Abstract
Background
HSP90 inhibition leads to proteosomal degradation of activated KIT and has in vitro activity against gastrointestinal stromal tumors (GIST). BIIB021 is an oral non-ansamycin HSP90 inhibitor. We carried out a phase II study of BIIB021 in patients with GIST refractory to imatinib and sunitinib.
Patients and methods
The primary end-point was metabolic partial response (mPR) as assessed by fluorodeoxyglucose positron emission tomography (FDG-PET). The secondary end-points were pharmacokinetic assessments of BIIB021 and pharmacodynamic assessments of HSP70. Twenty-three patients were treated on two schedules: 12 pts received 600 mg twice a week (BIW) and 11 patients received 400 mg three times a week (TIW). All had prior imatinib and sunitinib but stopped >14 days before starting BIIB021.
Results
The median age was 59 years (33–88 years), 61% male, 44% Eastern Cooperative Oncology Group 1 (ECOG1). The best response was PR by FDG-PET for five patients (3/12 at 600 mg BIW and 2/9 at 400 TIW) for an overall response rate of 22%. The response duration was 25–138 days. Adverse events (AEs) were mild to moderate. The mean Cmax was 1.5 µmol and the mean AUC was 2.9 µmol h. Cmax >1.5 µmol was associated with a decrease in standardized uptake value (SUVmax). HSP70 increased substantially following treatment.
Conclusions
This study met its primary end-point. BIIB021 leads to objective responses in refractory GIST patients. Pharmacodynamic studies confirmed HSP90 inhibition. Further evaluation of BIIB021 in GIST is warranted.
Keywords: gastro-intestinal stromal tumors, hSP90 inhibitors, phase II trials, pharmacokinetics and pharmacodynamics, sarcoma/soft-tissue malignancies
introduction
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal cancers of the digestive tract and perhaps most common connective tissue malignancy [1]. Activating mutations in the genes encoding receptor tyrosine kinases KIT and PDGFRα are believed to be the key drivers in the development and progression of GIST. Multiple mutations in KIT have been described and specific mutations correlate with therapeutic response to imatinib and sunitinib, the two Food and Drug Administration-approved drugs for GIST. The development of secondary kinase mutations often accounts for the development of secondary resistance to these drugs [2].
KIT and PDGFRα are client proteins of the molecular chaperone heat shock protein 90 (HSP90). Treatment with an HSP90 inhibitor results in proteosomal degradation of mutated KIT and PDGFRα [3]. With imatinib and sunitinib, the antitumor activity is dependent on the presence or absence of a specific kinase specific mutation. HSP90 inhibitors, in contrast, are expected to result in the degradation of any form of mutationally activated KIT and PDGFRα. Thus, HSP90 inhibitors may have activity in GIST in the both the first-line setting and in patients with acquired resistance to imatinib and sunitinib.
BIIB021 is an oral fully synthetic HSP90 inhibitor that binds competitively with geldanamycin, the prototypical HSP90 inhibitor, in the ATP-binding pocket of HSP90 [4]. BIIB021 is not an ansamycin derivative and has not demonstrated any substantial hepatotoxicity. A phase I study has been completed and the maximum tolerated dose (MTD) determined. The drug was well-tolerated and pharmacodynamic studies demonstrated that HSP90 was effectively inhibited [5]. In addition, preclinical data suggest that synthetic HSP90 inhibitors such as BIIB021 may have activity against tumors with acquired multidrug resistance [6].
Based on these results, we carried out a phase II study of BIIB021 in patients with GIST refractory to imatinib and sunitinib. 18-Fluorodeoxyglucose positron emission tomography (FDG-PET) was used to optimize the dose and schedule of BIIB021. FDG-PET is a sensitive and highly predictive marker of response in GIST. A dramatic reduction in FDG-PET has been observed within 24 h of treatment with imatinib, suggesting that in GIST changes in FDG-PET may be used as a rapid marker of tumor response [7]. HSP90 inhibition can decrease FDG-PET uptake in patients with GIST, as demonstrated in a phase I study of the HSP90 inhibitor IPI-504 [8].
The primary objective of the study was to assess changes in FDG-PET imaging to guide the dose and schedule of BIIB021 in patients with GIST. The secondary objectives were to assess the safety profile, pharmacokinetics and pharmacodynamics, and clinical activity using Response Evaluation Criteria in Solid Tumors (RECIST) and Choi criteria [9, 10].
patients and methods
patient selection
Eligible patients were ≥18 years of age with a diagnosis of pathologically confirmed GIST and were refractory to, or intolerant of, both imatinib and sunitinib. Prior treatment with other TKIs was permitted but not required. Eligible patients had evaluable disease by FDG-PET, defined as SUVmax (averaged over a maximum of five lesions) ≥2. Eligible patients had Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, absolute neutrophil count ≥1500/mm3, platelet count of ≥100 000/mm3, hemoglobin ≥9 g/dl, bilirubin ≤1.5 × upper limit of normal (ULN), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤2.5 × ULN (or ≤5 × ULN if liver metastases present), creatinine ≤2.0 × ULN. Patients must have stopped prior TKIs at least 14 days before study entry. Prior treatment with an HSP90 inhibitor was not allowed. The protocol was approved by the Institutional Review Board of both institutions and all patients provided a written informed consent (NCT00618319).
study design and treatment
This was an open-label, non-randomized study. The starting dose was 600 mg oral twice weekly, the MTD that was determined in the phase I study [5]. The study drug was administered on Days 1, 4, 8, 11, 15, 18, 22, and 25 of each 28-day cycle. FDG-PET assessments were carried out at baseline and again on Day 5 and Day 8 of cycle 1, and then Day 29 (the first day of cycle 2). The Day 5 time point, 24 h after the second dose of study drug, was chosen to determine the dose of BIIB021 which is sufficient to result in a substantial decrease in FDG-PET uptake. The Day 8 time point was chosen to inform decisions on the schedule since this represents the trough of the second dose of the study drug. The Day 29 time point was selected to reflect the cumulative effect of BIIB021 treatment and was used to adjudicate response.
A preliminary review of the results of the first 10 patients treated on the twice a week (BIW) schedule showed that pathway inhibition was observed. On Day 5, 24 h after the second dose of BIIB021, many patients had a decrease in standardized uptake value (SUVmax). However, pathway inhibition did not appear to be sustained between doses. Between Day 5 and Day 8 (trough of the second dose), mean SUVmax increased in most of the patients treated on the BIW schedule (supplementary Figure S1, available at Annals of Oncology online, black bars). Therefore, given that pathway inhibition was observed with BIIB021 but did not appear to be sustained on a BIW schedule, the treatment plan was changed to evaluate the same total weekly dose given on the more frequent schedule of 400 mg three times a week (TIW). Enrollment continued on this new schedule (Days 1, 3, 5, 8, 10, 12, 15, 17, 19, 22, 24, and 26 of each 28-day cycle). FDG-PET scans were obtained as before, except that the Day 5 time point was changed to Day 6, 24 h after the third dose of study drug.
assessment of response and AEs
The assessments of response and toxic effects consisted of history and physical examinations, complete blood counts, serum chemistry and electrocardiograms every 4 weeks. FDG-PET scans were carried out in accordance with EORTC guidelines [11]. Specifically, scans were performed when patients were fasting. The SUV was corrected for the blood glucose level. Up to five indicator lesions were chosen at baseline and were followed throughout the study. Radiographic responses were assessed by central review. Toxic effect was graded in accordance with the National Cancer Institute Common Toxicity Criteria version 4.0.
end-points and statistical analysis
The primary assessment of antitumor activity using FDG-PET is the change in SUVmax from baseline to Day 1 of cycle 2 (Day 29). Subjects with a >25% reduction in mean SUVmax (over a maximum of five lesions) at Day 1 of cycle 2 (Day 29) were considered to have a metabolic partial response (mPR) [11]. The secondary end-points included response using standard RECIST and response using Choi criteria, defined as a 10% decrease in tumor size or a 15% decrease in tumor density [9].
The study was designed to test the hypothesis that treatment with BIIB021 would result in 20% of patients achieving an mPR (defined as a 25% reduction in SUVmax of FDG-PET).
pharmacokinetics and pharmacodynamics
Blood samples were obtained on Day 1 before the first dose, and 1, 2, 4 and 6 h after the dose. The serum concentration of BIIB021 was measured by high-performance liquid chromatography. Pharmacokinetic parameters were estimated using a non-compartmental model.
Additional blood samples were collected for pharmacodynamic assessment of HSP90 inhibition. Induction of HSP70 has been studied as a biomarker of HSP90 inhibition [12]. Serum and peripheral blood mononuclear cells (PBMC) were collected at the following time points: Day 1 before the first dose, 6 h after the dose, Day 2, Day 5 or 6 (depending on BIW or TIW treatment schedule), Day 8, and Day 29 (cycle 2, Day 1). HSP70 was measured in serum using the enzyme-linked immunosorbent assay as previously described [12, 13]. HSP70 in PBMC was measured by western blot.
results
Between February 2008 and September 2010, 25 subjects were enrolled and 23 were treated. The characteristics of the treated patients are shown in Table 1. Sixty-one percent of the patients were male. The median age was 59 (range 33–88) and the median time since the initial diagnosis was 5 years (range 1–10). All patients received prior treatment with both imatinib and sunitinib. 61% received prior sorafenib and 9% received nilotinib in addition to the other three drugs. Mutation status was KIT exon 11 (7 patients); KIT exon 9 (1 patient); no detected PDGFRA or KIT mutation (1 patient); and unknown mutation status (14 patients).
Table 1.
Patient characteristics
| Characteristic | No. of patients (%) |
|---|---|
| Total | 23 |
| Assessable for response | |
| Male | 14 (61) |
| Female | 9 (39) |
| Age, years | |
| Median | 59 |
| Range | 33–88 |
| ECOG | |
| 0 | 13 |
| 1 | 10 |
| Time since the initial diagnosis (years) | |
| Median | 5 |
| Range | 1–10 |
| Prior treatment | |
| Imatinib | 23 (100) |
| Sunitinib | 23 (100) |
| Sorafenib | 14 (61) |
| Nilotinib | 2 (9) |
ECOG, Eastern Cooperative Oncology Group.
activity
The radiographic responses are summarized in Table 2. Responses by FDG-PET were determined based on the change in mean SUVmax from baseline (before Day 1) to Day 29 (end of cycle 1). The best response by FDG-PET was partial response for 3 of 12 subjects (25%) who received 600 mg BIW and 2 of 11 subjects (18%) who received 400 mg TIW. Responses were observed in patients treated on both the schedules and the duration of response was not apparently different by schedule. The sample size is too small to determine whether either schedule was associated with a higher response rate. Responses were also observed in patients with KIT exon 9 mutations and exon 11 mutations, as well as in patients with unknown mutation status.
Table 2.
Best response by cohort
| Best response | Cohort 1 |
Duration on drug | Cohort 2 |
Duration on drug | Overall | ||
|---|---|---|---|---|---|---|---|
| 600 mg BIW |
400 mg TIW |
||||||
| N = 12 | N = 11 | N = 23 | |||||
| FDG-PET | PR | 3 (25%) | 25–109 days | PR | 2 (18%) | 36–138 days | 5 (22%) |
| RECIST | PR | 0 | PR | 0 | 0 | ||
| SD | 4 (33%) | 53–109 days | SD | 6 (55%) | 35–138 days | 10 (43%) | |
| Choi | PR | 1 (8%) | 82 days | PR | 0 | 1 (4%) | |
| SD | 2 (17%) | 81–109 days | SD | 4 (36%) | 36–138 days | 6 (26%) | |
FDG-PET, 18-fluorodeoxyglucose positron emission tomography; PR, partial response; SD, stable disease.
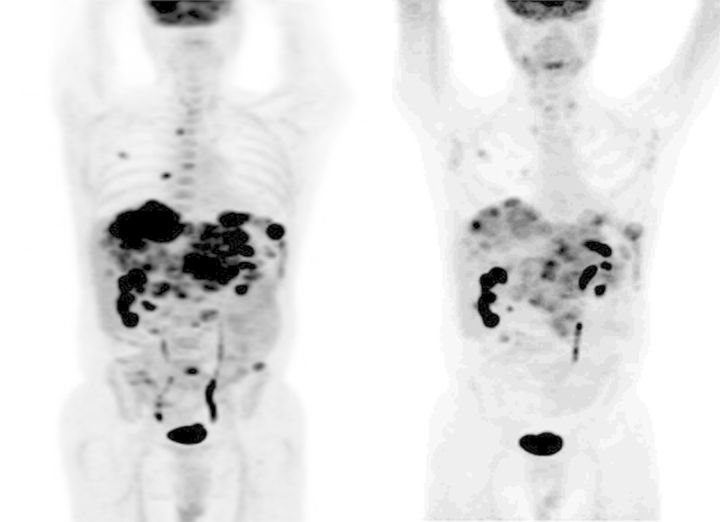
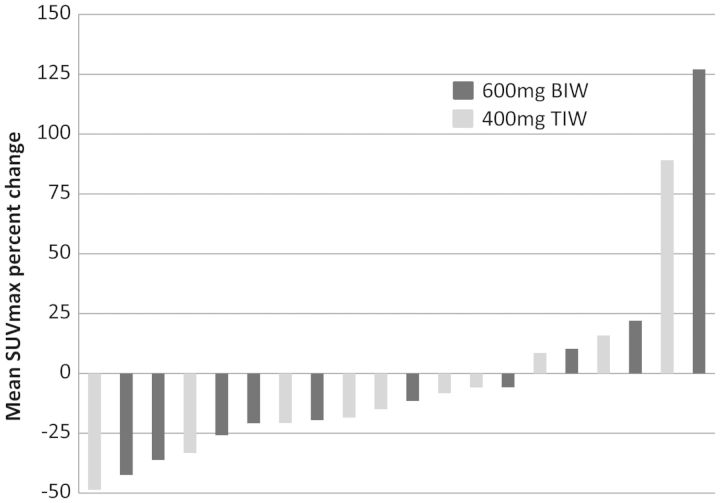
Figure 1 shows an example of a patient who had a mPR with a substantial reduction in the FDG avidity of multiple liver metastases after treatment with BIIB021 at the 400 mg TIW dose for 4 weeks. Figure 2 shows a waterfall plot of the change in mean SUVmax for the 20 patients who remained on study at least until Day 29. Not included in this figure are three patients who had disease progression during cycle 1 and therefore did not have Day 29 PET scans carried out. Of the 20 patients in Figure 2, 5 patients had a decrease of >25% and thus achieved a mPR. A further 9 patients had a lesser decline in SUVmax that did not meet the criterion for mPR.
Figure 1.
18-Fluorodeoxyglucose positron emission tomography (FDG-PET) scans at baseline (left) and after one cycle (4 weeks) of treatment with BIIB021 400 mg TIW. The patient had an metabolic partial response (mPR) showing a substantial reduction in multiple FDG-avid liver metastases.
Figure 2.
Waterfall plot showing the change in the mean standardized uptake value (SUVmax) for patients from baseline (before Day 1) to Day 29 (end of cycle 1). Five patients had a decrease of >25% and thus achieved a metabolic partial response (mPR). A further nine patients had a lesser decline in SUVmax that did not meet the criterion for the mPR.
The best response by RECIST was stable disease for 10 of 23 subjects (43%): 4 of 12 subjects (33%) who received 600 mg BIW and 6 of 11 subjects (55%) who received 400 mg TIW.
The best responses by Choi criteria [9] were a partial response in 1 subject (4%) and stable disease in 6 subjects (26%) of 23 subjects: 1 of 12 subjects (8%) with a partial response and 2 of 12 subjects (17%) with stable disease who received 600 mg BIW and 4 of 11 subjects (36%) with stable disease who received 400 mg TIW.
The median time of study was 35 days (range 8–138 days) for all patients.
safety and tolerability
Grade 2 and higher AEs are listed in Table 3. AEs were generally mild to moderate. The majority of treatment-related AEs were no greater than Grade 2 in both the dose schedules. Although many patients experienced at least one AE, many were adjudicated as being related to progressive GIST (including liver enzyme abnormalities and abdominal symptoms). The median number of days on treatment was 35 (range 8–138 days) with most patients discontinuing treatment due to disease progression. In the phase I study of BIIB021, grade 3 dizziness and syncope were considered dose-limiting toxicities (DLTs) [5]. In the current study, only three patients had grade 2 dizziness and there were no grade 3 or 4 dizziness events.
Table 3.
Incidence of Grade 2–4 adverse events possible, probably, or definitely related to treatment
| AE | 600 mg BIW |
400 mg TIW |
All patients |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G2 | G3 | G4 | Total | G2 | G3 | G4 | Total | G2 | G3 | G4 | Total | |
| Leukopenia | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Neutropenia | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Anemia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Vitamin A deficiency | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Dizziness | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 3 | 0 | 0 | 3 |
| Night blindness | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Hypertension | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Nausea | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 2 |
| Abdominal pain | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Diarrhea | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| Vomiting | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 2 |
| Abdominal distension | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Constipation | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 2 |
| Fatigue | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| AST increased | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 2 | 2 | 1 | 0 | 3 |
| ALT increased | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Alk phos increased | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 1 | 2 | 0 | 3 |
| γ-Glutamyltransferase increased | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 2 |
| Amylase increased | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Lipase increased | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Bilirubin increased | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Tumor necrosis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
AE, adverse event.
pharmacokinetics
The results for BIIB021 pharmacokinetic parameters are summarized in Table 4. These values were calculated on Day 1 only and thus may reflect changes in dose (400 mg versus 600 mg) but not schedule. The drug was rapidly absorbed. The mean Cmax was 1.5 µg/ml and the AUC was 2.9 µg/ml h. Both the doses were associated with similar Cmax and AUC results, although there was substantial inter-patient variability and the total sample size is small.
Table 4.
Pharmacokinetic parameters for BIIB021 on Day 1
|
Cmax (ng/ml) |
AUC 0–6 (ng/ml h) |
Tmax (h) |
||||
|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | Mean | Range | |
| 600 mg | 1439 | 1025 | 2942 | 1977 | 1.33 | 1–2 |
| 400 mg | 1528 | 1256 | 2802 | 2035 | 1.27 | 1–4 |
| All | 1482 | 1115 | 2875 | 1960 | 1.30 | 1–4 |
For each patient, the mean SUVmax at the beginning of cycle 2 was compared with baseline. Supplementary Figure S2, available at Annals of Oncology online, shows mean SUVmax % change at Day 29 for each patient as a function of BIIB021 Cmax. Patients who achieved a higher Cmax tended to have a larger decrease in SUVmax. Specifically, all patients with Cmax above the mean (>1500 ng/ml) had a decrease in SUVmax, whereas patients with Cmax below the mean (<1500 ng/ml) had either an increase or a decrease in SUVmax.
pharmacodynamics
The results of pharmacodynamic analyses are summarized in Supplementary Figure S3, available at Annals of Oncology online. All changes in pharmacodynamic markers are normalized to a baseline level of 1 for each patient. The mean results for all patients are shown with the standard error bars. HSP70 in PBMC increased following the first dose of BIIB021 (supplementary Figure S3, available at Annals of Oncology online top) and reached 30-fold higher levels than baseline (with large variation) 24 h after treatment. HSP70 levels remained elevated but fluctuated through Days 5 to 8; however, these remained roughly ninefold higher than the baseline at the beginning of cycle 2 (P = 0.10 by T-test). HSP70 in serum also increased following treatment (supplementary Figure S3, available at Annals of Oncology online bottom) but, in contrast, the change was gradual throughout the cycle, reaching a fourfold increase at the beginning of cycle 2 (P < 0.05).
discussion
HSP90 inhibition represents a promising new strategy for targeted therapy of GIST. Notably, this approach does not rely on the presence of particular mutations to predict sensitivity to a given tyrosine kinase inhibitor, but rather may target oncogene-driven tumors more broadly. Preclinical data have already suggested that HSP90 inhibition may be active in GIST that are inherently imatinib-resistant [14].
This study demonstrates that treatment with the HSP90 inhibitor BIIB021 is feasible in patients with refractory GIST. We observed an objective response rate of 22% by FDG-PET criteria, demonstrating that HSP90 inhibition can alter the metabolic activity of GIST. Compared with IPI-504, the other HSP90 inhibitor extensively tested in GIST, the formulation of BIIB021 tested here was orally bioavailable and relatively well-tolerated, although in a smaller group of patients [15].
Treatment with BIIB021 can lead to metabolic responses in patients who have previously progressed on imatinib and sunitinib. However, progression-free survival was relatively short. Pharmacokinetic studies show that therapeutic blood levels can be achieved in patients. The mean Cmax of 1.5 µg/ml is equivalent to 3.6 µM, which substantially exceeds the IC50 in a broad range of cell lines [6]. These levels are also well above those associated with HSP90 client protein degradation in preclinical models [13]. Pharmacodynamic studies suggest that HSP90 inhibition was achieved, demonstrated by substantial rise in HSP70.
Treatment with BIIB021 leads to a rapid decrease in FDG uptake in many patients, as measured by PET scan carried out after just two doses. Optimizing dose and schedule will be critical in further developing HSP90 inhibitors for GIST. When BIIB021 is given on BIW schedule, there is a substantial increase in FDG activity in 64% of patients between day 5 (just after the second dose) and day 8 (just before the next dose), suggesting that although target inhibition is achieved, it is not sustained (supplementary Figure S1, available at Annals of Oncology online, dark bars). We attempted to address this by reducing the interval between doses on a new schedule of 400 mg TIW. However, even on this schedule most patients (64%) had an increase in mean SUVmax between day 6 (after the third dose) and day 8 (before the next dose), as shown in Supplementary Figure S1, available at Annals of Oncology online (light bars). This suggests that more frequent dosing with this agent (perhaps daily) may be required for optimal antitumor activity.
In addition to optimal schedule, we attempted to identify optimal dose. Both doses studied here led to similar pharmacokinetic parameters but with substantial inter-patient variability. The cause of this variability is unclear. All patients took BIIB021 in the fasted state and drugs known to interfere with its absorption (such as proton-pump inhibitors) were prohibited. Nevertheless, our results suggest that patients who achieve higher Cmax are more likely to have a decline in SUVmax (supplementary Figure S2, available at Annals of Oncology online). Thus, higher doses may be required to optimize antitumor activity. In the phase I study of BIIB021, the maximum administered dose was 800 mg twice a week which results in two DLT's (Grade 3 syncope and Grade 3 dizziness) [5]. Thus, this dose was considered to exceed the MTD and the recommend phase II dose was 600 mg twice a week. In addition, a dose of 600 mg twice weekly was expected to be potentially efficacious because the exposure (AUC) achieved in subjects administered 600 mg BIIB021 twice weekly is greater than the exposure (AUC) resulting in at least 90% of the antitumor activity in xenograft models. More frequent dosing has not been tested in solid tumors because preclinical data indicate that the drug accumulates in tumors and has a prolonged effect despite a short plasma half-life. Actual intratumoral levels of the drug in patients are not known. Inadequate penetration of the drug could explain the lack of prolonged response. Future studies could include tumor biopsies to address this. In addition, an MTD for continuous daily dosing could be determined in a new phase I study.
This clinical trial met its primary end-point. Treatment with BIIB021 led to metabolic responses in >20% of patients and compared with ansamycin derivatives such as IPI-504 there was no substantial hepatotoxicity. These results provide a strong foundation for future development of non-ansamycin HSP90 inhibitors in GIST.
funding
This work was supported by Biogen Idec.
disclosure
DRD served as a consultant for Pfizer. RGM served on a Data and Safety Monitoring Board for Infinity. All the remaining authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–1741. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- 2.Wardelmann E, Thomas N, Merkelbach-Bruse S, et al. Acquired resistance to imatinib in gastrointestinal stromal tumours caused by multiple KIT mutations. Lancet Oncol. 2005;6:249–251. doi: 10.1016/S1470-2045(05)70097-8. [DOI] [PubMed] [Google Scholar]
- 3.Bauer S, Yu LK, Demetri GD, et al. Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Res. 2006;66:9153–9161. doi: 10.1158/0008-5472.CAN-06-0165. [DOI] [PubMed] [Google Scholar]
- 4.Lundgren K, Zhang H, Brekken J, et al. BIIB021, an orally available, fully synthetic small-molecule inhibitor of the heat shock protein Hsp90. Mol Cancer Ther. 2009;8:921–929. doi: 10.1158/1535-7163.MCT-08-0758. [DOI] [PubMed] [Google Scholar]
- 5.Elfiky A, Saif MW, Beeram M, et al. BIIB021, an oral, synthetic non-ansamycin Hsp90 inhibitor: Phase I experience. J Clin Oncol. 2008;26 Abstr 2503. [Google Scholar]
- 6.Zhang H, Neely L, Lundgren K, et al. BIIB021, a synthetic Hsp90 inhibitor, has broad application against tumors with acquired multidrug resistance. Int J Cancer. 2010;126:1226–1234. doi: 10.1002/ijc.24825. [DOI] [PubMed] [Google Scholar]
- 7.Van den Abbeele AD. The lessons of GIST––PET and PET/CT: a new paradigm for imaging. Oncologist. 2008;13(Suppl 2):8–13. doi: 10.1634/theoncologist.13-S2-8. [DOI] [PubMed] [Google Scholar]
- 8.Wagner AJ, Morgan JA, Chugh R, et al. Inhibition of heat shock protein 90 (Hsp90) with the novel agent IPI-504 in metastatic GIST following failure of tyrosine kinase inhibitors (TKIs) or other sarcomas: Clinical results from phase I trial. J Clin Oncol. 2008;26 Abstr 10503. [Google Scholar]
- 9.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET study group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 12.Dakappagari N, Neely L, Tangri S, et al. An investigation into the potential use of serum Hsp70 as a novel tumour biomarker for Hsp90 inhibitors. Biomarkers. 2010;15:31–38. doi: 10.3109/13547500903261347. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Chung D, Yang YC, et al. Identification of new biomarkers for clinical trials of Hsp90 inhibitors. Mol Cancer Ther. 2006;5:1256–1264. doi: 10.1158/1535-7163.MCT-05-0537. [DOI] [PubMed] [Google Scholar]
- 14.Dewaele B, Wasag B, Cools J, et al. Activity of dasatinib, a dual SRC/ABL kinase inhibitor, and IPI-504, a heat shock protein 90 inhibitor, against gastrointestinal stromal tumor-associated PDGFRAD842V mutation. Clin Cancer Res. 2008;14:5749–5758. doi: 10.1158/1078-0432.CCR-08-0533. [DOI] [PubMed] [Google Scholar]
- 15.Demetri GD, Le Cesne A, Von Mehren M, et al. Final results from a phase III study of IPI-504 (retaspimycin hydrochloride) versus placebo in patients (pts) with gastrointestinal stromal tumors (GIST) following failure of kinase inhibitor therapies. ASCO Gastrointest Cancer Symp. 2010 Abstr 64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.