Abstract
This study examined the catecholaminergic and serotonergic innervation of the foregut of Aplysia californica, a model system in which the control of feeding behaviors can be investigated at the cellular level. Similar numbers (15-25) of serotonin-like-immunoreactive (5HTli) and tyrosine hydroxylase-like-immunoreactive (THli) fibers were present in each (bilateral) esophageal nerve (En), the major source of pregastric neural innervation in this system. The majority of En 5HTli and THli fibers originated from the anterior branch (En2), which innervates the pharynx and the anterior esophagus. Fewer fibers were present in the posterior branch (En1), which innervates the majority of the esophagus and the crop. Backfills of the two En branches toward the central nervous system (CNS) labeled a single, centrifugally projecting serotonergic fiber, originating from the metacerebral cell (MCC). The MCC fiber projected only to En2. No central THli neurons were found to project to the En. Surveys of the pharynx and esophagus revealed major differences between their patterns of catecholaminergic (CA) and serotonergic innervation. Whereas THli fibers and cell bodies were distributed throughout the foregut, 5HTli fibers were present in restricted plexi, and no 5HTli somata were detected. Double-labeling experiments in the periphery revealed THli neurons projecting toward the buccal ganglion via En2. Other afferents received dense perisomatic serotonergic innervation. Finally, qualitative and quantitative differences were observed between the buccal motor programs (BMPs) produced by stimulation of the two En branches. These observations increase our understanding of aminergic contributions to the pregastric regulation of Aplysia feeding behaviors.
Indexing terms: dopamine, serotonin, pharynx, esophagus, buccal ganglion
The feeding behaviors of most animals are strongly influenced by signals that originate from the alimentary tract (Pavlov, 1910). The diversity of such signals is reflected by the varied effects they exert on consummatory behaviors, ranging from enhancement of ingestion to development of satiation (Novin et al., 1976; Smith, 1998). Because of the structural and functional complexity of feeding control in vertebrates, the contributions of specific afferent pathways and neurotransmitter systems are difficult to assess (Norgren, 1978; Grill, 2006). They may, however, be effectively studied in certain invertebrate systems, where the consequences of preabsorbtive signals can be evaluated on simpler neural networks and behaviors (Gelperin, 1967; Beenhakker et al., 2004; Melcher and Pankratz, 2005).
One system in which pregastric regulation of feeding can be examined in the context of well-defined neural circuits is the marine mollusc Aplysia californica (Kupfermann, 1974a,b). Intensive study of molluscan feeding behavior and its neural control has contributed to our general understanding of motor system organization (for reviews see Murphy, 2001; Elliott and Susswein, 2002; Cropper et al., 2004). As in other repetitive actions, the consummatory behaviors of Aplysia are largely controlled by a central pattern generator (CPG) network. Fundamental design features that this network appears to share with CPGs found in more complex nervous systems include 1) the capacity to accomplish multiple behaviors via reconfiguration of its constituent modules (i.e., multifunctionality; see Getting, 1989; Morton and Chiel, 1994; Kupfermann and Weiss, 2001), 2) the ability to be activated and influenced by signals originating from a variety of sources (Rosen et al., 1991; Nargeot et al., 1997; Horn and Kupfermann, 2002); and 3) the capacity to modify its cycle-by-cycle activity in response to sensory stimuli (Evans and Cropper, 1998; Borovikov et al., 2000; Shetreat-Klein and Cropper, 2004).
The central feeding network of Aplysia is strongly influenced by afferent signals originating from pharyngeal and esophageal sources (Susswein and Kupfermann, 1975a,b; Kuslansky et al., 1978, 1987). The neural pathways that convey these signals access the central nervous system via the esophageal nerve, which has direct access to feeding CPG elements present in the buccal ganglion (see Fig. 1; see Chiel et al., 1986; Nargeot et al., 1999; Jing et al., 2007; Proekt et al., 2007). In the periphery, the esophageal nerve bifurcates into two major branches (Schwarz and Susswein, 1986), 1) a long posterior branch (designated En1 by Nargeot et al., 1997) that can be traced down the length of the crop and 2) a shorter anterior branch (En2 of Nargeot et al., 1997) that preferentially innervates the pharynx (see also Scott et al., 1991; Xin et al., 1995). Lesion experiments indicate that esophageal afferents exert diverse actions on feeding behavior, including signaling satiation (Susswein and Kupfermann, 1975a,b; Kuslansky et al,, 1978, 1987), learning about food palatability (Schwarz and Susswein, 1986), and reinforcement of both operant and classically conditioned consummatory behaviors (Nargeot et al., 1997,1999; Lechner et al., 2000). Esophageal nerve stimulation is commonly used to activate coordinated buccal motor programs (Chiel et al., 1986; Morgan et al., 2002; Proekt et al., 2004) and is also employed as an unconditioned stimulus (US) in in vitro and in vivo conditioning paradigms (Brembs et al., 2002; Mozzachiodi et al., 2003; Baxter and Byrne, 2006). Although some observations suggest that signals originating from the two esophageal nerve branches may affect the feeding CPG differentially (see Discussion), few comparisons of their anatomical and physiological properties have been reported.
Figure 1.
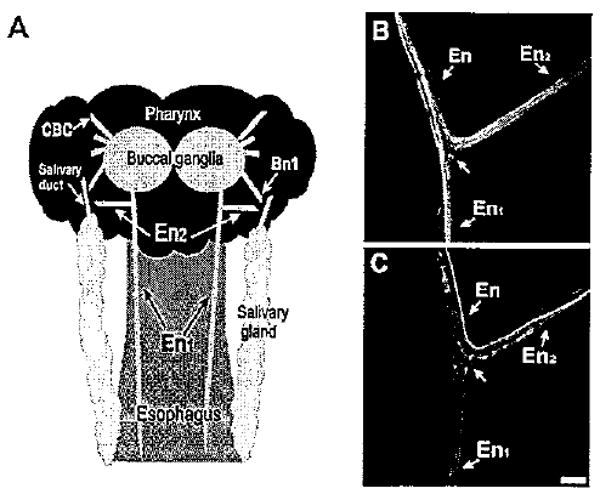
Aminergic fibers in the esophageal nerve. A: Schematic diagram (not drawn to scale) of the structures examined in this study. The posterior region of the buccal mass, to which the paired buccal ganglia adhere, corresponds to the pharynx. One esophageal nerve emerges from each buccal hemiganglion. Near its origin, the esophageal nerve bifurcates into a posterior branch (En1), which projects along the length of the esophagus, and a shorter anterior branch (En2), which projects laterally toward the pharynx and salivary gland. In the region of the salivary duct, the En2 projection converges with buccal nerve 1 (Bn1). CBC, cerebral buccal connective. B: THli in the esophageal nerve. At the En bifurcation, THli fibers could be traced to both the En1 and En2 branches. C: Serotonin-like immunoreactivity in the En. Several 5HTli fibers were observed in each En branch. Scale bar = 100 μm.
The biogenic amines dopamine (DA) and serotonin (5HT) are known to regulate the neural circuits that generate feeding-related behaviors of mollusks (Wieland and Gelperin, 1983; Trimble and Barker, 1984; Kyriakides and McCrohan, 1989; Quinlan et al., 1997; Kabotyanski et al., 2000). In Aplysia, DA and serotonin are present in specific interneurons that exert broad and coordinated actions on the feeding circuitry (Weiss et al., 1978; Rosen et al., 1991; Teyke et al., 1993; Alexeeva et al., 1998; Kabotyanski et al., 1998). Potential peripheral sources of these neurotransmitters have also been described, but their participation in the control of feeding is not well understood (Goldstein et al., 1984; Susswein et al., 1993; Xin et al., 1995; Croll, 2001). This investigation explored the aminergic innervation of the foregut with an aim toward increasing our understanding of the neurotransmitter systems that contribute to preabsorbtive regulation of feeding in Aplysia.
Materials and Methods
Specimens and solutions
Experiments were conducted on specimens of Aplysia californica (150–250 g) that were purchased from the Aplysia Resource Facility and Experimental Hatchery (University of Miami, Coral Gables, FL) or from Marinus Inc. (Pasadena CA). Animals were maintained in refrigerated aquaria (14–16°C) and fed dried seaweed twice per week. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Puerto Rico Medical Sciences Campus.
Electrophysiological experiments were performed in normal artificial seawater (ASW) consisting of 460 mM NaCl, 10 mM KCl, 55 mM MgCl2,11 mM CaCl2, and 10 mM HEPES. Fixation was achieved with a 4% paraformaldehyde solution containing 27% sucrose. Antibody incubations and washes were conducted in 80 mM phosphate buffer (PB: 24 mM KH2PO4, 56 mM Na2HPO4, pH 7.4) containing 2% Triton X-100 and 0.1% sodium azide (solution referred to below as PTA).
Nerve backfills
Ganglia were positioned with minutien pins near a small Vaseline well on the surface of a Sylgard-lined Petri dish. The esophageal nerve was cut, and one of its branches was drawn into the well. The ASW inside the well was withdrawn and replaced with a saturated aqueous solution (1.6 mg/30 μl) of biocytin (Sigma Chemical, St. Louis, MO). The preparation was covered and incubated overnight at 14°C. The well was then removed, and the ganglia were washed three to five times, repinned, and fixed in paraformaldehyde. The fixed ganglia were transferred to microcentrifuge tubes, washed five times (30 minutes each) with PTA solution, and incubated overnight (room temperature, with shaking) in Alexa avidin 488 (Molecular Probes, Eugene, OR) diluted 1:2,000 in PTA (24–48 hours, room temperature). The ganglia were then washed five times with PTA, and the quality of the backfill was assessed prior to further immunohistochemical processing. In the double-labeling experiments, immunolabeling was visualized with Alexa 546-conjugated second antibodies. Nerve fills toward the periphery were conducted by using the same methods.
Immunohistochemistry
Standard whole-mount immunohistochemical protocols were followed (for details of buffer composition, incubation, and wash procedures see Díaz-Ríos et al., 1999). Tissues were dissected, secured to a Sylgard-lined Petri dish with minutien pins, and fixed in chilled 4% paraformaldehyde. Tissues being processed for TH-like immunoreactivity were fixed for 1 hour, whereas those being tested for serotonin-like immunoreactivity were fixed for 4 hours. When antibodies against both amines were examined on the same tissue, an intermediate fixation time (3 hours) was employed. After preincubation with normal goat serum (0.8%), tissues were immersed (48 hours, room temperature) in the primary antibody.
Catecholaminergic neurons were detected with a mouse monoclonal antibody (Diasorin, Stillwater MN; product No. 22941) generated against rat tyrosine hydroxylase [lot LNC1 purified from rat pheochromocytoma (PC12) cells]. Primary antibody concentrations ranged from 1:50 to 1:200 (see Díaz-Ríos et al., 2002). This antibody is reported to possess wide species cross-reactivity, which is proposed to reflect its recognition of a highly conserved epitope in the midportion of the TH molecule (Diasorin specification sheet 22914). It specifically labels neurons that are stained with several independent catecholaminergic (CA) markers, including the glyoxylic acid (Rathouz and Kirk, 1988; Kabotyanski et al., 1998) and the formaldehyde (Fa)-glutaraldehyde (Glu) histofluorescent techniques (Goldstein et al., 1989; Croll, 2001; Díaz-Ríos et al., 2002). These CA mapping studies were conducted on specimens spanning a range of developmental stages and exhibit some variance with respect to details concerning small or weakly staining cells. They collectively agree, however, on the presence of five strongly staining CA neurons (the paired B20 cells, the paired B65 cells, and one unpaired interneuron) in the Aplysia buccal ganglion, as observed in this study. Additional biochemical and pharmacological studies indicate that dopamine is the major catecholamine neurotransmitter in Aplysia and that DA mediates synaptic signaling by the neurons labeled with this antibody (Carpenter et al., 1971; McCaman et al., 1973, 1979; Teyke et al., 1993; Due et al., 2004; Díaz-Ríos and Miller, 2005). The synaptic actions of one THli neuron (B20) were shown to be blocked by the dopaminergic antagonists ergonovine (Teyke et al., 1993) and sulpiride (Díaz-Ríos et al., 2005), and excitatory postsynaptic potentials (EPSPs) produced by another THli buccal neuron (B65) were blocked by sulpiride (Due et al., 2004). Finally, DA was shown to mimic and occlude the postsynaptic actions of both B20 and B65 when applied directly to identified follower neurons (Due et al., 2004; Díaz-Ríos et al., 2005). Collectively, these observations support the utility of this antibody for labeling neurons with a catecholaminergic phenotype in this system.
For serotonin, a rabbit polyclonal antiserum (Sigma-Aldrich, St. Louis MO; product No. S5545) was used at a dilution of 1:200. The immunogen serotonin creatinine complex was conjugated with formaldehyde to bovine serum abumin (BSA). Labeling was eliminated after preincubation of the antibody (1:200) with serotonin creatinine complex (1 mM; overnight at 4°C). It was not reduced following preabsorbtion with BSA (0.25%; overnight at 4°C). The results obtained with this antibody were in agreement with numerous independent serotonin mapping studies in Aplysia (Ono and McCaman, 1984; Goldstein et al., 1984; Longley and Longley, 1986) and other mollusks (Croll, 1987; Sudlow et al., 1998; Newcomb et al., 2006).
After repeated PTA washes (five times for at least 30 minutes each, room temperature), ganglia were incubated in second antibodies conjugated to fluorescent markers [Alexa 488 goat anti-mouse IgG (H + L) conjugate or Alexa 546 goat anti-rabbit IgG (H + L); Molecular Probes]. The second antibody dilutions ranged from 1:500 to 1:3,000. Preparations were viewed on a Nikon Eclipse or on a Zeiss Pascal laser scaning confocal microscope (LSCM). Images were captured with the Nikon ACT-1 (version 2.10) software of the Eclipse or the Zeiss LSM 5 Image Browser (version 3.1.0.11) program of the Pascal. They were transported as BMP files to Adobe Photoshop for adjustment of overall contrast and brightness and then imported to Corel Draw 8 for addition of labels and organization of panels.
Electrophysiology
Neurons were identified in preparations consisting of the paired buccal and cerebral ganglia. Intracellular microelectrodes filled with 2 M KCl (10–20 MΩ) were used for DC recording of membrane potentials (NeuroProbe 1600; AM Systems). Extracellular signals were recorded with polyethylene suction electrodes and AC-coupled amplifiers (model 1700; AM Systems). The typical configuration consisted of two cut-end recordings from buccal nerve 1 (Bn1; see Fig. 1A). Experiments were conducted at room temperature (19–21°C) in normal ASW (see composition above).
Nerve stimulation was achieved with 3-msec 2-Hz pulses generated from an isolated pulse stimulator. Buccal motor program classification was based on the method of Morgan et al. (2002; see also Serrano et al., 2007). Intracellular recordings from the radula closer motor neuron B8a/b were used to determine the phase of radula closure. Ingestive-like programs were defined as those in which the ratio of the average firing frequency of B8 during protraction to its average firing frequency during retraction was less than 0.65. Egestive-like programs were defined as those in which the ratio of the average firing frequency during protraction to its average firing frequency during retraction was greater than 2.0. Programs that did not fulfill either criterion were classified as intermediate.
Data analysis
All results reported in this study were observed in a minimum of three specimens. Measurements are reported as the mean ± SD or SEM, as noted. Parametric (Student's t-test, two tailed) or nonparametric statistical tests were performed by comparing measurements obtained prior to drug application with those attained at the peak of the response. Multiple group tests were performed with ANOVA followed by Tukey-Kramer pairwise comparisons. A value of P < 0.05 was established as the criterion for significance.
Results
The esophageal nerve of Aplysia californica bifurcates into two major branches near its emergence from the buccal ganglion (Schwarz and Susswein, 1986; shown schematically in Fig. 1A). A posterior branch (En1; Nargeot et al., 1997) projects in the longitudinal direction, innervating the entire length of the crop to the anterior gizzard. A shorter anterior branch (Schwarz and Susswein, 1986; Scott et al., 1991; En2 of Nargeot et al., 1997) projects laterally toward pharyngeal and salivary structures. Whole-mount immunohistochemical methods revealed similar total numbers of TH-like-immunoreactive (-li) and 5HTli fibers in the esophageal nerve branches (En1 + En2; THli: 18.5 ± 2.1; 5HTli: 22.5 ± 1.6; Fig. 1B1,2, Table 1). Both amines exhibited an unbalanced distribution of fibers in the two En branches (Table 1). In both cases, the number of fibers in En2 (THli: 14.3 ± 1.3; 5HTli: 16.8 ± 1.8) exceeded the number in En1 (THli 4.3 ± 0.9; 5HTli: 5.8 ± 1.0; Table 1).
Figure 2.
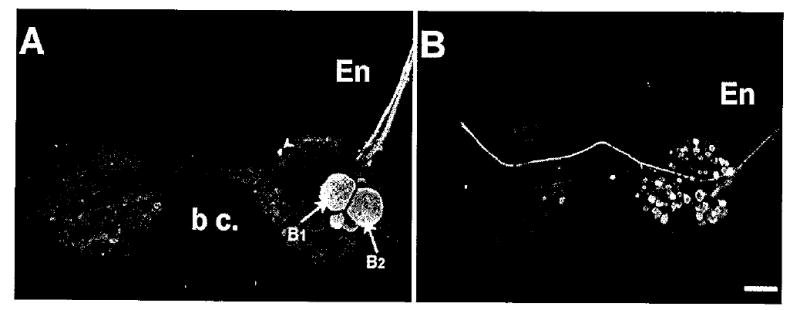
Backfills of the two esophageal nerve branches labeled distinct and nonoverlapping populations of buccal neurons. A: Backfill of En1 labeled the B1, B2 cluster of motor neurons on the caudal surface of the ipsilateral hemiganglion. A single small neuron in the S-cell cluster (arrowhead) was also usually filled. B: Backfills of En2 did not label neurons in the B1, B2 cluster. Many moderately sized neurons spanning the central regions of both hemiganglia were labeled. Scale bar = 200 μm.
Table 1.
Quantification of THli and 5HTli Fibers in En1 and En2, the Two Major Branches of the Esophageal Nerve
| N | Total (En1 + En2) | En1 | En2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (S.E.) | Max # fibers | Min # fibers | Mean (± S.E.) | Max # fibers | Min # fibers | Mean (± S.E.) | Max # fibers | Min # fibers | ||
| TH | 4 | 18.5 ± 2.1 | 18 | 2 | 4.3 ± 0.9 | 6 | 2 | 14.3 ± 1.3 | 18 | 12 |
| 5-HT | 4 | 34.0 ± 1.6 | 21 | 3 | 5.8 ± 1.0 | 8 | 3 | 16.8 ± 1.8 | 21 | 13 |
The differential distributions of aminergic fibers in the two En branches prompted us to explore further the composition of these nerves. Previous investigations showed that central neurons project to the esophageal nerve (Lloyd et al., 1988; Scott et al., 1991), so backfill methods were used to compare systematically the projections from the buccal ganglion to the two En branches (Fig. 2). Projections to En1 were chiefly limited to a single cluster of large neurons (6.0 ± 1.5 cell bodies; n = 4) on the caudal surface of the ipsilateral buccal hemiganglion (Fig. 2A, Table 2). Neurons in this cluster, which includes the largest cell bodies in the ganglion (B1 and B2; Fig. 2A), have been characterized previously as peptidergic motor neurons that promote gut motility (Lloyd et al., 1988). Backfills of En2 labeled a more numerous (42.3 ± 9.5 total cell bodies, n = 4) and dispersed population of buccal neurons (Fig. 2B, Table 2). The cells projecting to En2 were distributed across the rostral and caudal surfaces of both hemiganglia. In contrast to En1, the neurons labeled with En2 backfills could not be readily identified as cells that were described in previous investigations.
Table 2.
Distribution of Buccal Ganglion Cell Bodies Labeled with Backfills of Esophageal Nerve Branches En1 and En2
| n | IPSILATERAL HEMIGANGLION | CONTRALATERAL HEMIGANGLION | TOTAL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean (± S.E.) | max # cells | min # cells | mean (± S.E.) | max # cells | min # cells | mean (± S.E.) | max # cells | min # cells | ||
| En1 | 4 | 6.0 ± 1.5 | 10 | 3 | 1.0 ± 0.4 | 2 | 0 | 7.0 ± 1.2 | 10 | 4 |
| En2 | 4 | 34.0 ± 7.1 | 52 | 20 | 7.5 ± 3.1 | 15 | 1 | 42.3 ± 9.5 | 62 | 24 |
Several central neurons within the buccal ganglion of Aplysia contain markers for catecholamines (Rathouz and Kirk, 1988; Goldstein and Schwartz, 1989; Teyke et al., 1993; Kabotyanski et al., 1998). Anatomical descriptions of CA buccal neurons that have been reported to date have not included projections to the En (Teyke et al., 1993; Kabotyanski et al., 1998; Díaz-Ríos et al., 2002). However, as the branching patterns of buccal CA neurons remain incompletely documented, biocytin backfills of the two En branches were conducted in conjunction with TH immunohistochemistry to determine whether centrifugal projections could contribute to the catecholaminergic fibers of the esophageal nerve (Fig. 3). Several CA neurons, including B65 (Kabotyanski et al., 1998), B20 (Teyke et al., 1993), and a pair of unidentified lateral neurons, were located near neurons with En1 projections, but none of them was double labeled (Fig. 3A1,2). Likewise, backfills of the En2 branch did not label THli buccal neurons in the ipsilateral (Fig. 3B1) or contralateral (Fig. 3B2) buccal hemiganglia. Together, these observations indicate that efferent axons from central neurons do not contribute to the catecholaminergic fiber systems of the En branches.
Figure 3.
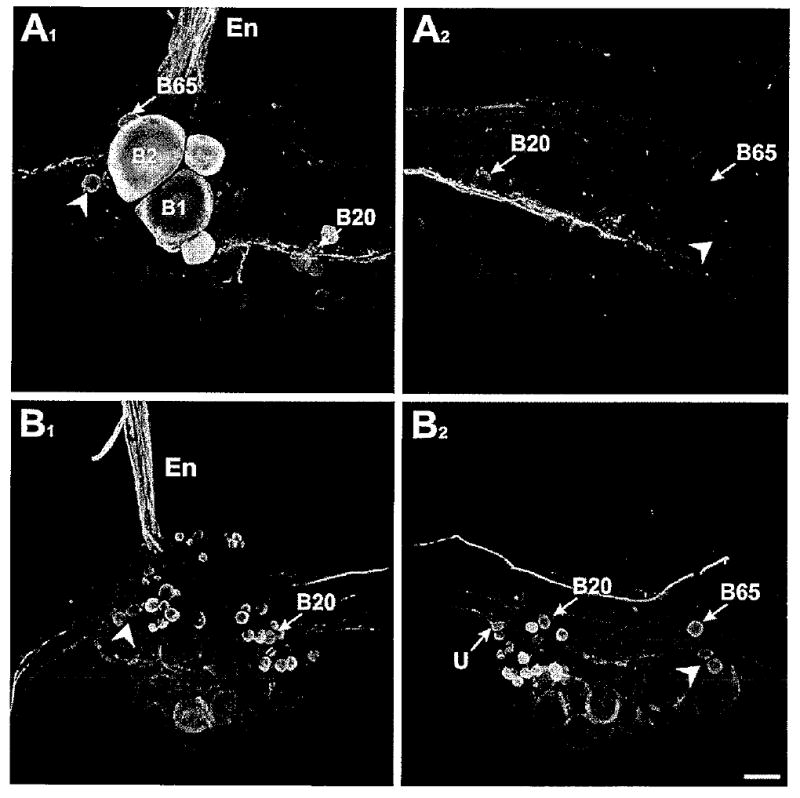
Double-labeling experiments demonstrating that THli neurons in the buccal ganglion do not project fibers to the esophageal nerve. A1: Biocytin backfill (green) of the En1 branch of the esophageal nerve (En) overlayed with THli (red) in the ipsilateral buccal hemiganglion. THli was present in a small number of buccal neurons, including B20, B65, and a pair of unidentified cells (arrowhead) on the caudal surface near the B1, B2 cluster. None of the THli neurons was double labeled. A2: Contralateral hemiganglion from the same preparation as in A1. None of the THli neurons, including B20, B65, or the unidentified lateral neurons (arrowhead), was labeled by the contralateral En1 backfill. B1: Biocytin backfill (green) of the En2 branch of the esophageal nerve (En) overlayed with THli (red) in the ipsilateral buccal hemiganglion. None of the THli neurons, including B20 and the pair of unidentified lateral cells (arrowhead), was double labeled. B2: Contralateral hemiganglion from the same preparation as in B1. None of the THli neurons, including an unpaired medial neuron (U; see Díaz-Ríos et al., 2002), B20, B65, or the unidentified lateral neurons (arrowhead), was labeled by the contralateral En2 backfill. Magenta/green image provided as Supporting Information Figure 1. Scale bar = 100 μm.
Analogous experiments were conducted to determine the contribution of efferent axons to the serotonergic fibers in the En branches (Fig. 4). Previous investigations showed that the buccal ganglion is devoid of serotonergic cell bodies but that it receives dense serotonergic innervation (Longley and Longley, 1986). Much of this innervation originates from the metacerebral cell (MCC), a large serotonergic neuron that projects from the cerebral ganglion (Gerschenfeld and Paupardin-Tritsch, 1974; Weiss et al., 1978; Alexeeva et al., 1998). In addition to its central ramifications, electrophysiological recording showed that MCC axon branches enter many buccal nerves, including the ipsilateral En (Weiss and Kupfermann, 1976). Our anatomical experiments confirmed this projection (Fig. 4). One markedly large-caliber serotonergic fiber was observed in the En (Fig. 4A1, arrow). At the En bifurcation, this fiber turned sharply to enter the En2 branch. Within the buccal ganglion, it could be traced back to the major site of MCC axon branching, near the junction of the cerebral-buccal connective (CBC) with the ganglion (Fig. 4A2, arrow). When backfills of En2 were combined with serotonin immunohistochemisty, a single, large double-labeled fiber was observed in the CBC (Fig. 4C1,2). No additional CBC fibers were double labeled. Moreover, no CBC serotonergic CBC fibers were labeled when the En1 branch was backfilled (not shown). Together, these findings indicate that the sole efferent serotonergic En fiber originates from the MCC and that this fiber projects only to En2.
Figure 4.
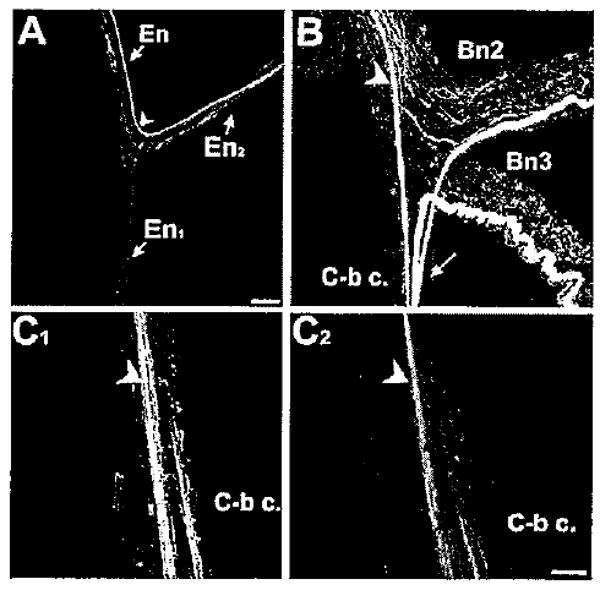
The serotonergic metacerebral cell projects to En2. A: One markedly large-caliber 5HTli fiber was observed in the En. At the En bifurcation (arrowhead), this fiber turned sharply to enter the En2 branch. B: Within the buccal ganglion, the 5HTli fiber could be traced back to the major site of MCC axon branching (arrow), near the junction of the cerebral-buccal connective (C-b c) with the ganglion. C1: Backfill of En2 labeled several fibers in the C-b c, including one large caliber axon (arrowhead). C2: When 5HTli was examined in the same preparation, the large fiber in the C-b c was labeled. No additional double labeling was observed in fibers backfilled from the En2. Scale bars = 50 μm in A; 50 μm in C (applies to B,C).
The limited contributions of central projections to the aminergic fiber systems of the En led us to examine their possible role as afferents. Upon close inspection, many THli and 5HTli fibers could be observed to taper and ramify upon entry into the ganglion (Fig. 5). They appeared to give rise to fine networks that terminated around the cell bodies of specific neurons and clusters within the ganglion. The densities of THli and 5HTli fibers within the buccal ganglion rendered it impossible to attribute specific regions of innervation to individual En afferents or to distinguish En projections from those originating from other sources. With both amines, however, the En appeared to be the predominant source of afferent input to the BG. No THli fibers were present in buccal nerves 1–3 or the radula nerve (see also Kabotyanski et al., 1998). Although the sheath regions surrounding these nerves were richly innervated by fine, irregular 5HTli networks, very few 5HTli fibers could be detected passing longitudinally within the nerve cores (Fig. 5B).
Figure 5.
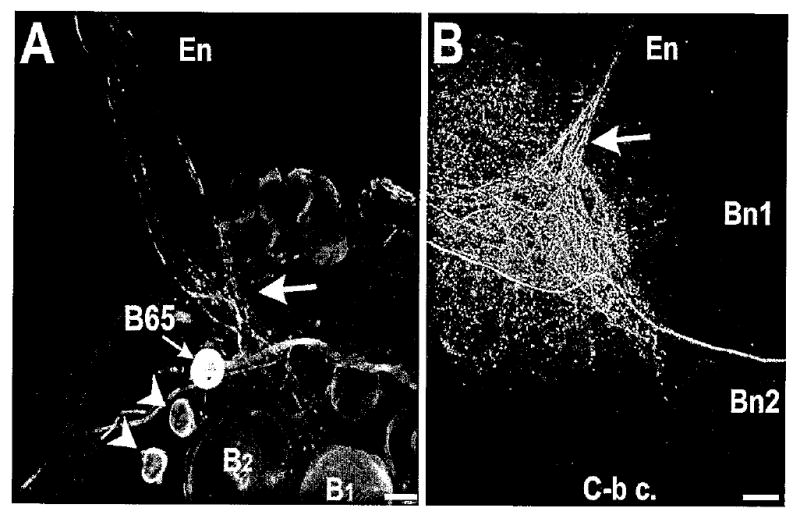
Extensive aminergic innervation of the buccal ganglion originating from the esophageal nerve. A: THli fibers from the En entered the buccal ganglion and projected in a loose bundle (arrow) toward the large motor neurons B1 and B2. The THli fibers crossed the axon of the dopaminergic interneuron B65. They did not project toward the soma of B65 or toward two additional unidentified THli cells (arrowheads) in the lateral region of the ganglion. B: 5HTli fibers from the En also projected in a loose bundle (arrow) toward the medial region of the ganglion. Comparatively, few serotonergic fibers projected toward the ganglion via the other buccal nerves (Bn1 and Bn2). Scale bars = 50 μm in A; 100 μm in B.
Results described thus far suggested a predominantly afferent function for the aminergic fiber systems of the esophageal nerve. Peripheral aminergic labeling was therefore examined to determine whether potential sources of THli and 5HTli En fibers could be identified. THli fiber systems were present throughout the pharynx and esophagus (Fig. 6). All major En branches contained brightly staining fibers. In addition, small spherical or ellipsoid (20.2 ± 1.6 μm length, 17.8 ± 1.4 μm width; n = 25) THli cell bodies were observed Fig. 6A–D). Most of the THli somata were located close to major En branches, with some appearing to be embedded within or appended to the nerves. The majority of THli cells possessed a single prominent process that was oriented toward the closest nerve (Fig. 6A, arrowheads).
Figure 6.
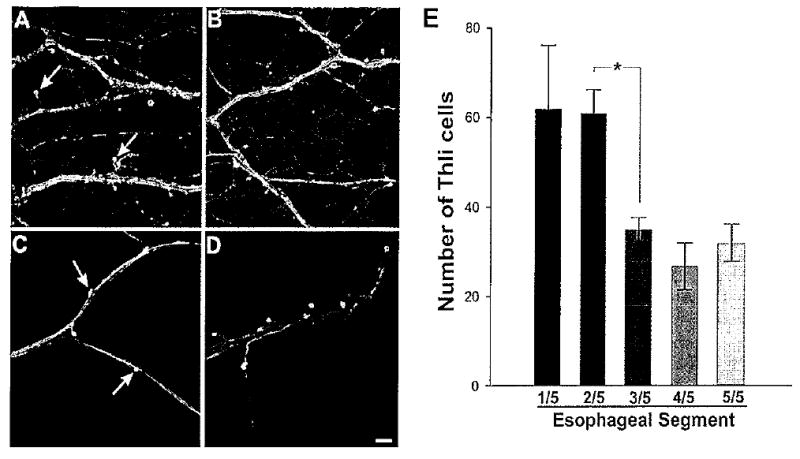
Tyrosine hydroxylase-like immunoreactivity in the pharynx and esophagus. A: Numerous THli fibers were present in each of the major nerves innervating the pharynx. Fine varicose fibers covered the entire surface of the pharynx. Cell bodies (arrows) were located near major fiber bundles. It was often possible to detect a single fine process projecting from each THli cell body toward the nearest fiber bundle. B: The pattern of THli in the anterior region of the esophagus was similar to that observed in the pharynx. Labeled cell bodies (arrows) were positioned close to major fiber bundles. C,D: Bundles of THli fibers coursed through more posterior regions of the esophagus, but the surface was not covered with the fine network observed in more anterior segments. Labeled cell bodies (arrows) were closely associated with, and often appeared to be embedded within, the THli fiber bundles. E: Group data (n = 5) from experiments in which the esophagus was cut into five segments along Its longitudinal axis prior to processing for THli. The number of labeled cell bodies decreased steeply between the second and the third segments (corresponding to B and C). The numbers of labeled neurons did not diminish over the remaining segments. *P < 0.05. Scale bar = 100 μm.
The distribution of THli neurons did not appear to be constant throughout the length of the esophagus (compare Fig. 6A–D). To quantify possible differences, the esophagus was divided into five sections along its length from the buccal mass to the triturating stomach. When the total numbers of THli cells in each segment were compared (n = 4 specimens; Fig. 6E), a significant decline in the total number of THli cells was observed (one-way ANOVA; F4,15 = 5.10, P < 0.01). This decline occurred fairly abruptly between the second segment (61.0 ± 10.4 cells) and the third segment (35.0 ± 5.4 cells; Student-Newman-Keuls pairwise multiple comparison procedure; q = 3.49, P < 0.05). The number of THli in the more posterior segments (4 and 5) did not decline further (pairwise multiple comparisons tests with segment 3: segment 4, q = 1.107, P > 0.05; segment 5, q = 0.40, P > 0.05).
The localization of serotonin-like immunoreactivity in the esophagus (Fig. 7) differed from that of TH. Most notable was a complete absence of 5HTli neuronal cell bodies (Fig. 7A,B). The pharynx and the region surrounding the buccal ganglion were completely covered with a dense network of fine fibers (Fig. 7A). More posterior regions of the crop contained large patches of immunoreactive fibers (Fig. 7B). The overall density of innervation within these patches was less than that in the pharynx. The fibers did not appear to ramify into fine terminals, and no varicosities were observed. The borders of the patches were very well defined and often gave the appearance of exact boundaries (Fig. 7B, arrows). The 5HTli fiber patches did not appear to correspond to underlying tissue structures.
Figure 7.
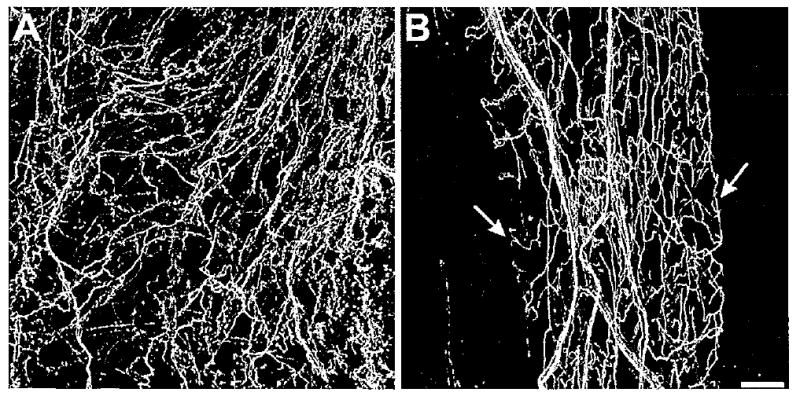
Serotonin-like immunoreactivity in the foregut. A: Serotonin-immunoreactive fiber network in the anterior esophagus. B: Serotonin-immunoreactive fibers in the crop. The fibers were more uniform in diameter than those seen in the pharynx and esophagus. Although the patches did not correspond to any discernible underlying structures, they sometimes had clearly defined borders (arrows). No 5HTli cell bodies were detected in the foregut. Scale bar = 100 μm.
Biocytin fills of the esophageal nerve toward the periphery were performed to identify foregut regions that may give rise to aminergic afferents. Examination of En1 at a distance of approximately 1 cm along its course along the esophagus revealed no THli (Fig. 8A1) or 5HTli (Fig. 8A2) fibers within the nerve. When En2 was examined in the region of the salivary gland, however, many THli (Fig. 8B1) and 5HTli (Fig. 8B2) fibers were present within the nerve. Occasionally, double-labeled fibers could be detected in En2 (arrowheads in Fig. 8B1,2), indicating that fibers labeled with the biocytin contained the amines. Together, these observations indicated that aminergic projections to the CNS originate from foregut regions innervated by En2, including the pharynx and most anterior portions of the esophagus.
Figure 8.
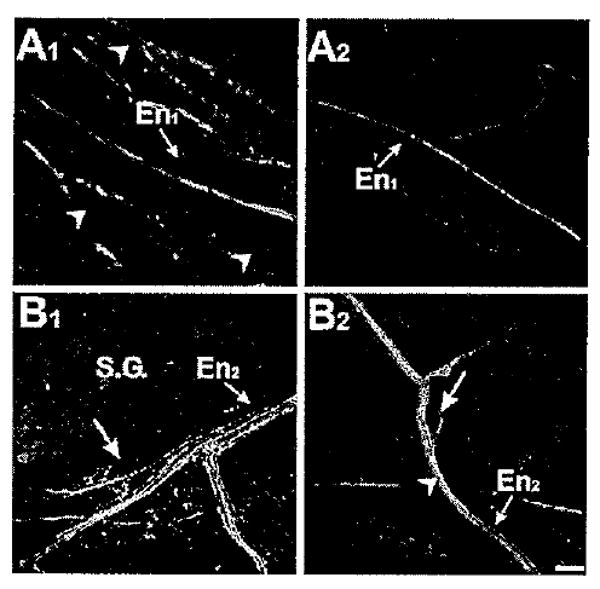
Fiber tract tracing indicates that aminergic afferents are associated with the proximal foregut. A1: Biocytin fill (red) of the En toward the periphery labeled a few fibers in En1. When examined approximately 1 cm from the buccal ganglion, THli fibers (green) and small cell bodies (arrowheads) were prevalent in the region surrounding En1, but no THli fibers were observed in the nerve itself. A2: Nerve tracing (red) experiment conducted as in A1, but with serotonin-like immunohistochemistry (green). Image was captured at approximately the same distance from the buccal ganglion as A1. Note comparatively little 5HTli on the esophagus (see Figs. 6, 7). No 5HTli fibers were detected in En1. B1: Biocytin fill (red) of the En toward the periphery labeled fibers in En2. Image was captured in the region of the salivary gland (S.G.), using the large peripheral salivary afferent neuron (arrow) as a landmark. Many THli fibers (green) were present in En2. Although the majority of these THli fibers were not filled by the biocytin, fibers with a yellow appearance (arrowhead) were observed, indicative of the presence of THli afferents in this region. B2: Forward-fill (red) conducted in combination with 5HTli labeling (green). Image captured in the same region as B1 (large peripheral salivary afferent neuron, arrow). Note the higher level of serotonergic innervation compared with more posterior foregut regions, e.g., A2. Several serotonergic fibers were present in En2, including a few (arrowhead) that had a yellow appearance, indicative of double labeling. Magenta/green images submitted as Supporting Information Figure 2. Scale bar = 100 μm.
These nerve-tracing experiments prompted a focused exploration of potential aminergic afferents within foregut regions innervated by En2. First, double-labeling experiments were performed to determine relations between THli and 5HTli in this region (Fig. 9A). Although no serotonergic neurons were detected (see also Fig. 7), numerous profiles were observed that were indicative of cell bodies enveloped by 5HTIi varicose fibers (two indicated by arrows in Fig. 9A; see also Goldstein et al., 1984). In addition to its dense catecholaminergic innervation, the pharynx was also stippled with THli cell bodies (two indicated by arrowheads in Fig. 9A). Serotonergic innervation of THli cell bodies was never detected (>30 THli cells examined in four double-labeled preparations).
Figure 9.
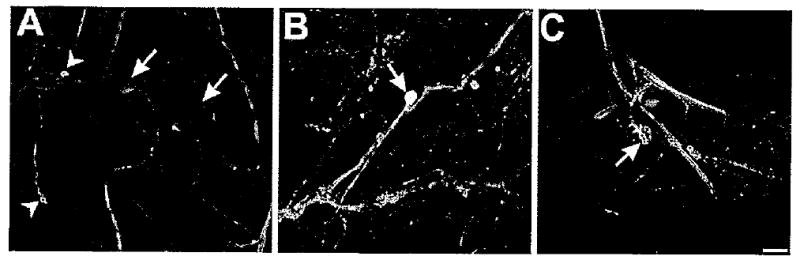
Double-labeling experiments illustrate relations between catecholaminergic and serotonergic innervation of the foregut. A: Image of the pharynx region labeled for THli (green) and 5HTIi (red). Several small THli cell bodies were observed (two indicated by arrowheads). Although no serotonergic somata were detected, several cell bodies were enveloped by varicose serotonergic fibers (two indicted by arrows). No TH-5HTIi double-labeled fibers or cell bodies were observed. Moreover, serotonergic somatic innervation was not found to occur on THli neurons. B: Overlay image of biocytin fill (red) of En2 toward the pharynx and THli (green). Although the majority of the THli cells in the pharynx did not exhibit double labeling, THli was occasionally detected in pharyngeal neurons that had afferent En projections (cell appears yellow in overlay image, arrow). C: Biocytin fill (red) of En2 toward the pharynx overlayed with 5HTli (green). No double labeling was observed, but several filled peripheral cell bodies were enveloped with serotonergic fibers (two indicated by arrow), indicative of serotonergic regulation of CNS afferents. Magenta/green images submitted as Supporting Information Figure 3 Scale bar = 100 μm.
The catecholaminergic and serotonergic innervations of the pharynx were further characterized in conjunction with biocytin fills of En2 toward the periphery. Such experiments revealed double-labeled THli cell bodies in the region of the pharynx (Fig. 9B, eight observed in six preparations). In the case of serotonin (Fig. 9C), varicose fiber networks were sometimes observed surrounding cell bodies that were labeled by the fills (Fig. 9C). Together, these findings indicate that THli peripheral cells contribute to afferent pathways originating from En2. Although serotoninergic fibers do not appear to contribute directly to such afferent pathways, they may regulate activity originating from other classes (i.e., non-THli-containing) of pharyngeal neurons that project to the CNS.
After we had established that the two major branches of the esophageal nerve differ with respect to their patterns of peripheral aminergic innervation, experiments were conducted to compare systematically the influence En1 vs. En2 on evoked motor programs (Fig. 10). In these experiments, an extracellular recording from buccal nerve 1 (Bn1) was used as a monitor of the protraction phase of BMPs (Neustadter et al., 2002), and intracellular recordings from the multifunctional neurons B4/5 (Jahan-Parwar et al., 1983) and motor neurons B8a/b (Morton and Chiel, 1993) were used as monitors of radula retraction and closure, respectively. As observed in previous studies (Chiel et al., 1986; Morgan et al., 2002; Proekt et al., 2004), most (86.6% ± 8.1%; Fig. 10A1,B) buccal motor programs produced in response to stimulation of En1 (total of 41 programs from five preparations) were functionally classified as egestive-like (see Materials and Methods for classification algorithms). Fewer programs were classified as intermediate (13.4% ± 8.1 %), and no ingestive-Iike programs were observed (one-way ANOVA; F = 49.86, P < 0.05). Stimulation of En2 (total of 48 programs from six preparations), on the other hand, produced a more balanced proportion of motor programs (egestive-like: 46.3% ± 16.1%; intermediate: 19% ± 7.5%; ingestive-Iike: 34.3% ± 14.7%; one-way ANOVA; F = 1.06, P > 0.05; Fig. 10B,C1–3).
Figure 10.
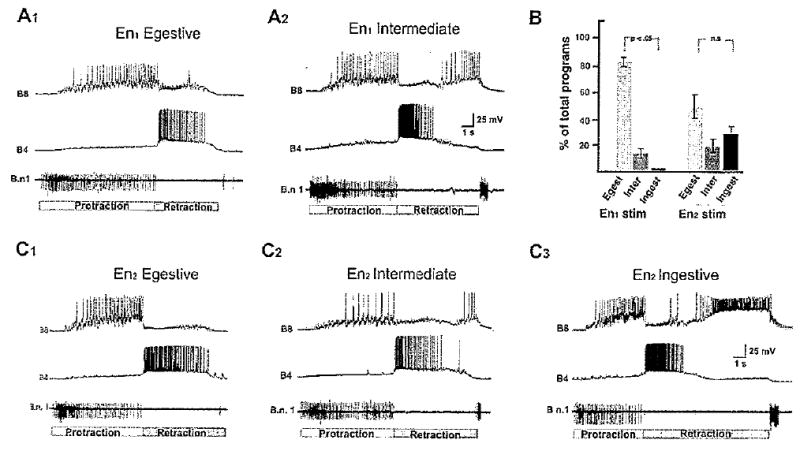
Comparison of buccal motor programs produced by stimulation of the two major esophageal nerve branches. A: Motor programs produced by stimulation (3-msec pulses, 2 Hz) of En1. Extracellular recordings from buccal nerve 1 (Bn1) were used to monitor the protraction phase of motor programs (Neustadter et al. 2002; duration of protraction indicated by the white bar below recordings). Intracellular recording from the multifunctional neuron B4 provided a monitor of the retraction phase (duration of retraction indicated by gray bar below recordings). Note that retraction phase duration corresponds to the entire period of B/4 depolarization, which typically outlasts its period of firing. Intracellular recording from B8 served as a monitor of radula closure. In A1, the closure phase is coincident with protraction, indicative of a fictive egestive-like program. A2 illustrates an intermediate program in which closure is not preferentially associated with either protraction or retraction (see Materials and Methods for classification algorithm). B: Group data (41 programs from five preparations) showed that the motor programs produced by En1 stimulation were biased toward the egestive-like classification. C: Motor programs produced by stimulation of En2. In C1, the closure phase is coincident with protraction, indicative of a fictive egestive-like program. C2 illustrates an intermediate program in which closure is not preferentially associated with either protraction or retraction. In C3, closure was predominantly associated with retraction, indicative of an ingestive-like program. Group data for En2 stimulation (48 programs from six preparations) are shown in B (right side). No significant bias toward any of the three program classifications was detected.
Discussion
This study contributes to our understanding of the aminergic innervation of the pregastric alimentary tract of Aplysia. Our findings disclosed several differences between the serotonergic and catecholaminergic patterns of foregut innervation. 1) Although no efferent CA projections could be attributed to central neurons, one serotonergic efferent projection originated from the MCC; 2) although no peripheral serotonergic cell bodies were observed, THli somata were abundantly distributed throughout the foregut; and, 3) although serotonergic fibers enveloped many peripheral somata, no peripheral THli cells were observed to receive serotonergic innervation. These experiments also revealed differences between the two major branches of the esophageal nerve. 1) Most THli and 5HTli fibers in the En were associated with the En2 branch; 2) the serotonergic efferent fiber originating from the MCC projected exclusively to En2; 3) THli afferents were detected only in En2; and 4) the functional profile of buccal motor programs produced in response to stimulation of En1 differed from that elicited by stimulation of En2.
Serotonin is a major regulator of gastrointestinal motility in vertebrates (Gershon and Tack, 2007; Beattie and Smith, 2008) and invertebrates (Orchard, 2006; Stern et al., 2007), including molluscs (Ajimal and Ram, 1981; Hernadí et al., 1998; Kiss et al., 2003). The localization of 5HTli observed in this study is in general agreement with previous reports in Aplysia (Goldstein et al., 1984) and related gastropods (Moroz et al., 1997; Hernadí et al., 1998). Notably, the absence of 5HTli peripheral neurons is consistent with observations in Helix pomatia (Hernadí et al., 1998), Pleurobranchaea californica, and Tritonia diomedea (Moroz et al., 1997), as well as juvenile Aplysia (Goldstein et al., 1984). Collectively, these studies leave unresolved the cellular origins of peripheral serotonin-immunoreactive fibers. One hypothesis proposes that all peripheral serotonergic fibers arise from central neurons (Moroz et al., 1997). If this is so, then the serotonergic afferent fibers that appear to enter the buccal ganglion via the En may originate from neurons in other central ganglia that take a circuitous peripheral route to reach their targets. Observations of serotonergic fibers on nonserotonergic cell bodies in the gut led previous investigators to postulate a role for 5HT in the regulation of reflexes or feedback to the CNS (Goldstein et al., 1984; Moroz et al., 1997). Our finding that peripheral neurons labeled with dye fills of the En were enveloped with 5HTli supports the hypothesis that serotonin can regulate direct feedback pathways to the buccal CPG.
The observed dopaminergic innervation of the Aplysia foregut was also in agreement with previous findings obtained with juvenile specimens (Susswein et at., 1993; Croll, 2001). Although dopamine has been reported to regulate gastrointestinal motility in molluscs (Hernadí et al., 1998), its function is best understood as a modulator of the neural networks that control feeding (Wieland and Gelperin, 1983; Trimble and Barker, 1984; Kyriakides and McCrohan, 1989; Quinlan et al., 1997). In Aplysia, dopamine is present in specific interneurons within the feeding system that are instrumental in the generation and specification of consummatory motor programs (Rosen et al., 1991; Teyke et al., 1993; Kabotyanski et al., 1998; Jing and Weiss, 2001). DA has not been localized to motor neurons in this system. The presence of dopaminergic afferents in the pharyngeal region reported here indicates that DA may mediate transmission of proprioceptive feedback from specific effector elements directly to the feeding CPG (see below).
Neuroregulation of the foregut: functional organization
The backfill and nerve stimulation experiments conducted in this study disclosed significant structural and functional differences between the two major esophageal nerve branches, En1 and En2. In mammals, diverse preabsorbtive signals are thought to exert influence on the regulation of consummatory behaviors, ranging from enhancement of ingestion to development of satiation (Novin et al., 1976; Zeigler, 1994; Smith, 1998). Considerable evidence indicates a comparable level of complexity in the pregastric signaling of mollusks (Croll et al., 1987; Schwarz et al., 1988; Elliott and Benjamin, 1989; Horn et al., 2001). In Aplysia, successful consumption of small quantities of food produces an enhancement of feeding, probably via activation of gut chemoreceptors (Susswein et al., 1984). Further filling the crop, however, with food or nonnutritive bulk produces a stimulus-graded satiation (Susswein and Kupfermann, 1975a,b; Susswein et al., 1976). Bilateral sectioning of the esophageal nerves results in a significant increase in the volume required to achieve satiation, indicating that the sensory afferents signaling gut distension reach the CNS via the En (Kuslansky et al., 1987). Direct support for the participation of the esophageal nerve in transmitting information concerning the presence of food in the foregut was obtained in free-feeding Aplysia, where bouts of high-frequency En activity were observed when bites resulted in the ingestion of food, but not when the animals were prevented from swallowing (Brembs et al., 2002).
The digestive system of Aplysia is richly innervated by a broad spectrum of neuroregulators that participate in both motor and sensory functions (Lloyd et al., 1988; Miller et al., 1991,1992; Fujisawa et al., 1999; Furukawa et al., 2001, 2003). Our observations indicate that the biogenic amines dopamine and serotonin are preferentially associated with En2, the esophageal nerve branch that innervates the pharynx and most anterior esophagus. With the exception of a single fiber originating from the metacerebral cell, DA and 5HT do not appear to participate directly in the efferent CNS control of foregut targets. Other neuroregulators are specifically associated with En1, the branch that innervates more posterior esophageal and crop regions (Lloyd et al., 1988; Jing et al., 2007). The small cardioactive peptides (SCPs) are present in the large caudal motor neurons, including B1 and B2, which project to the En (Lloyd et al., 1985, 1988). Stimulation of B1 was shown to produce peristaltic contractions in a relatively distal portion of the gut (Lloyd et al., 1988). These actions are consistent with our backfill experiments showing that neurons of the B1/B2 cluster project exclusively to En1. Although we obtained no evidence for motor projections to the pharynx via En2, this region is known to receive motor innervation via another buccal nerve (buccal nerve 1; Nagahama and Takata, 1987; Serrano et al., 2007).
Afferent signals from the alimentary tract contribute to satiation in molluscan species with diverse feeding strategies and preferences, including Pleurobranchea (Davis et al., 1977), Limax (Reingold and Gelperin, 1980), and Navanax (Susswein and Bennett, 1979). In Aplysia, a herbivore that consumes well-defined meals (Kupfermann, 1974a), recent experiments have provided evidence for the contribution of afferent signals mediated by Aplysia neuropeptide Y (apNPY; Rajpara et al., 1992) to producing the transition from hunger to satiety (Jing et al., 2007). That study and others implicating the participation of gut afferents in producing satiation (see above) have focused on the posterior branch (En1) of the esophageal nerve in signaling crop distension, a stimulus that is preabsorbtive but postingestive. Other studies have disclosed a more complex control of satiation that emphasizes sensory feedback from more anterior regions of the alimentary tract (Horn et al., 2001). The association of aminergic afferents with the En2 anterior esophageal nerve branch indicates that dopamine and serotonin may be signaling some parameter of the ingestive (or egestive) process itself, such as passage of substances between the pharynx and the esophagus. The distinct sources of motor (buccal nerve 1) vs. sensory (En2) innervation of this region coupled with the partitioning of certain neurotransmitter systems between the two En branches should facilitate further functional characterization of peripheral determinants of feeding and satiation in Aplysia.
Pxeabsorbtive signals and conditioning of feeding behaviors
Considerable interest has focused on esophageal nerve sensory signals in modifications of feeding behaviors that result from learning. In one conditioning paradigm, bilateral lesion of the En was found to attenuate the ability of Aplysia to cease responding to inedible food (Schwarz and Susswein, 1984,1986). En2 lesions were also found to impair learning in a classical conditioning protocol in which food was used as an unconditioned stimulus (US; Lechner et al., 2000). Such experiments led to the identification of En2 as a pathway that is both necessary and sufficient for transmission of US information in both classical and instrumental conditioning of feeding responses (Nargeot et al., 1997; Brembs et al., 2002; Mozzachiodi et al., 2003; Baxter and Byrne, 2006).
The efficacy of En2 stimulation as contingent reinforcement in a neuronal analogue of operant conditioning (Nargeot et al., 1997) led to an investigation of dopaminergic signaling in this pathway (Nargeot et al., 1999). In specific buccal neurons, En2 stimulation was shown to produce direct PSPs that were blocked by the dopamine antagonist methylergonovine. Importantly, these synaptic potentials were significantly reduced by a concentration of methylergonovine (1 nM) that also blocked the efficacy of En2 stimulation as a US. The demonstration of dopaminergic afferents projecting to the buccal ganglion via the En2 further supports the hypothesis that DA conveys the natural US in the operant conditioning of Aplysia feeding.
In summary, our findings indicate that dopamine (directly) and serotonin (indirectly) participate in feedback projections from the anterior alimentary tract to the feeding CPG of Aplysia. For action patterns that possess motivational or goal-directed determinants, such feedback could contribute to computing the value, or incentive salience, that is assigned to a particular stimulus (Berridge and Robinson, 1998; Alcaro et al., 2007). It can also participate in the comparative evaluation of actual vs. expected consequences of an organism's motor actions (Schultz, 1998; Schultz and Dickinson, 2000). Plasticity in the feeding motor network of Aplysia possesses a history dependence that exhibits features of intentions and expectation in higher organisms (Proekt et al, 2004). The presence of dopaminergic afferents on the pharynx and anterior esophagus is highly consistent with a system that provides feedback to the feeding CPG concerning the effectiveness of ingestive or egestive actions. In this respect, dopaminergic systems of molluscs may share critical functional attributes with their counterparts in vertebrate brains; i.e., they may provide information to central motor networks concerning the consequences of the actions that they generate.
Supplementary Material
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number RCMI RR-03051; Grant number: NIGMS MBRS GM-08224; Grant number: NIGMS RISE G12 RR-03051; National Science Foundation; Grant number: DBI-0115825.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Literature Cited
- Ajimal GS, Ram JL. Aplysia gastrointestinal tract motility: spontaneous activity and pharmacological sensitivity. Comp Biochem Physiol. 1981;C68:133–144. [Google Scholar]
- Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev. 2007;56:283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeeva V, Borovikov D, Miller MW, Rosen SC, Cropper EC. Effect of a serotonergic extrinsic modulatoy neuron (MCC) on radula mechanoafferent function in Aplysia. J Neurophysiol. 1998;80:1609–1622. doi: 10.1152/jn.1998.80.4.1609. [DOI] [PubMed] [Google Scholar]
- Baxter DA, Byrne JH. Feeding behavior of Aplysia: a model system for comparing cellular mechanisms of classical and operant conditioning. Learning & Memory. 2006;13:669–680. doi: 10.1101/lm.339206. [DOI] [PubMed] [Google Scholar]
- Beattie DT, Smith JA. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:181–203. doi: 10.1007/s00210-008-0276-9. [DOI] [PubMed] [Google Scholar]
- Beenhakker MP, Blitz DM, Nusbaum MP. Long-lasting activation of rhythmic neuronal activity by a novel mechanosensory system in the crustacean stomatogastric nervous system. J Neurophysiol. 2004;91:78–91. doi: 10.1152/jn.00741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Borovikov D, Evans CG, Jing J, Rosen SC, Cropper EC. A proprioceptive role for an exteroceptive mechanoafferent neuron in Aplysia. J Neurosci. 2000;20:1990–2002. doi: 10.1523/JNEUROSCI.20-05-01990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembs B, Lorenzetti FD, Reyes FD, Baxter DA, Byrne JH. Operant reward learning in Aplysia: neuronal correlates and mechanisms. Science. 2002;296:1706–1709. doi: 10.1126/science.1069434. [DOI] [PubMed] [Google Scholar]
- Carpenter D, Breese G, Schanberg S, Kopin I. Serotonin and dopamine: distribution and accumulation in Aplysia nervous and nonnervous tissues. Int J Neuroci. 1971;2:49–56. doi: 10.3109/00207457109146992. [DOI] [PubMed] [Google Scholar]
- Chiel HJ, Weiss KR, Kupfermann I. An identified histaminergic neuron modulates feeding motor circuitry in Aplysia. J Neurosci. 1986;6:2427–2450. doi: 10.1523/JNEUROSCI.06-08-02427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll RP. Distribution of monoamines in the central nervous system of the nudibranch gastropod, Hermissenda crassicornis. Brain Res. 1987;405:337–347. doi: 10.1016/0006-8993(87)90303-9. [DOI] [PubMed] [Google Scholar]
- Croll RP. Catecholamine-containing cells in the central nervous system and periphery of Aplysia californica. J Comp Neurol. 2001;441:91–105. doi: 10.1002/cne.1399. [DOI] [PubMed] [Google Scholar]
- Croll RP, Albuquerque T, Fitzpatrick L. Hyperphagia resulting from gut denervation in the sea slug, Pleurobranchaea. Behav Neural Biol. 1987;47:212–218. doi: 10.1016/s0163-1047(87)90341-4. [DOI] [PubMed] [Google Scholar]
- Cropper EC, Evans CG, Hurwitz I, Proekt A, Romero A, Rosen SC. Feeding neural networks in the mollusc Aplysia. Neurosignals. 2004;13:70–86. doi: 10.1159/000076159. [DOI] [PubMed] [Google Scholar]
- Davis WJ, Mpitsos GJ, Pinneo M, Ram J. Modification of the behavioral hierarchy in Pleurobranchaea. I. Satiation and feeding motivation. J Comp Physiol. 1977;117:99–125. [Google Scholar]
- Díaz-Ríos M, Miller MW. Rapid dopaminergic signaling by interneurons that contain markers for catecholamines and GABA in the feeding circuitry of Aplysia. J Neurophysiol. 2005;93:2142–2156. doi: 10.1152/jn.00003.2004. [DOI] [PubMed] [Google Scholar]
- Díaz-Ríos M, Suess E, Miller MW. Localization of GABA-like immunoreactivity in the central nervous system of Aplysia californica. J Comp Neurol. 1999;413:255–270. doi: 10.1002/(sici)1096-9861(19991018)413:2<255::aid-cne7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Díaz-Ríos M, Oyola E, Miller MW. Colocalization of γ-aminobutyric acid-like immunoreactivity and catecholamines in the feeding network of Aplysia californica. J Comp Neurol. 2002;445:29–46. doi: 10.1002/cne.10152. [DOI] [PubMed] [Google Scholar]
- Due MR, Jing J, Weiss KR. Dopaminergic contributions to modulatory functions of a dual-transmitter interneuron in Aplysia. Neurosci Lett. 2004;358:53–57. doi: 10.1016/j.neulet.2003.12.058. [DOI] [PubMed] [Google Scholar]
- Elliott CJH, Benjamin PR. Esophageal mechanoreceptors in the feeding system of the pond snail Lymnaea stagnalis. J Neurophysiol. 1989;61:727–736. doi: 10.1152/jn.1989.61.4.727. [DOI] [PubMed] [Google Scholar]
- Elliott CJ, Susswein AJ. Comparative neuroethology of feeding control in molluscs. J Exp Biol. 2002;205:877–896. doi: 10.1242/jeb.205.7.877. [DOI] [PubMed] [Google Scholar]
- Evans CG, Cropper EC. Proprioceptive input to feeding moto neurons in Aplysia. J Neurosci. 1998;18:8016–8031. doi: 10.1523/JNEUROSCI.18-19-08016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa Y, Furukawa Y, Ohta S, Ellis TA, Dembrow NC, Li L, Floyd PD, Sweedler JV, Minakata H, Nakamaru K, Morishita F, Matsushima O, Weiss KR, Vilim FS. The Aplysia mytilus inhibitory peptide-related peptides: identification, cloning, processing, distribution, and action. J Neurosci. 1999;19:9618–9634. doi: 10.1523/JNEUROSCI.19-21-09618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y, Nakamaru K, Wakayama H, Fujisawa Y, Minakata H, Ohta S, Morishita F, Matsushima O, Li L, Romanova E, Sweedler JV, Park JH, Romero A, Cropper EC, Dembrow NC, Jing J, Weiss KR, Vilim FS. The enterins: a novel family of neuropeptides isolated from the enteric nervous system and CNS of Aplysia. J Neurosci. 2001;21:8247–8261. doi: 10.1523/JNEUROSCI.21-20-08247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y, Nakamaru K, Sasaki K, Fujisawa Y, Minakata H, Ohta S, Morishita F, Matsushima O, Li L, Alexeeva V, Ellis TA, Dembrow NC, Jing J, Sweedler JV, Weiss KR, Vilim FS. PRQFVamide, a novel pentapeptide identified from the CNS and gut of Aplysia. J Neurophysiol. 2003;89:3114–3127. doi: 10.1152/jn.00014.2003. [DOI] [PubMed] [Google Scholar]
- Gelperin A. Stretch receptors in the foregut of the blowfly. Science. 1967;157:208–210. doi: 10.1126/science.157.3785.208. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld HM, Paupardin-Tritsch D. On the transmitter function of 5-hydroxytryptamine at excitatory and inhibitory monosynaptic junctions. J Physiol. 1974;243:457–481. doi: 10.1113/jphysiol.1974.sp010762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Getting PA. Emerging principles governing the operation of neural networks. Annu Rev Neurosci. 1989;12:185–204. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- Goldstein RS, Schwartz JH. Catecholamine neurons in Aplysia: improved light-microscopic resolution and ultastructural study using paraformaldehyde and glutaraldehyde (FaGlu) cytochemistry. J Neurobiol. 1989;20:203–218. doi: 10.1002/neu.480200404. [DOI] [PubMed] [Google Scholar]
- Goldstein R, Kistler HB, Jr, Steinbusch HW, Schwartz JH. Distribution of serotonin-immunoreactivity in juvenile Aplysia. Neuroscience. 1984;11:535–547. doi: 10.1016/0306-4522(84)90043-5. [DOI] [PubMed] [Google Scholar]
- Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity. 2006;14:216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- Hernadí L, Erdély L, Hiripi L, Elekes K. The organization of serotonin-, dopamine-, and FMRFamide-containing neuronal elements and their possible role in the regulation of spontaneous contraction of the gastrointestinal tract in the snail Helix pomatia. J Neurocytol. 1998;27:761–775. doi: 10.1023/a:1006955018882. [DOI] [PubMed] [Google Scholar]
- Horn CC, Kupfermann I. Egestive feeding responses in Aplysia persist after sectioning of the cerebral-buccal connectives: evidence for multiple sites of control of motor programs. Neurosci Lett. 2002;323:175–178. doi: 10.1016/s0304-3940(02)00155-6. [DOI] [PubMed] [Google Scholar]
- Horn CC, Geizhals CR, Kupfermann I. Further studies of bulk and orosensory decrement in producing satiation of feeding in Aplysia. Brain Res. 2001;918:51–59. doi: 10.1016/s0006-8993(01)02919-5. [DOI] [PubMed] [Google Scholar]
- Jahan-Parwar B, Wilson AH, Fredman SM. Role of proprioceptive reflexes in control of feeding muscles of Aplysia. J Neurophysiol. 1983;49:1469–1480. doi: 10.1152/jn.1983.49.6.1469. [DOI] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Neural mechanisms of motor program switching in Aplysia. J Neurosci. 2001;21:7349–7362. doi: 10.1523/JNEUROSCI.21-18-07349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Horn CC, Alexeeva V, Hatcher NG, Sasaki K, Yashina I, Zhurov Y, Kupfermann I, Sweedler JV, Weiss KR. From hunger to satiety: reconfiguration of a feeding network by Aplysia neuropeptide Y. J Neurosci. 2007;27:3490–3502. doi: 10.1523/JNEUROSCI.0334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabotyanski EA, Baxter DA, Byrne JH. Identification and characterization of catecholaminergic neuron B65, which initiates and modifies patterned activity in the buccal ganglia of Aplysia. J Neurophysiol. 1998;79:605–621. doi: 10.1152/jn.1998.79.2.605. [DOI] [PubMed] [Google Scholar]
- Kabotyanski EA, Baxter DA, Cushman SJ, Byrne JH. Modulation of fictive feeding by dopamine and serotonin in Aplysia. J Neurophysiol. 2000;83:374–392. doi: 10.1152/jn.2000.83.1.374. [DOI] [PubMed] [Google Scholar]
- Kiss T, Hiripi L, Papp N, Elekes K. Dopamine and serotonin receptors mediating contractions of the snail, Helix pomatia, salivary duct. Neuroscience. 2003;116:775–790. doi: 10.1016/s0306-4522(02)00754-6. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Feeding behavior in Aplysia: a simple system for the study of motivation. Behav Biol. 1974a;10:1–26. doi: 10.1016/s0091-6773(74)91644-7. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Dissociation of the appetitive and consummatory phases of feeding behavior in Aplysia: a lesion study. Behav Biol. 1974b;10:89–97. doi: 10.1016/s0091-6773(74)91694-0. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, Weiss KR. Motor program selection in simple model systems. Curr Opin Neurobiol. 2001;11:673–677. doi: 10.1016/s0959-4388(01)00267-7. [DOI] [PubMed] [Google Scholar]
- Kuslansky B, Weiss KR, Kupfermann I. A neural pathway mediating satiation of feeding behavior in Aplysia. Behav Biol. 1978;23:230–237. doi: 10.1016/s0091-6773(78)91862-x. [DOI] [PubMed] [Google Scholar]
- Kuslansky B, Weiss KR, Kupfermann I. Mechanisms underlying satiation of feeding behavior of the mollusc Aplysia. Behav Neural Biol. 1987;48:278–303. doi: 10.1016/s0163-1047(87)90836-3. [DOI] [PubMed] [Google Scholar]
- Kyriakides MA, McCrohan CR. Effect of putative neuromodulators on rhythmic buccal motor output in Lymnaea stagnalis. J Neurobiol. 1989;20:635–650. doi: 10.1002/neu.480200704. [DOI] [PubMed] [Google Scholar]
- Lechner HA, Baxter DA, Byrne JH. Classical conditioning of feeding in Aplysia: I. Behavioral analysis. J Neurosci. 2000;20:3369–3376. doi: 10.1523/JNEUROSCI.20-09-03369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd PE, Mahon AC, Kupfermann I, Cohen JL, Scheller RH, Weiss KR. Biochemical and immunocytological localization of molluscan small cardioactive peptides in the nervous system of Aplysia californica. J Neurosci. 1985;5:1851–1861. doi: 10.1523/JNEUROSCI.05-07-01851.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd PE, Kupfermann I, Weiss KR. Central peptidergic neurons regulate gut motility in Aplysia. J Neurophysiol. 1988;59:1613–1626. doi: 10.1152/jn.1988.59.5.1613. [DOI] [PubMed] [Google Scholar]
- Longley RD, Longley AJ. Serotonin immunoreactivity of neurons in the gastropod Aplysia californica. J Neurobiol. 1986;17:339–358. doi: 10.1002/neu.480170408. [DOI] [PubMed] [Google Scholar]
- McCaman MW, Weinreich D, McCaman RE. The determination of picomole levels of 5-HT and dopamine in Aplysia, Tritonia, and leech nervous tissues. Brain Res. 1973;53:129–137. doi: 10.1016/0006-8993(73)90772-5. [DOI] [PubMed] [Google Scholar]
- McCaman MW, Ono JK, McCaman RE. Dopamine measurements in molluscan ganglia using a new, sensitive assay. J Neurochem. 1979;32:1111–1113. doi: 10.1111/j.1471-4159.1979.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Melcher C, Pankratz MJ. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol. 2005;3:e305. doi: 10.1371/journal.pbio.0030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Alevizos A, Cropper EC, Vilim FS, Karagogeos D, Kupfermann I, Weiss KR. Localization of myomodulin-like immunoreactivity in the cental nervous system and peripheral tissues of Aplysia californica. J Comp Neurol. 1991;314:627–644. doi: 10.1002/cne.903140402. [DOI] [PubMed] [Google Scholar]
- Miller MW, Alevizos A, Cropper EC, Kupfermann I, Weiss KR. Distribution of buccalin-like immunoreactivity in the central nervous system and peripheral tissues of Aplysia californica. J Comp Neurol. 1992;320:182–195. doi: 10.1002/cne.903200204. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Jing J, Vilim FS, Weiss KR. Interneuronal and peptidergic control of motor pattern switching in Aplysia. J Neurophysiol. 2002;87:49–61. doi: 10.1152/jn.00438.2001. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Sudlow LC, Jing J, Gillette R. Serotonin-immunoreactivity in peipheral tissues of the opisthobranch molluscs Pleurobranchaea californica and Tritonia diomedea. J Comp Neurol. 1997;382:176–188. doi: 10.1002/(sici)1096-9861(19970602)382:2<176::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Morton DW, Chiel HJ. The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol. 1993;A173:519–536. doi: 10.1007/BF00197761. [DOI] [PubMed] [Google Scholar]
- Morton DW, Chiel HJ. Neural architectures for adaptive behavior. Trends Neurosci. 1994;17:413–420. doi: 10.1016/0166-2236(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Mozzachiodi R, Lechner HA, Baxter DA, Byrne JH. In vitro analog of classical conditioning of feeding behavior in Aplysia. Learn Mem. 2003;10:478–494. doi: 10.1101/lm.65303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AD. The neuronal basis of feeding in the snail, Helisoma, with comparisons to selected gastropods. Prog Neurobiol. 2001;63:383–408. doi: 10.1016/s0301-0082(00)00049-6. [DOI] [PubMed] [Google Scholar]
- Nagahama T, Takata M. Food-induced firing patterns in motoneurons innervating the pharynx of Aplysia kurodai. J Comp Physiol A Sens Neural Behav Physiol. 1987;161:799–809. doi: 10.1007/BF00193459. [DOI] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, Byrne JH. Contingent-dependent enhancement of rhythmic motor patterns: an in vitro analog of operant conditioning. J Neuroscience. 1997;17:8093–8105. doi: 10.1523/JNEUROSCI.17-21-08093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot R, Baxter DA, Patterson GW, Byrne JH. Dopaminergic synapses mediate neuronal changes in an analogue of operant conditioning. J Neurophysiol. 1999;81:1983–1987. doi: 10.1152/jn.1999.81.4.1983. [DOI] [PubMed] [Google Scholar]
- Neustadter DM, Drushel RF, Crago PE, Adams BW, Chiel HJ. A kinematic model of swallowing in Aplysia californica based on radula/odontophore kinematics and in vivo magnetic resonance images. J Exp Biol. 2002;205:3177–3206. doi: 10.1242/jeb.205.20.3177. [DOI] [PubMed] [Google Scholar]
- Newcomb JM, Fickbohm DJ, Katz PS. Comparative mapping of serotonin-immunoreactive neurons in the central nervous systems of nudibranch mollusks. J Comp Neurol. 2006;499:485–505. doi: 10.1002/cne.21111. [DOI] [PubMed] [Google Scholar]
- Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience. 1978;3:207–218. doi: 10.1016/0306-4522(78)90102-1. [DOI] [PubMed] [Google Scholar]
- Novin D, Wyrwicka W, Bray GD, editors. Hunger: basic mechanisms and clinical implications. New York: Raven Press; 1976. [Google Scholar]
- Ono JK, McCaman RE. Immunocytochemical localization and direct assays of serotonin-containing neurons in Aplysia. Neuroscience. 1984;11:549–560. doi: 10.1016/0306-4522(84)90044-7. [DOI] [PubMed] [Google Scholar]
- Orchard I. Serotonin: a coordinator of feeding-related physiological events in the blood-gorging bug, Rhodnius prolixus. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:316–324. doi: 10.1016/j.cbpa.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. The work of the digestive glands. 2nd. London: Charles Griffin & Company, Ltd.; 1910. [Google Scholar]
- Proekt A, Brezina V, Weiss KR. Dynamical basis of intentions and expectations in a simple neuronal network. Proc Natl Acad Sci U S A. 2004;101:9447–9452. doi: 10.1073/pnas.0402002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt A, Jing J, Weiss KR. Multiple contributions of an input-representing neuron to the dynamics of the Aplysia feeding network. J Neurophysiol. 2007;97:3046–3056. doi: 10.1152/jn.01301.2006. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Arnett BC, Murphy AD. Feeding stimulants activate an identified dopaminergic interneuron that induces the feeding motor program in Helisoma. J Neurophysiol. 1997;78:812–824. doi: 10.1152/jn.1997.78.2.812. [DOI] [PubMed] [Google Scholar]
- Rajpara SM, Garcia PD, Roberts R, Eliasen JC, Owens DF, Maltby D, Myers RM, Mayeri E. Identification and molecular cloning of a neuropeptide Y homolog that produces prolonged inhibition in Aplysia neurons. Neuron. 1992;9:505–513. doi: 10.1016/0896-6273(92)90188-j. [DOI] [PubMed] [Google Scholar]
- Rathouz MM, Kirk MD. Localization of catecholamines in the buccal ganglia of Aplysia californica. Brain Res. 1988;458:170–175. doi: 10.1016/0006-8993(88)90512-4. [DOI] [PubMed] [Google Scholar]
- Reingold SC, Gelperin A. Feeding motor programme in Limax. II. Modulation by sensory inputs in intact animals and isolated central nervous systems. J Exp Biol. 1980;85:1–19. doi: 10.1242/jeb.85.1.1. [DOI] [PubMed] [Google Scholar]
- Rosen SC, Teyke T, Miller MW, Weiss KR, Kupferman I. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J Neurosci. 1991;11:3630–3655. doi: 10.1523/JNEUROSCI.11-11-03630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Wintie RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hébert TE, van der Kooy D, Schafer WR, Culotti JG, Van Tol HH. Dopamine modulates plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J. 2004;23:473–482. doi: 10.1038/sj.emboj.7600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal prediction of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Susswein AJ. A neural pathway for learning that food is inedible in Aplysia. Brain Res. 1984;294:363–366. doi: 10.1016/0006-8993(84)91051-5. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Susswein AJ. Identification of the neural pathway for reinforcement of feeding when Aplysia learn that food is inedible. J Neurosci. 1986;6:1528–1536. doi: 10.1523/JNEUROSCI.06-05-01528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M, Markovich S, Susswein AJ. Parametric features of inhibition of feeding in Aplysia by associative learning, satiation, and sustained lip stimulation. Behav Neurosci. 1988;102:124–133. doi: 10.1037//0735-7044.102.1.124. [DOI] [PubMed] [Google Scholar]
- Scott ML, Govind CK, Kirk MD. Neuromuscular organization of the buccal system in Aplysia californica. J Comp Neurol. 1991;312:207–222. doi: 10.1002/cne.903120204. [DOI] [PubMed] [Google Scholar]
- Serrano GE, Martinez-Rubio C, Miller MW. Endogenous motor neuron properties contribute to a program-specific phase of activity in the multifunctional feeding central pattern generator of Aplysia. J Neurophysiol. 2007;98:29–42. doi: 10.1152/jn.01062.2006. [DOI] [PubMed] [Google Scholar]
- Shetreat-Klein AN, Cropper EC. Afferent-induced changes in rhythmic motor programs in the feeding circuitry of Aplysia. J Neurophysiol. 2004;92:2312–2322. doi: 10.1152/jn.00137.2004. [DOI] [PubMed] [Google Scholar]
- Smith GP, editor. Satiation: from gut to brain. New York: Oxford University Press; 1998. [Google Scholar]
- Stern M, Knipp S, Bicker G. Embryonic differentiation of serotonin-containing neurons in the enteric nervous system of the locust (Locusta migratoria) J Comp Neurol. 2007;501:38–51. doi: 10.1002/cne.21235. [DOI] [PubMed] [Google Scholar]
- Sudlow LC, Jing J, Moroz LL, Gillette R. Serotonin-immunoreactivity in the central nervous system of the marine moliuscs Pleurobranchaea californica and Tritonia diomedea. J Comp Neurol. 1998;395:466–480. [PubMed] [Google Scholar]
- Susswein AJ, Bennett MVL. Plasticity of feeding behavior in the opisthobranch mollusk Navanax. J Neurobiol. 1979;10:521–534. doi: 10.1002/neu.480100603. [DOI] [PubMed] [Google Scholar]
- Susswein AJ, Kabotyanski EA, Hurwitz I, Sakharov DA. Catecholaminergic neurons in the esophagus of Aplysia: a peripheral brain? Soc Neurosci Abstr. 1993;19:350. [Google Scholar]
- Susswein AJ, Kupfermann I. Bulk as a stimulus for satiation in Aplysia. Behav Biol. 1975a;13:203–209. doi: 10.1016/s0091-6773(75)91903-3. [DOI] [PubMed] [Google Scholar]
- Susswein AJ, Kupfermann I. Localization of bulk stimuli underlying satiation in Aplysia. J Comp Physiol. 1975b;101:309–328. [Google Scholar]
- Susswein AJ, Kupfermann I, Weiss KR. The stimulus control of biting in Aplysia. J Comp Physiol. 1976;108:75–96. [Google Scholar]
- Susswein AJ, Weiss KR, Kupfermann I. Internal stimuli enhance feeding behavior in the moliusk Aplysia. Behav Neural Biol. 1984;41:90–95. doi: 10.1016/s0163-1047(84)90784-2. [DOI] [PubMed] [Google Scholar]
- Teyke T, Rosen SC, Weiss KR, Kupfermann I. Dopaminergic neuron B20 generates rhythmic neuronal activity in the feeding motor circuitry of Aplysia. Brain Res. 1993;630:226–237. doi: 10.1016/0006-8993(93)90661-6. [DOI] [PubMed] [Google Scholar]
- Trimble DL, Barker DL. Activation by dopamine of patterned motor output from the buccal ganglia of Helisoma trivolvis. J Neurobiol. 1984;15:37–48. doi: 10.1002/neu.480150105. [DOI] [PubMed] [Google Scholar]
- Weiss KR, Kupfemann I. Homology of the giant serotonergic neurons (metacerebral cells) in Aplysia and pulmonale mollusks. Brain Res. 1976;117:33–49. doi: 10.1016/0006-8993(76)90554-0. [DOI] [PubMed] [Google Scholar]
- Weiss KR, Cohen JL, Kupfermann I. Modulatory control of buccal musculature by a serotonergic neuron (metacerebal cell) in Aplysia. J Neurophysiol. 1978;41:181–203. doi: 10.1152/jn.1978.41.1.181. [DOI] [PubMed] [Google Scholar]
- Wieland SJ, Gelperin A. Dopamine elicits feeding motor program in Limax maximus. J Neurosci. 1983;3:1735–1745. doi: 10.1523/JNEUROSCI.03-09-01735.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y, Weiss KR, Kupfermann I. Distribution in the central nervous system of Aplysia of afferent fibers arising from cell bodies located in the periphery. J Comp Neurol. 1995;359:627–643. doi: 10.1002/cne.903590409. [DOI] [PubMed] [Google Scholar]
- Zeigler HP. Brainstem orosensorimotor mechanisms and the neural control of ingestive bahavior. In: Legg CR, Booth DA, editors. Appetite: neural and behavioural bases. New York: Oxford University Press; 1994. pp. 54–85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


