Abstract
Defects within apoptotic pathways have been implicated in prostate cancer (PCa) tumorigenesis, metastatic progression and treatment resistance. A hallmark of cancers is the ability to derail apoptosis by inhibiting the apoptotic signal, reducing the expression of apoptotic proteins and/or amplifying survival signals through increased production of antiapoptotic molecule. This review describes associations between heat shock proteins (HSPs) and the human androgen receptor (AR), the role of HSPs and other stress-induced proteins in PCa development and emerging strategies in targeting these protective proteins to treat PCa.
Keywords: apatorsen, apoptosis, bcl-2 homologous antagonist-killer protein, clusterin, custirsen, ganetespib, heat-shock proteins, prostatic neoplasms
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed non-skin cancer in men and the second leading cause of cancer death among men in the United States.1 At diagnosis, PCa growth is uniformly androgen-dependent. Therefore, androgen deprivation therapy (ADT) is used as first-line therapy in the setting of metastatic disease. ADT is very effective initially in reducing tumor burden; however, eventually tumor will grow in the presence of castrate levels of testosterone (<50 ng dl−1) and develop into castration-resistant prostate cancer (CRPC). With multiple new agents demonstrating proven overall survival (OS) advantages, treatment approaches to CRPC are rapidly evolving. A major challenge moving forward is to define how to best manage primary and secondary treatment resistance. One approach to overcome resistance to hormonal and chemotherapies is to target mediators of apoptosis. A dysfunctional apoptotic pathway has been identified as one of the hallmarks of human cancers,2 and evasion of apoptosis frequently contributes to treatment resistance. Overexpression of Bcl-2, chaperone proteins and clusterin (CLU) is common in PCa, and these mediators have been implicated as contributors to therapeutic resistance, disease recurrence and shortened survival.3,4,5
APOPTOTIC PATHWAY IN CANCER
A hallmark of cancers is the ability to derail apoptosis by inhibiting the apoptotic signal, reducing the expression of apoptotic proteins and/or amplifying survival signals through increased production of anti-apoptotic molecules.6 Apoptosis is a complex biological process that initiates with an instruction from either an extrinsic pathway (death receptor) and/or the intrinsic pathway (mitochondrial), and concludes with the cellular degradation by activated caspase enzymes.
Heat shock proteins
HSPs are highly conserved stress-induced factors that play an essential role as molecular chaperones by regulating protein folding, stability, transport and aggregation. HSPs have cytoprotective roles and are essential for cancer cell survival. HSPs are often upregulated in cancer and this constitutive expression is necessary for cancer cells’ survival.7 Several of these proteins have demonstrated a direct interaction with components of the cell signaling pathways. For example, the androgen receptor (AR) is a major player in PCa growth and progression and is a well-known interacting factor of HSPs. Since AR function is very dependent on HSP activity, many emerging compounds address AR-associated HSPs as novel drug targets.8
HSPs have been classified into four families according to their molecular weight: HSP90, HSP70, HSP60 and small HSPs (15–30 kDa) that include HSP27.9 HSPs are powerful regulators of apoptosis through an ability to interact with key components of the apoptotic signaling pathway, in particular, those involved in caspase activation. HSP90 is a molecular chaperone involved in the conformational maturation and function of a large number of ‘client’ proteins that have been implicated in oncogenesis. The AR, a key driver of PCa growth and treatment resistance, is an HSP90 client and its function is dependent on HSP90 chaperone activity. HSP27 and HSP70 are the most strongly induced chaperones during cellular stress.
HSP27 is an ATP-independent, small HSP that, once phosphorylated, forms a chaperoning oligomer that regulates multiple cell survival and signaling pathways.10 At the post-mitochondrial level, HSP27 binds to cytochrome C and inhibits caspase activation and apoptotic cell death.11,12 HSP27 and CLU act together to stabilize the cell against apoptotic stressors.
HSP and AR
HSP27 has been shown to form a complex with the AR, thus resulting in AR regulated gene transactivation (Figures 1 and 2). Overexpression of HSP27 and CLU has been reported in human PCas. Accumulating evidence links rising HSP27 levels with the development of CRPC. The HSP27 expression as detected by immunohistochemistry (IHC) is highest in CRPC, suggesting an important role in the development of castration resistance disease.5 Treatment of AR-positive PCa cell lines (LNCaP) with HSP27-specific siRNA resulted in a down-regulation of AR levels. This down-regulation of protein was paralleled by a decrease in AR mRNA. Overexpression of HSP27 in PC-3 cells led to a significant increase in AR mRNA.13 CLU overexpression in response to hormonal ablation, chemotherapy and radiotherapy has been implicated in treatment resistance.5,14
Figure 1.
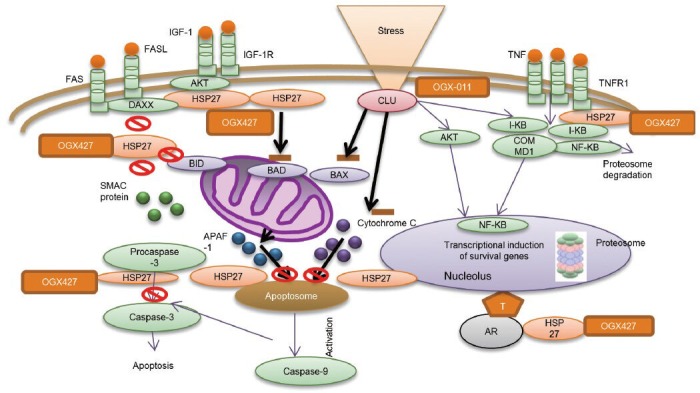
Role of Heat shock protein-27 (HSP27) and clusterin (CLU) in cancer cell survival. CLU increases cell survival through mechanisms involving inhibition of ER stress, suppressing Bax activation with mitochondrial sequestration of cytochrome C, and transcriptional induction of survival genes. Custirsen (OGX-011) acts at each of these points by decreasing clusterin expression. HSP27 inhibits apoptosis by integrating different signaling pathways, including extrinsic and intrinsic apoptosis pathways, as well as growth factor pathways. It enhances androgen receptor signaling and insulin-like growth factor 1-induced Bad phosphorylation. HSP27 inhibits the extrinsic and the intrinsic apoptosis pathway. OGX-427 reduces HSP27 expression leading to appoptosis. APAF1: apoptotic protease-activating factor-1; ER: endoplasmic reticulum. With permission from Zielinski et al.6
Figure 2.
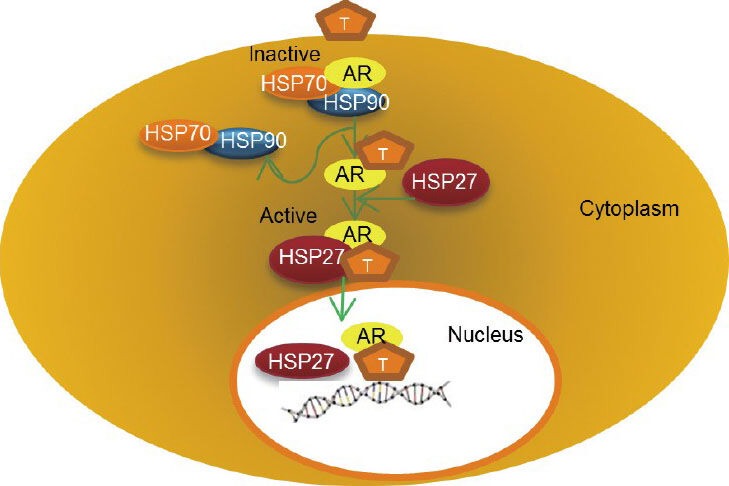
Schematic diagram shows the relationship between heat shock protein-27 (HSP27) and the androgen receptor (AR). Binding of Androgen (T) to AR causes a conformational change in the AR-HSP90/HSP70 complex in the cytoplasm. HSP27 undergoes a rapid phosphorylation and then it displaces HSP90 from the complex with AR. HSP27 chaperon AR from the cytoplasm into the nucleus. Once in the nucleus, the complex disassembles releasing AR, which binds to DNA where it facilitates transcription of DNA into mRNA.
AR is maintained in an inactive state in the absence of ligand by a complex of protein including HSPs and co-chaperones. Ligand binding leads to a conformational change in the AR and dissociation from the large HSP complex. Subsequently, the AR translocates to the nucleus, and binds to its androgen response elements to transactivate target gene expression. HSPs are important players in the AR activation.10 Androgen-bound AR induces rapid HSP27 phosphorylation, then HSP27 displaces HSP90 from a complex with AR to chaperone AR into the nucleus and interact with its response elements to enhance its genomic activity (Figure 2). In vitro study has shown that inhibition of HSP27 phosphorylation or knockdown using the antisense drug OGX-427, shifted the association of AR with HSP90 to murine double minute 2, increased proteasome-mediated AR degradation, decreased AR transcriptional activity and increased PCa LNCaP cell apoptotic rates.10
Clusterin
CLU is an ATP-independent chaperone protein with structural similarity to the heat-shock proteins, and is known to be overexpressed in many solid tumors including PCa. CLU overexpression was found to correlate with higher pathological grade on both biopsy and radical prostatectomy specimens.15 CLU is thought to have multiple functions in the stress response and in cell survival pathways, but its mechanism of action is not yet fully understood.16,17 CLU was found to inhibit apoptosis by interfering with Bax activation in mitochondria. It specifically interacts with conformation-altered Bax in response to chemotherapy. This interaction impedes Bax oligomerization, which leads to the release of cytochrome C from mitochondria and caspase activation (Figure 1).18
Anti-chaperone agents
Strategies targeting chaperone proteins have emerged as an important target in PCa therapy, especially in the context of delaying treatment resistance. The most promising and mature results to date are HSP27 and CLU inhibitors.
HSP27 inhibitor
Apatorsen (OGX-427) is an antisense oligonucleotide that inhibits expression of HSP27 (Figure 1). A phase I study evaluated 36 patients treated with OGX-427 as a single agent and 12 with OGX-427 in combination with docetaxel who had failed prior chemotherapy. Weekly OGX-427 as a single agent was evaluated at doses from 200 to 1000 mg in five cohorts. Two further cohorts tested OGX-427 at the 800 and 1000 mg doses combined with docetaxel in various solid tumors. OGX-427 was safe and well-tolerated as a monotherapy as well as in combination with docetaxel. In addition, OGX-427 when used as a single agent demonstrated declines in circulating tumor cells at all doses and in all diseases evaluated. In 9 of 26 evaluable patients, circulating tumor cells which were positive for HSP27 had decreased significantly in all diseases evaluated and in 89% of patients treated. When OGX-427 was combined with docetaxel, 5 of 10 patients had a decrease in measurable disease of 20% or greater. Five of nine patients with PCa had a decrease of 30% or greater in PSA.19
In a subsequent phase II study, chemotherapy-naive mCRPC patients with no/minimal symptoms were randomized to receive apatorsen 600 mg IV ×3 loading doses then 1000 mg IV weekly with prednisone or prednisone alone. Crossover to apatorsen was allowed for patients progressing on prednisone alone. The primary endpoint was proportion of patient's progression-free survival (PFS) at 12 weeks. Secondary endpoints included PSA decline and response rate. After enrollment of the first 32 evaluable patients, the 12-week progression-free rate was 40% in patients treated with prednisone and 71% in those randomized to receive apatorsen. In addition, 50% of the apatorsen-treated patients experienced a 50% decline in PSA compared with 20% in the prednisone-alone patients. An objective response rate of 40%, including a patient with a complete response, was observed in the apatorsen arm; whereas, no objective responses were observed in patients treated with prednisone alone.20
A second phase II trial with apatorsen has been initiated in patients with CRPC with asymptomatic PSA progressive disease while on abiraterone acetate. Patients are randomized to receive ongoing abiraterone acetate alone or combination of abiraterone acetate plus apatorsen.
Clusterin inhibitor
Custirsen (OGX-011) is a second-generation antisense oligonucleotide with high affinity to CLU mRNA (Figure 1). In a novel phase I study, Chi et al. administered custirsen in escalating doses in combination with neoadjuvant hormonal therapy to men with localized PCa before radical prostatectomy. This study demonstrated a proof-of concept dose-dependent relationship between tissue levels of custirsen and suppression of its target, CLU, in prostate tissues; and established a relationship between decreased prostate tissue CLU and an increased apoptotic index. CLU levels decreased in a dose-dependent manner, with 92% knockdown of CLU protein and mRNA at the 640 mg dose; the mean apoptotic indices increased threefold.21
A subsequent phase II trial randomized 82 chemonaïve mCRPC patients to docetaxel, prednisone and custirsen (Arm A) or docetaxel, prednisone alone (Arm B). This study showed an improved OS of 23.8 months in Arm A vs 16.9 months. However, the study did not show statistical difference in the primary endpoint of PSA decline of ≥50% (58% of patients in arm A and in 54% of patients in arm B). Also there was no difference in the secondary endpoints of median PFS (7.3 months) in patients who received docetaxel and prednisone along with custirsen vs PFS (6.1 months) in those who received docetaxel and prednisone alone.22 In another phase II trial in 42 post-docetaxel mCRPC patients randomized to receive docetaxel and prednisone with custirsen (DPC, n = 20) or mitoxantrone and prednisone with custirsen (MPC, n = 22). The results showed improved OS and time to pain progression in the DPC arm compared with the MPC arm of 15.8 and 10 months vs 11.5 and 5.2 months, respectively, despite a lack of significant PSA or objective responses.23 This study confirmed that treatment with custirsen can significantly decrease its target protein, CLU, when compared with pretreatment baseline levels. A low serum CLU level during custirsen plus chemotherapy was shown to be associated with a 70% reduction in the hazard of death at the start of the serum CLU response (P < 0.001).
There are two randomized phase III trials ongoing of custirsen in combination with chemotherapy in patients with mCRPC. The SYNERGY study (NCT01188187) randomized 1000 chemotherapy-naive patients with mCRPC to receive standard docetaxel and prednisone with or without custirsen. The primary outcome was OS. This study completed accrual in November 2012. The AFFINITY trial (NCT01578655) is another phase III trial, which is comparing the combination of custirsen with cabazitaxel versus cabazitaxel alone in the second-line chemotherapy setting with OS as the primary endpoint. Results of these studies are eagerly awaited.
HSP90 inhibitor
HSP90 inhibition has a particular importance for PCa as HSP90 is overexpressed in PCa cells compared with normal prostate tissue and thus provides a potential selective target. Despite strong preclinical data establishing antitumor activity of first-generation HSP90 inhibitors in PCa, poor clinical responses initially cast uncertainty over the clinical utility of this class of agents. However, more recent advances in compound design and development have now stimulated reappearance for these drugs as a therapeutic option in PCa.24
The first HSP90 inhibitors to be studied were geldanamycin and radicicol, both naturally occurring antibiotics.25,26 The first geldanamycin was tanespimycin (17-AAG). This agent underwent extensive preclinical studies in PCa cell lines and animal models.27 However, a multicenter phase II trial in patients with mCRPC showed no responses and as a result further studies were halted.28 Other HSP90 inhibitors have shown activity in vitro and are being evaluated in phase I and II clinical trials in chronic myelogenous leukemia, multiple myeloma, refractory non-small cell lung cancer, breast, gastric and PCa.29,30,31,32
AT13387 is a novel second generation non-ansamycin HSP90 inhibitor. It is a potent inhibitor of HSP90 and has previously shown potent anti-proliferative activity in a panel of 30 tumor cell lines including four different PCa cell lines32 as well as inhibiting tumor growth in a number of xenograft models. HSP90 inhibition disrupted AR nuclear localization and AR transcriptional activity and resulted in down-regulation of AR-regulated genes in vitro. AT13387 showed substantially greater potency than the first generation, natural product-based HSP90 inhibitor 17-AAG in modulating HSP90 client proteins, inhibiting cell growth and inducing cell death. The antitumor activity of AT13387 was demonstrated in vivo in a human xenograft model of castration-resistant PCa.33 A Phase I study performed in 53 patients with refractory solid tumors identified an AT13387 maximum tolerated dose of 260 mg m−2 administered as a once weekly IV. All drug-related toxicities were generally reversible.34 These results led to the design of a currently accruing phase I/II clinical trial of AT13387 with and without abiraterone acetate in CRPC no longer responding to abiraterone (NCT01685268).
Ganetespib (STA-9090) is a synthetic small molecule that binds to the ATP pocket in the N-terminal of HSP90 causing degradation of cellular proteins and ultimately death of cancer cells dependent on these proteins. In a phase I study, ganetespib was well-tolerated with dose-limiting toxicities of amylase elevation, fatigue and diarrhea.35,36 In a phase II study, 18 heavily pretreated post-docetaxel CRPC patients were treated with single agent ganetespib 200 mg m−2 IV weekly for 3 weeks in 28 days cycle. The trial was terminated early due to lack of activity (NCT01270880). Median PFS was 1.9 months and median OS was 10.2 months.37
BCL-2 in prostate cancer
The Bcl-2 gene encoding the BCL-2 family of proteins plays a central role in the regulation of apoptosis, and can be divided into death antagonists, such as Bcl-2 and death agonists, such as Bak (Bcl-2 homolgogous antagonist/killer) and Bax (Bcl-2 associated X). Bcl-2 is an inner mitochondrial membrane protein that inhibits apoptosis, conferring a survival advantage on cells expressing this oncoprotein.38 On the other hand, Bak and Bax are proapoptotic. Increased expression of Bcl-2 is associated with PCa tumorigenesis and the development of castration-resistant state. Overexpression of Bcl-2 has been clearly documented as contributing to therapeutic resistance, disease recurrence and poor outcome in CRPC. Conversely, downregulation of Bcl2 related proteins results in increased sensitivity to docetaxel in PCa cell lines.3,6 HSP27 interact with the intrinsic pathway and inhibit Bax and prevent the release of cytochrome C and thus inhibit apoptosis.
The first drug tested in clinical trials targeting Bcl2 was oblimersen, a synthetically modified DNA antisense oligonucleotide that hybridizes to mRNA, preventing translation of Bcl-2 protein. A phase II trial in CRPC randomized 115 men to docetaxel alone or in combination with oblimersen.39 This was a negative study with PSA responses observed in 46% of patients in the docetaxel arm and 37% of patients in the docetaxel-oblimersen arm. Eighteen percent of patients on the docetaxel arm achieved partial objective response vs 24% of patients on the docetaxel-oblimersen arm.
AT-101, a natural compound derived from the cotton plant, is considered a pan-Bcl2 inhibitor and potent inducer of proapoptotic proteins. AT-101 was studied in chemotherapy-naive and chemotherapy-refractory PCa patients. A phase II study of 221 chemotherapy-naive mCRPC patients randomized study participants to docetaxel plus AT-101 or docetaxel alone. Unfortunately, there were no statistical differences between study arms with respect to PSA response, PFS or median OS endpoints.40
Several other candidate compounds have been developed to inhibit Bcl2 family members. These compounds are different by their binding specificity and the number of Bcl2 family members they inhibit and are being currently tested in clinical trials.
CONCLUSION
A hallmark of PCa is its ability to escape apoptosis by inhibiting the apoptotic pathways. Androgen deprivation and chemotherapy in PCa relies on an intact apoptotic process to cause cellular death following the initial insult. Therefore, targeting apoptosis represents a crucial opportunity to overcome treatment resistance in PCa. Despite the disappointing results of many of HSP inhibitors, currently OGX-427 and custirsen (OGX-011) hold clinical promise and results from phase III trials are eagerly awaited.
AUTHOR CONTRIBUTIONS
Both authors contributed equally to writing, editing and proof reading the manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Yoshino T, Shiina H, Urakami S, Kikuno N, Yoneda T, et al. Bcl-2 expression as a predictive marker of hormone-refractory prostate cancer treated with taxane-based chemotherapy. Clin Cancer Res. 2006;12:6116–24. doi: 10.1158/1078-0432.CCR-06-0147. [DOI] [PubMed] [Google Scholar]
- 4.Khor LY, Moughan J, Al-Saleem T, Hammond EH, Venkatesan V, et al. Bcl-2 and Bax expression predict prostate cancer outcome in men treated with androgen deprivation and radiotherapy on radiation therapy oncology group protocol 92-02. Clin Cancer Res. 2007;13:3585–90. doi: 10.1158/1078-0432.CCR-06-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocchi P, So A, Kojima S, Signaevsky M, Beraldi E, et al. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res. 2004;64:6595–602. doi: 10.1158/0008-5472.CAN-03-3998. [DOI] [PubMed] [Google Scholar]
- 6.Zielinski RR, Eigl BJ, Chi KN. Targeting the apoptosis pathway in prostate cancer. Cancer J. 2013;19:79–89. doi: 10.1097/PPO.0b013e3182801cf7. [DOI] [PubMed] [Google Scholar]
- 7.Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, et al. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12:743–61. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hessenkemper W, Baniahmad A. Targeting heat shock proteins in prostate cancer. Curr Med Chem. 2013;20:2731–40. doi: 10.2174/0929867311320220001. [DOI] [PubMed] [Google Scholar]
- 9.Jego G, Hazoume A, Seigneuric R, Garrido C. Targeting heat shock proteins in cancer. Cancer Lett. 2013;332:275–85. doi: 10.1016/j.canlet.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Zoubeidi A, Zardan A, Beraldi E, Fazli L, Sowery R, et al. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 2007;67:10455–65. doi: 10.1158/0008-5472.CAN-07-2057. [DOI] [PubMed] [Google Scholar]
- 11.Samali A, Robertson JD, Peterson E, Manero F, van Zeijl L, et al. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 2001;6:49–58. doi: 10.1379/1466-1268(2001)006<0049:hpmotc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, et al. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol. 2002;22:816–34. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stope MB, Schubert T, Staar D, Ronnau C, Streitborger A, et al. Effect of the heat shock protein HSP27 on androgen receptor expression and function in prostate cancer cells. World J Urol. 2012;30:327–31. doi: 10.1007/s00345-012-0843-z. [DOI] [PubMed] [Google Scholar]
- 14.Miyake H, Nelson C, Rennie PS, Gleave ME. Acquisition of chemoresistant phenotype by overexpression of the antiapoptotic gene testosterone-repressed prostate message-2 in prostate cancer xenograft models. Cancer Res. 2000;60:2547–54. [PubMed] [Google Scholar]
- 15.Steinberg J, Oyasu R, Lang S, Sintich S, Rademaker A, et al. Intracellular levels of SGP-2 (Clusterin) correlate with tumor grade in prostate cancer. Clin Cancer Res. 1997;3:1707–11. [PubMed] [Google Scholar]
- 16.Jones SE, Jomary C. Clusterin. Int J Biochem Cell Biol. 2002;34:427–31. doi: 10.1016/s1357-2725(01)00155-8. [DOI] [PubMed] [Google Scholar]
- 17.So A, Hadaschik B, Sowery R, Gleave M. The role of stress proteins in prostate cancer. Curr Genomics. 2007;8:252–61. doi: 10.2174/138920207781386951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, et al. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7:909–15. doi: 10.1038/ncb1291. [DOI] [PubMed] [Google Scholar]
- 19.OncoGenex Pharmaceuticals announces final results from Phase 1 Trial evaluating OGX-427 as a treatment for solid tumors. 2010. Available from: http://filesshareholder.com/downloads/SNUS/2851718935x0x380358/4d11a629-02af-4784-b154-116a2ca1ffc4/OGXI_News_2010_6_7_OncoGenex_Pharmaceuticals.pdf .
- 20.Chi KN, Hotte SJ, Ellard S, Gingerich JR, Joshua AM, et al. A randomized Phase II study of OGX-427 plus prednisone (P) versus P alone in patients (pts) with metastatic castration resistant prostate cancer (CRPC) J Clin Oncol. 2012;30(Suppl):4514. [Google Scholar]
- 21.Chi KN, Eisenhauer E, Fazli L, Jones EC, Goldenberg SL, et al. A phase I pharmacokinetic and pharmacodynamic study of OGX-011, a 2’-methoxyethyl antisense oligonucleotide to clusterin, in patients with localized prostate cancer. J Natl Cancer Inst. 2005;97:1287–96. doi: 10.1093/jnci/dji252. [DOI] [PubMed] [Google Scholar]
- 22.Chi KN, Hotte SJ, Yu EY, Tu D, Eigl BJ, et al. Randomized phase II study of docetaxel and prednisone with or without OGX-011 in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:4247–54. doi: 10.1200/JCO.2009.26.8771. [DOI] [PubMed] [Google Scholar]
- 23.Saad F, Hotte S, North S, Eigl B, Chi K, et al. Randomized phase II trial of Custirsen (OGX-011) in combination with docetaxel or mitoxantrone as second-line therapy in patients with metastatic castrate-resistant prostate cancer progressing after first-line docetaxel: CUOG trial P-06c. Clin Cancer Res. 2011;17:5765–73. doi: 10.1158/1078-0432.CCR-11-0859. [DOI] [PubMed] [Google Scholar]
- 24.Centenera MM, Fitzpatrick AK, Tilley WD, Butler LM. Hsp90: still a viable target in prostate cancer. Biochim Biophys Acta. 2013;1835:211–8. doi: 10.1016/j.bbcan.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma SV, Agatsuma T, Nakano H. Targeting of the protein chaperone, HSP90, by the transformation suppressing agent, radicicol. Oncogene. 1998;16:2639–45. doi: 10.1038/sj.onc.1201790. [DOI] [PubMed] [Google Scholar]
- 27.Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–93. [PubMed] [Google Scholar]
- 28.Heath EI, Hillman DW, Vaishampayan U, Sheng S, Sarkar F, et al. A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2008;14:7940–6. doi: 10.1158/1078-0432.CCR-08-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin Cancer Res. 2011;17:1561–70. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh WK, Galsky MD, Stadler WM, Srinivas S, Chu F, et al. Multicenter phase II trial of the heat shock protein 90 inhibitor, retaspimycin hydrochloride (IPI-504), in patients with castration-resistant prostate cancer. Urology. 2011;78:626–30. doi: 10.1016/j.urology.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sessa C, Shapiro GI, Bhalla KN, Britten C, Jacks KS, et al. First-in-human phase I dose-escalation study of the HSP90 inhibitor AUY922 in patients with advanced solid tumors. Clin Cancer Res. 2013;19:3671–80. doi: 10.1158/1078-0432.CCR-12-3404. [DOI] [PubMed] [Google Scholar]
- 32.Graham B, Curry J, Smyth T, Fazal L, Feltell R, et al. The heat shock protein 90 inhibitor, AT13387, displays a long duration of action in vitro and in vivo in non-small cell lung cancer. Cancer Sci. 2012;103:522–7. doi: 10.1111/j.1349-7006.2011.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferraldeschi R, Hedayat S, Smyth T, Wallis N, Lyons J, et al. In vitro and in vivo antitumor activity of the next generation HSP90 inhibitor, AT13387, in both hormone-sensitive and castration-resistant prostate cancer models. Cancer Res. 2013;73(8 Suppl) Abstract 2433. [Google Scholar]
- 34.Mahadevan D, Rensvold DM, Kurtin SE, Cleary JM, GAndhi L, et al. First-in-human phase I study: results of a second-generation non-ansamycin heat shock protein 90 (HSP90) inhibitor AT13387 in refractory solid tumors. J Clin Oncol. 2012;30(Suppl):A3028. [Google Scholar]
- 35.Goldman JW, Raju RN, Gordon GA, El-Hariry I, Teofilivici F, et al. A first in human, safety, pharmacokinetics, and clinical activity phase I study of once weekly administration of the Hsp90 inhibitor ganetespib (STA-9090) in patients with solid malignancies. BMC Cancer. 2013;13:152. doi: 10.1186/1471-2407-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho D, Heath EI, Cleary JM, Kwak EL, Gandhi L, et al. A phase I doseescalation study of the Hsp90 inhibitor ganetespib (STA-9090) administered twice weekly in patients with solid tumors: updated report. J Clin Oncol. 2011;29:A3051. [Google Scholar]
- 37.Elisabeth I, Heath MN, Vaishampayan UN, Emmanuel S, Antonarakis, et al. Phase II trial of single-agent ganetespib (STA-9090), a heat shock protein 90 (Hsp90) inhibitor in heavily pretreated patients with metastatic castration-resistant prostate cancer (mCRPC) post docetaxel-based chemotherapy: results of a Prostate Cancer Clinical Trials Consortium (PCCTC) study. J Clin Oncol. 2013;31(suppl):A5085. [Google Scholar]
- 38.Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sternberg CN, Dumez H, Van Poppel H, Skoneczna I, Sella A, et al. Docetaxel plus oblimersen sodium (Bcl-2 antisense oligonucleotide): an EORTC multicenter, randomized phase II study in patients with castration-resistant prostate cancer. Ann Oncol. 2009;20:1264–9. doi: 10.1093/annonc/mdn784. [DOI] [PubMed] [Google Scholar]
- 40.Sonpavde G, Matveev V, Burke JM, Caton JR, Fleming MT, et al. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Ann Oncol. 2012;23:1803–8. doi: 10.1093/annonc/mdr555. [DOI] [PubMed] [Google Scholar]


