Abstract
Excessive amounts of reactive oxygen species (ROS) cause a state of oxidative stress, which result in sperm membrane lipid peroxidation, DNA damage and apoptosis, leading to decreased sperm viability and motility. Elevated levels of ROS are a major cause of idiopathic male factor infertility, which is an increasingly common problem today. Lycopene, the most potent singlet oxygen quencher of all carotenoids, is a possible treatment option for male infertility because of its antioxidant properties. By reacting with and neutralizing free radicals, lycopene could reduce the incidence of oxidative stress and thus, lessen the damage that would otherwise be inflicted on spermatozoa. It is postulated that lycopene may have other beneficial effects via nonoxidative mechanisms in the testis, such as gap junction communication, modulation of gene expression, regulation of the cell cycle and immunoenhancement. Various lycopene supplementation studies conducted on both humans and animals have shown promising results in alleviating male infertility—lipid peroxidation and DNA damage were decreased, while sperm count and viability, and general immunity were increased. Improvement of these parameters indicates a reduction in oxidative stress, and thus the spermatozoa is less vulnerable to oxidative damage, which increases the chances of a normal sperm fertilizing the egg. Human trials have reported improvement in sperm parameters and pregnancy rates with supplementation of 4–8 mg of lycopene daily for 3–12 months. However, further detailed and extensive research is still required to determine the dosage and the usefulness of lycopene as a treatment for male infertility.
Keywords: antioxidants, lycopene, male infertility, oxidative stress, reactive oxygen species, sperm parameters
INTRODUCTION
There has been increasing evidence in recent years that oxidative stress plays a vital role in the pathogenesis of idiopathic male factor infertility. Elucidating the value of antioxidant supplementation as a treatment option for infertility has therefore become a goal for many researchers. Extensive research has been conducted to show that antioxidants like vitamins E and C and carnitines help in reducing oxidative stress by quenching free radicals.1 However, less is known about the effectiveness of carotenoids, especially that of lycopene—a potent antioxidant and singlet oxygen quencher.2 In this review, we will (i) explain how oxidative stress can cause infertility, (ii) synthesize pertinent information on lycopene, (iii) discuss the possibility of lycopene supplementation as a treatment option for idiopathic male factor infertility and (iv) give a detailed analysis of various human and animal studies involving lycopene that have been conducted both in vivo and in vitro.
OXIDATIVE STRESS AND ITS EFFECTS ON MALE REPRODUCTION
A free radical refers to a molecule that has at least one unpaired electron,3,4 which is responsible for the molecule's short-lived high energy state that causes instability and extreme reactivity.5 These free radicals will take part in propagative chain reactions and generate even more free radicals until two such radicals react and the unpaired electrons are neutralized.4 In the process, membrane lipids, amino acids and carbohydrates in nucleic acids will be attacked by the free radicals and undergo oxidation.4 Examples of highly reactive oxygen radicals include superoxide anions, hydroxyl radicals and hypochlorite radicals; and these are collectively known as reactive oxygen species (ROS).3 ROS are byproducts of oxygen metabolism and physiological amounts play important roles in sperm function, such as in capacitation, acrosome reaction, hyperactivation and sperm-oocyte fusion.5 Under normal conditions, there are natural antioxidants—both enzymatic and nonenzymatic—present in the seminal plasma to ensure that ROS concentrations remain low. Enzymatic antioxidants include glutathione reductase, superoxide dismutase and catalase; while nonenzymatic oxidants include vitamins C, E and B, carotenoids and carnitines. When ROS levels are greatly increased or antioxidant levels substantially decreased such that the delicate balance between ROS and antioxidants is disturbed, oxidative stress occurs.3,5
Several studies have shown a link between oxidative stress and idiopathic male factor infertility.6,7 This is largely due to the fact that infertile patients have been found to produce a greater amount of abnormal spermatozoa, which generate more ROS and less antioxidants, therefore leading to oxidative stress.4,8,9 Oxidative stress affects spermatozoa in three main ways—membrane lipid peroxidation, DNA damage and induction of apoptosis.10,11,12 However, the extent of damage caused depends on the nature of the ROS involved and the environment of the sperm.11
Cell membranes of spermatozoa are rich in polyunsaturated fatty acids, especially docosahexaenoic acid, which makes them more susceptible to oxidative damage by free radicals.11,13 Polyunsaturated fatty acids consist of numerous unconjugated double bonds containing many electrons. These electrons are donated to ROS upon reaction and lead to the generation of lipid peroxides.5 As a result, the fluidity of the spermatozoal cell membrane is disrupted, thus negatively affecting sperm motility and viability.11,13 Sperm motility will be affected because of the decrease in axonemal protein phosphorylation,10 while viability will decrease due to modification of important membrane proteins and abnormal acrosome reaction that compromises the ability of the sperm to fuse with the oocyte.3 Since this is a self-propagating cycle, the process also results in amplification, further exacerbating all the associated problems.5
Another effect of ROS on spermatozoa is that of DNA damage.10,11 This occurs via direct attack on the bases (especially guanine) or the phosphodiester backbones, hence destabilizing the DNA molecule and causing anomalies including, but not limited to, point mutations, polymorphisms, deletions, translocations and even double-stranded breaks.5,11 DNA fragmentation will result in abnormal fertilization, reduced implantation and poor embryonic development such that the offspring is likely to have a shorter lifespan and an increased risk of developing cancer.5,13 In cases of more severe damage, spermatozoa may undergo apoptosis, resulting in low sperm counts characteristic of idiopathic male factor infertility.10 However, sperm DNA is less prone to ROS-induced damage than the plasma membrane due to its highly condensed structure, which offers less surface area for attack by ROS.13
LYCOPENE
Properties
Lycopene is one of the many compounds that make up the carotenoid family. Carotenoids are naturally found in fruits and vegetables, and give plants their bright yellow, orange and red colors.14,15 They are essential for photosynthesis and provide protection from excessive light. Hence, they are only synthesized by plants and microorganisms, but not humans.2,16 Consumption of fruits and vegetables is the only way humans take in carotenoids. Carotenoids are vital components of human diet not only because they are sources of vitamin A, but also because they have antioxidant properties.15
Lycopene (C40H56) is a red-pigmented unsaturated linear carotenoid with a molecular weight of 536.85 Da, containing 11 conjugated and two non-conjugated double bonds.15,17 It is lipophilic and hence more soluble in organic solvents.15,18 The presence of double bonds allows for both cis- and trans-isomeric forms,2 and conversion between the forms occurs when it is exposed to light, heat or chemical reactions.15,16 Although most red-colored fruits and vegetables are lycopene sources, not all red-colored foods contain lycopene.15 Some common dietary sources of lycopene include tomatoes and processed tomato products, pink grapefruits, watermelons, apricots, guavas, papayas and rosehips, with processed tomato products containing the highest amount of lycopene.19,20 The main carotenoid found in humans is lycopene, which has a half-life of approximately 2–3 days when consumed.15,19 It may also interact with other dietary components to result in enhanced effects.14 However, unlike the other carotenoids, lycopene does not have a beta-ionic ring at either end and therefore lacks vitamin A activity.2,16,17 Despite the non-toxicity and proven beneficial effects of lycopene, it is yet to be considered an essential dietary component. As such, there is no official recommended amount for the daily intake of lycopene.2,17
Pharmacokinetics
Absorption
Humans absorb about 10%–30% of lycopene present in their diet,2,17 while the rest is excreted.15 Like other lipophilic compounds, lycopene is absorbed in the small intestine, and together with other lipids and bile acids, contribute to the formation of micelles.14,15 These micelles are passively transported into the mucosa cells of the gastrointestinal tract and subsequently incorporated into chylomicrons destined for the liver via the lymphatic system.17,19
Many factors influence the absorption of lycopene, and these include age, gender, hormonal status, smoking, alcohol and other components present in the diet.15,17 For instance, the bioavailability of lycopene decreases as healthy individuals age, probably due to age-related changes in the gastrointestinal tract which lowers its absorption.17,21 Smoking and alcohol consumption are also known to decrease lycopene concentration in the body.15
Previous reports have shown that lycopene from processed and heated tomato products are better absorbed than lycopene from raw tomatoes.2 Several factors contribute to this improved absorption, namely (i) heating and processing results in the disintegration of the food matrix, hence making lycopene more bioavailable;15,20,22 (ii) the conversion of all-trans lycopene to the cis-isomers during processing increases absorption of lycopene into the body by up to 2.5 times2,17,18 and (iii) due to its lipophilic nature, absorption of lycopene is improved when it is consumed with other lipids in the diet18,20 or cooked in an oil medium.16,19
Distribution
After being absorbed, lycopene is transported by low density lipoproteins and very low density lipoproteins15,18,19 and distributed via the circulatory system, resulting in its accumulation in various tissues.2,14 Lycopene preferentially accumulates in the testes, adrenal glands, liver and prostate, with concentrations in the testes as high as 10 times that of other tissues.15,17 Although the exact biochemical mechanisms have yet to be elucidated, this higher concentration of lycopene could possibly be either due to the presence of a large number of lipoprotein receptors, the relatively higher uptake of lipoproteins or the higher metabolic/oxidation rates in these tissues.23,24,25 The uneven distribution of lycopene is therefore suggestive of its exclusive biological role in certain tissues.15,17,19
Metabolism
Information concerning the in vivo metabolism of lycopene is lacking.19 Lycopene can be cleaved enzymatically or oxidatively to yield apolycopenals,15,22 which could be responsible for some biological activity as well.14
Mechanism of action
Several mechanisms of action have been proposed as an explanation of how lycopene works to reduce the risk of oxidative stress-mediated/chronic diseases such as cancer, hypertension, cardiovascular disease (CVD), neurodegenerative disease and osteoporosis.15,17,19 These mechanisms of action can be categorized into oxidative and nonoxidative mechanisms,15 of which the former is more pertinent and more likely to be the mechanism by which lycopene works to alleviate male infertility. An overview of lycopene's mechanism of action is shown in Figure 1.
Figure 1.
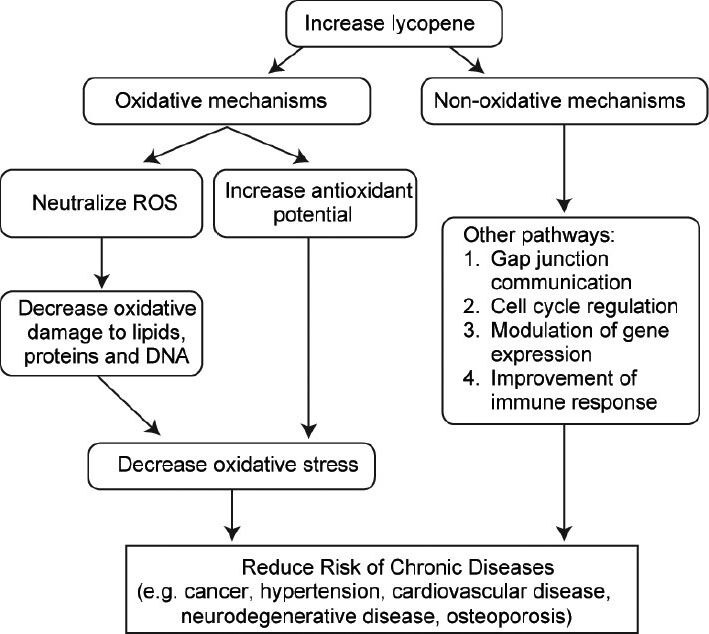
General mechanisms of action of lycopene. The proposed mechanisms of action of lycopene (oxidative and nonoxidative) that decreases the risk of oxidative stress-mediated diseases. Lycopene most likely acts via the oxidative mechanism of action to prevent oxidative stress and its detrimental effects on male infertility. ROS: reactive oxygen species.
Oxidative mechanisms
Due to its 11 conjugated double bonds, lycopene contains many electrons which can be donated to free radicals, resulting in their neutralization.16,17,26 In this way, lycopene acts as an antioxidant to trap free radicals and halt the propagative chain reactions,20 reducing the ROS burden and alleviating oxidative stress, thus preventing oxidative damage to lipids, proteins and DNA.15,26
Lycopene is regarded as one of the most potent singlet oxygen quenchers in the carotenoid family17,19,27 because it is twice as effective as β-carotene and up to 10 times more effective than α-tocopherol.15,16,26 However, it has been reported that a mixture of carotenoids gives a more marked effect than any individual compound, and this synergism was most clearly seen when lycopene or lutein was included in the mixture.27 Besides quenching singlet molecular oxygen, lycopene is also known to act on other free radicals like hydrogen peroxide, nitrogen dioxide and hydroxyl radicals.19,28 Furthermore, since lycopene is lipophilic, it tends to accumulate in cell membranes and lipoproteins, thus exerting a more noticeable effect within such components of a cell.19
In addition to directly neutralizing ROS by acting as a singlet oxygen quencher, hence causing the overall amount of ROS to decrease, lycopene also indirectly decreases oxidative stress by activating other mechanisms that increase antioxidant potential.
Nonoxidative mechanisms
Other nonoxidative mechanisms by which lycopene could exert its effects include the following: aiding in gap junction communication, modulating gene expression, regulating the cell cycle and enhancing the immune system.15,17,26
It is projected that tumor cells lack gap junction communication and therefore, continue to proliferate without inhibition. By improving communication between cells, lycopene could possibly prevent tumor formation and hence cancer, especially in the prostate, breast and lung.2,15,27 Lycopene also prevents unwanted cell proliferation by disrupting insulin-like growth factor-1 signaling and preventing cell cycle progression.17,22,27
Lycopene has been shown to have hypocholesterolemic properties because it inhibits hydroxyl-methly-glutaryl coenzyme A reductase, an important rate-limiting enzyme responsible for cholesterol production.19,27 A decrease in cholesterol also contributes to the alleviation of CVD because less atherosclerotic plaque will be present.27
The aforementioned nonoxidative mechanisms of action could possibly apply to male infertility too, but as far as the authors are aware, no studies have been conducted in this area. Thus, more research is still required to ascertain the exact mechanism of action by which lycopene exerts its effects in reducing the risk of chronic diseases22 and that of male infertility.
MALE INFERTILITY AND LYCOPENE AS A POSSIBLE TREATMENT STRATEGY
According to the World Health Organization, infertility is defined as ‘the inability of a sexually active couple (at least three times per month), not using contraception, to achieve pregnancy within one year’.12 About 10%–15% of couples worldwide are affected by this problem, and approximately half of the cases are due to the male factor.5,29 The most common cause of male factor infertility is varicocele (approximately 35%),5 while 25% of the patients are idiopathically infertile.29 Other causes include urogenital infections, congenital and genetic anomalies, immunologic factors and endocrine disorders.5,12
Although the precise mechanism of action by which lycopene exerts its effect is hitherto unknown,13,30 several studies have shown some evidence that lycopene can help to alleviate male infertility. A study that was performed to investigate the effects of lycopene on sperm parameters showed that the testes contained relatively high lycopene concentrations compared to other parts of the body. This suggests that lycopene is likely to play a major physiological role as an antioxidant in the process of spermatogenesis.13 A separate study conducted to qualify and quantify the antioxidants present in human seminal plasma further revealed that the concentration of lycopene was significantly lower in infertile men.31 With less antioxidants in the seminal plasma, there will be more free radicals available to cause oxidative damage, therefore resulting in abnormal spermatozoa that cause infertility. Goyal et al.30 proved that lycopene concentration in seminal plasma increases with oral supplementation of lycopene. It can therefore be postulated that the intake of lycopene will offer protection from ROS in seminal plasma and decrease oxidative stress, one of the main causes of idiopathic male factor infertility. Hence, there is a strong indication that lycopene, a natural antioxidant, may contribute to the treatment of male infertility.13,31
A few mechanisms have been proposed, but the main one whereby lycopene is thought to aid in the treatment of infertility is via the antioxidation pathway. Antioxidants are usually reducing agents that donate an electron to free radicals in order to quench ROS.12,32 In this way, lycopene reduces the amount of ROS and decreases lipid peroxidation, thus retaining the integrity of the spermatozoal cell membrane.28,33 Moreover, since lycopene is lipophilic and frequently found in cell membranes,19 it is likely to be present in sufficient amounts to protect spermatozoa from damage by oxidative stress.34 Other mechanisms that have been suggested include that of indirectly increasing the amount of antioxidant enzymes in the body by activation of the antioxidant system,28,35 and decreasing the transcription of proinflammatory factors.36 Figure 2 shows a summary of the proposed mechanism of action of lycopene in restoring male fertility.
Figure 2.
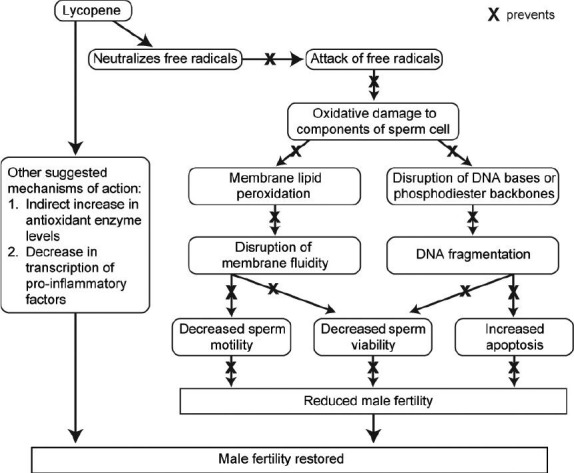
Proposed mechanisms of action of lycopene in treating idiopathic male infertility. Flow chart depicting the pathway by which increased concentrations of lycopene in the seminal plasma could reduce the risk of oxidative stress-induced idiopathic male factor infertility.
Several studies have been performed on humans and animals, both in vivo and in vitro, in an attempt to elucidate the true value of lycopene supplementation as a possible treatment strategy for idiopathic male factor infertility. In this section, we will evaluate these 12 studies, half of which were conducted on human subjects and the other half on animals, by analyzing the outcomes that are related to oxidative stress (Table 1) and sperm parameters (Table 2).
Table 1.
Summary of studies measuring the effect of lycopene supplementation on oxidative stress-related parameters
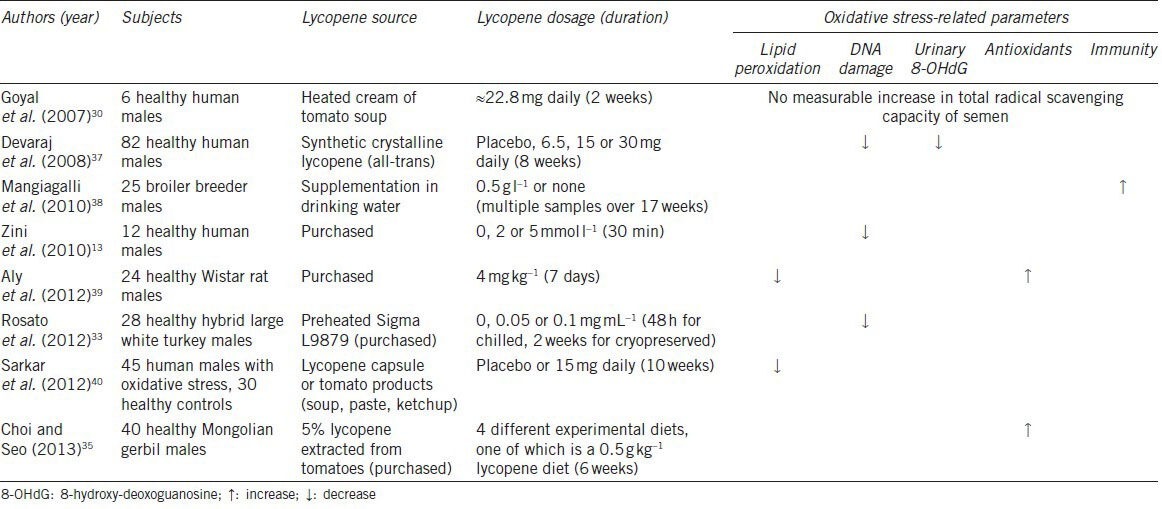
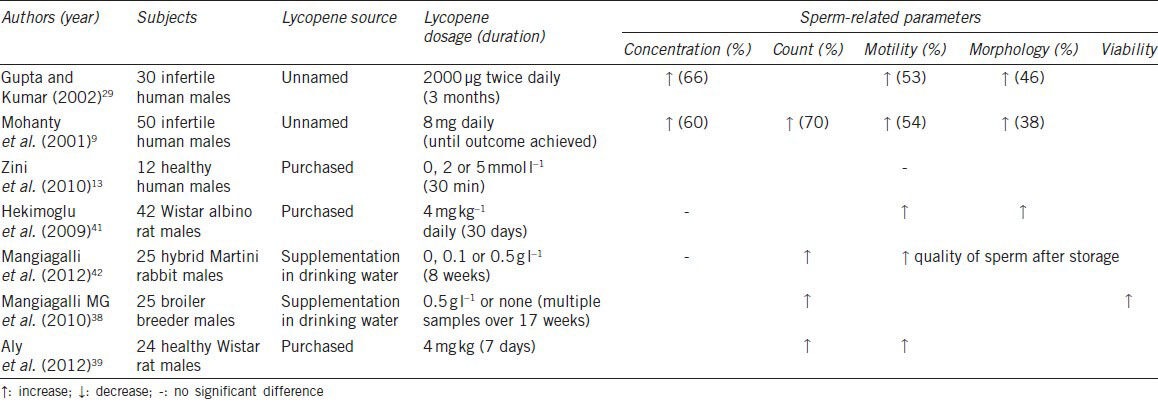
Table 2.
Summary of studies measuring the effects of lycopene supplementation on sperm-related parameters
Oxidative stress-related parameters
In order to measure the efficacy of lycopene, studies have measured biomarkers of oxidative stress such as the amount of lipid peroxidation, the DNA fragmentation index which is representative of the extent of DNA damage, and the amount of 8-hydroxy-deoxoguanosine in the urine.
Since malondialdehyde is produced as an end product of lipid peroxidation, by using the thiobarbituric acid reactive substances test, malondialdehyde concentrations can be an indicator of the damage caused to lipid membranes.39 Two studies have reported a decrease in lipid peroxidation after lycopene was given to the subjects. Sarkar et al.40 conducted a study where 45 patients and 30 healthy controls were given lycopene from various sources (tomato, synthetic or placebo) as part of their diet for 10 weeks, after 2 weeks of lycopene-restricted diet. Their blood samples were analyzed to quantify the amounts of biomarkers of oxidative stress before and after supplementation. Regardless of the source of lycopene, all patients showed a decrease in lipid peroxidation and oxidative stress. Aly et al.39 also showed that pretreatment with lycopene protected mitochondrial membranes from lipid peroxidation in rats. Hence, both these studies show that lycopene is effective in reducing lipid peroxidation, therefore reducing the damage caused by oxidative stress.
Another parameter measured was the extent of damage that occurred to spermatozoal DNA, which is expressed as percent DNA fragmentation index and measured with different methods such as the comet assay and sperm chromatin structure assay.13,37 Three studies specifically measured this outcome. While Zini and coworkers found that pretreatment with 5 mmol L−1 lycopene was needed to lower DNA fragmentation index in an in vitro study,13 Devaraj's group performed an in vivo study which showed that a daily dose of 30 mg of lycopene was required for a 9% decrease in DNA damage.37 In another study, Rosato et al.33 showed that semen extenders containing lycopene reduced the damage sustained by DNA when turkey semen samples were refrigerated or cryopreserved. Taken together, these studies imply that lycopene is able to aid in the reduction of DNA damage in spermatozoa, therefore increasing the chances of successful fertilization of the oocyte and better embryo development.5,13
Urinary 8-hydroxy-deoxoguanosine, another biomarker of oxidative stress, was only tested for in the study conducted by Devaraj and his coworkers, who evaluated the effects of various doses of lycopene (0, 6.5, 15 or 30 mg daily) on oxidative stress. Urinary 8-hydroxy-deoxoguanosine was quantified with a competitive enzyme-linked immunosorbent assay and showed a 23% decrease after 8 weeks of a daily dose of 30 mg of lycopene;37 hence, indicating that DNA damage was significantly reduced with lycopene supplementation.
Other oxidative stress-related parameters that were discussed in the studies we evaluated are the level of antioxidants and overall immunity. Studies conducted by Choi and Seo35 and Aly et al.39 revealed an increase in antioxidants, such as catalase and glutathione peroxidase, after lycopene supplementation. By measuring bactericidal activity in serum, a study on broiler breeder males also proved that lycopene supplementation improved overall immunity.38 Raised immunity and a higher level of antioxidants will help to reduce oxidative stress and improve both the quality and quantity of spermatozoa, therefore improving fertility outcomes.
On the other hand, a study performed by Goyal et al.30 indicated that there was no noteworthy increase in the capacity of seminal plasma to scavenge free radicals. In this experiment, six healthy volunteers were instructed to have 400 g of heated cream of tomato soup daily for 2 weeks. Blood and semen samples were analyzed before and after the experiment. The 2,2’azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) assay was used to assess the total radical-trapping antioxidant potential of the semen samples, but no significant change was observed. This could be due to the fact that lycopene is hydrophobic and therefore trapped in the lipid membranes in seminal plasma. Since the experimental conditions were aqueous, lycopene might have been unable to act as it would in vivo. Moreover, the small size of the study could have affected the reliability of the results as well.
As explained earlier, oxidative stress has a negative effect on the male reproductive system by inducing lipid peroxidation and DNA damage, which may eventually lead to apoptosis. Hence, an improvement in the biomarkers of oxidative stress would show a decrease in oxidative stress-related problems, and is likely to aid in the treatment of infertility.
Sperm parameter-related parameters
In addition to biomarkers of oxidative stress, other studies also observed sperm parameters to make a more direct and specific evaluation of the effectiveness of lycopene on treating male factor infertility. Sperm parameters generally include sperm count and concentration, motility, viability and morphology.
Sperm count was found to increase in four different studies, with Mohanty's group reporting a significant 70% increase with the administration of 8 mg of lycopene daily.9,38,39,42 It can therefore be seen that sperm count will increase with lycopene supplementation.
Of the four studies that measured sperm concentration, only two of them, Gupta and Kumar29 and Mohanty et al.9 showed an improvement of 66% and 60%, respectively.9,29 However, Gupta and Kumar also noted that a baseline sperm concentration of less than 5 × 106 mL−1 did not show substantial improvement in concentration.29 The other two studies did not find any difference in sperm concentration before and after lycopene supplementation. Hence, there is no conclusive evidence for an improvement in sperm concentration of the ejaculation.
Sperm motility was analyzed by five studies, with a majority of them showing that lycopene had a beneficial effect on this parameter. Gupta and Kumar29 and Mohanty et al.9 conducted studies that showed a marked improvement in patients’ sperm motility of 53% and 54%, respectively, with the former administering 2 mg of lycopene twice a day for 3 months and the latter giving 8 mg of lycopene once daily. Two other studies that were conducted on rats also produced similar positive results.39,41 However, Zini et al.13 reported that pretreatment with 0, 2 or 5 μmol l−1 lycopene for 30 min at 25 °C did not preserve sperm motility after oxidative stress was induced. Although different from the other studies, these results are supported in theory, as mentioned earlier, DNA is less susceptible to damage by oxidative stress than membrane lipids due to its highly condensed and compacted structure. Hence, it would be comparatively more difficult to prevent membrane lipid peroxidation with antioxidants. As such, more extensive studies have to be performed in order to determine if lycopene supplementation will improve sperm motility.
Sperm viability was only assessed by a single study, which indicated that lycopene supplementation improved this parameter. In this study, broiler breeders were separated into two groups, and only one group was given lycopene supplementation of 0.5 g l−1 in their drinking water. At 42 weeks of age, semen samples were analyzed and it was shown that the group which received lycopene was almost 6% more viable than the control group that did not receive lycopene.38 This therefore indicates that lycopene does help in improving sperm viability.
The last sperm parameter analyzed is sperm morphology. Although all three studies that measured this outcome reported improved morphology after lycopene supplementation, the improvement was less than expected and not as significant as that of sperm concentration and motility.9,29 Hekimoglu et al.41 also showed that lycopene was successful in normalizing the amount of abnormal sperm produced in rat testes.
In general, the human trials analyzed in this review showed that 4–8 mg of daily lycopene supplementation for 3–12 months is sufficient to treat male infertility.9,29 This translates to the intake of approximately 150 g of raw tomatoes or 80 g of watermelon daily.15 However, more research and clinical trials have to be conducted on humans to determine the most accurate therapeutic dosage.
CONCLUSION AND FUTURE DIRECTIONS
As demonstrated by the analyses of the various studies above, the only parameters that are conclusively improved with lycopene supplementation are: a decrease in lipid peroxidation and DNA damage, an increase in antioxidants and therefore general immunity, and improved sperm count and viability. These improvements are vital in tackling the problem of oxidative stress, which affects sperm viability, motility and DNA, and therefore causes infertility. The conflicting results of different studies could be due to the lack of standardized protocols and outcome measurements, and further compounded by the relatively small study sizes which could have introduced some bias into the outcomes. Although the results are generally promising, it is evident that more detailed and extensive research has to be done on the efficacy of lycopene, a potent singlet oxygen quencher, in the treatment of idiopathic male factor infertility. Therefore, in order to prove this, a large-scale placebo-controlled clinical trial must be carried out for statistically significant results. Patients should be randomly assigned to receive different daily dosages of lycopene over a specific time period, and the outcomes measured should not only include sperm parameters, but pregnancy rates as well.
AUTHOR CONTRIBUTIONS
DD conceived of the study, participated in its design, carried out the literature search, participated in the analysis and interpretation of the information, drafted and revised the manuscript; AA provided critical review of the manuscript, participated in the study coordination and gave the final approval of the version to be published; CO participated in the study design, carried out the literature search, participated in the compilation, analysis and interpretation of the information, drafted and revised the manuscript; and PP drafted the manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by funds from the Center for Reproductive Medicine, Cleveland Clinic. DD's research fellowship was supported by the Fulbright Visiting Scholar Program and MARA University of Technology, Malaysia.
REFERENCES
- 1.Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8:616–27. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 2.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55:207–16. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–50. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 4.Tremellen K. Oxidative stress and male infertility-a clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: Part 1. Nat Rev Urol. 2012;9:678–90. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 6.Gomez E, Buckingham DW, Brindle J, Lanzafame F, Irvine DS, et al. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J Androl. 1996;17:276–87. [PubMed] [Google Scholar]
- 7.Said TM, Agarwal A, Sharma RK, Mascha E, Sikka SC, et al. Human sperm superoxide anion generation and correlation with semen quality in patients with male infertility. Fertil Steril. 2004;82:871–7. doi: 10.1016/j.fertnstert.2004.02.132. [DOI] [PubMed] [Google Scholar]
- 8.Aitken RJ, Irvine DS, Wu FC. Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am J Obstet Gynecol. 1991;164:542–51. doi: 10.1016/s0002-9378(11)80017-7. [DOI] [PubMed] [Google Scholar]
- 9.Mohanty N, Kumar S, Jha A, Arora R. Management of idiopathic oligoasthenospermia with lycopene. Indian J Urol. 2001;18:57–61. [Google Scholar]
- 10.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–43. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 11.Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011;26:1628–40. doi: 10.1093/humrep/der132. [DOI] [PubMed] [Google Scholar]
- 12.Hamada AJ, Montgomery B, Agarwal A. Male infertility: a critical review of pharmacologic management. Expert Opin Pharmacother. 2012;13:2511–31. doi: 10.1517/14656566.2012.740011. [DOI] [PubMed] [Google Scholar]
- 13.Zini A, San Gabriel M, Libman J. Lycopene supplementation in vitro can protect human sperm deoxyribonucleic acid from oxidative damage. Fertil Steril. 2010;94:1033–6. doi: 10.1016/j.fertnstert.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Stahl W, Sies H. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys. 1996;336:1–9. doi: 10.1006/abbi.1996.0525. [DOI] [PubMed] [Google Scholar]
- 15.Rao AV, Ray MR, Rao LG. Lycopene. Adv Food Nutr Res. 2006;51:99–164. doi: 10.1016/S1043-4526(06)51002-2. [DOI] [PubMed] [Google Scholar]
- 16.Atasoy N. Biochemistry of lycopene. J Anim Vet Adv. 2012;11:2605–10. [Google Scholar]
- 17.Chauhan K, Sharma S, Agarwal N, Chauhan B. Lycopene of tomato fame: its role in health and disease. IJPSR. 2011;10:99–115. [Google Scholar]
- 18.Kaur A, Dhari J, Sharma OP, Gupta GD, Kharb V. Lycopene. IJPT. 2011;3:1605–22. [Google Scholar]
- 19.Rao AV, Agarwal S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: A review. Nutr Res. 1999;19:305–23. [Google Scholar]
- 20.Bohm V, Frohlich K, Bitsch R. Rosehip-a “new” source of lycopene? Mol Aspects Med. 2003;24:385–9. doi: 10.1016/s0098-2997(03)00034-7. [DOI] [PubMed] [Google Scholar]
- 21.Cardinault N, Tyssandier V, Grolier P, Winklhofer-Roob BM, Ribalta J, et al. Comparison of the postprandial chylomicron carotenoid responses in young and older subjects. Eur J Nutr. 2003;42:315–23. doi: 10.1007/s00394-003-0426-2. [DOI] [PubMed] [Google Scholar]
- 22.Wang XD. Lycopene metabolism and its biological significance. Am J Clin Nutr. 2012;96:1214–22S. doi: 10.3945/ajcn.111.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan LA, Lau JM, Stein EA. Carotenoid composition, concentrations, and relationships in various human organs. Clin Physiol Biochem. 1990;8:1–10. [PubMed] [Google Scholar]
- 24.Schmitz HH, Poor CL, Wellman RB, Erdman JW., Jr Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue. J Nutr. 1991;121:1613–21. doi: 10.1093/jn/121.10.1613. [DOI] [PubMed] [Google Scholar]
- 25.Erdman JW., Jr How do nutritional and hormonal status modify the bioavailability, uptake, and distribution of different isomers of lycopene? J Nutr. 2005;135:2046–7S. doi: 10.1093/jn/135.8.2046S. [DOI] [PubMed] [Google Scholar]
- 26.Palozza P, Catalano A, Simone R, Cittadini A. Lycopene as a guardian of redox signalling. Acta Biochim Pol. 2012;59:21–5. [PubMed] [Google Scholar]
- 27.Heber D, Lu QY. Overview of mechanisms of action of lycopene. Exp Biol Med (Maywood) 2002;227:920–3. doi: 10.1177/153537020222701013. [DOI] [PubMed] [Google Scholar]
- 28.Krishnamoorthy G, Selvakumar K, Elumalai P, Venkataraman P, Arunakaran J. Protective role of lycopene on polychlorinated biphenyls (aroclor 1254)-induced adult rat sertoli cell dysfunction by increased oxidative stress and endocrine disruption. Biomed Prev Nutr. 2011;1:116–25. [Google Scholar]
- 29.Gupta NP, Kumar R. Lycopene therapy in idiopathic male infertility--a preliminary report. Int Urol Nephrol. 2002;34:369–72. doi: 10.1023/a:1024483520560. [DOI] [PubMed] [Google Scholar]
- 30.Goyal A, Chopra M, Lwaleed BA, Birch B, Cooper AJ. The effects of dietary lycopene supplementation on human seminal plasma. BJU Int. 2007;99:1456–60. doi: 10.1111/j.1464-410X.2007.06804.x. [DOI] [PubMed] [Google Scholar]
- 31.Palan P, Naz R. Changes in various antioxidant levels in human seminal plasma related to immunoinfertility. Arch Androl. 1996;36:139–43. doi: 10.3109/01485019608987090. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal A, Sekhon LH. The role of antioxidant therapy in the treatment of male infertility. Hum Fertil (Camb) 2010;13:217–25. doi: 10.3109/14647273.2010.532279. [DOI] [PubMed] [Google Scholar]
- 33.Rosato MP, Centoducati G, Santacroce MP, Iaffaldano N. Effects of lycopene on in vitro quality and lipid peroxidation in refrigerated and cryopreserved turkey spermatozoa. Br Poult Sci. 2012;53:545–52. doi: 10.1080/00071668.2012.716508. [DOI] [PubMed] [Google Scholar]
- 34.Erdman JW, Jr, Ford NA, Lindshield BL. Are the health attributes of lycopene related to its antioxidant function? Arch Biochem Biophys. 2009;483:229–35. doi: 10.1016/j.abb.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi SK, Seo JS. Lycopene supplementation suppresses oxidative stress induced by a high fat diet in gerbils. Nutr Res Pract. 2013;7:26–33. doi: 10.4162/nrp.2013.7.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oborna I, Malickova K, Fingerova H, Brezinova J, Horka P, et al. A randomized controlled trial of lycopene treatment on soluble receptor for advanced glycation end products in seminal and blood plasma of normospermic men. Am J Reprod Immunol. 2011;66:179–84. doi: 10.1111/j.1600-0897.2011.00984.x. [DOI] [PubMed] [Google Scholar]
- 37.Devaraj S, Mathur S, Basu A, Aung HH, Vasu VT, et al. A dose-response study on the effects of purified lycopene supplementation on biomarkers of oxidative stress. J Am Coll Nutr. 2008;27:267–73. doi: 10.1080/07315724.2008.10719699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangiagalli MG, Martino PA, Smajlovic T, Guidobono Cavalchini L, Marelli SP. Effect of lycopene on semen quality, fertility and native immunity of broiler breeder. Br Poult Sci. 2010;51:152–7. doi: 10.1080/00071660903401540. [DOI] [PubMed] [Google Scholar]
- 39.Aly HA, El-Beshbishy HA, Banjar ZM. Mitochondrial dysfunction induced impairment of spermatogenesis in LPS-treated rats: Modulatory role of lycopene. Eur J Pharmacol. 2012;677:31–8. doi: 10.1016/j.ejphar.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 40.Sarkar PD, Gupt T, Sahu A. Comparative analysis of lycopene in oxidative stress. J Assoc Physicians India. 2012;60:17–9. [PubMed] [Google Scholar]
- 41.Hekimoglu A, Kurcer Z, Aral F, Baba F, Sahna E, et al. Lycopene, an antioxidant carotenoid, attenuates testicular injury caused by ischemia/reperfusion in rats. Tohoku J Exp Med. 2009;218:141–7. doi: 10.1620/tjem.218.141. [DOI] [PubMed] [Google Scholar]
- 42.Mangiagalli MG, Cesari V, Cerolini S, Luzi F, Toschi L. Effect of lycopene supplementation on semen quality and reproductive performance in rabbit. World Rabbit Sci. 2012;20:141–8. [Google Scholar]


