Abstract
Abnormal diffusing capacity is common in HIV-infected individuals including never smokers. Etiologies for diffusing capacity impairment in HIV are not understood, particularly in those without a history of cigarette smoking.
A cross-sectional analysis of 158 HIV-infected individuals without acute respiratory symptoms or infection to determine associations between a DLCO % predicted and participant demographics, pulmonary spirometric measures (FEV1 and FEV1/FVC), radiographic emphysema (fraction of lung voxels <-950 Hounsfield units), pulmonary vascular/cardiovascular disease (echocardiographic tricuspid regurgitant jet velocity [TRV], N-terminal pro-brain natriuretic peptide), and airway inflammation (induced sputum cell counts), stratified by history of smoking.
Mean DLCO was 65.9% predicted, and 55 (34.8%) participants had a significantly reduced DLCO (<60 % predicted). Lower DLCO % predicted in ever smokers was associated with lower post-bronchodilator FEV1 % predicted (p<0.001) and greater radiographic emphysema (p=0.001). In never smokers, mean (standard deviation) DLCO was 72.7% (13.4%) predicted, and DLCO correlated with post-bronchodilator FEV1 (p=0.02), sputum neutrophils (p=0.03), and sputum lymphocytes (p=0.009), but not radiographic emphysema.
Airway obstruction, emphysema, and inflammation influence DLCO in HIV. Never smokers may have a unique phenotype of diffusing capacity impairment. The interaction of multiple factors may account for the pervasive nature of diffusing capacity impairment in HIV infection.
Keywords: HIV, Pulmonary function, Diffusing capacity, AIDS
Introduction
Lung disease is an important cause of morbidity and mortality in the HIV-infected population even in the current era of combination antiretroviral therapy (ART). A greater incidence of several non-infectious lung diseases has been found in HIV-infected persons compared to HIV-uninfected persons,[1] and death from obstructive lung disease has increased in the HIV-infected population since the introduction of ART.[2] Abnormal diffusing capacity and an accelerated form of emphysema were associated with HIV infection prior to ART.[3-6] A recent study showed that abnormal diffusing capacity remains extremely common with over 64% of HIV-infected persons having an impaired diffusing capacity for carbon monoxide (DLCO) (<80% predicted). Diffusing capacity impairment is not limited to smokers with HIV as over 47% of never-smokers have been reported to have a DLCO below 80% percent predicted.[7] Additionally, diffusing capacity impairment in the HIV-uninfected population has been associated with increased mortality,[8] highlighting the importance of this abnormality in lung function.
DLCO can be decreased by multiple mechanisms including parenchymal destruction, interstitial lung disease, loss of alveolar surface, or primary pulmonary vascular processes. The contributors to decreased diffusing capacity in HIV are not well-known, but studies prior to ART found the majority of HIV-infected individuals had impairments in DLCO related to advanced HIV, infections, and emphysema.[3-6] One study from a pre-ART cohort of participants without AIDS-defining lung disease demonstrated impairment in diffusing capacity was related to a decrease in capillary blood volume and not an increase in the membrane component of gas diffusion, suggesting emphysema or pulmonary vascular disease to be the significant contributors.[4] Although recent studies have found that abnormal DLCO remains common in HIV infection in the post-ART era, specific contributors to this abnormality has not been examined, and associations in non-smokers who may represent a distinct phenotype have not been specifically investigated. Understanding causes of diffusion impairment in HIV might lead to development of novel therapies.
We investigated contributors to impaired diffusing capacity in an HIV-infected cohort. Factors examined included mechanical lung function, computed tomography (CT) assessed emphysema, echocardiographic pulmonary hypertension, markers of cardiac strain, and lung inflammation. We also investigated the same relationships to impaired diffusing capacity in the subset of participants who had never smoked.
Methods
Participants
Details of the methods can be found in the online data repository. In brief, participants were 158 HIV-infected outpatients and were a subset of an established cohort recruited from the University of Pittsburgh HIV/AIDS clinic who had a study visit between February 2009 and August 2011.[7] All participants signed written informed consent, and the University of Pittsburgh IRB approved the protocol. Standardized questionnaires were used to obtain demographic and clinical data including smoke exposure and smoking history, any occupational exposures to vapors, gases, dusts, or fumes, and prior pneumonia. Medical record review was used to obtain CD4+ T-lymphocyte count and plasma HIV RNA levels within the past six months.
Testing procedures
Participants performed spirometry and single breath CO diffusing capacity (DLCO) measurement per American Thoracic Society/European Respiratory Society guidelines.[9, 10] Race-adjusted predicted values for spirometry were determined using Hankinson formulas and for DLCO using Neas formulas adjusted for carboxyhemoglobin and hemoglobin concentrations [9, 11, 12]. Standardized non-contrast CT scans of the entire thorax at end-inspiration were obtained in individuals who had less than approximately 10 rad exposure to radiation in the prior year. Percentage of lung voxels associated with emphysema defined as voxels below −950 Hounsfield units was calculated[13]. Scans were reviewed independently without knowledge of the participant’s lung function or clinical characteristic by two pulmonologist (MG and CW) and a radiologist (CF) to determine the presence of interstitial lung disease or fibrosis defined by the presence of diffuse, peripheral, or subpleural patchy ground glass opacities, reticular opacities, honeycomb changes, or signs of volume loss (traction bronchiectasis, displaced fissure)[14]. Echocardiography was performed to determine peak tricuspid regurgitant jet velocity (TRV),[15] left ventricular (LV) ejection fraction, LV hypertrophy, and diastolic dysfunction. A TRV ≥3.0 m/sec was defined as elevated.[16] Echocardiograms were added to the study protocol as of July 1, 2009 and were available in 126 individuals. Percentages of neutrophils, lymphocytes, and eosinophils were determined from sputum induced via inhalation of nebulized 3% saline.[17] Sputum samples were considered suitable for analysis if they contained fewer than 30% squamous cells (n=128). Plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) was measured in participants who had a Modification of Diet in Renal Disease[18] estimated glomerular filtration rate ≥60 ml/min per 1.73 m2 (n=143).[19] CT scans and echocardiograms were administered by trained clinical technicians. Pulmonary function tests were administered by either of 2 trained research nurses. Sputum analysis and NT-proBNP measurement were done in a research lab.
Statistical approach
Because it is likely that causes of diffusion impairment are different in those who had ever smoked and never smokers, analysis was stratified by smoking status. The dependent variable DLCO % predicted was analyzed as a dichotomous variable, identifying those with more significantly impaired diffusing capacity (≤60% predicted) vs. >60%, and also as a continuous variable. This cut-off was chosen because it is used clinically to define moderate impairment of DLCO and we were interested in a more severe phenotype. Participant characteristics were summarized and compared between participants with a DLCO >60% predicted and ≤60% predicted using t-tests, Wilcoxon rank-sum, chi-squared, or Fisher’s exact tests where appropriate with a p-value less than 0.05 considered significant. Variables of interest associated with DLCO were assessed from four categories: measures of obstructive lung disease (forced expiratory volume in 1 second [FEV1] % predicted, forced vital capacity [FVC] % predicted, FEV1/FVC, and percent of radiographic emphysema), interstitial lung disease (presence of interstitial changes on CT scan), pulmonary vascular/cardiovascular disease (TRV, elevated TRV, NT-proBNP, left ventricular function), and airway inflammation (induced sputum cell counts). To approximate normality, NT-proBNP was transformed using the natural logarithm and sputum lymphocyte counts using the square root. Sputum eosinophils were dichotomized for analysis to detectable versus absent. Associations between variables of interest and DLCO % predicted were determined using simple linear regression.
Two linear regression models, one for ever smokers and another for never smokers, were created to determine independent associations between DLCO % predicted and participant demographics, obstructive lung disease, pulmonary vascular disease, and inflammation. Variables were selected for the model that had univariate associations of p<0.1. Models were selected using stepwise regression.[20] The multivariable models were assessed for excess colinearity by checking variance inflation factors.[21] The effect of secondhand smoke exposure was assessed in the multivariate models and no significant confounding was noted.
Results
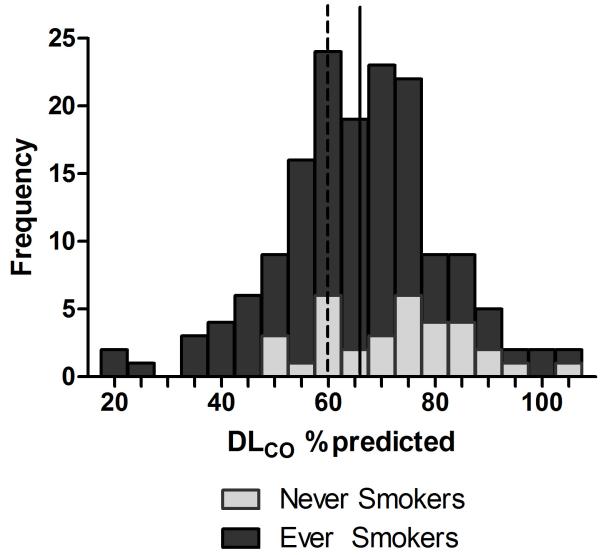
One hundred and fifty-eight participants completed pulmonary function testing, 125 who had ever smoked and 33 who had never smoked. The majority of participants (71.3%) had undetectable plasma HIV RNA levels, and CD4 counts at the most recent assessment within 6 months prior to study visit were generally high (median CD4 cells/μL, 561; range, 24-2094). The mean (standard deviation [SD]) DLCO was 64.1% (15.0%) predicted in ever smokers and 72.7% (13.4%) in never smokers (Table 1). Eighty-four percent of participants had a DLCO <80% predicted. Forty-seven (37.6%) smokers had a more significantly reduced DLCO (≤60% predicted), and even 8 (24.2%) never smokers had a DLCO ≤60% predicted (Figure 1). Among smokers, those with a reduced DLCO had lower BMI, smoked more pack-years, and were more likely to have used cocaine (Table 1). In 125 participants with data collected on secondhand smoke exposure, secondhand smoke exposure during childhood was more common in ever smokers with significantly reduced DLCO.
Table 1.
| Ever Smokers (n=125) |
DLCO >60% predicted (n=78) |
DLCO ≤60% predicted (n=47) |
p- value |
Never Smokers (n=33) |
DLCO >60% predicted (n=25) |
DLCO ≤60% predicted (n=8) |
p- value |
|
|---|---|---|---|---|---|---|---|---|
| Age, mean (SD) | 46.1 (9.2) | 45.4 (10.0) | 47.3 (7.6) | 0.25 | 48.8 (10.7) | 48.4 (11.6) | 50.0 (8.2) | 0.71 |
| Female, n (%) | 38 (30.4) | 25 (32.1) | 13 (26.7) | 0.61 | 7 (21.2) | 4 (16.0) | 3 (37.5) | 0.32 |
| African American, n (%) | 70 (56.0) | 43 (55.1) | 27 (57.5) | 0.37 | 10 (30.3) | 7 (28.0) | 3 (37.5) | 0.61 |
| HIV risk factor, n (%) | 0.26 | 0.28 | ||||||
| MSM | 60 (48.4) | 39 (50.0) | 21 (44.7) | 18 (54.6) | 15 (60.0) | 3 (37.5) | ||
| Heterosexual contact | 41 (30.1) | 28 (35.9) | 13 (27.7) | 10 (30.3) | 7 (28.0) | 3 (37.5) | ||
| Intravenous drug use | 15 (12.1) | 6 (7.7) | 9 (19.1) | 0 (0) | -- | -- | ||
| Blood transfusion/unknown/refused | 9 (7.2) | 5(6.4) | 3 (6.4) | 5 (15.2) | 3 (12.0) | 2 (20.0) | ||
| Body mass index, mean (SD) | 26.7 (5.7) | 27.5 (5.7) | 25.4 (5.5) | 0.02 | 27.3 (5.2) | 26.6 (4.9) | 29.3 (6.1) | 0.21 |
| Smoking Status, n (%) | 0.14 | na | ||||||
| Never | -- | -- | -- | 33 (100) | 25 (100) | 8 (100) | ||
| Former | 39 (31.2) | 28 (35.9) | 11 (23.4) | |||||
| Current | 86 (68.8) | 50 (64.1) | 36 (76.6) | |||||
| Pack-years smoked, median (range) | 14.2 (0- 75) |
10 (0-75) | 17.4 (0-70) | 0.03 | 0 (0-0) | 0 (0-0) | 0 (0-0) | na |
| Secondhand smoke exposure, n (%)* | ||||||||
| Childhood home | 77 (78.6) | 40 (69.0) | 37 (92.5) | 0.01 | 19 (70.4) | 14 (70.0) | 5 (71.4) | 0.94 |
| Adult home | 83 (84.7) | 48 (82.8) | 35 (87.5) | 0.52 | 20 (74.1) | 14 (70.0) | 6 (85.7) | 0.63 |
| Adult outside of home | 85 (86.7) | 50 (86.2) | 35 (87.5) | 0.85 | 18 (66.7) | 13 (65) | 5 (71.4) | 0.76 |
| Marijuana use ever, n (%) | 80 (64.0) | 51 (65.4) | 29 (61.7) | 0.68 | 8 (24.2) | 5 (20.0) | 3 (37.5) | 0.37 |
| Cocaine use ever, n (%) | 34 (27.2) | 16 (20.5) | 18 (38.3) | 0.03 | 3 (9.1) | 2 (8.0) | 1 (12.5) | 1.0 |
| Hepatitis C, n (%) | 19 (15.2) | 11 (14.1) | 8 (17.2) | 0.68 | 5 (15.2) | 4 (16.0) | 1 (12.5) | 1.0 |
| Occupational exposure, n (%) | 30 (24.0) | 18 (23.1) | 12 (25.5) | 0.76 | 6 (18.2) | 5 (20.0) | 1 (12.5) | 0.63 |
| Using pneumonia prophylaxis, n (%) | 38 (30.4) | 23 (29.5) | 15 (31.9) | 0.85 | 7 (21.2) | 6 (24.0) | 1 (12.5) | 0.65 |
| Prior pneumonia, n (%) | 32 (25.6) | 21 (20.6) | 17 (31.5) | 0.13 | 6 (18.2) | 4 (16.0) | 2 (25.0) | 0.62 |
| Prior Pneumocystis pneumonia, n (%) | 3 (2.4) | 2 (2.6) | 1 (2.1) | 0.81 | 0 | -- | -- | na |
| Antiretroviral use, n (%) | 110 (88.0) | 67 (85.9) | 43 (91.5) | 0.35 | 27 (81.8) | 20 (80.0) | 7 (87.5) | 1.0 |
| CD4+ T-lymphocytes/μL, median (range) |
561.5 (24- 2094) |
571 (33-2094) | 528.5 (24- 1798) |
0.59 | 533 (179- 1327) |
513 (179-1140) | 685.5 (265- 1327) |
0.48 |
| Plasma HIV RNA - ln copies/mL, median (range) |
UD (UD- 13.2) |
UD (UD-13.2) | UD (UD-12.1) | 0.88 | UD (UD- 13.7) |
UD (UD-13.7) | UD (UD-8.9) | 0.65 |
| DLCO % predicted, mean (SD) | 64.1 (15.0) | 73.0 (9.5) | 49.4 (10.0) | na | 72.7 (13.4) | 78.1 (10.5) | 55.8 (3.5) | na |
Data on secondhand smoke exposure was collected for 125 participant, 98 smokers and 27 never smokers.
P-values represent comparisons between the participants with DLCO>60% predicted and those with a DLCO≤60% predicted.
n = number; SD = standard deviation; HIV = human immunodeficiency virus; MSM = men who have sex with men; RNA = ribonucleic acid; UD = undetectable; EGFR = estimated glomerular filtration rate; DLCO diffusing capacity for carbon monoxide.
Figure 1.
Distribution of DLCO % predicted. Dashed line represents 60% predicted DLCO. Solid line represents the mean. (light gray) = never smokers; (dark gray) = ever smokers.
Measures of obstructive lung disease
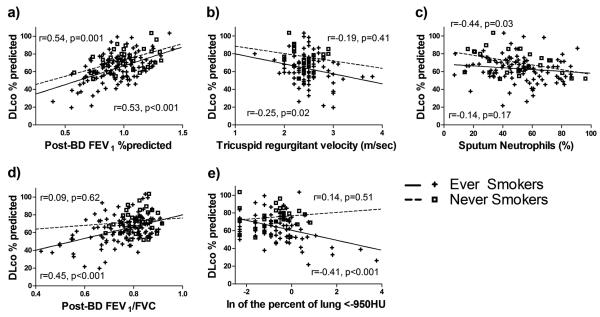
Diffusing capacity was associated with several measures of obstructive lung disease. In ever smokers, a lower DLCO % predicted was associated with lower post-bronchodilator FEV1, FVC, and FEV1/FVC (Table 2), but in never smokers, lower DLCO % predicted was only associated with FEV1 and FVC. In 117 participants who had CT scans, a lower DLCO % predicted was seen with a higher fraction of lung voxels <-950 HU in ever smokers but not never smokers (Figure 2).
Table 2.
Univariate associations of markers of airway obstruction,pulmonary hypertension, and airway inflammation with DLCO % predicted
| Ever Smokers | Never Smokers | |||
|---|---|---|---|---|
| DLCO % predicted | p-value | DLCO % predicted | p-value | |
| β-coefficient | β-coefficient | |||
| Obstructive lung disease | ||||
| Post-BD FEV1 % predicted | 0.4245 | <0.001 | 0.3679 | 0.001 |
| Post-BD FVC % predicted | 0.3255 | <0.001 | 0.4743 | <0.001 |
| Post-BD FEV1/FVC | 0.6748 | <0.001 | 0.2084 | 0.62 |
| Log Fraction <950 HU | −0.0564 | <0.001 | 0.0191 | 0.52 |
| Pulmonary vascular and LV disease | ||||
| TRV, per m/sec | −0.1123 | 0.02 | −0.0827 | 0.41 |
| TRV ≥3.0 m/sec | −0.1572 | 0.002 | --- | --- |
| LV ejection fraction | 0.0024 | 0.512 | 0.0205 | 0.02 |
| LV diastolic dysfunction | −0.0290 | 0.46 | 0.0971 | 0.13 |
| LV hypertrophy | −0.0182 | 0.65 | −0.0113 | 0.91 |
| NT-proBNP, per log units/mL | −0.0153 | 0.14 | 0.0036 | 0.91 |
| Airway inflammation | ||||
| Sputum neutrophils | −0.1025 | 0.17 | −0. 2684 | 0.03 |
| Sputum eosinophils (detectable vs. none) | −0.0321 | 0.27 | −0.0105 | 0.86 |
| Square root - sputum lymphocytes | 0.0546 | 0.75 | 1.3000 | 0.003 |
BD = bronchodilator; FEV1 = forced expiratory volume in the first second; FVC = forced vital capacity; HU = Hounsfield units; TRV = tricuspid regurgitant jet velocity; LV = left ventricle; NT-proBNP = N-terminal pro-brain natriuretic peptide; DLCO = diffusing capacity for carbon monoxide.
Figure 2.
Scatterplots and regression lines for DLCO % predicted by a) post-bronchodilator (BD) FEV1 % predicted, b) Tricuspid regurgitant velocity, c) sputum neutrophil percent, d) post-BD FEV1/FVC, and e) natural logarithm (ln) of the fraction of lung <-950 for ever smokers (positive symbols,+, and solid lines) and never smokers (open squares, □, and dashed lines). Pearson correlation coefficients (r) and significance values (p) are shown nearest to the regression lines of smokers (bottom) and never smokers (top) for correlations between the independent variable in each graph and DLCO % predicted.
Measures of interstitial lung disease
None of the CT exams reviewed were rated as positive for the presence of interstitial lung disease or fibrosis.
Measures of pulmonary vascular/cardiovascular disease
Lower DLCO % predicted was seen with a higher TRV and higher NT-proBNP levels in ever smokers. Ten participants (7.9%), all ever smokers, had echocardiograms with an abnormal TRV (≥3.0m/sec), and an abnormal TRV was associated with a lower DLCO % predicted. Measures of left ventricular function were not associated with DLCO in ever smokers, but lower LVEF was associated with lower DLCO % predicted in never smokers (Table 2).
Measures of airway inflammation
The mean (SD) percent neutrophils in 128 participants with adequate induced sputum was 50.5% (19.8%). None of the sputum cell counts were associated with diffusing in ever smokers, but higher sputum neutrophil percent was associated with a lower DLCO % predicted in never smokers (Figure 2). The median (range) lymphocyte percentage was 0.7% (0-9.0%), and lower sputum lymphocyte percents were associated with lower DLCO in never smokers.
While the associations between DLCO and post-BD FEV1 % predicted, TRV, and sputum neutrophils (Figures 2a, 2b, 2c) were similar in smokers and never smokers, the associations appeared different between smokers and never smokers for post-BD FEV1/FVC and radiographic emphysema (Figures 2d and 2e).
Multivariable regression
Regression models were evaluated to determine specific factors independently associated with impaired diffusion in ever smokers and never smokers separately (Table 3). In ever smokers, the final model showed that lower post-bronchodilator FEV1 % predicted and greater percent emphysema measured by the fraction of lung <950 HU were independently associated with worse DLCO % predicted. Opposed to this, in never smokers, lower DLCO % predicted was associated with lower post-bronchodilator FVC % predicted, a greater percent of neutrophils in sputum, and a lower percent of lymphocytes in sputum.
Table 3.
Multivariable regression models showing independent associations for DLCO % predicted in ever smokers and never smokers.
| Ever Smokers* | Never Smokers | |||
|---|---|---|---|---|
| β-coefficient | p-value | β-coefficient | p-value | |
| Post-FEV1 % predicted | 0.3940 | <0.001 | ||
| Post-BD FVC % predicted | 0.3323 | 0.02 | ||
| Log Fraction <−950 HU | −0.0423 | 0.001 | ||
| Sputum % Neutrophils | −0.1967 | 0.03 | ||
| Sputum % Lymphocyte (square root) | 0.9407 | 0.009 | ||
Body Mass Index, pack-years smoking, and cocaine use were considered for model construction.
BD – bronchodilator; FEV1 – forced expiratory volume in 1 second; TRV – tricuspid regurgitant jet velocity
Discussion
In an HIV-infected cohort, we found that impaired diffusing capacity is common, even in never smokers, and may result from different mechanisms in smokers and non-smokers. Overall, in smokers, impaired diffusing capacity was associated with measures related to obstruction and emphysema (lower FEV1 % predicted and greater radiographic emphysema), and in never smokers, diffusing capacity impairment was associated with FVC and airway inflammation, but not lower FEV1/FVC or radiographic emphysema.
Degree of diffusing capacity impairment in this cohort is comparable to findings in prior studies of diffusing capacity in HIV-infected persons.[3-6] We found a mean DLCO of 65.9% predicted, and most previous studies find low DLCO in HIV-infected individuals. In our cohort, 34.3% had a DLCO less than 60% predicted and 84.8% had a DLCO less than 80% predicted, while prior studies found 55% had a DLCO below 80% predicted[3, 6] or 25% had a DLCO below 72% predicted.[4]
The pathogenesis for diffusing capacity impairment in the HIV-infected population is not completely understood. Early in the HIV epidemic, it was thought primarily due to HIV-related inflammation or infection[3, 5], and diffusing capacity seemed to worsen with HIV progression.[3] Prior work was performed before widespread use of effective ART, and many participants had acute pulmonary processes.[3-6] In contrast, 87% of participants in the current study were using ART and the majority had CD4+ T-cell counts greater than 500 cells/μl and undetectable viral loads (< 50 copies/ml by ultrasensitive assays). None of the participants had acute pulmonary infections, and these factors were not associated with DLCO impairment in the cohort.
The impairment in diffusing capacity in HIV in the pre-ART era was also related to a decrease in the capillary blood volume in the lung and an accelerated form of emphysema.[4] The current study supports emphysema contributing to diffusing capacity impairment in smokers; however, the prevalence of diffusing capacity impairment in never smokers suggests diffusing capacity impairment is not entirely smoking-related.
Prior work has also demonstrated reductions in diffusing capacity in HIV related to lung infection or inflammation.[3, 5] In the HIV-uninfected population, increased sputum neutrophils are a hallmark of COPD and correlate with disease severity.[22, 23] In our cohort, airway inflammatory markers were associated with reduced diffusing capacity, only in never smokers. However, because we do not see an association between measures of emphysema and diffusing impairment in never smokers, other mechanisms linking increased neutrophils and diffusing impairment may also be important.
Pulmonary vascular disease (or pulmonary arterial hypertension) is more prevalent in HIV-infected persons[16, 24] and could also contribute to impairment in diffusing capacity. We found that elevated pulmonary artery pressures (as measured by increased TRV on echocardiography) correlated with decreases in diffusing capacity. We have previously shown that lower diffusing capacity is associated with elevated TRV, but our analysis demonstrates that there is an independent contribution of pulmonary artery pressures to diffusing capacity.[25] It is also possible that COPD contributed to the increased pulmonary artery pressures seen, and longitudinal or animal studies will be helpful in examining cause and effect of these abnormalities.
Finally, it is possible that there are extra-pulmonary causes for reduced diffusing capacity. We corrected for anemia, but hyperglycemia is under-appreciated in HIV-infected persons, and chronic hyperglycemia can reduce diffusing capacity.[26, 27] We did not have measures of hemoglobin A1C to assess chronic glucose levels. Cardiac function might also impact diffusing capacity, but there was no correlation between LV function (both systolic or diastolic) or hypertrophy and DLCO except in the never smokers, although this association was quite modest. Cardiac strain, as indicated by NT-proBNP levels, was associated with DLCO abnormalities and may reflect right heart strain in smokers given the association of elevated pulmonary artery pressures with DLCO or left heart strain in non-smokers given the association with LV function.
The degree of diffusing capacity impairment in never smokers is striking (over ¼ with a DLCO less than 60% predicted) and may indicate causes for diffusing capacity impairment that are independent of smoking. Although a small sample, we found that impaired diffusing capacity in never smokers was associated with reduced FVC, but not a reduced FEV1/FVC or radiographic emphysema. Because decreased FEV1/FVC ratio and radiographic emphysema are suggestive of an emphysema phenotype,[28] emphysema may play a large role in diffusing capacity impairment in smokers, but in never smokers, diffusing capacity impairment may be driven by a process other than emphysema or a process that might later develop into emphysema. We speculate that a component of restrictive lung physiology and inflammation or immune reconstitution may be in part responsible for impaired diffusing capacity in HIV-infected individuals. These mechanisms may be overshadowed by smoking, but still could be important contributors to diffusing capacity impairment.
Taken together, these data suggest diffusing capacity impairment in HIV infection is multifactorial and not solely the result of accelerated COPD that has been reported. The associations of diffusing capacity impairment with measures of airflow obstruction, pulmonary vascular disease, and airway inflammation indicate that several pathologic processes contribute to the reduction in diffusing capacity commonly seen in HIV-infected individuals. Various pathways may be contributing to these pathologies such as smoking leading to airway obstruction and emphysema, HIV-viral proteins contributing to pulmonary vascular disease,[29] or immune reconstitution leading to lung inflammation.[30] Vascular dysfunction may also link smoking and emphysema.[31] However, it is possible these are tied together by a single underlying mechanism such as immune activation related to HIV infection.[32] Chronic immune stimulation is believed to cause premature aging and immune senescence in HIV-infected persons[33] and has been implicated in both emphysema[34] and pulmonary vascular disease in the HIV-uninfected population.[35] Understanding individual factors important in diffusing capacity abnormalities in this population could have direct implications for guiding appropriate diagnostic evaluation in HIV-infected patients with respiratory symptoms and an abnormal diffusing capacity and influencing treatment modalities pursued.
This study has several limitations. It is a single-center study with a high prevalence of smoking and drug use in the cohort and may not be applicable to the entire HIV population, although smoking and drug use are quite common in HIV-infected individuals.[36] We did not evaluate other diseases that could affect diffusing capacity and have been reported more frequently in HIV such as primary pulmonary hypertension or lymphocytic interstitial pneumonia.[1] However, these diseases are still rare even in HIV infection, and they would be unlikely to be responsible for the great degree of impaired diffusing capacity seen. We have cross-sectional data on CD4+ T-cell and plasma HIV RNA levels, but peak and nadir levels or changes over time may be important factors in the development of impaired diffusing capacity. Pre-therapy HIV RNA levels, HIV specific immune responses, host genetic factors, or immune activation/chronic inflammation may also be important. We have a relatively small sample of never smokers which may limit the power to detect meaningful associations. Additionally, the degree and magnitude of secondhand smoke exposure is difficult to quantify, and with over 70% of never smokers reporting some secondhand smoke exposure it raises the question as to whether they are truly without smoke-related injury.
In conclusion, abnormal diffusing capacity is common in HIV-infected persons, even in non-smokers. While smoking and illicit drug use are associated with abnormal diffusing capacity, independent contributions of obstructive lung disease, primary pulmonary vascular disease, and inflammation suggest either a multifactorial cause of diffusing capacity impairment in HIV-infected individuals or a common, underlying pathway linking these processes. Given that reduced DLCO also predicts mortality,[8] the driving forces resulting in accelerated emphysema, pulmonary vascular disease, and inflammation in HIV infection are important to understand in order to ameliorate this significant impairment in lung function.
Acknowledgements, Competing interests, Funding
Sources of support: NIH T32 HL007563 and K23 HL108697 (MG); P50 HL084948 and N01 HR46163R01 (FS); R01 HL083461, HL083461S, and HL090339 (AM); the University of Pittsburgh CTSI (UL1 RR024153)
References
- 1.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, Oursler KK, Rimland D, Gibert CL, Butt AA, Justice AC. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388–95. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louie JK, Hsu LC, Osmond DH, Katz MH, Schwarcz SK. Trends in causes of death among persons with Acquired Immunodeficiency Syndrome in the era of highly active antiretroviral therapy, San Francisco, 1994-1998. J Infect Dis. 2002;186(7):1023–7. doi: 10.1086/343862. [DOI] [PubMed] [Google Scholar]
- 3.Nieman RB, Fleming J, Coker RJ, Harris JR, Mitchell DM. Reduced carbon monoxide transfer factor (TLCO) in human immunodeficiency virus type I (HIV-I) infection as a predictor for faster progression to AIDS. Thorax. 1993;48(5):481–5. doi: 10.1136/thx.48.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Neal D, Nagaraja HN, Drake J, Clanton TL. The pathophysiology of pulmonary diffusion impairment in human immunodeficiency virus infection. Am J Respir Crit Care Med. 1999;160(1):272–7. doi: 10.1164/ajrccm.160.1.9812089. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell DM, Fleming J, Pinching AJ, Harris JR, Moss FM, Veale D, Shaw RJ. Pulmonary function in human immunodeficiency virus infection. A prospective 18-month study of serial lung function in 474 patients. Am Rev Respir Dis. 1992;146(3):745–51. doi: 10.1164/ajrccm/146.3.745. [DOI] [PubMed] [Google Scholar]
- 6.Shaw RJ, Roussak C, Forster SM, Harris JR, Pinching AJ, Mitchell DM. Lung function abnormalities in patients infected with the human immunodeficiency virus with and without overt pneumonitis. Thorax. 1988;43(6):436–40. doi: 10.1136/thx.43.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gingo MR, George MP, Kessinger CJ, Lucht L, Rissler B, Weinman R, Slivka WA, McMahon DK, Wenzel SE, Sciurba FC, Morris A. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182(6):790–6. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neas LM, Schwartz J. Pulmonary function levels as predictors of mortality in a national sample of US adults. Am J Epidemiol. 1998;147(11):1011–8. doi: 10.1093/oxfordjournals.aje.a009394. [DOI] [PubMed] [Google Scholar]
- 9.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–35. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 10.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 11.Neas LM, Schwartz J. The determinants of pulmonary diffusing capacity in a national sample of U.S. adults. Am J Respir Crit Care Med. 1996;153(2):656–64. doi: 10.1164/ajrccm.153.2.8564114. [DOI] [PubMed] [Google Scholar]
- 12.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 13.Leader JK, Zheng B, Rogers RM, Sciurba FC, Perez A, Chapman BE, Patel S, Fuhrman CR, Gur D. Automated lung segmentation in X-ray computed tomography: development and evaluation of a heuristic threshold-based scheme. Acad Radiol. 2003;10(11):1224–36. doi: 10.1016/s1076-6332(03)00380-5. [DOI] [PubMed] [Google Scholar]
- 14.Raghu G, Nicholson AG, Lynch D. The classification, natural history and radiologic/histologic appearance of idiopathic fibrosis and the other idiopathic interstitial pneumonias. Eur Respir Rev. 2008;17(109):108–15. [Google Scholar]
- 15.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66(4):493–6. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 16.Hsue PY, Deeks SG, Farah HH, Palav S, Ahmed SY, Schnell A, Ellman AB, Huang L, Dollard SC, Martin JN. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. AIDS. 2008;22(7):825–33. doi: 10.1097/QAD.0b013e3282f7cd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahy JV, Boushey HA, Lazarus SC, Mauger EA, Cherniack RM, Chinchilli VM, Craig TJ, Drazen JM, Ford JG, Fish JE, Israel E, Kraft M, Lemanske RF, Martin RJ, McLean D, Peters SP, Sorkness C, Szefler SJ. Safety and reproducibility of sputum induction in asthmatic subjects in a multicenter study. Am J Respir Crit Care Med. 2001;163(6):1470–5. doi: 10.1164/ajrccm.163.6.9901105. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Tsutamoto T, Wada A, Sakai H, Ishikawa C, Tanaka T, Hayashi M, Fujii M, Yamamoto T, Dohke T, Ohnishi M, Takashima H, Kinoshita M, Horie M. Relationship between renal function and plasma brain natriuretic peptide in patients with heart failure. Journal of the American College of Cardiology. 2006;47(3):582–6. doi: 10.1016/j.jacc.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 20.Hosmer DW, Jr., Wang CY, Lin IC, Lemeshow S. A computer program for stepwise logistic regression using maximum likelihood estimation. Comput Programs Biomed. 1978;8(2):121–34. doi: 10.1016/0010-468x(78)90047-8. [DOI] [PubMed] [Google Scholar]
- 21.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16(9):965–80. doi: 10.1002/(sici)1097-0258(19970515)16:9<965::aid-sim509>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Singh D, Edwards L, Tal-Singer R, Rennard S. Sputum neutrophils as a biomarker in COPD: findings from the ECLIPSE study. Respir Res. 2010;11:77. doi: 10.1186/1465-9921-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright JL, Churg A. Current concepts in mechanisms of emphysema. Toxicol Pathol. 2007;35(1):111–5. doi: 10.1080/01926230601059951. [DOI] [PubMed] [Google Scholar]
- 24.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177(1) doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 25.Morris A, Gingo MR, George MP, Lucht L, Kessinger C, Singh V, Hillenbrand M, Busch M, McMahon D, Norris KA, Champion HC, Gladwin MT, Zhang Y, Steele C, Sciurba FC. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS. 2011 doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glesby MJ, Hoover DR, Shi Q, Danoff A, Howard A, Tien P, Merenstein D, Cohen M, Golub E, Dehovitz J, Nowicki M, Anastos K. Glycated haemoglobin in diabetic women with and without HIV infection: data from the Women’s Interagency HIV Study. Antiviral therapy. 2010;15(4):571–7. doi: 10.3851/IMP1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marvisi M, Bartolini L, del Borrello P, Brianti M, Marani G, Guariglia A, Cuomo A. Pulmonary function in non-insulin-dependent diabetes mellitus. Respiration. 2001;68(3):268–72. doi: 10.1159/000050509. [DOI] [PubMed] [Google Scholar]
- 28.Park KJ, Bergin CJ, Clausen JL. Quantitation of emphysema with three-dimensional CT densitometry: comparison with two-dimensional analysis, visual emphysema scores, and pulmonary function test results. Radiology. 1999;211(2):541–7. doi: 10.1148/radiology.211.2.r99ma52541. [DOI] [PubMed] [Google Scholar]
- 29.Marecki JC, Cool CD, Parr JE, Beckey VE, Luciw PA, Tarantal AF, Carville A, Shannon RP, Cota-Gomez A, Tuder RM, Voelkel NF, Flores SC. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. Am J Respir Crit Care Med. 2006;174(4):437–45. doi: 10.1164/rccm.200601-005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipman M, Breen R. Immune reconstitution inflammatory syndrome in HIV. Curr Opin Infect Dis. 2006;19(1):20–5. doi: 10.1097/01.qco.0000200543.80712.01. [DOI] [PubMed] [Google Scholar]
- 31.Gordon C, Gudi K, Krause A, Sackrowitz R, Harvey BG, Strulovici-Barel Y, Mezey JG, Crystal RG. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med. 2011;184(2):224–32. doi: 10.1164/rccm.201012-2061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Path. 2008;214(2):231–41. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 33.Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infection. Exp Gerontol. 2007;42(5):432–7. doi: 10.1016/j.exger.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Muller KC, Welker L, Paasch K, Feindt B, Erpenbeck VJ, Hohlfeld JM, Krug N, Nakashima M, Branscheid D, Magnussen H, Jorres RA, Holz O. Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res. 2006;7:32. doi: 10.1186/1465-9921-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noureddine H, Gary-Bobo G, Alifano M, Marcos E, Saker M, Vienney N, Amsellem V, Maitre B, Chaouat A, Chouaid C, Dubois-Rande JL, Damotte D, Adnot S. Pulmonary artery smooth muscle cell senescence is a pathogenic mechanism for pulmonary hypertension in chronic lung disease. Circ Res. 2011 Aug 19;109(5):543–53. doi: 10.1161/CIRCRESAHA.111.241299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prevalence of risk behaviors for HIV infection among adults--United States, 1997. MMWR Morb Mortal Wkly Rep. 2001;50(14):262–5. [PubMed] [Google Scholar]