room; ICU: Intensive care unit; IPD: In-patient department; LAU: Low acuity; SU: Surgical unit.
aAt risk defined as admission duration >48 hours.
bThe definition of devices includes: peripheral intravenous catheter, urinary catheter, endotracheal tube, chest drain, wound drain, nasogastric/orogastric tube, peritoneal dialysis catheter, external fixator.
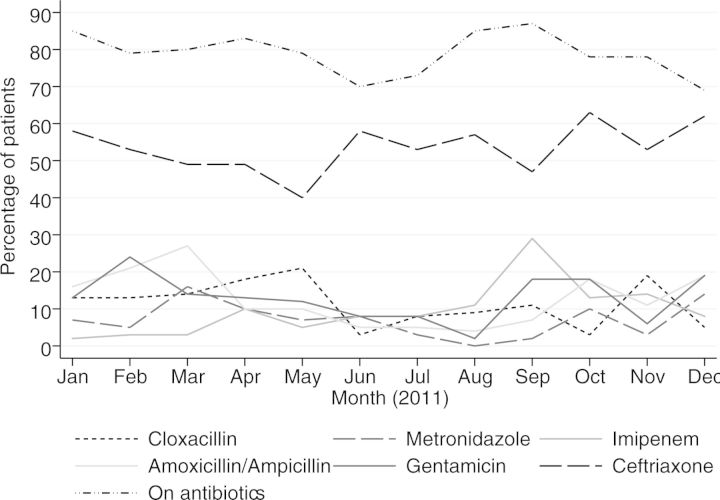
Overall antimicrobial usage and usage for commonly prescribed antimicrobials is shown in Figure 1; ceftriaxone was most frequently prescribed. Imipenem use increased significantly from 2% to 29% by September (p = 0.04), and then declined. Trends in consumption of other antibiotics were not significant.
Figure 1.
Percentage of patients on antibiotics by month (calculated as a proportion of all patients surveyed) and on commonly prescribed antibiotics (calculated as a proportion of all patients on antibiotics), January–December 2011.
In total, 356/428 (83.2%) at-risk children had a peripheral intravenous catheter, 34 (7.9%) a urinary catheter, and 43 (10.0%) an endotracheal tube (ETT); 66 (15.4%) had other medical devices, including 56 (13.1%) with nasogastric tubes, four (0.9%) with chest drains, three (0.7%) with wound drains, and one each (0.2%) with an external fracture fixation device, peritoneal dialysis catheter, and orogastric tube.
Fifty-nine patients had evidence of an HCAI acquired at AHC (13.8/100 patients at-risk); six others had been transferred with HCAIs acquired elsewhere. A significant decline in HCAI prevalence was noted, from a median of 15.8% in the first half of the year to 11.1% in the second (p = 0.01; Figure 2A). No significant trends were observed by ward. The median (IQR) prevalence of HCAI in patients at-risk was higher in the ICU/ER setting (50%; 25–67%) versus the non-ICU/ER setting (3.1%; 0–12.9%) (p < 0.001). Univariable logistic regression analyses were used to identify possible risk factors for the presence of HCAI; on multivariable modelling, four factors remained significant (Table 2).
Figure 2.

(A) Patients with healthcare associated infections (HCAI) as a proportion of those at-risk at each monthly surveillance time-point, January–December 2011. (B) Healthcare associated infection case mix observed at each monthly time-point. The total number of patients at-risk at each monthly time-point is expressed above each bar; hospital-acquired pneumonias (HAP); urinary tract infection (UTI); ventilator-associated pneumonia (VAP); skin/soft tissue infection (SSTI). (B) Healthcare associated infection case mix observed at each monthly time-point. The total number of patients at-risk at each monthly time-point is expressed above each bar. HAP: hospital-acquired pneumonias; SSTI: ; UTI: urinary tract infection; VAP: ventilator-associated pneumonia.
Table 2.
Univariable and multivariablea analysis of risk factors for the presence of HCAI in patients at-risk of HCAI
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| Factor | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value |
| Age (per increase of one year) | 0.90 (0.84–0.97) | 0.004 | 0.91 (0.84–0.98) | 0.01 |
| Duration of admission (per increase of one day) | 1.04 (1.02–1.06) | <0.001 | 1.05 (1.03–1.08) | <0.001 |
| Current antibiotic therapy | 8.87 (2.12–37.07) | 0.003 | 22.34 (3.84–129.95) | 0.001 |
| At least one chronic comorbidity | 2.32 (1.11–3.41) | 0.02 | a | NS |
| Intravenous catheter | 6.65 (1.58–27.89) | 0.01 | a | NS |
| Urine catheter | 3.53 (1.62–7.71) | 0.002 | a | NS |
| Endotracheal tube | 11.5 (5.75–22.99) | <0.001 | 6.47 (2.92–14.30) | <0.001 |
| Ward location | ||||
| ICU | 7.1 (2.78–18.14) | <0.001 | a | NS |
| ER | 6.97 (2.38–20.45) | <0.001 | a | NS |
| IPD | 0.86 (0.36–2.09) | NS | a | NS |
| SU | 0.21 (0.04–1.02) | NS | a | NS |
ER: Emergency room; ICU: Intensive care unit; IPD: In-patient department; NS: Not significant; SU: Surgical unit.aIndependent risk factors for the presence of HCAI were identified using multivariable logistic regression analysis of all statistically significant (p < 0.05) univariable risk factors with backwards elimination (exit p-value = 0.05); odds ratios for non-significant risk factors are therefore not calculated.
Respiratory HCAIs predominated (22 VAPs, 14 hospital-acquired pneumonias [HAPs]; 61% of all HCAIs), of which seven had concomitant bloodstream infections and one a urinary tract infection (UTI). Ten children had skin/soft tissue/wound infections, one also with gastroenteritis. Four children had a bacteraemia without an obvious source, one an isolated UTI, one gastroenteritis, and six had systemic infection without a clear focus or bacteraemia. There was one case of nosocomially-acquired measles. Details of the HCAI case mix at each monthly time-point are depicted in Figure 2B.
Thirty-nine organisms deemed potentially clinically significant were cultured from 30 specimens from 23 patients. Nine specimens cultured mixed pathogens (seven respiratory site specimens, one skin/wound sample, one blood culture). Klebsiella pneumoniae was most commonly isolated (nine blood cultures, five respiratory and one skin/wound specimen), followed by Pseudomonas aeruginosa (one blood culture, five respiratory and two skin/wound specimens). Two of three S. aureus isolated (ETT, skin/wound) were MRSA and 11 of 13 (85%) K. pneumoniae isolates with susceptibility data were extended-spectrum beta-lactamase (ESBL) producers. There was one imipenem-resistant P. aeruginosa isolate.
Ventilator-associated pneumonia surveillance data
Ventilator-associated pneumonia numbers decreased significantly, from 30 to 10/1000 patient-ventilator days (p = 0.02).
Microbiological screening data
Of 54 children, 51 (94%) had nasal swabs and five (10%) were colonised with S. aureus; one with MRSA (2% of patients swabbed; 20% of carriers). The MRSA strain was resistant to ciprofloxacin, gentamicin and clindamycin. The four methicillin-susceptible S. aureus (MSSA) strains were resistant to penicillin; one was additionally resistant to ciprofloxacin.
Of 54 patients, 29 (54%) submitted faecal samples, 25 (86%) of whom were colonised with 31 cefpodoxime-resistant GNBs as follows: Escherichia coli (n = 16), K. pneumoniae (9), Enterobacter spp. (2), Edwardsiella tarda (2) and two organisms which remained unspeciated. Sixteen (52%) of these strains were additionally resistant to co-amoxiclav, ciprofloxacin and gentamicin. No imipenem resistance was noted.
Hand hygiene programme results
The response rate for the baseline HH/HCAI perception survey was 53.3%, and 50.5% a year later. At baseline, 80.7% of respondents stated they routinely used hand-rub between 50–100% of the time; at re-survey this figure was 88.8%. The commonest reason for non-compliance was skin irritation perceived to be associated with hand-rubs. More than 75% of respondents rated hospital education and accessibility of hand-rub as ≥5 (scale from 0 = ‘not effective’ to 7 = ‘very effective’). About a third (34.0%) of respondents were unclear on the institutional prevalence of HCAI compared with 23.9% at re-survey (p = 0.09). More than half perceived the dangers associated with HCAI as ‘low/very low’ at both time-points (54.8 and 58.3% respectively); 31.1% felt that HH had ‘low/very low’ efficacy in preventing HCAI, which only marginally dropped at re-survey (26.4%).
The results of HH audits are presented in Figure 3. Overall compliance doubled from 26.0% to 51.6% (p < 0.001).
Figure 3.
Hand hygiene audit results, June–December 2011.
Discussion
This paper describes the first Cambodian study of its kind, to our knowledge. Several studies have investigated aspects of HCAI and IC in the region, but these are mostly: from countries with more advanced healthcare infrastructures and IC;7,8 singular point-prevalence surveys;9 in the critical care setting only;10 or undertaken with respect to specific infectious pathogens11 or syndromes.12 Cambodia has only recently adopted national IC guidelines, and in this respect is several years behind Thailand and Vietnam.13,14
Perception of the risks of HCAI and associated outcomes were relatively poor, despite on-going education. Self-reported HH compliance was high, and contrasted with the observed rate at audit (26.0–51.6%), a discordance described elsewhere.15 A third of staff erroneously felt HH was ineffective in preventing HCAI. The lack of improvement in understanding at the repeat survey was disappointing and may have been partly related to staff turnover, as well as to cultural perceptions of hospital hygiene, including an emphasis on visual cleanliness.16 However, monitored HH compliance has doubled, and is in line with institutional targets. Future interventions to achieve the >90% compliance target by July 2013 include improving signage reminding staff/carers of the importance of HH, and having a dedicated staff member policing HH on a daily basis. We are also discussing the possibility of encouraging patient carers to remind staff to clean their hands, although the cultural barriers in overcoming the status of healthcare professionals may be too substantial at present.
The prevalence of HCAI (13.8%) in our institution and critical care setting (50.0%) is within the range observed in other resource-limited countries.1 Respiratory HCAIs are most common (61.0% of HCAIs), and the decline in overall HCAI prevalence was mirrored by that of VAP prevalence. Independent risk factors for HCAI in our institution are similar to those elsewhere.17 Attempts to mitigate risk have included implementing antimicrobial and ventilation guidelines.
The epidemiology of HCAI at our institution may reflect the current lack of central venous or arterial vascular devices, and low rates of urinary catheterisation (7.0%) in contrast to those in hospitalised adults.18 Rates of wound infection on the surgical unit (used here as a proxy for surgical site infection) are relatively low (2.3/100 at risk patients) in comparison to other published data,1,3 which may relate to setting, case-mix and/or underlying co-morbidities. We are currently switching to antisepsis with alcohol-based chlorhexidine as opposed to aqueous iodine, and will be introducing specific surgical site infection-based surveillance.
Likely microbiological pathogens were isolated from clinical samples in 39.0% of cases, and were mostly Gram-negative species, as observed in several Asian countries.1 Interestingly, K. pneumoniae, and not P. aeruginosa or Acinetobacter spp., was most commonly isolated. The majority of K. pneumoniae were multi-drug resistant, susceptible only to carbapenems, colistin and variably to chloramphenicol. Imipenem is available but expensive, and the global spread of carbapenem resistance is of concern. The transient increase in imipenem usage observed during the study period may have been in response to an increased awareness of the high prevalence of ESBL-associated infections, and peak use coincided with a cluster of cases of ESBL-producing K. pneumoniae bloodstream infections, which may have influenced prescribing patterns.
There are limited data on S. aureus carriage in hospitalised children in Asia, with most studies undertaken in healthy and/or community-based children. Prevalence ranges from 6.0–26.0% for MSSA and 2.7–12.0% for MRSA,19,20 and is consistent with our findings, albeit with small numbers. There were extremely high rates of faecal carriage of third-generation cephalosporin-resistant GNB (86.0%), but isolation of these patients to limit transmission is not feasible given the lack of available facilities to do so. In addition, although we have made no assessment of carriage in the community, high rates of ESBL carriage (29–51% of healthy community populations) have been reported in Thailand,21 suggesting that these organisms are probably widely disseminated in both hospital and community settings in the region. The selection pressure for these organisms exerted by nosocomial antibiotic use in our institution is substantial, with 40–60% of patients on ceftriaxone at any time-point.
Limitations
There are several limitations to this survey. The numbers in our study were small and we collected a restricted set of epidemiological data. Serial point-prevalence monitoring is not as comprehensive as longitudinal surveillance; however, it is recognised as an acceptable surveillance method in the context of resource/staffing constraints,22 and has been shown to be an acceptable approach in the monitoring of paediatric HCAI elsewehere.17,23 Criticisms of the point-prevalence methodology include the inability to capture outbreaks of HCAI occurring between surveillance time-points, and the possibility that there may have been important demographic differences in the cohorts of children surveyed which were not identified as a result of the limited dataset collected, and therefore not controlled for in the risk factor analyses. In addition, we were not able to capture specific outcomes in relation to HCAI.
Our IC programme is hospital-wide, which meant that we were unable to make controlled comparisons assessing the impact of infection control interventions; in addition, it was not possible to determine the efficacy of individual interventions given the similar timeframes of introduction. There were no policy changes in waste management, cleaning (undertaken in accordance with Cambodian National Infection Control Guidelines)24 or antibiotic prescribing during the study period, but infection control and HCAI were highlighted as issues of importance in meetings and on noticeboards. We were not able to make an assessment of external factors, such as seasonality, which may have influenced the epidemiology of HCAI.25
Finally, microbiological techniques may not determine the true causative pathogens – especially for respiratory and skin/soft tissue/wound infections.
Conclusions
Despite the limitations, we have broadly defined issues relevant to HCAI in a Cambodian regional paediatric hospital including perceptions related to HH, HCAI surveillance and risk factors for HCAI, and antimicrobial susceptibility in clinical and carried organisms, and correlated these with various IC interventions. In particular, point-prevalence surveys have been a feasible means through which we have been able to monitor trends in response to the introduction of IC efforts, and could be deployed in similar resource-restricted settings. This has been important in highlighting local issues for concern, such as antimicrobial resistance, in defining areas for intervention, and in contributing to the body of knowledge on the subject from a country with minimal currently published data.
Acknowledgments
Authors' contributions: CMP, KE, CEM, S Soklin, KPA, S Sophal, SC, PN, YP, S Sona, NC, VK and NS conceived the surveillance and screening protocols. CMP, KE, S Soklin, KPA, SC and NS collected data for the point-prevalence surveys. YP, PN and SC collected data for the ventilator-associated pneumonia surveillance. CEM and S Sona carried out the microbiological analyses. NS and CMP carried out the data analysis and interpretation, and drafted the manuscript. CEM, NPJD, VK and DL critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. NS and CMP are guarantors of the paper.
Acknowledgements: The authors wish to thank Mr Sun Sopheary for providing hospital denominator data.
Funding: The AHC infection control programme is funded by the Ptarmigan Charitable Foundation, Calgary, Alberta, Canada. The microbiology service at AHC is part-funded by the Wellcome Trust, London, UK and the Li Ka Shing-University of Oxford Global Health Programme, Oxford, UK.
Competing interests: None declared.
Ethical approval: The AHC Institutional Review Board (IRB) did not feel formal ethical review was needed since surveillance was part of the hospital's infection control programme.
References
- 1.Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377:228–41. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 2.Raza MW, Kazi BM, Mustafa M, Gould FK. Developing countries have their own characteristic problems with infection control. J Hosp Infect. 2004;57:294–9. doi: 10.1016/j.jhin.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Posfay-Barbe KM, Zerr DM, Pittet D. Infection control in paediatrics. Lancet Infect Dis. 2008;8:19–31. doi: 10.1016/S1473-3099(07)70310-9. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Geneva: World Health Organization; 2012. World Health Statistics 2012. [Google Scholar]
- 5.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 6.CLSI. CLSI Document M100–S22, Approved Standard – 9th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. [Google Scholar]
- 7.Korbkitjaroen M, Vaithayapichet S, Kachintorn K, et al. Effectiveness of comprehensive implementation of individualized bundling infection control measures for prevention of health care-associated infections in general medical wards. Am J Infect Control. 2011;39:471–6. doi: 10.1016/j.ajic.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Apisarnthanarak A, Thongphubeth K, Yuekyen C, et al. Effectiveness of a catheter-associated bloodstream infection bundle in a Thai tertiary care center: a 3-year study. Am J Infect Control. 2010;38:449–55. doi: 10.1016/j.ajic.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Thu TA, Hung NV, Quang NN, et al. A point–prevalence study on healthcare-associated infections in Vietnam: public health implications. Infect Control Hosp Epidemiol. 2011;32:1039–41. doi: 10.1086/661915. [DOI] [PubMed] [Google Scholar]
- 10.Johansson M, Phuong DM, Walther SM, Hanberger H. Need for improved antimicrobial and infection control stewardship in Vietnamese intensive care units. Trop Med Int Health. 2011;16:737–43. doi: 10.1111/j.1365-3156.2011.02753.x. [DOI] [PubMed] [Google Scholar]
- 11.Lien LT, Hang NT, Kobayashi N, et al. Prevalence and risk factors for tuberculosis infection among hospital workers in Hanoi, Viet Nam. PLoS One. 2009;4:e6798. doi: 10.1371/journal.pone.0006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CK, Chua YP, Saw A. Antimicrobial gauze as a dressing reduces pin site infection: a randomized controlled trial. Clin Orthop Relat Res. 2012;470:610–5. doi: 10.1007/s11999-011-1990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danchaivijitr S, Rongrungruang Y, Assanasen S, et al. Development of national guidelines for the prevention and control of nosocomial infections. J Med Assoc Thai. 2005;88:S54–8. [PubMed] [Google Scholar]
- 14.To KG, Graves N, Huynh VAN, Le ATT. Structure of infection control and prevention in Cho Ray Hospital: An analysis of the current situation. Int J Infect Control. 2011 doi:10.3396/ijic.v8i1.004.12. [Google Scholar]
- 15.Jenner EA, Fletcher BC, Watson P, et al. Discrepancy between self-reported and observed hand hygiene behaviour in healthcare professionals. J Hosp Infect. 2006;63:418–22. doi: 10.1016/j.jhin.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Hancart-Petitet P, Dumas C, Faurand-Tournaire AL, et al. Social and cultural dimensions of hygiene in Cambodian health care facilities. BMC Public Health. 2011;11:83. doi: 10.1186/1471-2458-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciofi Degli Atti ML, Cuttini M, Ravà L, et al. Trend of healthcare-associated infections in children: annual prevalence surveys in a research hospital in Italy, 2007–2010. J Hosp Infect. 2012;80:6–12. doi: 10.1016/j.jhin.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Saint S, Wiese J, Amory JA, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109:476–80. doi: 10.1016/s0002-9343(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 19.Lo WT, Wang CC, Lin WJ, et al. Changes in the nasal colonization with methicillin-resistant Staphylococcus aureus in children: 2004–2009. PLoS One. 2010;5:e15791. doi: 10.1371/journal.pone.0015791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pathak A, Marothi Y, Iyer RV, et al. Nasal carriage and antimicrobial susceptibility of Staphylococcus aureus in healthy preschool children in Ujjain, India. BMC Pediatr. 2010;10:100. doi: 10.1186/1471-2431-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luvsansharav UO, Hirai I, Niki M, et al. Analysis of risk factors for a high prevalence of extended-spectrum β-lactamase-producing Enterobacteriaceae in asymptomatic individuals in rural Thailand. J Med Microbiol. 2011;60:619–24. doi: 10.1099/jmm.0.026955-0. [DOI] [PubMed] [Google Scholar]
- 22.Pottinger JM, Herwaldt LA, Perl TM. Basics of surveillance–an overview. Infect Control Hosp Epidemiol. 1997;18:513–27. doi: 10.1086/647659. [DOI] [PubMed] [Google Scholar]
- 23.Burgner D, Dalton D, Hanlon M, et al. Repeated prevalence surveys of paediatric hospital–acquired infection. J Hosp Infect. 1996;34:163–70. doi: 10.1016/s0195-6701(96)90062-6. [DOI] [PubMed] [Google Scholar]
- 24.Ministry of Health, Kingdom of Cambodia. Phnom Penh: Ministry of Heath; 2010. Infection Prevention and Control Guidelines for Healthcare Facilities. [Google Scholar]
- 25.Richet H. Seasonality in Gram-negative and healthcare-associated infections. Clin Microbiol Infect. 2012;18:934–40. doi: 10.1111/j.1469-0691.2012.03954.x. [DOI] [PubMed] [Google Scholar]