Abstract
Several studies have reported that C-reactive protein (CRP), an inflammation biomarker, may be associated with the prognosis of prostate cancer (PCa). The objective of this systematic review is to summarize the predictive role of CRP for survival in PCa as reported in previous studies. Related studies were identified, and evaluated for quality through multiple search strategies. Data was collected from studies comparing overall and cancer-specific survival (CSS) in patients with elevated CRP levels and those having lower levels. However, for progression-free survival (PFS), data were collected according to the log of CRP. The hazard ratio (HR) and its 95% confidence interval (CI) were used to assess the strength of associations. A total of nine studies (n = 1,497) were evaluated in this meta-analysis (five for overall survival (OS), four for CSS and two for PFS). For OS and PFS, the pooled HR of CRP was statistically significant at 1.51 (95% CI, 1.28–1.79) and 1.50 (95% CI, 1.25–1.81), respectively. For CSS, the pooled HR was 1.91 (95% CI, 1.36–2.69) with higher CRP expression in PCa, which strongly indicates poorer survival in PCa. This study demonstrates that CRP may have a critical prognostic value in patients with prostatic cancer.
Keywords: C-reactive protein, meta-analysis, prognosis, prostate cancer
INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed cancer and second leading cause of death in men.1 While, many men present with localized and potentially curable disease, a large number of deaths from PCa is due to the development of metastatic disease. Therefore, more accurate prognosis and predictive markers should be applied for PCa to guide therapy and monitor disease progress in individual patients. It has been shown that pain, Gleason score, Eastern Cooperative Oncology Group Performance Status, presence of visceral metastases, hemoglobin, albumin and alkaline phosphatase are prognostic factors for overall survival (OS),2,3,4,5 and several other prognostic algorithms have also been proposed.3,4,6,7
Recently, the presence of a systemic inflammatory response, which is measured by an acute-phase reactant has been identified to be associated with a poor prognosis in various types of cancers such as lung cancer, gastric cancer, colorectal cancer, renal cell carcinoma and others.8,9 C-reactive protein (CRP) is a typical acute-phase protein that is mainly produced by the hepatocytes,10 and it can precipitate C-polysaccharide of Streptococcus pneumonia.11 The association of CRP with survival was stronger than all other predictors, such as serum prostate-specific antigen, Eastern Cooperative Oncology Group Performance Status and age.
While some of the previous studies have reported that in PCa patients a higher CRP level was significantly associated with worse outcome in PCa,12,13 some other studies did not show any significant link between CRP and survival in PCa patients.14 Therefore, it is essential to carry out a systematic meta-analysis to summarize the global results and address the inconsistencies in the literature. Here, we seek to conduct a systematic review and meta-analysis to evaluate the overall risk of elevated CRP and survival in PCa.
MATERIALS AND METHODS
This meta-analysis was carried out as per the guidelines of the MOOSE (Meta-analysis of Observational Studies in Epidemiology Group).15
Search strategy
A systematic literature search was performed in MEDLINE and EMBASE up to August 2013 to identify the relevant studies. An initial search strategy using recognized search terms (CRP or C-reactive protein, prognosis and prostate cancer or PCa) was conducted. Studies were considered eligible if they met the following criteria: (i) they measured pretreatment CRP values, (ii) they evaluated the potential association between pretreatment CRP and the survival outcome of PCa and (iii) prospective or retrospective study design. Articles were excluded based on the following criteria: (i) letters or review articles, (ii) laboratory studies and (iii) missing key information such as hazard ratio (HR) and 95% confidence interval (CI).
All searches were conducted independently by two reviewers. The identified studies were double-checked by both. Disagreements were resolved by consensus between the two readers or consultation with a third reviewer. Additionally, a manual search was performed using references from the relevant literature, including all of the identified studies, reviews and editorials. When duplicate studies were found, the study with reported HR or that involved more patients (usually the most recent), was used for the meta-analysis. This step was carried out to prevent overlapping between cohorts and overestimation of the overall HR.
Quality assessment
We systematically assessed the quality of all the studies included as per the crucial review checklist of the Dutch Cochrane Centre proposed by MOOSE.15 The key points of the current checklist include: (i) clear definition of study population and origin of country; (ii) clear definition of study design; (iii) clear definition of outcome assessment, OS, cancer-specific survival (CSS) and progression-free survival (PFS); (iv) clear definition of cutoff for CRP or clear definition of log of CRP and (v) sufficient period of follow-up. Those studies which do not mention all these 5 points were excluded to avoid compromising the quality of the meta-analysis. A flow diagram of the study selection process is shown in Figure 1.
Figure 1.
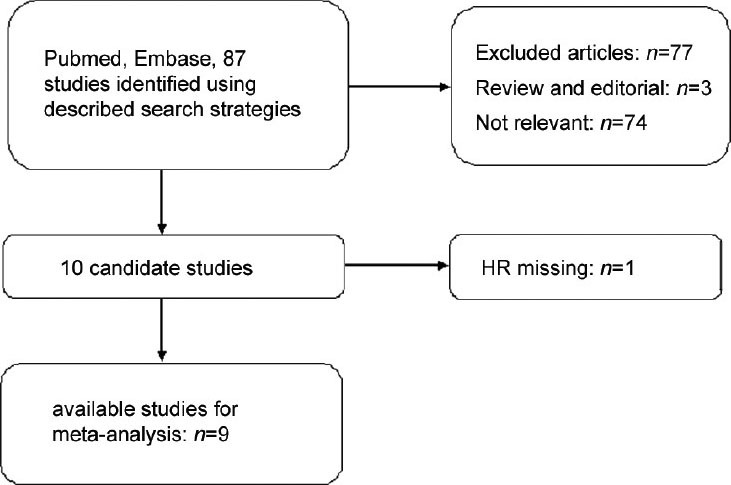
Flow diagram of the study selection process. HR: hazard ratio.
Data extraction and conversion
The following data were collected: (i) publication details, including first author's last name, year of publication, study population and country in which the study was conducted; (ii) study design; (iii) characteristics of the studied population, including sample size, age and stage of disease and (iv) HR of elevated CRP for OS, CSS and PFS and their 95% CIs. The simplest method consisted of the direct collection of HR and their 95% CIs from the original literature, with an HR of > 1 being associated with a poorer outcome. When these data were not directly reported, we extracted the total number of observed deaths and the number of patients in each group to calculate HR.16 Data were extracted from the graphical survival plots when data were available only as Kaplan-Meier curves, and then estimation of the HR was performed by the described method.16
Statistical analysis
The heterogeneity of the combined HRs was performed using Cochran's Q test and Higgins I-squared statistics. P < 0.05 was considered significant. We used the random effects model (Der Simonian and Laird method) if heterogeneity was observed (P < 0.05). The random effects model was also applied in the absence of between-study heterogeneity (P ≥ 0.05). Sensitivity analyses were performed to explore the reasons for heterogeneity among these studies. Publication bias was evaluated by the funnel plot with the Egger's bias indicator test.17 Statistical analyses were carried out using the statistical software Stata version 12.0 (Stata CorpLP, College Station, TX, USA).
RESULTS
Data retrieval
Eighty-three records for CRP were identified after a primary search of PubMed and EMBASE. After reading the titles and abstractions, 73 studies were excluded for being review articles, letters, laboratory studies, studies with important data missing and studies irrelevant to the current analysis. For example, some studies analyzed the predictive value of other inflammatory factors such as interleukin-6, but not CRP. Some studies analyzed the CRP levels at one time point without follow-up to observe the outcome. One study was excluded because HR was missing.14 The final meta-analysis for CRP was performed for the remaining nine studies (Figure 1).12,13,18,19,20,21,22,23,24 Notably, there were three publications that involved two studies.21,23,24
Study characteristics
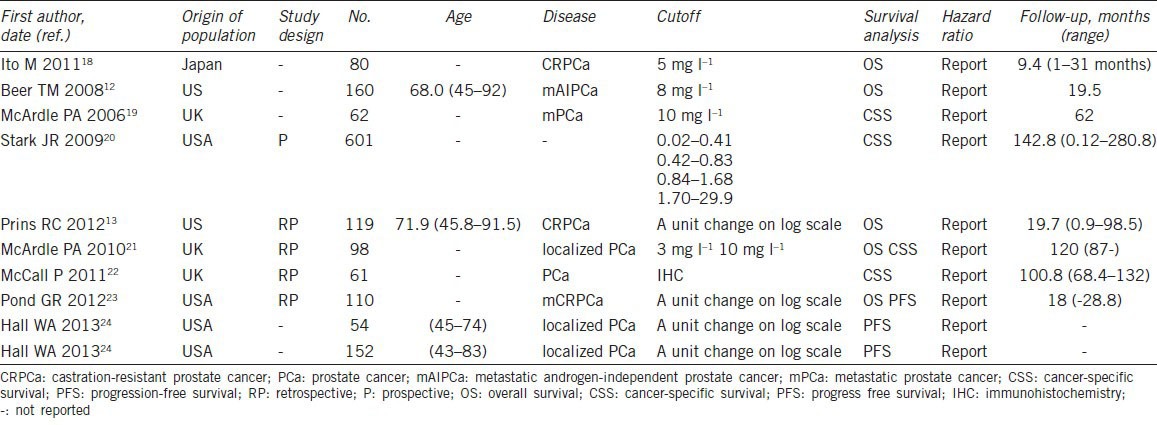
The characteristics of selected studies are summarized in Table 1. We collected the data from nine studies, which involved a total of 1497 patients from the United Kingdom, Japan, Canada and the United States. In these nine studies, CRP values were analyzed by different means in each study. Five studies dealt with CRP as a dichotomous variable, with different cutoff values. One study dealt with CRP as a trichotomous variable and compared the outcome of the highest tertile with the lowest tertile. In the remaining three studies, CRP was considered as a continuous variable and HR was calculated as a unit change on a log scale. All the nine selected studies presented HRs. The median follow-up period in all the studies ranged from 9.4 to 142.8 months.
Table 1.
Summery table of the meta-analysis
Overall survival
In the studies evaluating the OS, there was no evidence for significant heterogeneity between studies for categorized CRP (P = 0.317). The random model was applied to calculate the pooled HR and its 95% CI. The increased serum CRP level was significantly correlated to OS with a pooled HR estimate of 1.51 (95% CI, 1.28–1.79) (Figure 2). For log CRP, there was some evidence for heterogeneity (I2 = 73.5, P = 0.052), and the pooled HR and 95% CI were not significant at 1.21 (0.98–1.49) (Figure 2).
Figure 2.
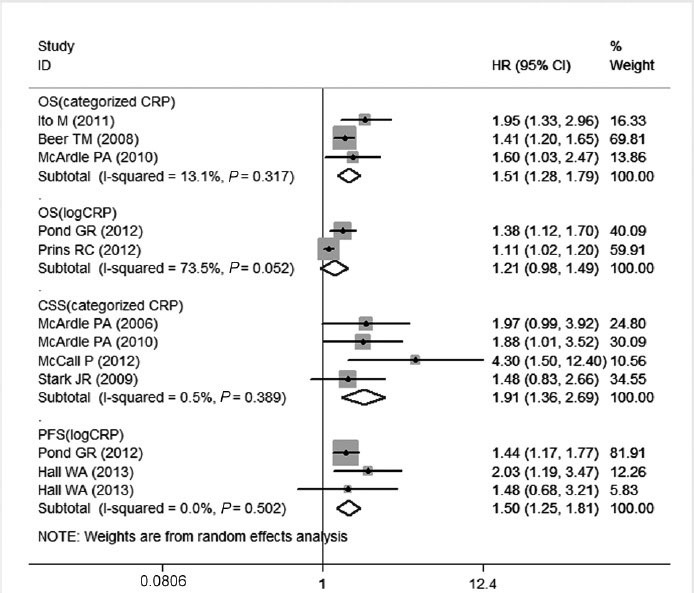
Forest plots of studies evaluating hazard ratios (HR) with 95% confidence interval (95% CI) for high C-reactive protein (CRP) levels as compared with low levels. Survival data are reported as overall survival (OS), cancer-specific survival (CSS) and progression-free survival (PFS).
Cancer-specific survival and progression-free survival
For CSS and PFS, a random effects model was applied. The P values of between-study heterogeneity were 0.389 and 0.502 for CSS and PFS analyses, respectively. As illustrated in Figure 2, the combined HR of 1.91 (95% CI, 1.36–2.69) showed that the high CRP level had significant relationship with CSS in PCa patients. As for the ability to evaluate PFS, the pooled HR was 1.50 (95% CI, 1.25–1.81) for each unit increase in CRP.
From the above data, CRP proved to be a prognostic biomarker for OS, CSS and PFS in PCa. However, as an inflammatory marker, CRP was not significantly associated with OS, CSS and PFS when a risk cutoff of 2.0 was used.27
Publication bias
Finally, we applied funnel plots and Egger's test to evaluate publication bias of the studies included in this meta-analysis. As shown in Figure 3, two of the funnel plots were symmetrical. There was no evidence for significant publication bias for OS (categorized) and PFS, since the P value for Egger's regression intercepts were > 0.05 (P = 0.379 and P = 0.568). However, in the analyses of CSS, significant publication bias existed, with a P value of 0.020.
Figure 3.
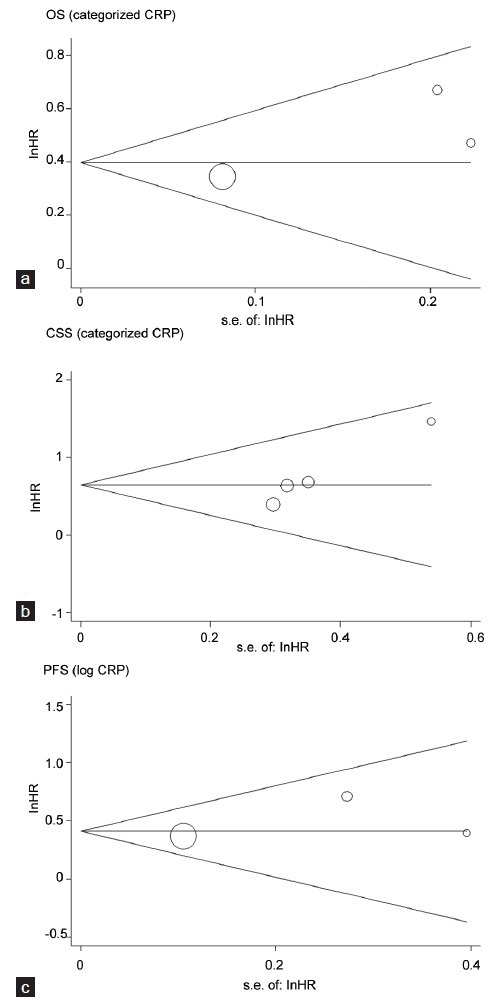
Funnel plots of studies included in the three meta-analyses: (a) overall survival (OS), (b) cancer-specific survival (CSS) and (c) progression-free survival (PFS). HR: hazard ratio; S.E.: standard error.
DISCUSSION
In recent decades, a variety of predictors have been identified and applied for predicting PCa outcomes. We currently use prostate-specific antigen, prostate-specific membrane antigen and prostate stem cell antigen in routine pathological assessment of PCa. Many clinical studies have indicated that epithelial growth factor receptor, pAKT, nuclear factor-kappa B, macrophage inhibitory cytokine-1, matrix metalloproteinase-1 and matrix metalloproteinase-9 are associated with survival in PCa patients.26,27 In addition, microRNAs have become novel members of the predictive family with the development of molecular biology. For example, miQ, a novel microRNA has been shown to be closely associated with survival in PCa patients,28 so as to microRNA 224.29 However, the above-mentioned biomarkers should be examined in cancerous tissues. Thus, it is difficult to monitor their levels continuously in the process of disease progression. In contrast, the inflammation indicators can be easily assayed in plasma or serum, which is widely applied in a clinic set-up. In order to improve their prognostic accuracy, it is suggested that biomarkers should be carefully selected and integrated to develop a prognostic system. Recently, the Glasgow Prognostic Score has been developed to evaluate the value of an inflammation-based score in patients with metastatic PCa. The Glasgow Prognostic Score, evaluating elevated CRP (>10 mg l−1) and hypoalbuminemia (<35 g l−1), appears to be a useful indicator of worse outcome, independent of treatment in patients with metastatic PCa.30
There are a number of possible mechanisms by which CRP is associated with worse outcome in patients with cancer. Firstly, chronic inflammation can promote carcinogenesis, which can contribute to the onset or progression of cancer and circulating concentrations of vascular endothelial growth factor are directly associated with CRP.31 Secondly, immune response can be invoked by rapid tumor growth, and thus many inflammatory factors are released. Inflammation can cause tumorigenesis by supplying the tumor microenvironment with bioactive molecules, including growth factors that induce proliferation, survival factors that reduce cell death, proangiogenic factors, extracellular matrix-modifying enzymes that stimulate angiogenesis, invasion and metastasis, inductive signals that facilitate epithelial-to-mesenchymal transition and other effects.32 Previous studies have reported that other inflammatory markers such as serum interleukin (IL-6), soluble tumor necrosis factor receptor II and Epac1 as other PCa prognostic factors.33,34 An elevated CRP identifies those patients with an impaired T-lymphocytic response, since poor infiltration of tumor appears to be associated with poor outcome35,36 and an elevated CRP concentration has recently been shown to be inversely associated with T-lymphocyte subset infiltration.37 Our meta-analysis has proven the prognostic value of CRP, which is a most commonly used and a representative inflammatory marker in PCa.
Our study showed that an elevated CRP level could predict poor survival in patients with PCa. Our data showed that CRP level was associated with OS, CSS and PFS. The pooled risks of CRP for OS, CSS and PFS, although, were statistically significant (P < 0.05), were not strong when an HR of more than two was considered as the cutoff for a strong predictor.25
However, the above conclusion should be tempered for several reasons. First, in the OS group, there was some heterogeneity of subjects for log CRP. Heterogeneity might be contributed by the baseline characteristics of the patients, such as age, differentiation or disease stage, adjuvant treatment they might have received, the duration of follow-up and adjustments for other cofactors. It is possible that the results of this meta-analysis could have been influenced by the heterogeneity. The treatment methods of the nine studies included in our study are different, and thus it can affect the positive associations between CRP and PCa prognosis. For example, in one study, those patients were treated with docetaxel and corticosteroid,18 three studies used docetaxel-based chemotherapy,12,13,23 three studies did not state the exact treatment method,20,22,23 one study used androgen-deprived therapy19 and in one study, the patients were divided into two groups in which one group of patients received radiation therapy after undergoing radical prostatectomy, while the other group patients were managed with definitive radiation therapy alone.24 Taking into account that such differences might have a confounding effect with this study, we chose to apply a random model to minimize the effect. Since there were only two such studies, we did not assess the source of heterogeneity by sensitivity analyses. The CRP is usually regarded as a prognostic marker in several diseases which are related to survival, such as cardiovascular diseases. Thus, we cannot consider CRP as a ‘predictor’ for survival unless the involved patients do not have other severe diseases related to CRP. Because the presence or absence of concomitant severe diseases was not mentioned in the selected studies, we should be careful while considering CRP as a predictor of survival in cancer patients.
Meanwhile, there are other limitations in this study. The technique of detecting CRP may lack comparability among the studies. One study in the meta-analysis used immunohistochemistry staining to study expressions of CRP. Although immunohistochemistry staining is simple and cost-effective to perform, results are highly dependent on a variety of methodological factors. Finally, only eligible studies were included in the meta-analysis, which could explain the obvious publication bias for CSS. Thus, we need further studies with larger sample sizes to confirm the positive associations.
Our meta-analysis also had some advantages. First, the quality of studies included in the meta-analysis was satisfactory and strictly met the inclusion criteria. Second, all the extrapolated HRs were directly obtained from published statistics, and this method is more reliable than the calculated values from the data included in the article or extrapolated from the survival curves. Third, there were no significant heterogeneity for OS (categorized CRP), CSS and PFS. Fourth, we adopted Begg's funnel plot and Egger's test to assess the publication bias and the results failed to show any obvious evidence of publication bias either for OS or PFS.
CONCLUSIONS
In conclusion, our meta-analysis, including a quantified synthesis of all published studies, indicated that elevated CRP expression is significantly associated with worse PCa survival, and CRP is a strong predictor for all three survival outcomes, especially for CSS. The critical role of CRP in cancer prognosis may contribute to its clinical utility. Considering the limitations of the present meta-analysis, further research with standardized unbiased methods and larger, worldwide sample sizes are expected to confirm our results.
AUTHOR CONTRIBUTIONS
ZQL, LC and QX conceived and designed the study. JMF and XZ collected the data. HXZ and YJC performed the statistical analyses. ZQL and LC drafted and revised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study is supported by the National Natural Science Foundation of China (No.: 30872591) and Shanghai Science and Technology Commission (No.: 11411950602).
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Halabi S, Vogelzang NJ, Kornblith AB, Ou SS, Kantoff PW, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol. 2008;26:2544–9. doi: 10.1200/JCO.2007.15.0367. [DOI] [PubMed] [Google Scholar]
- 3.Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 4.Smaletz O, Scher HI, Small EJ, Verbel DA, McMillan A, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–82. doi: 10.1200/JCO.2002.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong AJ, Garrett-Mayer ES, Yang YC, de Wit R, Tannock IF, et al. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007;13:6396–403. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong AJ, Tannock IF, de Wit R, George DJ, Eisenberger M, et al. The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. Eur J Cancer. 2010;46:517–25. doi: 10.1016/j.ejca.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong AJ, Garrett-Mayer E, de Wit R, Tannock I, Eisenberger M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16:203–11. doi: 10.1158/1078-0432.CCR-09-2514. [DOI] [PubMed] [Google Scholar]
- 8.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–63. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Fu X, Zhu X, He X, Zou C, et al. Prognostic role of systemic inflammatory response in renal cell carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2011;137:887–96. doi: 10.1007/s00432-010-0951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurlimann J, Thorbecke G, J, Hochwald GM. The liver as the site of C-reactive protein formation. J Exp Med. 1966;123:365–78. doi: 10.1084/jem.123.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tillett WS, Francis T., Jr Serological reactions in pneumonia with a non-protein fraction of pneumococcus. J Exp Med. 1930;52:561–71. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beer TM, Lalani AS, Lee S, Mori M, Eilers KM, et al. C-reactive protein as a prognostic marker for men with androgen-independent prostate cancer: results from the ASCENT trial. Cancer. 2008;112:2377–83. doi: 10.1002/cncr.23461. [DOI] [PubMed] [Google Scholar]
- 13.Prins RC, Rademacher BL, Mongoue-Tchokote S, Alumkal JJ, Graff JN, et al. C-reactive protein as an adverse prognostic marker for men with castration- resistant prostate cancer (CRPCa): confirmatory results. Urol Oncol. 2012;30:33–7. doi: 10.1016/j.urolonc.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsberger B, Lankston L, McMillan DC, Underwood MA, Edwards J. Presence of tumoural C-reactive protein correlates with progressive prostate cancer. Prostate Cancer Prostatic Dis. 2011;14:122–8. doi: 10.1038/pcan.2011.5. [DOI] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito M, Saito K, Yasuda Y, Sukegawa G, Kubo Y, et al. Prognostic impact of C-reactive protein for determining overall survival of patients with castration-resistant prostate cancer treated with docetaxel. Urology. 2011;78:1131–5. doi: 10.1016/j.urology.2011.07.1416. [DOI] [PubMed] [Google Scholar]
- 19.McArdle PA, Mir K, Almushatat AS, Wallace AM, Underwood MA, et al. Systemic inflammatory response, prostate-specific antigen and survival in patients with metastatic prostate cancer. Urol Int. 2006;77:127–9. doi: 10.1159/000093905. [DOI] [PubMed] [Google Scholar]
- 20.Stark JR, Li H, Kraft P, Kurth T, Giovannucci EL, et al. Circulating prediagnostic interleukin-6 and C-reactive protein and prostate cancer incidence and mortality. Int J Cancer. 2009;124:2683–9. doi: 10.1002/ijc.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McArdle PA, Qayyum T, McMillan DC. Systemic inflammatory response and survival in patients with localised prostate cancer: 10-year follow-up. Urol Int. 2010;85:482. doi: 10.1159/000320242. [DOI] [PubMed] [Google Scholar]
- 22.McCall P, Catlow J, McArdle PA, McMillan DC, Edwards J. Tumoral C-reactive protein and nuclear factor kappa-B expression are associated with clinical outcome in patients with prostate cancer. Cancer Biomark. 2011–2012;10:91–9. doi: 10.3233/CBM-2012-0236. [DOI] [PubMed] [Google Scholar]
- 23.Pond GR, Armstrong AJ, Wood BA, Leopold L, Galsky MD, et al. Ability of C-reactive protein to complement multiple prognostic classifiers in men with metastatic castration resistant prostate cancer receiving docetaxel-based chemotherapy. BJU Int. 2012;110:E461–8. doi: 10.1111/j.1464-410X.2012.11148.x. [DOI] [PubMed] [Google Scholar]
- 24.Hall WA, Nickleach DC, Master VA, Prabhu RS, Rossi PJ, et al. The association between C-reactive protein (CRP) level and biochemical failure-free survival in patients after radiation therapy for nonmetastatic adenocarcinoma of the prostate. Cancer. 2013;119:3272–9. doi: 10.1002/cncr.28185. [DOI] [PubMed] [Google Scholar]
- 25.Hayes DF, Isaacs C, Stearns V. Prognostic factors in breast cancer: current and new predictors of metastasis. J Mammary Gland Biol Neoplasia. 2001;6:375–92. doi: 10.1023/a:1014778713034. [DOI] [PubMed] [Google Scholar]
- 26.Mimeault M, Johansson SL, Batra SK. Pathobiological implications of the expression of EGFR, pAkt, NF-kB and MIC-1 in prostate cancer stem cells and their progenies. PLoS ONE. 2012;7:e31919. doi: 10.1371/journal.pone.0031919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozden F, Saygin C, Uzunaslan D, Onal B, Durak H, et al. Expression of MMP-1, MMP-9 and TIMP-2 in prostate carcinoma and their influence on prognosis and survival. J Cancer Res Clin Oncol. 2013;139:1373–82. doi: 10.1007/s00432-013-1453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larne O, Martens-Uzunova E, Hagman Z, Edsjö A, Lippolis G, et al. miQ-a novel microRNA based diagnostic and prognostic tool for prostate cancer. Int J Cancer. 2013;132:2867–75. doi: 10.1002/ijc.27973. [DOI] [PubMed] [Google Scholar]
- 29.Mavridis K, Stravodimos K, Scorilas A. Downregulation and prognostic performance of microRNA 224 expression in prostate cancer. Clin Chem. 2013;59:261–9. doi: 10.1373/clinchem.2012.191502. [DOI] [PubMed] [Google Scholar]
- 30.Shafique K, Proctor MJ, McMillan DC, Qureshi K, Leung H, et al. Systemic inflammation and survival of patients with prostate cancer: evidence from the Glasgow Inflammation Outcome Study. Prostate Cancer Prostatic Dis. 2012;15:195–201. doi: 10.1038/pcan.2011.60. [DOI] [PubMed] [Google Scholar]
- 31.Xavier P, Belo L, Beires J, Rebelo I, Martinez-de-Oliveira J, et al. Serum levels of VEGF and TNF-alpha and their association with C-reactive protein in patients with endometriosis. Arch Gynecol Obstet. 2006;273:227–31. doi: 10.1007/s00404-005-0080-4. [DOI] [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Misra UK, Pizzo SV. Evidence for a pro-proliferative feedback loop in prostate cancer: the role of Epac1 and COX-2-dependent pathways. PLoS One. 2013;8:e63150. doi: 10.1371/journal.pone.0063150. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Hoyt MA, Stanton AL, Bower JE, Thomas KS, Litwin MS, et al. Inflammatory biomarkers and emotional approach coping in men with prostate cancer. Brain Behav Immun. 2013;32:173–9. doi: 10.1016/j.bbi.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet. 1987;1:1303–6. doi: 10.1016/s0140-6736(87)90552-6. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner N, et al. The RANX05 Study Group Independent prognostic value of eosinophil and mastcell infiltration in colorectal cancer tissue. J Pathol. 1999;189:487–95. doi: 10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 37.Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92:651–4. doi: 10.1038/sj.bjc.6602419. [DOI] [PMC free article] [PubMed] [Google Scholar]


