Abstract
Men with prostate cancer suffer substantially from bone-related complications. Androgen deprivation therapy itself is a cause of loss of bone mineral density and is associated with an increased incidence of osteoporotic fractures. In advanced disease, bone is by far the most common site of metastasis. Complications of bone metastases prominently include pain and the potential for skeletal events such as spinal cord compression and pathologic fractures. Elevated osteoclast activity is an important aspect of the pathophysiology of both treatment-related osteoporosis and skeletal complications due to metastases. The osteoclast is therefore a therapeutic target. Denosumab is a fully human monoclonal antibody to receptor activator of nuclear factor-κ-B ligand that was designed to potently inhibit osteoclast activity and is the central focus of this review. Bisphosphonates, radiopharmaceuticals and systemically-active hormonal agents such as abiraterone acetate and enzalutamide have each been shown to improve skeletal morbidity in specific clinical situations. Denosumab is the only agent that has been shown to prevent osteoporotic fractures in men receiving androgen deprivation therapy and at elevated risk for fracture. It has also demonstrated superiority to the potent bisphosphonate zoledronic acid for the prevention of skeletal-related events in men with castration-resistant prostate cancer metastatic to bone. Efficacy and toxicity data will be discussed.
Keywords: denosumab, osteoporosis, osteopenia, osteoclast, RANK (receptor activator of nuclear factor-kappa B), skeletal-related events
INTRODUCTION
Skeletal complications are a potential direct or treatment-related hazard of prostate cancer. Men without bone metastases can be burdened by therapy itself as androgen deprivation leads to loss of bone mineral density (BMD) and an increased risk for osteoporotic fractures. Men with castration-resistant prostate cancer (CRPC) that does not yet involve bone are known to have substantial risk for the development of bone metastases at some point in the disease process. Those who do have bone metastases have an increased risk for pain or for skeletal events such as pathologic fractures or spinal cord compression due to epidural extension of tumor. Each of these clinical situations represents a potential point of therapeutic intervention for the prevention of skeletal morbidity and mortality.
These prominent and clinically-burdensome risks have led to considerable research related to the pharmacologic prevention of skeletal complications in men with prostate cancer. Given the important role that osteoclasts play in the pathogenesis of these processes, osteoclast inhibition is a rational strategy for bone targeted therapies in prostate cancer. The two approved classes of agents include the bisphosphonates and more recently, denosumab the monoclonal antibody to receptor activator of nuclear factor-kappa B (RANKL). Other molecular targets for bone-related systemic therapies under past or ongoing study have included cathepsin K,1 endothelin A,2,3 SRC4,5,6 and hepatocyte growth factor (MET).7,8 Anticancer agents not specifically targeted to bone such as abiraterone acetate and enzalutamide have also recently been shown to prevent skeletal complications, arguing that disease control is important to the prevention of skeletal events. Further, the radiopharmaceutical radium-223 represents a hybrid of anticancer therapy and bone-targeted therapy.
The role of denosumab in the prevention of skeletal morbidity in men with prostate cancer is a central focus of this review.
DENOSUMAB MECHANISM AND PHARMACOLOGY
Osteoclasts are tissue-specific macrophages that are responsible for bone resorption.9 In physiologic bone remodeling, osteoclast-mediated bone resorption is in balance with new bone formation by osteoblasts. Osteoporosis can be the result of an excess of osteoclast activity relative to osteoblast activity. Distinct from their role in osteoporosis, osteoclasts can contribute to the pathophysiology of bone metastases through the resorption-mediated liberation of growth factors10 that may stimulate tumor growth within the bone microenvironment. Bone turnover and osteoclast activity in particular are elevated in the presence of bone metastases.11,12,13 The role of osteoclasts in these processes forms a basis for osteoclast inhibition as a therapeutic strategy.
RANK is a cell surface receptor on osteoclasts and is a central regulator of their maturation, activation and survival.9 Denosumab is a fully human monoclonal antibody that is conceptually similar to osteoprotegerin, an endogenous decoy receptor for RANKL.14 Denosumab potently (Kd 3 × 10− 12 M) and specifically binds to RANKL, thereby competitively inhibiting RANK binding and downstream signaling. It is highly bioavailable at typical doses when given by subcutaneous injection. It suppresses markers of bone turnover (N-telopeptide) within hours, an effect that can persist for over 6 months in some clinical situations.15 Typical of a monoclonal antibody, its plasma half-life is approximately 1 month with clearance rates similar to those of endogenous IgG.16,17
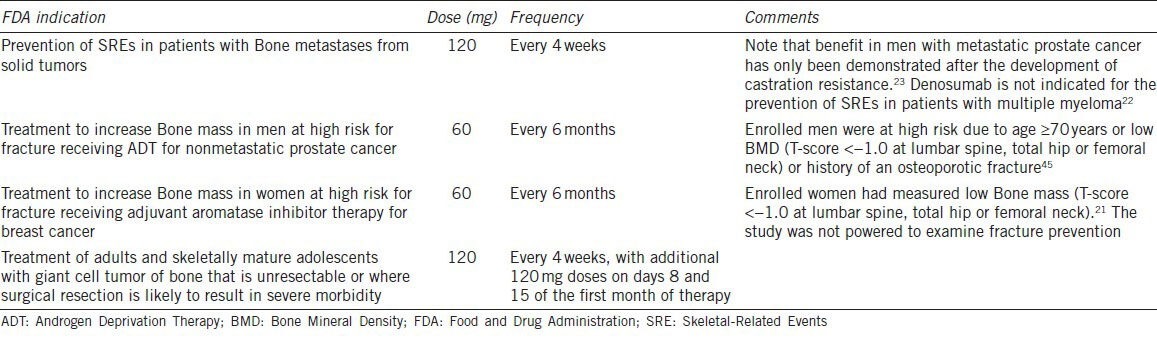
Denosumab has been the subject of broad study for both benign and malignant indications. In the non-cancer population, it has been shown to improve osteoporotic fracture risk in postmenopausal women with osteoporosis18 and to improve BMD in men.19 In the cancer population, it has been shown to improve BMD in women receiving aromatase inhibitor therapy for breast cancer20,21 and to reduce fracture risk in men at high risk of fracture receiving androgen deprivation therapy (ADT) for prostate cancer. It has also been shown to reduce skeletal-related events (SREs; a composite endpoint that includes pathological fracture, spinal cord compression and surgery or radiation therapy to bone) in patients with a broad range of solid tumors metastatic to bone.22,23,24 Finally, it has been shown to reduce or eliminate RANK-positive tumor cells in patients with giant cell tumor of the bone.25,26 Cancer-related indications for denosumab are summarized in Table 1. Dose and frequency vary considerably by clinical indication (e.g. 60 mg every 6 months for osteoporosis, 120 mg every 4 weeks for cancer metastatic to bone).
Table 1.
Cancer-related indications and dose schedules for denosumab
In notable contrast to denosumab, bisphosphonates are pyrophosphate analogs that feature a central carbon bonded to two phosphate groups and two organic side chains. They adsorb to calcium phosphate (hydroxyapatite) crystals within new bone matrix and exhibit inhibitory effects on osteoclasts within the bone microenvironment. The relative potency of a given bisphosphonate is determined by the structure of the two organic side chains bonded to the central carbon atom. Zoledronic acid is the most potent available bisphosphonate. Its serum half-life is 146 h with renal elimination, but once incorporated in bone it persists there and can durably suppress markers of osteoclast activity and bone turnover in some clinical situations.
OSTEOPOROSIS
Osteoporosis is a prevalent comorbid condition among men in the age range typical for prostate cancer. In the general population, osteoporotic fractures cause considerable morbidity and mortality. One-fourth of all hip fractures occur in men,27 with incidence rising steeply with age. It is within this context that ADT causes deleterious effects on bone health.
ADT for prostate cancer causes marked changes in the hormonal environment most notable for a drop in circulating androgens. As male estrogen production takes place through the aromatization of testosterone, ADT-induced severe hypogonadism reduces circulating estrogen levels as well. This leads to a loss of BMD28 and is associated with an increased risk for clinical fractures.29,30 For comparison, men in the general population gain BMD with age.31 Selective estrogen receptor modulators raloxifene and toremifene has been shown to improve BMD in men receiving ADT,32,33 an effect that can reasonably be taken to serve as proof of principle of the importance of estrogen to bone health in men.
BMD is an important marker of fracture risk, but is just one among a number of well-described risk factors. Meta-analyses have demonstrated the importance of a number of clinical factors including tobacco smoking,34 personal history of fracture,35 alcohol intake,36 chronic glucocorticoid use,37 parental history of fracture,38 and rheumatoid arthritis.39 Each of those factors is associated with elevated fracture risk independent of its effect on BMD. Risk of osteoporotic fracture in men rises markedly with age in the general population even as BMD rises modestly, arguing for the importance of clinical factors. Some, such as smoking status and history of glucocorticoid use can be easily assessed. Others such as frailty are more difficult to quantitate.
Given the comorbid hazards of osteoporosis and treatment-induced adverse effects, several classes of pharmacologic agents have been tested in men receiving ADT for prostate cancer. Bisphosphonate treatment of men receiving ADT has clearly and reproducibly been shown to improve BMD, a surrogate for fracture risk. BMD benefits have been demonstrated with alendronate,39 pamidronate,28,41 zoledronic acid,42,43 and neridronate44 Denosumab was studied in a trial that enrolled over 1400 men and is the only approved agent that has been shown to improve both BMD and fracture risk for men receiving ADT for prostate cancer.45 The trials involving bisphosphonates each enrolled 21–112 participants and were not powered to study effects on the rate of fractures. The effect of bisphosphonates on fracture risk is therefore simply not known. It is notable that the BMD effects of osteoporosis-treatment doses of zoledronic acid and denosumab are similar (approximately 4% gain in lumbar spine BMD at 12 months43,45,46).
Denosumab was studied in a double-blind trial that randomized 1468 men receiving ADT for nonmetastatic prostate cancer to denosumab treatment (60 mg subcutaneously every 6 months) or to placebo.45 Inclusion criteria required at least one of the following two risk factors for fracture: (i) age ≥70 years and (ii) low BMD (T score ≤1.0 at the lumbar spine, total hip or femoral neck). The primary endpoint was percent change in BMD at the lumbar spine at 24 months. Key secondary endpoints included incidence of new vertebral fractures and change in BMD at other sites. The trial was positive as the denosumab treatment group was found to have significantly improved 24 month BMD of the lumbar spine (5.6% gain vs 1.0% loss; P < 0.001) and a lower incidence of new vertebral fractures at 36 months (1.5% vs 3.9%; relative risk 0.38; 95% CI 0.19–0.78; P = 0.006).
Toremifene and raloxifene are selective estrogen receptor modulators that have been studied in men receiving ADT for prostate cancer. Each has been shown to improve BMD,32,33 and toremifene has been shown in a large phase III study to reduce fracture risk.47 One prominent adverse effect of toremifene was the observation of more frequent venous thromboembolic events (2.6% with toremifene vs 1.1% with placebo). Neither agent is approved for use in men with prostate cancer.
Given the availability of these agents and the data supporting their use in men with prostate cancer, screening and selection of treatment candidates is essential. Supplementation of calcium and vitamin D in all men receiving ADT is recommended by current National Comprehensive Cancer Network guidelines. A subset of those men will have risk sufficient to justify pharmacologic therapy. Appropriate candidates for therapy should be identified by predictive models that take clinical factors beyond BMD into account. The World Health Organization fracture risk assessment model FRAX (http://www.shef.ac.uk/FRAX/) is one such model. Clinical inputs include gender, age, height, weight, history of fracture, parental history of hip fracture, smoking status, use of glucocorticoids, daily consumption of at least 3 units of alcohol, rheumatoid arthritis and other causes of secondary osteoporosis. National Osteoporosis Foundation guidelines recommend the use of drug therapy to reduce fracture risk if 10 year risk exceeds either of two thresholds (>20% risk of major osteoporotic fracture or > 3% risk of hip fracture).48
Synthesis
ADT causes loss of BMD and is associated with an increased incidence of osteoporotic fracture. Osteoporosis therefore merits screening and management among men who receive ADT for prostate cancer. Measurement of BMD can aid risk assessment, but is not adequately sensitive in the absence of clinical factors. The online World Health Organization/FRAX fracture risk assessment tool is one method of more comprehensive risk assessment and is recommended by National Comprehensive Cancer Network guidelines. For those who merit treatment, denosumab is the only approved agent that is supported by level 1 evidence of fracture prevention. Several bisphosphonates have been shown to improve BMD and are also reasonable choices among treatment candidates.
CASTRATION-RESISTANT NONMETASTATIC PROSTATE CANCER
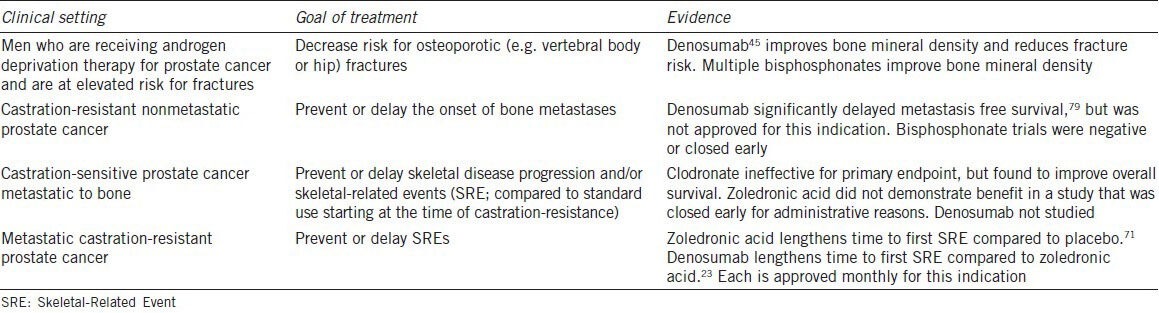
The natural history of advanced prostate cancer strongly features risk for metastases to bone. Recent phase III trials of systemic agents in men with metastatic CRPC have enrolled populations with 80%–90% baseline prevalence of bone metastases.49,50,51 This propensity for the disease to metastasize to bone has led to efforts to prevent bone metastases in men who have not yet developed. Denosumab is the only agent that has been shown to delay the onset of bone metastases. No bone-targeted agent has been approved for the prevention of bone metastases. See Table 2 for a summary of data related to osteoclast inhibition in men with prostate cancer.
Table 2.
Osteoclast-targeted therapy for men with prostate cancer
Bisphosphonates have failed to demonstrate benefit for the prevention of bone metastases. Clodronate is a relatively weak bisphosphonate that was studied in a well-designed phase III trial that did not demonstrate a significant difference relative to placebo in time to first bone metastasis.52,53 Zoledronic acid is more potent and was the subject of a phase III trial that closed early due to poor accrual and a lower than expected rate of bone metastases.54 Analysis of the placebo group of that trial revealed that time to first metastasis was shorter in men with prostate-specific antigen (PSA) > 10 ng ml−1 (relative risk (RR) 3.18) and elevated PSA velocity (RR 4.34 for each 0.01 increase in PSA velocity).54
Denosumab was then examined in a randomized phase III trial that met its primary endpoint, but did not led to approval of the agent for this indication. The trial enrolled 1432 men with CRPC not metastatic to bone who were at elevated risk for bone metastases as indicated by short PSA doubling time (≤10.0 months) and/or an absolute PSA value ≥8.0 ng dl− 1. They were randomized 1: 1 to receive denosumab (120 mg) or placebo every 4 weeks. The primary endpoint was bone-metastasis-free survival. The trial was positive as denosumab significantly increased bone-metastasis-free survival by 4.2 months (29.5 vs 25.2 months; hazard ratio (HR) 0.85; 95% CI 0.73–0.98; P = 0.028). Overall survival (OS) did not differ between the study arms. To date, it is the only positive trial of an osteoclast targeted agent for this indication.
Regulatory review by the United States Food and Drug Administration Oncology Drug Advisory Committee led to a recommendation against approval of denosumab for metastasis prevention. According to the briefing document, the recommendation was based on several factors including the absence of an effect on OS, an uncertain effect on symptoms and quality of life and treatment-related risk for osteonecrosis of the jaw (ONJ).
CASTRATION-SENSITIVE PROSTATE CANCER METASTATIC TO BONE
Cancer control is important to the prevention of skeletal morbidity in men with prostate cancer. First-line ADT for metastatic prostate cancer produces responses commonly and often for durations measured in years. In this context, no bone-targeted agent has improved skeletal outcomes for men with castration-sensitive prostate cancer metastatic to bone.
The comparably weak bisphosphonate clodronate was studied in 311 men starting or responding to first-line ADT. Clodronate produced no significant benefit in skeletal disease progression or prostate cancer death relative to placebo.55 Long-term follow-up, however, revealed a significant OS benefit (8-year OS 22 vs 14%; HR for death 0.77, 95% CI 0.60–0.98, P = 0.03),53 suggesting the potential for benefit with a more potent bone targeted agent.
The subsequent randomized placebo-controlled cooperative group trial CALGB 90202 was designed to assess early use of zoledronic acid for men with prostate cancer metastatic to bone. It enrolled men with castration-sensitive disease metastatic to bone and within 6 months of initiation of ADT and randomized them to zoledronic acid (4 mg every 4 weeks) or placebo until progression to CRPC. The trial was halted early due to withdrawal of drug supply by the corporate supporter. At the time it was halted, the trial had enrolled 645 of 680 planned participants and observed 284 of 470 anticipated SRE events. Primary analysis of this truncated study did not demonstrate significant delay in time to first SRE with early use of zoledronic acid (32.5 months with zoledronic acid vs 29.8 months with placebo; HR 0.96, 95% CI 0.76–1.22).56
Zoledronic acid is under study for men in this population in an additional phase III trial that has not yet reported its results (NCT00242567). The primary endpoint is skeletal-event-free survival at 18 months. Secondary endpoints include OS, SRE-free survival and multiple-event analysis of SREs over 3 years. Estimated enrollment is 550.
Synthesis
No bone-targeted agent has been convincingly shown to improve outcomes among men with prostate cancer metastatic to bone that is responding to first-line ADT. Clodronate significantly improved survival in a study that was negative for its primary endpoint. Early zoledronic acid was then studied in a large cooperative group trial that was halted early for administrative reasons and did not demonstrate benefit. Disease control is clearly a central aspect of the prevention of skeletal morbidity. Robust initial responses to first-line ADT make SREs less common and therapeutic benefit more difficult to demonstrate in this population. Denosumab has not been studied for the prevention of SREs in castration-sensitive disease.
It is important to note that United States and European regulatory approvals of zoledronic acid and denosumab are worded broadly enough to include the use of either agent in castration-sensitive prostate cancer metastatic to bone. These approvals were granted on the strength of the accumulated data from a number of clinical trials that together demonstrated benefits in patients with metastatic CRPC (detailed below) as well as with breast cancer or other solid tumors metastatic to bone. Despite this broadly-worded approval, current evidence does not support the use of either agent in men with prostate cancer metastatic to bone prior to the development of castration resistance.
METASTATIC CASTRATION-RESISTANT PROSTATE CANCER
CRPC metastatic to bone is clinically hazardous. Without osteoclast inhibition, the median time to first SRE is in the range of 11 months and approximately half of patients experience an event within 2 years.57 CRPC metastatic to bone is an approved indication for monthly potent osteoclast inhibition, specifically with denosumab or zoledronic acid. After failures of less potent bisphosphonates clodronate58 and pamidronate59 in this population, zoledronic acid was the first agent to demonstrate evidence of benefit. When the denosumab and zoledronic acid were later compared directly, denosumab produced a significantly superior time to first SRE. Data from the positive trials will be reviewed.
Zoledronic acid was compared to placebo in a phase III randomized trial of 643 men with CRPC and asymptomatic to minimally symptomatic bone metastases. The trial initially included two dose levels of zoledronic acid (4 or 8 mg given every 3 weeks), but the higher dose was reduced to 4 mg after the observation of nephrotoxicity. The primary endpoint was the proportion of men who experienced one or more SRE. After 15 months of follow-up, 44% of the placebo group and 33% of the treatment group had experienced an SRE (P = 0.02). Median time to first SRE was also significantly improved with treatment (>420 vs 321 days; P = 0.01). Zoledronic acid did not improve OS.
Denosumab was later compared to standard-of-care zoledronic acid a phase III trial for men with CRPC metastatic to bone. A total of 1904 men were randomized to every 4 weeks treatment with denosumab (120 mg subcutaneous) or zoledronic acid (4 mg intravenous). The primary endpoint was time to first on-study SRE and was assessed for non-inferiority. Assessment for superiority was a secondary endpoint. The trial was positive as median time to first SRE was significantly longer with denosumab (20.7 vs 17.1 months; HR 0.82; 95% CI 0.71–0.95; P = 0.0002 for non-inferiority; P = 0.008 for superiority). Survival was not significantly different between the two arms of the trial.
It is notable that two additional similarly-designed phase III trials compared the two agents in patients with non-prostate cancers metastatic to bone. In 2046 women with breast cancer metastatic to bone, median time to first SRE was superior with denosumab (HR 0.82; 95% CI 0.71–0.95; P = 0.01 for superiority).24 In 1776 patients with non-breast, non-prostate solid tumors or multiple myeloma involving bone, median time to first SRE was non-inferior with denosumab (HR 0.84; 95% CI 0.71–0.98; P = 0.0007). Survival did not differ between the study arms of any of the phase III trials comparing zoledronic acid and denosumab for cancers metastatic to bone.
Several lines of evidence suggest that denosumab inhibits osteoclast activity more potently than zoledronic acid. First, it produces superior median time to first SRE in two of the three trials discussed above.23,24 Second, hypocalcemia is an on-target side effect that occurs more frequently with denosumab than with zoledronic acid (e.g. 13% vs 6% in one trial23 and 10.8% vs 5.8% in another22). Third, one biomarker-driven trial examined the use of denosumab among patients with cancer involving bone and with elevated bone turnover despite treatment with zoledronic acid or pamidronate. Participants were randomized to continue bisphosphonate therapy or to switch to denosumab (180 mg subcutaneous every 4 weeks). N-telopeptide, a marker of osteoclast activity, normalized more frequently with denosumab than with control therapy (71% vs 29%; P < 0.001).60
Synthesis
Zoledronic acid and denosumab are each approved for use in this population to prolong time to first SRE. The relevant phase III trials showed that zoledronic acid offers a 5.6 month advantage over placebo and that denosumab offers a 3.6 month advantage over zoledronic acid. Thus, either agent is reasonable, but denosumab is superior in time to first SRE. Cost and availability are largely beyond the scope of this review, but may be important factors in clinical practice. Clinical and cost effectiveness analyses comparing the two agents have yielded varied results. Some have suggested that denosumab is not cost effective61,62,63 relative to zoledronic acid, while others have suggested that it is cost effective.64 In addition, it is anticipated that cost for zoledronic acid will be reduced by its patent expiration in 2013.
The dosing schedule of either agent is also a subject of some scientific and practical uncertainty. Zoledronic acid has been studied on an every 3 weeks schedule and on an every 4 weeks schedule, giving some flexibility of evidence-based dosing. Denosumab has been studied only as frequently as every 4 weeks; more frequent dosing is therefore not known to be safe. It is therefore important to note that denosumab dosing schedule may not align well with every 3 weeks chemotherapy dosing for patients receiving docetaxel or cabazitaxel. Strict evidence-based use would require dys-synchronous dosing weeks. More relaxed dosing of either agent (taxane every 4 weeks or denosumab every 6 weeks) could promote convenience, but with unknown impact on efficacy.
TOXICITIES OF OSTEOCLAST INHIBITION
Potent osteoclast inhibition is associated with several adverse class effects. The most prominent are hypocalcemia and ONJ. Incidence and severity of these and other side effects vary by dose and schedule and are generally more common with treatment designed to prevent SREs due to bone metastases. For example, the incidence of hypocalcemia was 13% with monthly denosumab to prevent SREs but was less than 1% with every 6-months denosumab to treat osteoporosis.23,45 Side effects of monthly dosing are summarized in Table 3.
Table 3.
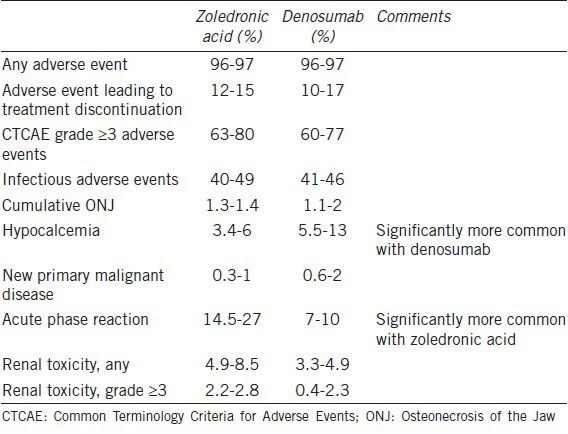
Hypocalcemia is common in response to either agent, but is most common with denosumab (13% with monthly denosumab and 6% with monthly zoledronic acid; P < 0.0001).23 Most cases are asymptomatic, but some are symptomatic and require hospitalization for intensive calcium repletion. Management of hypocalcemia is dependent on the clinical situation. First, it is likely important that all patients are vitamin D replete prior to initiation of therapy. Testing of serum 25-OH vitamin D is easily accomplished and can afford an opportunity to replete levels.
ONJ is an uncommon but potentially-morbid complication of osteoclast inhibition that was observed after the introduction of potent bisphosphonates such as pamidronate and zoledronic acid.65,66 Clinically, it presents as an exposed, non-healing area of bone. Risk factors identified in retrospective analyses prominently include invasive dental procedures after initiation of therapy, use of more potent agents and long duration of treatment.67 Published guidelines recommend preventive and management strategies that prominently include dental consultation and the completion of all anticipated procedures prior to initiation of potent osteoclast inhibition.68,69
ONJ risk related to the two agents may be different based on their differing serum and bone half-lives. Zoledronic acid has a serum half-life measured in days, but is present in bone for years. Denosumab has a serum half-life on the order of 1 month, but has been shown to suppress markers of bone turnover in some clinical settings for as long as 6 months in the wake of a single dose. Clinical trial evidence for a distinction in ONJ risk based on these pharmacologic differences is lacking. In the trio of phase III trials comparing monthly treatment with denosumab to zoledronic acid, the incidence was 1%–2% without significant difference between arms.70
An important distinction between denosumab and zoledronic acid is the potential for nephrotoxicity with zoledronic acid. All bisphosphonates are potentially nephrotoxic. Zoledronic acid in particular was found to cause acute kidney injury consistent with acute tubular necrosis55 in participants in early clinical study.71 Current guidelines for its use seek to minimize this risk by lengthening infusion time to ≥15 min, reducing doses for stable creatinine clearance 30 and 60 ml min− 1 and holding dosing for creatinine clearance < 30 ml min− 1 or acutely declining renal function.72 Nephrotoxicity due to denosumab has not been described.
One safety and efficacy concern particular to denosumab is the possibility of treatment-related immune dysfunction. RANKL and RANK are expressed by T-lymphocytes, B-cells and dendritic cells.73,74 Denosumab-mediated inhibition of RANK signaling may therefore inhibit dendritic cell function and has been associated with a small but measurable increase in the number of infectious serious adverse events.75 Sipuleucel T improves survival in men with metastatic CRPC, a therapeutic effect that is thought to depend on dendritic cell mediated antitumor immune effects. It is possible that these effects are blunted by concurrent or subsequent treatment with denosumab. Data to support or refute that theoretical concern are presently lacking.
It is notable that zoledronic acid is associated with a flu-like acute phase reaction that often features fever, malaise and myalgias. It is generally self-limited and resolves within 24–48 h.
Synthesis
Denosumab and zoledronic acid have similar but not identical toxicity profiles. Preventative dental work and vitamin D repletion are wise prior to the start of either therapy to avoid ONJ and hypocalcemia risks, respectively. Acute kidney injury and an acute phase reaction are each risks particular to zoledronic acid. The potential for suppression of dendritic cell function is a risk particular to denosumab. In patients with impaired renal function, denosumab is thought to be safe but has not been extensively studied.
OTHER AGENTS FOR THE PREVENTION OF SKELETAL MORBIDITY
The term SRE was created to refer to a group of bone-related clinical events (pathological fracture, spinal cord compression, surgery or radiation therapy to bone) that could be taken together as a composite endpoint for clinical trials of bone-targeted therapies. The pivotal trials that established zoledronic acid71 and denosumab23 for men with metastatic CRPC are among the important studies featured SRE-related primary endpoints. It is important to also note that any systemic agent that is active against prostate cancer likely has the potential to prevent skeletal morbidity. This realization has led to the recent inclusion of SREs as an endpoint for trials involving drugs such as hormonal agents that are not specifically targeted to bone. Active hormonal agents such as abiraterone and enzalutamide have been shown to prevent SREs.50,76
Radiopharmaceuticals have become a more prominent therapeutic consideration with the recent demonstration of a survival advantage with the alpha-emitting radium-223. In general, radiopharmaceuticals are systemically delivered bone-seeking agents that either emit radiation themselves or are linked to a radioactive source. Beta-emitting radiopharmaceuticals have long been approved for the palliation of pain due to bone metastases, but are limited by prominent marrow suppression and the absence of a demonstrated effect on survival.77 Radium-223 is a newer alpha emitting radiopharmaceutical that was shown in a randomized placebo controlled trial to improve OS and prevent SREs in men with CRPC metastatic to bone without visceral metastases.78 At present, there is limited data with regard to the safety and efficacy of combining radium-223 with other systemic anticancer agents or bone-targeted agents.
One important unanswered question is the amount of added benefit of osteoclast inhibition in the context of newer systemic therapies that are active in the setting of CRPC. Given that disease control reduces the risk for SREs, it is possible that the benefits previously demonstrated with osteoclast inhibition are now blunted by the benefits produced by abiraterone, enzalutamide, radium-223 and other agents. In the absence of level 1 evidence to guide-related decisions, it is likely important to be mindful of individualized risk for SRE. Those at highest risk for events have historically been most likely to benefit from bone-targeted therapy. For example, benefits have been demonstrated in the setting of CRPC metastatic to bone but not with castration-sensitive metastatic disease. Rising PSA, short PSA doubling time and elevated markers of bone turnover are among the prognostic factors indicating highest risk for SREs and could rationally be taken as particularly strong indications for osteoclast inhibition in the setting of metastatic CRPC regardless of the concurrent systemic regimen.
CONCLUSIONS
Osteoclast inhibition is a validated strategy in the management of men with prostate cancer. In those at elevated risk for osteoporotic fractures, denosumab as well as several bisphosphonates have been shown to improve BMD, a surrogate for fracture risk. Denosumab is the only approved agent that has also been shown to prevent fractures. In men with CRPC metastatic to bone, monthly therapy with either denosumab or zoledronic acid has been shown to reduce risk for SREs. When the two agents were compared directly, denosumab was superior. Prominent potential adverse effects of potent osteoclast inhibition include hypocalcemia and rarely, ONJ. Finally, there are a number of new classes of agents under current study for the prevention of skeletal morbidity in men with prostate cancer.
COMPETING INTEREST
The author declares no relevant competing interests.
ACKNOWLEDGEMENTS
Dr. Philip J Saylor is supported by a Young Investigator Award from the Prostate Cancer Foundation.
REFERENCES
- 1.Lewiecki EM. Odanacatib, a cathepsin K inhibitor for the treatment of osteoporosis and other skeletal disorders associated with excessive bone remodeling. IDrugs. 2009;12:799–809. [PubMed] [Google Scholar]
- 2.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3:110–6. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 3.Nelson JB, Love W, Chin JL, Saad F, Schulman CC, et al. Atrasentan Phase 3 Study Group. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478–87. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saad F, Lipton A. SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev. 2010;36:177–84. doi: 10.1016/j.ctrv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Yu EY, Massard C, Gross ME, Carducci MA, Culine S, et al. Once-daily dasatinib: Expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology. 2011;77:1166–71. doi: 10.1016/j.urology.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu EY, Wilding G, Posadas E, Gross M, Culine S, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7421–8. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain M, Smith MR, Sweeney C, Corn PG, Elfiky A, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): results from a phase II randomized discontinuation trial. J Clin Oncol. 2011;29(suppl) abstr 4516. [Google Scholar]
- 8.Smith DC, Smith MR, Small EJ, Sweeney C, Kurzrock R, et al. Phase II study of XL184 in a cohort of patients (pts) with castration-resistant prostate cancer (CRPC) and measurable soft tissue disease. J Clin Oncol. 2011;29(suppl 7) abstr 127. [Google Scholar]
- 9.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 10.Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J Biol Chem. 1986;261:12665–74. [PubMed] [Google Scholar]
- 11.Demers LM, Costa L, Lipton A. Biochemical markers and skeletal metastases. Cancer. 2000;88(12 Suppl):2919–26. doi: 10.1002/1097-0142(20000615)88:12+<2919::aid-cncr7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Cook RJ, Coleman R, Brown J, Lipton A, Major P, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006;12(11 Pt 1):3361–7. doi: 10.1158/1078-0432.CCR-06-0269. [DOI] [PubMed] [Google Scholar]
- 13.Brown JE, Cook RJ, Major P, Lipton A, Saad F, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 14.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 15.Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–66. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 16.Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93:2645–68. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- 17.Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–59. doi: 10.2165/11535960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, et al. Freedom Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 19.Orwoll E, Teglbjaerg CS, Langdahl BL, Chapurlat R, Czerwinski E, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97:3161–9. doi: 10.1210/jc.2012-1569. [DOI] [PubMed] [Google Scholar]
- 20.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, et al. Effect of denosumab on bone mineral density in women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer: subgroup analyses of a phase 3 study. Breast Cancer Res Treat. 2009;118:81–7. doi: 10.1007/s10549-009-0352-y. [DOI] [PubMed] [Google Scholar]
- 21.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875–82. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 22.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, et al. Randomized, double-blind study of denosumab versus zoledronic Acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2010;29:1125–32. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 23.Fizazi K, Carducci M, Smith M, Damião R, Brown J, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 25.Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, et al. Denosumab induces tumor reduction and bone formation in patients with giant-Cell tumor of bone. Clin Cancer Res. 2012;18:4415–24. doi: 10.1158/1078-0432.CCR-12-0578. [DOI] [PubMed] [Google Scholar]
- 26.Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, et al. Denosumab in patients with giant-Cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11:275–80. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 27.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407–13. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 28.Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–55. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 29.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 30.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 31.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int. 2002;13:105–12. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- 32.Smith MR, Malkowicz SB, Chu F, Forrest J, Price D, et al. Toremifene increases bone mineral density in men receiving androgen deprivation therapy for prostate cancer: Interim analysis of a multicenter phase 3 clinical study. J Urol. 2008;179:152–5. doi: 10.1016/j.juro.2007.08.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841–6. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 34.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155–62. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 35.Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–82. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 36.Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737–42. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 37.Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19:893–9. doi: 10.1359/JBMR.040134. [DOI] [PubMed] [Google Scholar]
- 38.Kanis JA, Johansson H, Oden A, Johnell O, De Laet C, et al. A family history of fracture and fracture risk: a meta-analysis. Bone. 2004;35:1029–37. doi: 10.1016/j.bone.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, et al. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–95. doi: 10.1007/s001980170006. [DOI] [PubMed] [Google Scholar]
- 40.Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on Bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146:416–24. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 41.Diamond TH, Winters J, Smith A, De Souza P, Kersley JH, et al. The antiosteoporotic efficacy of intravenous pamidronate in men with prostate carcinoma receiving combined androgen blockade: a double blind, randomized, placebo-controlled crossover study. Cancer. 2001;92:1444–50. doi: 10.1002/1097-0142(20010915)92:6<1444::aid-cncr1468>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 42.Smith MR, Eastham J, Gleason D, Shasha D, Tchekmedyian S, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men undergoing androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–12. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 43.Michaelson MD, Kaufman DS, Lee H, McGovern FJ, Kantoff PW, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–42. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morabito N, Gaudio A, Lasco A, Catalano A, Atteritano M, et al. Neridronate prevents bone loss in patients receiving androgen deprivation therapy for prostate cancer. J Bone Miner Res. 2004;19:1766–70. doi: 10.1359/JBMR.040813. [DOI] [PubMed] [Google Scholar]
- 45.Smith MR, Egerdie B, Hernandez Toriz N, Feldman R, Tammela TL, et al. Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–55. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith MR, Saad F, Egerdie B, Szwedowski M, Tammela TL, et al. Effects of denosumab on Bone mineral density in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2009;182:2670–5. doi: 10.1016/j.juro.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith MR, Morton RA, Barnette KG, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2010;184:1316–21. doi: 10.1016/j.juro.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Osteoporosis Foundation. Osteoporosis in Men. 2005 [Google Scholar]
- 49.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 50.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, et al. COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 52.Mason MD, Sydes MR, Glaholm J, Langley RE, Huddart RA, et al. Medical Research Council PR04 Collaborators. Oral sodium clodronate for nonmetastatic prostate cancer--results of a randomized double-blind placebo-controlled trial: medical Research Council PR04 (ISRCTN61384873) J Natl Cancer Inst. 2007;99:765–76. doi: 10.1093/jnci/djk178. [DOI] [PubMed] [Google Scholar]
- 53.Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10:872–6. doi: 10.1016/S1470-2045(09)70201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–25. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 55.Dearnaley DP, Sydes MR, Mason MD, Stott M, Powell CS, et al. Mrc Pr05 Collaborators. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial) J Natl Cancer Inst. 2003;95:1300–11. doi: 10.1093/jnci/djg038. [DOI] [PubMed] [Google Scholar]
- 56.Smith MR, Halabi S, Ryan CJ, et al. Efficacy and safety of zoledronic acid in men with castration-sensitive prostate cancer and Bone metastases: results of CALGB 90202 (Alliance) J Clin Oncol. 2013;31(suppl 6) doi: 10.1200/JCO.2013.51.6500. abstr 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, et al. Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–82. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 58.Ernst DS, Tannock IF, Winquist EW, Venner PM, Reyno L, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21:3335–42. doi: 10.1200/JCO.2003.03.042. [DOI] [PubMed] [Google Scholar]
- 59.Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of Bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21:4277–84. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 60.Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–71. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 61.Ford J, Cummins E, Sharma P, Elders A, Stewart F, et al. Systematic review of the clinical effectiveness and cost-effectiveness, and economic evaluation, of denosumab for the treatment of bone metastases from solid tumours. Health Technol Assess. 2013;17:1–386. doi: 10.3310/hta17290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie J, Namjoshi M, Wu EQ, Parikh K, Diener M, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with Bone metastases. J Manag Care Pharm. 2011;17:621–43. doi: 10.18553/jmcp.2011.17.8.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snedecor SJ, Carter JA, Kaura S, Botteman MF. Denosumab versus zoledronic acid for treatment of Bone metastases in men with castration-resistant prostate cancer: A cost-effectiveness analysis. J Med Econ. 2013;16:19–29. doi: 10.3111/13696998.2012.719054. [DOI] [PubMed] [Google Scholar]
- 64.Stopeck A, Rader M, Henry D, Danese M, Halperin M, et al. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and Bone metastases in the United States. J Med Econ. 2012;15:712–23. doi: 10.3111/13696998.2012.675380. [DOI] [PubMed] [Google Scholar]
- 65.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–7. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 66.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–34. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–36. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, et al. American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg. 2009;67(5 Suppl):2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 69.Edwards BJ, Hellstein JW, Jacobsen PL, Kaltman S, Mariotti A, et al. American Dental Association Council on Scientific Affairs Expert Panel on Bisphosphonate-Associated Osteonecrosis of the Jaw. Updated recommendations for managing the care of patients receiving oral bisphosphonate therapy: an advisory statement from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2008;139:1674–7. doi: 10.14219/jada.archive.2008.0110. [DOI] [PubMed] [Google Scholar]
- 70.Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2011;23:1341–7. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 71.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, et al. Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–68. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 72.Novartis. Zometa full prescribing information. Package insert. revised 10/2009 [Google Scholar]
- 73.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–9. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 74.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 75.Watts NB, Roux C, Modlin JF, Brown JP, Daniels A, et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: coincidence or causal association? Osteoporos Int. 2012;23:327–37. doi: 10.1007/s00198-011-1755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, et al. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 77.Paes FM, Serafini AN. Systemic metabolic radiopharmaceutical therapy in the treatment of metastatic bone pain. Semin Nucl Med. 2010;40:89–104. doi: 10.1053/j.semnuclmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, et al. ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 79.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, et al. Denosumab and Bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebo-controlled trial. Lancet. 2011;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


