Abstract
In recent years, immunotherapy has emerged as a viable and attractive strategy for the treatment of prostate cancer. While there are multiple ways to target the immune system, therapeutic cancer vaccines and immune checkpoint inhibitors have been most successful in late-stage clinical trials. The landmark Food and Drug Administration approval of sipuleucel-T for asymptomatic or minimally symptomatic metastatic prostate cancer set the stage for ongoing phase III trials with the cancer vaccine PSA-TRICOM and the immune checkpoint inhibitor ipilimumab. A common feature of these immune-based therapies is the appearance of improved overall survival without short-term changes in disease progression. This class effect appears to be due to modulation of tumor growth rate kinetics, in which the activated immune system exerts constant immunologic pressure that slows net tumor growth. Emerging data suggest that the ideal population for clinical trials of cancer vaccines is patients with lower tumor volume and less aggressive disease. Combination strategies that combine immunotherapy with standard therapies have been shown to augment both immune response and clinical benefit.
Keywords: androgen deprivation, immunotherapy, prostate cancer, therapeutic cancer vaccines
INTRODUCTION
Results of recent clinical trials have intensified interest in immunotherapy for prostate cancer. The primary aim of immunotherapy is to harness the immune system's ability to recognize and destroy tumor cells. Prostate cancer is particularly well suited for immunotherapeutic approaches for three reasons.1 First, early detection, along with the relatively indolent clinical course of prostate cancer, allows sufficient time to generate immune responses that may take weeks or months to mount. Second, prostate cancer cells express several tumor-associated antigens (TAAs), such as prostate-specific antigen (PSA), prostatic acid phosphatase (PAP) and prostate-specific membrane antigen, which can serve as targets for activated immune cells.2,3,4 Finally, since the prostate is a nonessential organ, eradication of residual normal prostate tissue as a result of the immune response has no clinical sequelae.
Current strategies at the forefront of immunotherapy for prostate cancer include therapeutic vaccines and immune checkpoint inhibitors. Therapeutic cancer vaccines are designed to stimulate immune cells to target specific TAAs overexpressed on cancer cells and are associated with minimal toxicity. Antigen-presenting cells (APCs) present antigens to the immune system via major histocompatibility complex molecules, which bind to appropriate T-cell receptors. Activated T cells travel to the tumor, which they recognize by way of the TAAs presented in the context of the major histocompatibility complex, leading to T cell-mediated killing of tumor cells or immunogenic cell death. Unlike standard cancer treatments, immunotherapeutic effects may persist well beyond tumor-cell death. Over time, the immune response may broaden to target multiple TAAs not found in the initial vaccine construct. As T cells lyse tumor cells, additional TAAs may be taken up by APCs and presented to immune cells as potential new targets. This expanded T-cell response is known as antigen spreading or antigen cascade,5 a process that can broaden and become more clinically relevant over time. In fact, emerging data show improved clinical outcomes in patients who mount a broad immunologic response.6,7,8
Immune checkpoint inhibitors interfere with the immune system's autoregulatory mechanisms, allowing for an expanded T-cell response and greater antitumor effects.9 However, inhibiting the body's mechanism for protecting against autoimmunity can create immune-related toxicities. Ipilimumab, a fully human monoclonal antibody, inhibits negative signals sent to T cells through the cell-surface molecule cytotoxic T lymphocyte antigen-4 (CTLA-4), thus blocking a negative checkpoint and removing the physiologic brake on the immune system. This first-in-class immune checkpoint inhibitor was Food and Drug Administration (FDA)-approved for the treatment of metastatic melanoma, based on overall benefit seen in clinical trials. Ipilimumab is currently being evaluated in late-stage clinical trials in patients with metastatic castration-resistant prostate cancer (mCRPC).10
SIPULEUCEL-T (PROVENGE®)
Sipuleucel-T is an autologous dendritic-cell vaccine designed to target PAP. APCs collected by leukapheresis are transported to a central processing facility where CD54+ APCs are pulsed with PA2024, a fusion protein consisting of PAP linked to the immunomodulatory cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF).11,12 The vaccine product must meet a minimum threshold of CD54 expression before it can be released for use. The vaccine is then infused into the patient three times at biweekly intervals. Promising early clinical data led to a pair of small phase III trials in which the primary endpoint was time to progression (TTP), with overall survival (OS) as a planned secondary endpoint.13,14 In each trial, men with asymptomatic or minimally symptomatic mCRPC were randomly assigned 2:1 to receive either sipuleucel-T or a placebo not pulsed with PA2024. Analysis of the 225 men from both trials showed no improvement in TTP, but a consistent benefit in OS relative to placebo (23.2 vs 18.9 months, hazard ratio (HR), 0.67, 95% confidence interval (CI) 0.49–0.91).
These results led to a larger phase III trial (IMPACT; n = 512) with OS as the primary endpoint rather than TTP. At a median follow-up of 34 months, patients treated with sipuleucel-T showed significantly improved OS compared with placebo (25.8 vs 21.7 months; HR, 0.78; 95% CI, 0.61–0.98). As in the earlier trials, there was no significant change in time to radiographic or PSA progression and few declines in PSA.15 Patients treated with sipuleucel-T had less disease-related pain; a retrospective data analysis detected delayed median time to first use of opioid analgesics compared to patients given placebo (11.9 vs 8.3 months; HR, 0.73; 95% CI, 0.54–0.99).16 A subgroup analysis showed activation of APCs through upregulation of CD54, as well as antibodies and T-cell proliferation responses to PAP and PA2024. An immunologic assessment of patients in the three sipuleucel-T trials suggested that patients with more potent immune responses post-vaccination showed the greatest improvement in OS.17
Research demonstrating the biologic activity of neoadjuvant sipuleucel-T preradical prostatectomy has been revealing. Prostatectomy specimens from vaccinated patients showed a greater than twofold increase in CD3+ and CD4+ T cells at the tumor interface, suggesting that immune cells travel to the prostate and are active in disease control.18 Furthermore, a retrospective analysis of the IMPACT trial found that patients in the lowest quartile of baseline PSA values received the greatest benefit from the vaccine, with a 13-month improvement in median OS (41.3 months with sipuleucel-T vs 28.3 months with placebo; HR, 0.51; 95% CI, 0.31–0.85). In contrast, patients in the highest baseline PSA quartile had a median OS of 18.4 vs 15.6 months for placebo (HR, 0.84; 95% CI, 0.55–1.29), an improvement of only 2.8 months.19 These results support the use of sipuleucel-T in earlier-stage disease.20 In all three trials, sipuleucel-T was well-tolerated, with minimal toxicity. Injection-site reactions, transient fever and flu-like symptoms were most frequently reported.
PSA-TRICOM (PROSTVAC -VF®)
PSA-TRICOM is a poxviral vector-based vaccine consisting of a priming dose of recombinant vaccinia followed by five or six recombinant fowlpox boosts.21,22 Both the vaccinia and fowlpox vectors are engineered to express PSA and three costimulatory molecules (TRICOM) designed to enhance the immune response. PSA-TRICOM is an off-the-shelf vaccine that can be generated in large quantities. Frozen doses are simply thawed and injected into the patient, making PSA-TRICOM a logistically simple yet immunologically advanced vaccine. Several early trials demonstrated that the prime-and-boost regimen was well-tolerated, with toxicities consisting mainly of fevers and injection-site reactions.23,24,25,26,27 In a single-arm phase II trial of PSA-TRICOM, patients with mCRPC (n = 32) had a median OS of 26.6 months. Thirteen of 29 evaluable patients had a greater than twofold increase in PSA-specific T-cell immune response by ELISPOT assay (interferon-γ secretion in response to PSA) and an association between magnitude of immune response and improved OS was observed (P = 0.055). The study also suggested that patients with more indolent disease characteristics based on predicted survival derived the most benefit from vaccine.28 In a second multicenter phase II trial, 125 men were randomized 2:1 to receive vaccine or placebo, respectively. As in the sipuleucel-T trials, there was no difference in terms of TTP. However, a mature follow-up showed that PSA-TRICOM conferred significantly improved OS (25.1 vs 16.6 months; HR 0.56; 95% CI 0.37–0.85) and a 3-year survival of 30 vs 17% for placebo.29 In addition, the presence of a preexisting antibody to a glycoprotein antigen in the vector was associated with improved outcome in patients who received PSA-TRICOM.30 This may result, in part, from the presence of a blood group A-like glycan on the surface of poxviruses that is presumably transferred from the chicken embryo dermal cells used as host cells in vaccine production. This glycan may help to optimize uptake of the poxviral vaccine by APCs.
These phase II trials suggested tumor-specific CTL responses and prolonged OS in patients treated with PSA-TRICOM. An international phase III randomized, placebo-controlled trial of PSA-TRICOM is currently enrolling 1200 patients with asymptomatic or minimally symptomatic mCRPC, with OS as the primary endpoint.31 Patients are randomized to receive either PSA-TRICOM with adjuvant GM-CSF, PSA-TRICOM with placebo GM-CSF or double placebo. Results of this trial are eagerly anticipated.
GVAX
Two phase III clinical trials of the allogeneic cell-based vaccine GVAX have had disappointing results.32,33 The vaccine is based on a platform of irradiated hormone-sensitive (LNCaP) and hormone-resistant (PC-3) prostate cancer cell lines genetically modified to secrete GM-CSF. Early clinical data suggesting clinical benefit led to two phase III trials of GVAX. VITAL-1 randomized patients with asymptomatic mCRPC to vaccine or docetaxel-prednisone, while VITAL-2 randomized symptomatic men to receive docetaxel or docetaxel plus GVAX. At a planned interim analysis, VITAL-2 demonstrated a trend towards more deaths in the experimental arm and shorter median OS, leading to the trial's closure. These findings prompted a futility analysis of VITAL-1. When results suggested a <30% chance of improved OS with GVAX, that trial was terminated as well. It is unclear whether the failure to demonstrate clinical benefit reflects a flaw in trial design or a true lack of vaccine efficacy. Preclinical data suggesting that combining vaccines with immune checkpoint inhibitors may substantially augment a limited antitumor response34 led to a recent trial on the safety and feasibility of combining GVAX and ipilimumab.35 Exploratory T-cell monitoring of the 28 patients enrolled on this phase I/II dose escalation/expansion trial found that patients with higher CTLA-4 expression on CD4 cells prior to treatment was associated with prolonged OS after therapy (46.5 vs 21 months, HR, 0.271, 95% CI, 0.079–0.931, P = 0.036).36 It is unclear whether this survival advantage is due to the specific use of antibodies against CTLA-4, versus a reflection of a more activated T-cell state, which would lead to improved clinical outcome regardless of the type of immunotherapy. However, the identification of potential biomarkers for clinical efficacy is a key consideration given the unique profile of immune-related adverse events (irAEs).
IPILIMUMAB
Ipilimumab, a fully human anti-CTLA-4 monoclonal antibody, is a first-in-class immune checkpoint inhibitor. CTLA-4, the most extensively studied immune checkpoint molecule, is expressed on CTLs after activation by APCs. The CTLA-4 receptor on CTLs is a negative regulator of T-cell activation that outcompetes CD28 for binding to B7 on APCs. In contrast to CD28/B7 binding, which acts as a costimulatory signal, the binding of CTLA-4 by ipilimumab removes the physiologic brake, augmenting the immune response by blocking the interaction of CTLA and B7.9
Ipilimumab was FDA-approved for the treatment of relapsed metastatic melanoma after demonstrating improved OS in a phase III trial.10 Patients were randomized to receive ipilimumab plus a glycoprotein vaccine (gp100), ipilimumab alone or gp100 alone. OS significantly increased in patients given ipilimumab. Patients in the ipilimumab-alone arm showed improved median OS relative to gp100 alone (10.1 vs 6.4 months, HR, 0.68; 95% CI, 8.0–13.8), while patients receiving ipilimumab plus gp100 had a median OS of 10.0 months (HR, 0.66; 95% CI 8.5–11.5). Interestingly, there was no significant difference in median TTP among the three arms, similar to results from the sipuleucel-T and PSA-TRICOM trials. Consistent with earlier clinical trials, ipilimumab's toxicity profile included a wide range of irAEs, thought to be caused by a breaking of tolerance to other host tissues, allowing for collateral damage by activated CTLs.37,38 Common irAEs included enterocolitis, hepatitis, dermatitis and endocrinopathies, most of which could be medically managed.
Phase III trials of ipilimumab are ongoing in both chemotherapy-naïve and chemotherapy-refractory mCRPC, based on the results of early-phase studies. In an initial phase I/II study, patients with mCRPC received ipilimumab with or without radiation therapy. In the phase I component, with safety as the primary endpoint, side effects were similar to those seen with ipilimumab alone in patients with melanoma. In the phase II component, which examined preliminary evidence of efficacy, 26% of patients had a PSA decline of ≥50% over the course of the study. One patient had a complete response wherein PSA normalized and an initially large prostatic lesion became undetectable.39 These results led to two ongoing phase III trials of ipilimumab in patients with mCRPC. The first randomizes chemotherapy-naïve patients to ipilimumab alone vs. placebo.40 The second compares limited radiation plus ipilimumab vs limited radiation plus placebo in patients previously treated with chemotherapy.41 Results of this trial were recently presented at the European Cancer Congress, with median OS favoring ipilimumab over placebo (11.2 vs 10 months; HR 0.85, 95% CI 0.72–10.00), though statistical significance was not achieved (P = 0.053). Interestingly, median progression-free survival also favored ipilimumab over placebo (HR 0.70, 95% CI 0.61–0.82), as did PSA declines of ≥50% in evaluable patients (13.1% vs 5.3%).42
A PARADIGM SHIFT
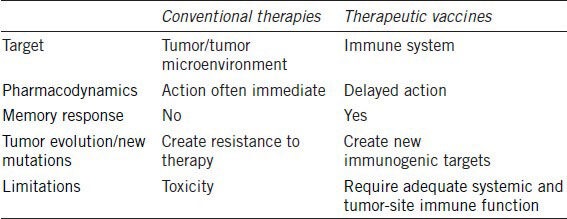
Phase III trials of sipuleucel-T and ipilimumab in melanoma, along with the randomized phase II trial of PSA-TRICOM, demonstrated significant improvement in OS with no evidence of short-term clinical benefit (Figure 1), although preliminary data from a trial of ipilimumab suggests a possible improvement in progression-free survival, a secondary endpoint in that study.10,15,29,42 This class effect highlights a key difference between the mechanisms of action of immunotherapy and traditional cytotoxic therapies. While conventional therapies directly target the tumor and its microenvironment, therapeutic vaccines and immune checkpoint inhibitors target the immune system, which subsequently targets the tumor. Following administration of therapeutic vaccines and other immunotherapies, the combination of immunogenic tumor targeting and antigen cascade can lead to a delayed yet prolonged clinical response that lasts over a period of weeks to months.43 While the initial immune response to a single TAA is quite brisk, the activated immune response may in turn lead to the development of long-lived memory cells that can sustain clinical benefit beyond the period of treatment.44 This phenomenon allows for a broadened immune response over time, even as the tumor mutates to present antigens not included in the vaccine. This is another key contrast to conventional therapies, to which the body does not mount an evolving response and may even become resistant (Table 1).
Figure 1.
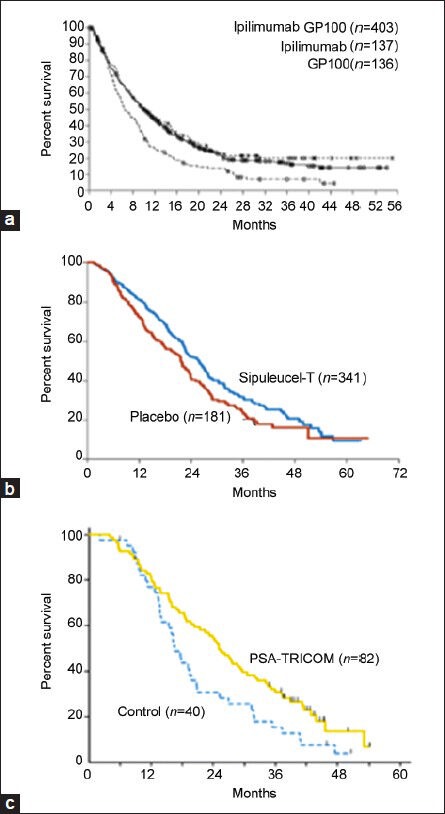
Overall survival curve for (a) ipilimumab (reproduced from Hodi et al.101), (b) sipuleucel-T (reproduced from Kantoff et al.115) and (c) PSA-TRICOM (reproduced from Kantoff et al.29)
Table 1.
Comparisons between conventional therapies and therapeutic vaccines
Alternative strategies for assessing the delayed yet prolonged response to immunotherapy are emerging, based on a new understanding of tumor-growth equilibrium in response to vaccine. Tumor growth rate is influenced by intrinsic tumor biology as well as extrinsic factors such as chemotherapy. While conventional cytoreductive therapy reduces tumor burden during treatment, the response is transient. When chemotherapy is discontinued, tumor growth typically returns to its pretreatment rate. On the other hand, immunotherapy may reset the tumor-growth equilibrium. The continued immunologic pressure exerted by effector cells slows the rate of tumor growth over time.45 Recent data from prostate cancer vaccine trials at the National Cancer Institute suggest that tumor growth slows after treatment with vaccine.46 A retrospective analysis evaluated patients receiving chemotherapy, hormonal therapy and immunotherapy. In patients who received chemotherapy, tumor burden decreased for a variable period of time, but the tumor growth rate returned to pretreatment levels when treatment was stopped. Patients who received vaccine did not experience an immediate decrease in tumor burden or initial slowing of tumor growth rate, but over time, immunologic pressure appeared to slow tumor growth rates, resulting in longer OS.
The concept that immunotherapy has the potential to induce a sustained immune response and slow the tumor growth rate without short-term benefit in TTP is supported by results from late-phase clinical trials of sipuleucel-T and PSA-TRICOM in mCRPC and ipilimumab in melanoma. Two trials in earlier-stage disease have also provided supportive evidence that vaccine slows the rate of tumor growth, as measured by PSA doubling time (PSA DT). In the PROTECT trial, patients with nonmetastatic castration-sensitive prostate cancer and normal testosterone were randomized 2:1 to receive sipuleucel-T or placebo after 3–4 months of androgen-deprivation therapy (ADT).20 Patients who received sipuleucel-T had a 48% longer PSA DT after testosterone normalization (155 vs 105 days; P = 0.038), supporting the notion that therapeutic cancer vaccines modulate tumor growth rates over time. A second trial evaluated patients with recurrent prostate cancer but no metastasis (stage D0). Patients were treated with PSA-TRICOM monthly for 3 months and then once every 3 months. In a post hoc analysis, patients treated with vaccine had an improvement in PSA DT of 4.4–7.7 months (P = 0.002), further suggesting the ability of vaccine to reset tumor-growth equilibrium.47
Evidence of immunogenic modulation of tumor growth over time suggests that the greatest clinical benefit accrues to patients who receive vaccine early in the disease course. This is supported by a retrospective analysis of baseline PSA values from the IMPACT trial, which found that patients in the lowest PSA quartiles (≤22) had the greatest improvements in survival (HR 0.51).19 HRs increased with PSA, further suggesting that greater benefit was seen in patients treated earlier in the course of disease. Conversely, administering immunotherapy in later-stage disease is associated with worse outcomes. Analyses of OS curves in randomized, controlled studies of therapeutic vaccines have shown a delayed separation, with no evidence of benefit for the first 6–12 months. Thus, patients destined to die within the first 6 months to a year after receiving vaccine derived no benefit.1 These data influenced the decision to enroll in the ongoing phase III trial of PSA-TRICOM only those patients with a life expectancy of >1 year. Analysis of retrospective data from the IMPACT trial detected a delayed separation in the time to first opioid analgesic use in patients who received vaccine vs those who did not,16 further supporting the notion that starting immunotherapy earlier has a greater impact on more disease-specific outcomes such as OS.
There is also evidence to suggest that changes in PSA kinetics may impact clinical endpoints such as metastasis-free survival. In a post hoc analysis of 146 men treated in four phase II trials investigating nonhormonal agents for biochemically recurrent nonmetastatic prostate cancer, changes in PSA DT and change in (log) PSA slope were associated with metastasis-free survival. Specifically, men whose PSA DT increased after study entry or whose PSA slope decreased had improved metastasis-free survival. While retrospective, this study gives credence to the value of PSA kinetics in determining clinical endpoints in trial design.48
BIOMARKERS
While our understanding of the fundamental differences between immunotherapies and standard cytoreductive treatments has grown, there is still an urgent need for definitive biomarkers to assess the short-term benefits of immunotherapies. Without clear markers of short-term benefit, such as reduced tumor volume or PSA decline, practitioners are faced with difficult choices as they integrate immunotherapy into treatment for prostate cancer. The absence of objective measures of a vaccine's effectiveness makes deciding whether to continue treatment or move on to the next course of therapy difficult at best.49
Theoretically, markers of immune activation may be evaluable in blood samples weeks or months after administration of vaccine. Retrospective data evaluating immune parameters in patients treated with sipuleucel-T suggest a correlation between the magnitude of cumulative APC activation and OS.17 An ongoing open-label phase II trial (OpenAct) will extensively evaluate cellular and humoral activation following treatment with sipuleucel-T.50 Similarly, improved clinical outcomes have been associated with CTL response in patients treated with PSA-TRICOM.28 Subsequent analysis also noted a significant correlation with survival in patients with prevaccination antibodies to a glycoprotein antigen in the vector.30 The ongoing phase III trial of PSA-TRICOM in mCRPC aims to evaluate immune endpoints and provide further data supporting vaccines’ ability to alter growth rate while providing clinically significant improvement in OS. However, it must be noted that, to date, no surrogate biomarkers for clinical outcomes in patients treated with immunotherapy have been identified.
Next steps: optimal combinations and sequencing of therapy
One apparent drawback of immunotherapy is the fact that other treatment modalities produce measurable tumor response in addition to improving OS. Thus, the maximum clinical benefit of immunotherapies may only be realized when they are combined or sequenced with conventional treatments for prostate cancer, preferably early in the course of disease when both tumor burden and tumor-induced tolerance are minimal.51,52
Dead or dying cancer cells may release antigens that stimulate an immune response in a process known as immunogenic cell death.53 There is strong evidence that combining immunotherapies for prostate cancer with conventional treatments such as chemotherapy and radiation can enhance the immune response by altering the tumor phenotype. This immunogenic modulation renders cells more sensitive to CTL killing, further supporting the rationale for combined treatment regimens.54,55
Preclinical data have demonstrated that combining vaccine and docetaxel induces enhanced immune activity compared with either treatment alone.56 In a study of patients with metastatic breast cancer, docetaxel combined with a poxviral vaccine targeting mucin-1 and carcinoembryonic antigen prolonged TTP (6.6 vs 3.8 months, P = 0.12, HR 0.67).57 Radiation therapy can also enhance antigen expression and immune-mediated tumor-cell killing.58,59 The potential value of this effect in prostate cancer was demonstrated in a clinical trial that combined vaccine with definitive radiation therapy in 30 patients with newly diagnosed disease. Patients treated with vaccine plus radiation had a significantly enhanced prostate cancer-specific immune response compared with those who received standard radiation alone.60,61 An interim analysis of a radiation combination trial using the radioisotope samarium-153 (Sm-153) combined with PSA-TRICOM showed prolonged TTP compared to treatment with Sm-153 alone. The combination of vaccine and Sm-153 was well-tolerated, with similar toxicity to Sm-153 alone, further supporting the rationale for combining vaccine and radiation.62
ADT is a mainstay of treatment for prostate cancer, having been shown in preclinical models to potentiate antitumor immunity via immune conditioning.63 Increasing data suggest that ADT can augment the immune response by increasing CTL infiltration of the prostate.64 ADT has also been shown to increase thymic production of naïve T cells, which can be harnessed by immunotherapy and directed at PSA.65 Other data suggest that ADT mitigates immune tolerance, allowing immunotherapies to target cancer cells that overexpress self-antigens.66 While the optimal sequencing of immunotherapy with ADT is unclear, combining ADT with immunotherapy for a defined period of time may help to eradicate residual prostate cancer cells once ADT is discontinued.
To explore this possibility, a phase II randomized trial is ongoing in which 68 patients with biochemically recurrent prostate cancer are randomized to receive sipuleucel-T either 6 weeks before or 12 weeks after a year-long course of ADT.67 Analysis of antitumor immune response will establish which sequence is more effective. The study will also compare PSA progression and metastasis-free survival in the two arms. An interim analysis has suggested an increase in antigen-specific responses and in vivo cytokines in the arm in which ADT is followed by vaccine, providing evidence for a possible improvement in antitumor response when ADT is administered first.68 Final results will further elucidate the optimal sequencing of immunotherapy with ADT.
PSA-TRICOM has also been studied in combination with standard therapies. A phase II study randomized 42 men with nonmetastatic CRPC to receive PSA-TRICOM or ADT, with an option to cross over to combined therapy at disease progression.69 This study suggested improved survival in patients who received vaccine prior to ADT. Promising data have also emerged from an ongoing trial evaluating flutamide with PSA-TRICOM in nonmetastatic CRPC. Interim TTP analysis favored the combination of PSA-TRICOM and flutamide compared to flutamide alone (192 vs 108 days).70
GVAX is also being studied in combination with cyclophosphamide and ADT in the neoadjuvant setting, based on preclinical data suggesting augmented antitumor immune responses if low-dose cyclophosphamide precedes vaccine.1 The ongoing neoadjuvant trial randomizes patients to cyclophosphamide and GVAX followed by ADT versus ADT alone prior to radical prostatectomy.72 Primary outcome measures include comparison of intraprostatic CD8 + T-cell infiltration between treatment arms.
The advent of novel hormonal agents such as abiraterone and enzalutamide has sparked interest in combining these therapies with immune-based approaches. An ongoing phase II trial randomizes men with mCRPC to receive sipuleucel-T followed by abiraterone in combination with prednisone or to concurrent therapy with both of these agents.73 This addresses an important issue regarding sequencing, as preclinical data suggest that immunotherapy may be optimally effective when administered before androgen ablation.74 Abiraterone, a CYP17 inhibitor FDA-approved for use in mCRPC, suppresses levels of circulating androgen. Suppression of the androgen axis via abiraterone may be immunostimulatory, suggesting that abiraterone plus sipuleucel-T could be synergistic. At the same time, administering abiraterone with prednisone may theoretically blunt an immunologic response; however, previous data suggest that memory cells are relatively resistant to steroid-induced killing compared with naïve cells.75 An interim analysis of this study shows similar humoral and cellular responses to sipuleucel-T in both the concurrent and sequential arms, suggesting that the steroids when given with abiraterone did not appear to blunt the immune response. This is in line with previous observations about the addition of glucocorticoids and immune response.75,76 Additionally, preliminary findings suggest that sipuleucel-T is well-tolerated in combination with concurrent or sequential abiraterone with prednisone.77 Recently reported data on 64 patients from this phase II trial indicate that sipuleucel-T can be successfully manufactured during concurrent abiraterone and prednisone without affecting product potency and immunologic prime-boost response.78
FDA approval of enzalutamide for the treatment of chemotherapy-refractory mCRPC has stimulated interest in using this novel androgen receptor antagonist (ARA) in earlier-stage prostate cancer and in combination with immunotherapy, given its favorable side-effect profile.79 This next-generation ARA binds to the androgen receptor with greater affinity than first-generation ARAs and prevents downstream effects, including nuclear translocation, DNA binding and signaling to coactivators. Another important characteristic of this ARA is that, unlike the androgen-biosynthesis inhibitor abiraterone, enzalutamide does not require daily prednisone. Patients with nonmetastatic castration-sensitive prostate cancer are currently being enrolled in a phase II trial that randomizes men to receive a 3-month course of enzalutamide with or without PSA-TRICOM.80 The primary endpoint of this study is to determine the vaccine's effect on PSA growth kinetics after enzalutamide is discontinued. This proof-of-concept study will help to define the benefits of combining ADT and immunotherapy. A similar study is being conducted in chemotherapy-naïve patients with metastatic disease (Table 2).81
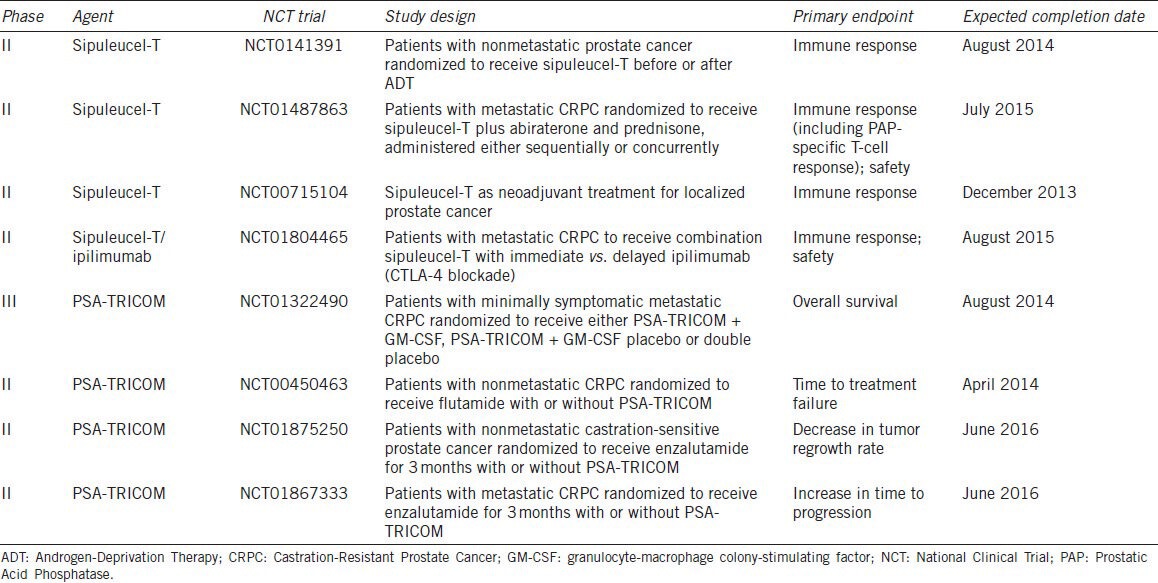
Table 2.
Selected immunotherapy trials for patients with prostate cancer
Combining immunotherapies (immunogenic intensification)
Combining immunotherapies could potentially generate a greater immune response and enhanced antitumor activity.82 Previous studies have shown evidence of enhanced clinical outcomes without increased toxicity. For example, combining ipilimumab and PSA-TRICOM provides directed T-cell activation while removing physiologic brakes on the immune system, allowing for a more robust CTL response. A phase I study treated 30 patients with docetaxel-refractory or chemotherapy-naïve mCRPC with a fixed dose of PSA-TRICOM in conjunction with escalating doses of ipilimumab given at monthly intervals. Rates of irAEs in patients receiving the combination therapy were similar to those reported with ipilimumab alone, including endocrinopathies and colitis, but no dose-limiting toxicities were observed. Only 1/6 patients previously treated with chemotherapy had a PSA decline from baseline. Of the 24 patients who were chemotherapy-naïve, 14 (58%) had PSA declines, six of which (25%) were >50%. In addition, the median OS of patients treated with this combination (34.4 months) appears to be greater than patients treated with vaccine alone.83 These hypothesis-generating data suggest that intensification of immune-based therapies may improve clinical outcomes.
Based on preclinical models suggesting synergy between ipilimumab and the whole tumor cell vaccine GVAX,84 a phase I dose-escalation trial enrolled chemotherapy-naïve patients with mCRPC and treated them with GVAX and concurrent intravenous ipilimumab for 24 weeks. Approximately 29% of patients experienced grade 3 irAEs; for one patient, 5 mg kg−1 of ipilimumab led to a dose-limiting toxicity. PSA declines of >50% were observed in 25% of men, with a median OS of 29.2 months.35 A recent preclinical study explored optimal sequencing of CTLA-4 blockade with cell-based immunotherapy.85
Early safety and efficacy studies of ipilimumab plus vaccine opened the door for future trials of combination therapy with immune checkpoint inhibitors, and provided the rationale for combining prostate cancer vaccines with monoclonal antibodies targeting programmed cell death protein 1. Early clinical trials of this novel immune checkpoint inhibitor have suggested antitumor activity with less frequent and severe irAEs than are reported with ipilimumab.86,87,88
CONCLUSIONS
Immunotherapies have contributed significantly to the evolving landscape of prostate cancer treatment. Ongoing phase III trials of PSA-TRICOM and the immune checkpoint inhibitor ipilimumab may soon broaden the scope of immunotherapies available to patients with mCRPC. Current strategies are also exploring optimal combinations and sequencing of immunotherapies with conventional treatments. Integration of immunotherapy into standard treatment regimens has been hampered by the lack of short-term measures of objective response. However, an evolving understanding of these agents’ effects on tumor growth rate equilibrium, coupled with well-designed clinical trials evaluating immune endpoints and growth rate kinetics, may soon provide answers to this dilemma.
COMPETING INTERESTS
The authors declare that there are no competing interests.
REFERENCES
- 1.Gulley JL, Drake CG. Immunotherapy for prostate cancer: recent advances, lessons learned, and areas for further research. Clin Cancer Res. 2011;17:3884–91. doi: 10.1158/1078-0432.CCR-10-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–61. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Goldfarb DA, Stein BS, Shamszadeh M, Petersen RO. Age-related changes in tissue levels of prostatic acid phosphatase and prostate specific antigen. J Urol. 1986;136:1266–9. doi: 10.1016/s0022-5347(17)45310-9. [DOI] [PubMed] [Google Scholar]
- 4.Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol. 1979;17:159–63. [PubMed] [Google Scholar]
- 5.Gulley JL. Therapeutic vaccines: the ultimate personalized therapy? Hum Vaccin Immunother. 2013;9:219–21. doi: 10.4161/hv.22106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–92. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardwick N, Chain B. Epitope spreading contributes to effective immunotherapy in metastatic melanoma patients. Immunotherapy. 2011;3:731–3. doi: 10.2217/imt.11.62. [DOI] [PubMed] [Google Scholar]
- 8.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–61. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 9.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel PH, Kockler DR. Sipuleucel-T: a vaccine for metastatic, asymptomatic, androgen-independent prostate cancer. Ann Pharmacother. 2008;42:91–8. doi: 10.1345/aph.1K429. [DOI] [PubMed] [Google Scholar]
- 12.Rini BI. Technology evaluation: APC-8015, Dendreon. Curr Opin Mol Ther. 2002;4:76–9. [PubMed] [Google Scholar]
- 13.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–94. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 14.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–9. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 15.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Eng J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 16.Small EJ, Higano C, Kantoff P, Whitmore JB, Frohlich MW, et al. Relationship of sipuleucel-T with time to first use of opioid analgesics (TFOA) in patients (pts) with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer (mCRPC) on the IMPACT trial [poster] J Clin Oncol. 2013;31(suppl 6) Abstr 74. [Google Scholar]
- 17.Sheikh NA, Petrylak D, Kantoff PW, Dela Rosa C, Stewart FP, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62:137–47. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong L, Weinberg VK, Corman JM, Amling CL, Stephenson RA, et al. Immune responses in prostate tumor tissue following neoadjuvant sipuleucel-T in patients with localized prostate cancer. J Clin Oncol. 2012;30(Supp 5) Abstr 181. [Google Scholar]
- 19.Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, et al. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81:1297–302. doi: 10.1016/j.urology.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 20.Beer TM, Bernstein GT, Corman JM, Glode LM, Hall SJ, et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin Cancer Res. 2011;17:4558–67. doi: 10.1158/1078-0432.CCR-10-3223. [DOI] [PubMed] [Google Scholar]
- 21.Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001–11. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longo DL. New therapies for castration-resistant prostate cancer. N Engl J Med. 2010;363:479–81. doi: 10.1056/NEJMe1006300. [DOI] [PubMed] [Google Scholar]
- 23.Arlen PM, Skarupa L, Pazdur M, Seetharam M, Tsang KY, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178:1515–20. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 24.Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–8. [PubMed] [Google Scholar]
- 25.Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–73. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 26.Gulley J, Chen AP, Dahut W, Arlen PM, Bastian A, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–17. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman HL, Wang W, Manola J, DiPaola RS, Ko YJ, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–32. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 28.Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–74. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell CT, Gulley JL, Oyelaran O, Hodge JW, Schlom J, et al. Serum antibodies to blood group A predict survival on PROSTVAC-VF. Clin Cancer Res. 2013;19:1290–9. doi: 10.1158/1078-0432.CCR-12-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.A Randomized, Double-blind, Phase 3 Efficacy Trial of PROSTVAC-V/F +/- GM-CSF in Men With Asymptomatic or Minimally Symptomatic Metastatic Castrate-Resistant Prostate Cancer (Prospect) [Last accessed on 2013 Aug 29]. Available from: http: //clinicaltrials.gov/ct2/show/NCT01322490 .
- 32.Cell Genesys Halts VITAL-2 GVAX Trial in Advanced Prostate Cancer. 2008. [Last accessed on 2013 Aug 29]. Available from: http://phx.corporate-ir.net/phoenix.zhtml?c=98399 and P=irol-newsArticle and ID=1191052 .
- 33.Cell Genesys Announces Termination of VITAL-1 Phase 3 Trial of GVAX Immunotherapy for Prostate Cancer. 2008. [Last accessed on 2013 Aug 29]. Available from: http://www.drugs.com/news/cell-genesys-announces-termination-vital-1-phase-3-trial-gvax-immunotherapyprostate-cancer-14159.html .
- 34.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–17. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 36.Santegoets SJ, Stam AG, Lougheed SM, Gall H, Scholten PE, et al. T cell profiling reveals high CD4+CTLA-4+T cell frequency as dominant predictor for survival after prostate GVAX/ipilimumab treatment. Cancer Immunol Immunother. 2013;62:245–56. doi: 10.1007/s00262-012-1330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber JS, O’Day S, Urba W, Powderly J, Nichol G, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–6. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 39.Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–21. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phase 3 Study of Immunotherapy to Treat Advanced Prostate Cancer. [Last accessed on 2013 August 29]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01057810 .
- 41.Study of Immunotherapy to Treat Advanced Prostate Cancer. [Last accessed on 2013 August 29]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00861614 .
- 42.Gerritsen WR, Fizazi A, Drake CG. Amsterdam, The Netherlands: European Cancer Congress 2013; A randomized, multicenter, double-blind phase 3 trial comparing overall survival (OS) in patients (pts) with post-docetaxel castration resistant prostate cancer (CRPC) and bone metastases treated with ipilimumab (ipi) vs placebo (pbo), each following single-dose radiotherapy (RT) Abst 2850. [Google Scholar]
- 43.Harty JT, Badovinac VP. Shaping and reshaping CD8+T-cell memory. Nat Rev Immunol. 2008;8:107–19. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 44.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res. 2007;13:3776–82. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–75. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stein WD, Gulley JL, Schlom J, Madan RA, Dahut W, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–17. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DiPaola RS, Chen Y, Bubley GJ. Orlando, Florida, USA: Genitourinary Cancers Symposium, February 26–28; 2009. A phase II study of PROSTVAC-V (vaccinia)/TRICOM and PROSTVAC-F (fowlpox)/TRICOM with GM-CSF in patients with PSA progression after local therapy for prostate cancer: results of ECOG 9802 [poster] abstr 108. [Google Scholar]
- 48.Antonarakis ES, Zahurak ML, Lin J, Keizman D, Carducci MA, et al. Changes in PSA kinetics predict metastasis- free survival in men with PSA-recurrent prostate cancer treated with nonhormonal agents: combined analysis of 4 phase II trials. Cancer. 2012;118:1533–42. doi: 10.1002/cncr.26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Open Label Study of Sipuleucel-T. [Last accessed on 2013 Aug 29]. Available from: http://clinicaltrials.gov/show/NCT00901342 .
- 51.Madan RA, Mohebtash M, Schlom J, Gulley JL. Therapeutic vaccines in metastatic castration-resistant prostate cancer: principles in clinical trial design. Expert Opin Biol Ther. 2010;10:19–28. doi: 10.1517/14712590903321421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antonarakis ES, Drake CG. Combining immunological and androgen-directed approaches: an emerging concept in prostate cancer immunotherapy. Curr Opin Oncol. 2012;24:258–65. doi: 10.1097/CCO.0b013e32835205a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–63. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 55.Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang KY, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013;133:624–36. doi: 10.1002/ijc.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–44. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.A phase 2 randomized trial of docetaxel alone or in combination with therapeutic cancer vaccine, CEA-, MUC-1-TRICOM. 2012. [Last accessed on 2013 Aug 29]. Available from: http://abstracts.webges.com/viewing/view.php?congress=esmo2012 and congress_id=370 and publication_id=3526 .
- 58.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 59.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 60.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–62. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 61.Nesslinger NJ, Ng A, Tsang KY, Ferrara T, Schlom J, et al. A viral vaccine encoding prostate-specific antigen induces antigen spreading to a common set of self-proteins in prostate cancer patients. Clin Cancer Res. 2010;16:4046–56. doi: 10.1158/1078-0432.CCR-10-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heery CR, Madan RA, Bilusic M, Kim JW, Singh NK, et al. A phase II randomized clinical trial of samarium-153 EDTMP (Sm-153) with or without PSA-TRICOM vaccine in metastatic castration-resistant prostate cancer (mCRPC) after docetaxel. J Clin Oncol. 2013;31(S6) Abstr 102. [Google Scholar]
- 63.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–71. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 64.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Natl Acad Sci USA. 2001;98:14565–70. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, et al. Effects of castration on thymocyte development in two different models of thymic involution. J Immunol. 2005;175:2982–93. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- 66.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–49. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sequencing of Sipuleucel-T and ADT in Men With Non-metastatic Prostate Cancer. [Last accessed on 2013 Sep 12]. Available from: http://clinicaltrials.gov/show/NCT01431391 .
- 68.Antonarakis E, Kibel A, Adams G, et al. A randomized phase II study evaluating the optimal sequencing of sipuleucel-T and androgen deprivation therapy (ADT) in biochemically recurrent prostate cancer (BRPC): immune results. J Clin Oncol. 2013;31S Sabstr 5016. [Google Scholar]
- 69.Madan RA, Gulley JL, Schlom J, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–31. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bilusic M, Gulley J, Heery C, et al. A randomized phase II study of flutamide with or without PSA-TRICOM in nonmetastatic castration-resistant prostate cancer (CRPC) J Clin Oncol. 2011;29(Suppl 7) abstr 163. [Google Scholar]
- 71.Wada S, Yoshimura K, Hipkiss EL, Harris TJ, Yen HR, et al. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–18. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.A Neoadjuvant Study of Androgen Ablation Combined With Cyclophosphamide and GVAX Vaccine for Localized Prostate Cancer. [Last accessed on 2013 Oct 4]. Available from: http://clinicaltrials.gov/show/NCT01696877 .
- 73.Concurrent Versus Sequential Treatment With Sipuleucel-T and Abiraterone in Men With Metastatic Castrate Resistant Prostate Cancer (mCRPC) [Last accessed on 2013 Oct 4]. Available from: http://clinicaltrials.gov/show/NCT01487863 .
- 74.Koh YT, Gray A, Higgins SA, Hubby B, Kast WM. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009;69:571–84. doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–9. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fairchok MP, Trementozzi DP, Carter PS, Regnery HL, Carter ER. Effect of prednisone on response to influenza virus vaccine in asthmatic children. Arch Pediatr Adolesc Med. 1998;152:1191–5. doi: 10.1001/archpedi.152.12.1191. [DOI] [PubMed] [Google Scholar]
- 77.Small EJ, Lance RS, Redfern CH, Millard FE, Gardner TA, et al. A randomized phase II trial of sipuleucel-T with concurrent or sequential abiraterone acetate (AA) plus prednisone (P) in metastatic castrate-resistant prostate cancer (mCRPC) J Clin Oncol. 2013;31S doi: 10.1158/1078-0432.CCR-15-0079. Abstr 5047. [DOI] [PubMed] [Google Scholar]
- 78.Small EJ, Lance R, Gardner TA, Karsh LI, Stubbs A, et al. Amsterdam, The Netherlands: European Cancer Congress 2013; A Phase 2 trial of sipuleucel-T in combination with concurrent or sequential abiraterone acetate in patients with metastatic castrate-resistant prostate cancer [poster] Abstr 2860. [Google Scholar]
- 79.USFDA: Enzalutamide (XTANDI Capsules) [Last accessed on 2013 Sep 12]. Available from: http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm317997.htm .
- 80.Enzalutamide in Combination With PSA-TRICOM in Patients With Non-Metastatic Castration Sensitive Prostate Cancer. [4 October 2013] [Last accessed on]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01875250 .
- 81.Enzalutamide With or Without Vaccine Therapy for Advanced Prostate Cancer. [4 October 2013] [Last accessed on]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01867333 .
- 82.Boehm AL, Higgins J, Franzusoff A, Schlom J, Hodge JW. Concurrent vaccination with two distinct vaccine platforms targeting the same antigen generates phenotypically and functionally distinct T-cell populations. Cancer Immunol Immunother. 2010;59:397–408. doi: 10.1007/s00262-009-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–8. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hurwitz AA, Foster BA, Kwon ED, Powderly JD, Picus J, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–8. [PubMed] [Google Scholar]
- 85.Wada S, Jackson CM, Yoshimura K, Powderly JD, Picus J, et al. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J Transl Med. 2013;11:89. doi: 10.1186/1479-5876-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]


