Key Points
ADAP interacts with talin and kindlin-3 in platelets.
ADAP is a hematopoietic component of the molecular machinery that promotes activation of and stable fibrinogen binding to αIIbβ3.
Abstract
ADAP is a hematopoietic-restricted adapter protein that promotes integrin activation and is a carrier for other adapter proteins, Src kinase–associated phosphoprotein 1 (SKAP1) and SKAP2. In T lymphocytes, SKAP1 is the ADAP-associated molecule that activates integrins through direct linkages with Rap1 effectors (regulator of cell adhesion and polarization enriched in lymphoid tissues; Rap1-interacting adapter molecule). ADAP also promotes integrin αIIbβ3 activation in platelets, which lack SKAP1, suggesting an ADAP integrin–regulatory pathway different from those in lymphocytes. Here we characterized a novel association between ADAP and 2 essential integrin-β cytoplasmic tail-binding proteins involved in αIIbβ3 activation, talin and kindlin-3. Glutathione S-transferase pull-downs identified distinct regions in ADAP necessary for association with kindlin or talin. ADAP was physically proximal to talin and kindlin-3 in human platelets, as assessed biochemically, and by immunofluorescence microscopy and proximity ligation. Relative to wild-type mouse platelets, ADAP-deficient platelets exhibited reduced co-localization of talin with αIIbβ3, and reduced irreversible fibrinogen binding in response to a protease activated receptor 4 (PAR4) thrombin receptor agonist. When ADAP was heterologously expressed in Chinese hamster ovary cells co-expressing αIIbβ3, talin, PAR1, and kindlin-3, it associated with an αIIbβ3/talin complex and enabled kindlin-3 to promote agonist-dependent ligand binding to αIIbβ3. Thus, ADAP uniquely promotes activation of and irreversible fibrinogen binding to platelet αIIbβ3 through interactions with talin and kindlin-3.
Introduction
Integrins engage in bidirectional signaling. Fibrinogen binding to integrins is regulated by inside-out signals initiated by agonist receptors, a process often called “integrin activation.” In turn, ligand-bound integrins transduce outside-in signals to regulate cellular responses, among them cytoskeletal reorganization.1 In platelets, integrin αIIbβ3 bidirectional signaling is required for efficient hemostatic platelet responses to vascular injury. Full inside-out signaling can promote increases in integrin affinity through conformational changes and increases in integrin avidity through receptor clustering; the relative contribution of each process can vary with the integrin and the cell studied.1,2 Recently, talin and kindlin, 2 adapter proteins that bind to integrin-β cytoplasmic tails, have emerged as essential regulators of integrin activation. Talin is a 280 kDa protein of the FERMT4 (4.1/ezrin/radixin, moesin domain T4) family composed of head (amino acids 1-433) and rod (482-2541) domains that are normally clasped but upon relief of auto-inhibition can bind via the Ferm3 phospho tyrosine-binding subdomain to regions of integrin β tails that include a central β-NPXY motif and a membrane-proximal motif.3-6 Talin-membrane association is promoted in platelets by talin’s interaction with Rap1 and its effector, Rap1-interacting adapter molecule (RIAM).7
The kindlin family of proteins is composed of kindlin-1, kindlin-2, and kindlin-3, which interact with the membrane-distal portion of β cytoplasmic tails that includes an NXXY motif. Deficiency of kindlin-3, the predominant isoform in hematopoietic cells, causes defects in integrin activation, resulting in a profound immune and hemostatic disorder, leukocyte adhesion deficiency syndrome (LAD-III syndrome).8-10 Talin is necessary and sufficient to activate αIIbβ3 in purified in vitro systems,11 but both talin and kindlin-3 are required for full integrin activation in platelets.8
A third adapter protein implicated in integrin regulation is the hematopoietic-restricted, alternatively-spliced 120/130 kDa adhesion and degranulation promoting adapter protein (ADAP).12,13 ADAP was previously implicated in αIIbβ3 activation in human and mouse platelets downstream of both tyrosine-kinase–coupled and G-protein–coupled receptors.14 Recent work has identified several ADAP binding partners (Figure 1A).15,16 In particular, ADAP stabilizes 2 Src kinase–associated phosphoproteins (SKAPs), SKAP1 and SKAP2, through ADAP residues 339 to 438, with only SKAP2 expressed in platelets.14,17,18 ADAP’s roles in signal transduction cascades have largely been explored in T lymphocytes, where ADAP and SKAP1 constitute a functional unit to enhance β2 integrin–mediated adhesion upon T-cell receptor stimulation. In these cells, SKAP1 is the relevant effector operating through interactions with the Rap1-guanosine triphosphate–binding proteins RIAM19 or regulator of cell adhesion and polarization enriched in lymphoid tissues (RapL).20 When lymphocytes are stimulated through chemokine (C-C motif) receptor 7, the ADAP-SKAP1 unit is recruited to separate complexes—one associated with RIAM, Mst1, talin, and kindlin-3, and the other associated with Rap1, RAPL, and Mst1.21 However, relationships between ADAP and talin or kindlin-3 have not been fully explored.
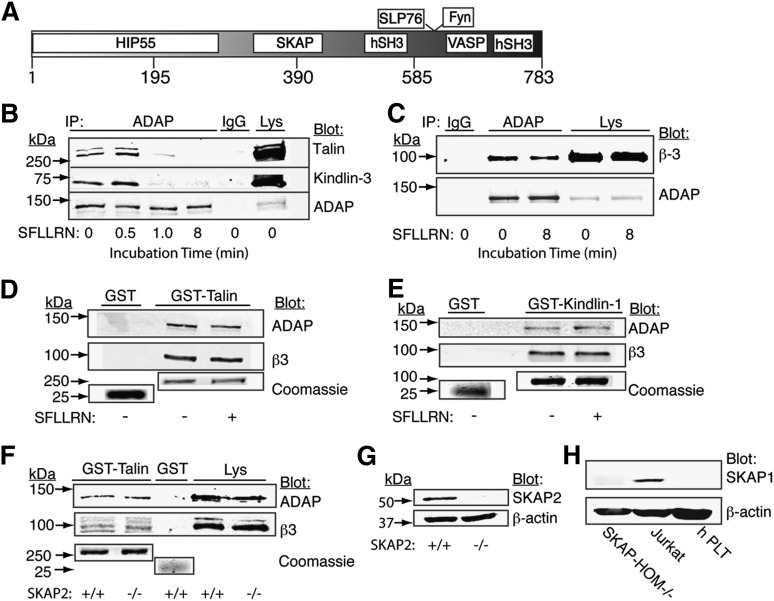
Figure 1.
Biochemical detection of ADAP in complex with talin, kindlin-3, and β3 in human and mouse platelets. (A) Shown is a primary sequence schematic of ADAP, illustrating regions known to be necessary for interaction with the indicated binding partners. (B-E) Washed human platelets were stimulated for the indicated times (or for 8 minutes if not indicated) with an activation peptide for human PAR1 (SFLLRN). Platelets were lysed with NP40 buffer containing protease inhibitors and used for immunoprecipitation (IP) (B-C) or pull-down assays (D-E), followed by Western blotting using the indicated antibodies. Unstimulated platelet lysate is shown as a positive control. For pull-down assays, lysates from human (D-E) or unstimulated SKAP2+/+ and SKAP2−/− murine (F) platelets were incubated with purified GST, GST-talin (D,F), or GST-kindlin-1 (E). Coomassie staining of 10% loading samples from the same gel indicated similar GST-fusion protein loading, (G) blotting of the murine platelet lysate with an antibody to SKAP2 confirmed the genotypes, and (H) blotting with an antibody recognizing human and mouse SKAP1 that confirmed SKAP1 expression in Jurkat cells, but not in human or SKAP2−/− platelets. Results are representative of at least 3 experiments.
In contrast to the relevance of SKAP1 in T cells, SKAP2-null platelets exhibit normal αIIbβ3 activation, excluding a necessary role for an ADAP-SKAP unit in this process.14,22 Therefore, it is unclear how ADAP interfaces with other cytoplasmic proteins in platelets to modulate αIIbβ3 activation. Given the key roles of talin and kindlin-3 in integrin function in platelets, we tested the hypothesis that ADAP may promote agonist-dependent αIIbβ3 activation through associations with either or both of these 2 integrin-proximal regulators. Using biochemical, molecular, and imaging techniques, our results are consistent with a novel cooperation between these 3 adapter molecules to regulate ligand binding to αIIbβ3.
Materials and methods
Reagents, mice, DNA constructs, and cell lines are described in supplemental Materials and methods. The institutional review board approved human studies, allowing the drawing of blood with donor consent. This study was conducted in accordance with the Declaration of Helsinki.
The Institutional Animal Care and Use Committee approved the animal strains and procedures used in the mouse studies described.
Co-immunoprecipitation and pull-down experiments
After stimulation with a human protease activated receptor 1 (PAR1)-activation peptide (SFLLRN), human platelets were lysed in NP-40 lysis buffer, and cleared lysates were immunoprecipitated with species-specific antibodies to ADAP.23 Pull-down experiments using cell lysates and glutathione S-transferase (GST)-talin or GST-kindlin-1 coupled to glutathione Sepharose beads (GE Healthcare, Buckinghamshire, UK) were performed as described.24 Immunoreactive bands were detected with the Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE).
Microscopic image acquisition and analyses
For imaging of adherent cells, washed human or mouse platelets in Walsh’s buffer14 or Chinese hamster ovary (CHO) cells in Dulbecco’s Modified Essential Medium were allowed to attach to fibrinogen-coated coverslips (100 μg/mL) for 1 hour at 37°C in the presence of a PAR receptor agonist peptide (SFLLRN for high-affinity human thrombin receptor, PAR1; AYPGKF for murine PAR4, because PAR1 is not expressed on mouse platelets) and then processed for imaging as described22 using a Deltavision deconvolution microscope (Applied Precision, Issaquah, WA) or a Zeiss LSM510 confocal microscope (Carl Zeiss Microscopy, Thornwood, NY) equipped with a ×60 1.3 numerical aperture objective for CHO cells or a ×100 1.4 numerical aperture oil objective for platelets. A Photometrics Sony Coolsnap HQ charge-coupled device camera system (Sony, ParkRidge, NJ) was used to capture images. All cells within an experiment were imaged under identical acquisition conditions. Acquired images were deconvolved using Softworks (Applied Precision) or Volocity (Perkin Elmer, Waltham, MA) and minimally processed using Adobe Photoshop, with linear manipulations applied identically for all images.
Platelet proximity ligation assays (PLA) that report on endogenous protein-protein associations at <40 nm25 were performed by following the manufacturer’s recommendations (O-Link Bioscience, Uppsala, Sweden) using oligonucleotide-conjugated secondary antibodies against mouse and rabbit primary antibodies. Protein-protein associations were detected by microscopy as bright red dots. Platelet F-actin was labeled with fluorescein iso-thiocyanate (FITC)-Phalloidin (Sigma Aldrich, St. Louis, MO). Image Pro Plus (Media Cybernetics Inc, Rockville, MD) was used to quantify the average PLA signal/platelet, with the PLA threshold set to the background signal in the absence of cells. A minimum of 200 platelets per experimental condition were examined in each experiment.
Bimolecular fluorescence complementation (BiFC) was used to determine whether ADAP could localize proximally to talin and αIIbβ3 complexes in CHO cells.26,27 Chimeric proteins VN-talin (Venus N-terminal moiety) and αIIb-VC (Venus C-terminal moiety)26 were inducibly expressed together with human PAR1 and wild-type β3 or a truncated β3, Δ724, that cannot bind to talin. Cells were then transiently-transfected with ADAP, stained with appropriate primary and secondary antibodies, and visualized by deconvolution microscopy.
To examine talin or kindlin-3 co-localization with αIIbβ3 in murine platelets, fibrinogen-adherent ADAP+/+ and ADAP−/− platelets were stimulated with the PAR4 agonist peptide, AYPGKF, and stained for talin or kindlin-3, and αIIbβ3. Eight 0.1-μm optical slices acquired by confocal microscopy, starting at the coverslip, were reconstructed to yield a 3-dimensional opacity image using Volocity software. For quantitative analysis of αIIbβ3 and talin co-localization, thresholding for peripheral αIIbβ3 was determined using a set of representative ADAP+/+ and ADAP−/− platelet images and applied to all acquired images. Associated talin fluorescence was determined and used for calculation of the Pearson correlation coefficient and voxel ratio. ADAP genotypes were verified by Western blotting of platelet lysates.
Flow cytometry
Inside-out activation of αIIbβ3-expressing CHO cells stimulated with SFLLRN was evaluated using the ligand-mimetic antibody PAC-1 after inducible expression of talin and PAR1.26,27 In parallel, PAC-1 binding was also studied after extrinsic activation of αIIbβ3 with MnCl2. Specific PAC-1 binding was defined as that inhibitable by 5 mM ethylenediaminetetraacetic acid (EDTA), and results were normalized for αIIbβ3 expression, using antibody D57. Results shown were calculated as follows for each transfectant: PAC-1 increase with agonist = Agonist [(FLPAC-1 − FLPAC-1 EDTA)/D57]/No Agonist [(FLPAC-1 − FLPAC-1/EDTA)/D57]. To test whether ADAP is involved in promoting changes in αIIbβ3 affinity and/or αIIbβ3 avidity in platelets, AYPGKF-stimulated ADAP+/+ and ADAP−/− platelets were incubated with 70 μg/mL FITC-fibrinogen in the presence or absence of POW-2 Fab, a recombinant monomeric Fab fragment that is a derivative of PAC-1 Fab28 and that can compete with fibrinogen by selectively binding to high-affinity murine αIIbβ3.29 Specific fibrinogen binding was defined as that inhibitable by 5 mM of EDTA. The amount of POW-2 chosen for these studies was based on the observation that a twofold higher POW-2 concentration did not further inhibit specific fibrinogen binding in response to 500 μM of AYPGKF.
To test for the stabilization of fibrinogen binding to αIIbβ3 over time,30,31 washed ADAP+/+ and ADAP−/− platelets were stimulated with ADP or AYPGKF for either 5 or 45 minutes in the presence of FITC-fibrinogen. Then 5 mM of EDTA or buffer was added, incubation continued for a further 10 minutes, and EDTA-resistant, irreversible fibrinogen binding was determined by flow cytometry. The percent of irreversibly bound FITC-fibrinogen for each strain was calculated relative to its respective maximum.
Statistical analysis
Variance analyses were performed using the Student t test for unpaired samples.
Results
ADAP forms a complex with kindlins and talin in platelets. With the aim of identifying integrin-regulatory pathways for ADAP in platelets, we asked whether ADAP can interact with talin and/or kindlin-3, because the latter are proximal regulators of αIIbβ3 activation.8-10,32 An antibody to ADAP specifically co-immunoprecipitated 3% to 5% of total talin and kindlin-3 from lysates of unstimulated human platelets, and these associations appeared to decrease with time after stimulation of unstirred platelets by the PAR1 agonist, SFLLRN (Figure 1B). ADAP also co-immunoprecipitated with β3 (Figure 1C). The dynamics of decreased association between ADAP, talin, and kindlin-3 could not be accounted for by calpain cleavage of these proteins under these experimental conditions (supplemental Figure 1). In an alternate approach, GST fusion constructs of talin and kindlin-1 were used to specifically capture ADAP from platelet lysates. Kindlin-1 was used here as bait instead of kindlin-3 because of its initial availability and the highly conserved domain structure among the kindlins.33 Both GST-talin and GST-kindlin-1, but not GST alone, pulled down ADAP as well as integrin β3 from resting and SFLLRN-stimulated platelets (Figure 1D-E). ADAP was also pulled down by His-kindlin-3 (supplemental Figure 2). Unlike the importance of SKAP1 in T cells for bridging ADAP to integrins through talin,19 the SKAP protein expressed in platelets (SKAP2) was not needed for ADAP association with talin because GST-talin specifically pulled down comparable amounts of ADAP from lysates of SKAP2+/+ and SKAP2−/− murine platelets (Figure 1F), independently of SKAP1 (Figure 1H). Thus ADAP can associate with talin and kindlins independently of SKAP proteins.
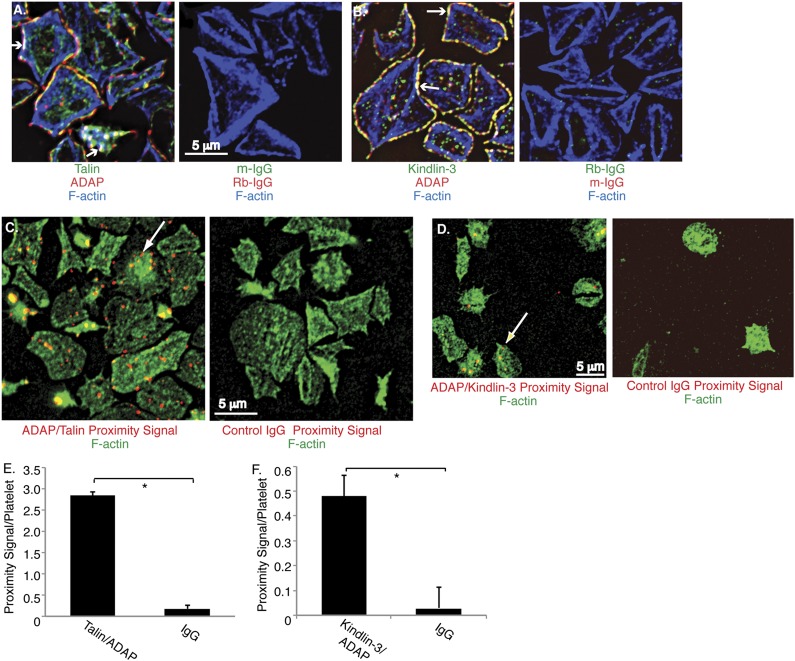
To localize ADAP in human platelets by deconvolution microscopy, SFLLRN-stimulated platelets were allowed to spread on fibrinogen. Under these conditions, ADAP co-localized with talin (Figure 2A) and kindlin-3 (Figure 2B), particularly at platelet peripheries, and often together with actin (arrows). To gain further insight into the proximity of these associations, we used a proximity ligation assay that detects native protein-protein associations within 40 nm.25 The strength of the output signal in PLA can vary as a function of antibody affinities, molecular orientations, and DNA amplification, but specificity is high. ADAP-talin associations produced a clear, specific PLA signal, with many bright red fluorescent dots indicating close proximity (Figure 2C). The specific signal for ADAP-kindlin-3 interactions was lower but nevertheless clearly present (Figure 2D and supplemental Figure 3). Quantification of PLA signals confirmed a statistically significant association between ADAP and talin and ADAP and kindlin-3 in human platelets (P < .01, n = 3; Figure 2E-F). Thus biochemical and microscopic analyses indicate that a pool of ADAP is proximal to talin and kindlin-3 in platelets.
Figure 2.
Microscopic detection of ADAP association with talin and kindlin-3 in human platelets. Washed human platelets were stimulated with SFLLRN, plated on fibrinogen-coated coverslips for 1 hour, and processed as described in Methods. (A-B) Permeabilized platelets were stained with antibodies against ADAP (red) and talin (green) (A), kindlin-3 (green) (B), or control immunoglobulin G (IgG) antibodies. Cells were counterstained with rhodamine-phalloidin to label F-actin (blue). Arrows indicate co-localization of all 3 stained proteins. (C-D) PLA to detect interactions between ADAP and talin (C) or ADAP and kindlin-3 (D). Specific PLA signals appear as bright red dots when ADAP-talin (C) and ADAP-kindlin (D) primary antibodies were used (arrows), but not when control rabbit (Rb-) or murine (m-) IgG antibodies were used. Platelets were counterstained with FITC-phalloidin (green). (E-F) Quantification of average proximity PLA signals/platelet between ADAP and talin (E) or ADAP and kindlin-3 (F) (*P < .01). Results represent the mean ± standard error of the mean of 3 experiments.
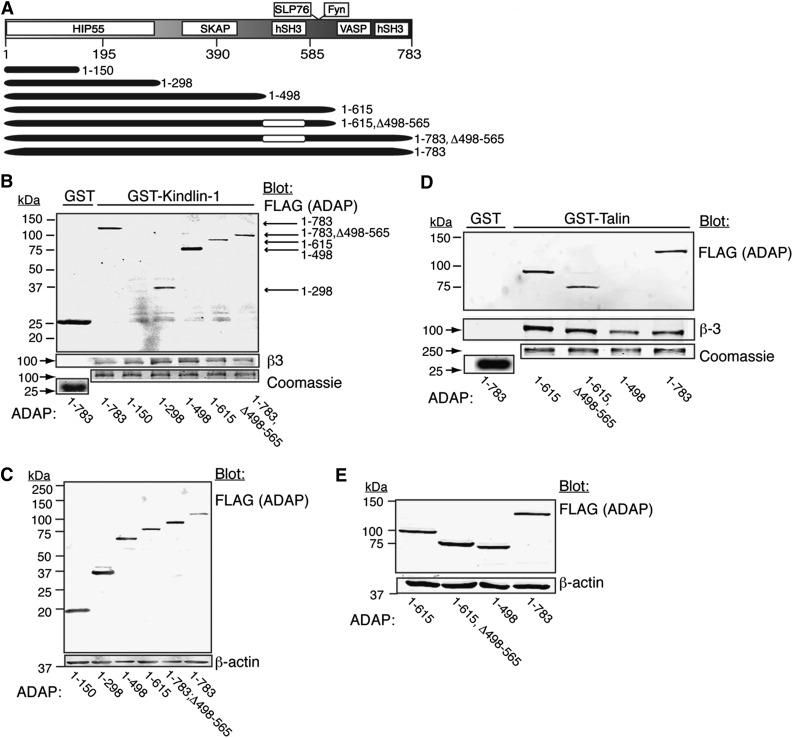
Distinct ADAP domains are required for association with talin and kindlins. To identify regions of ADAP that may be required for association with GST-talin or GST-kindlin-1 fusion proteins, ADAP truncation mutants (Figure 3A) were prepared with an amino-terminal FLAG tag, expressed in CHO cells, and tested in pull-down assays. Neither GST-kindlin-1 nor GST-talin pulled down the extreme N-terminal 1 to 150 amino acid fragment of ADAP, although it was well expressed (Figure 3 B-C). In contrast, GST-kindlin-1 pulled down ADAP fragment 1 to 298 and larger fragments containing this region, suggesting that the association minimally requires ADAP residues 150 to 298 (Figure 3B-C). GST-talin did not associate with ADAP fragments containing residues 1 to 498 but pulled down fragments containing residues between 1 to 615, even when lacking the N-terminal hSH3 region, 498 to 565, suggesting that residues between 565 and 615 mediated the talin-ADAP association (Figure 3D-E). Unlike in T cells, where ADAP interacts with RIAM and talin through SKAP1, neither SKAP1 nor RIAM was required for the ADAP associations studied here because CHO cells do not express SKAP proteins, and these associations were still observed in lysates from cells in which RIAM had been knocked down with shRNA (supplemental Materials and supplemental Figure 4). Thus 2 distinct primary amino acid sequence regions in ADAP are involved in associations with talin and kindlin, likely independent of SKAP1 and RIAM.
Figure 3.
GST-talin and GST-kindlin-1 pull-downs of ADAP and its truncation mutants. (A) Schematic map of ADAP truncation mutants. (B-E) CHO cells expressing PAR1, talin, and αIIbβ3 were transfected with the indicated FLAG-tagged ADAP (FLAG-ADAP) mutant constructs and harvested after 48 hours, and lysates were subjected to pull-down assays. (B) GST-kindlin-1 and GST pull-downs were probed for FLAG (ADAP). Gels were stained with Coomassie to assess loading, and blots were reprobed for β3 as a positive control. (C) Western blot of lysates from transfected cells in (B) showing truncated FLAG-ADAP protein expression. (D) Western blot of GST-talin and GST pull-downs probed for FLAG (ADAP). (E) Western blot of lysates from transfected cells in (D) showing FLAG-ADAP protein expression. Results are representative of at least 4 experiments. See the text for interpretations.
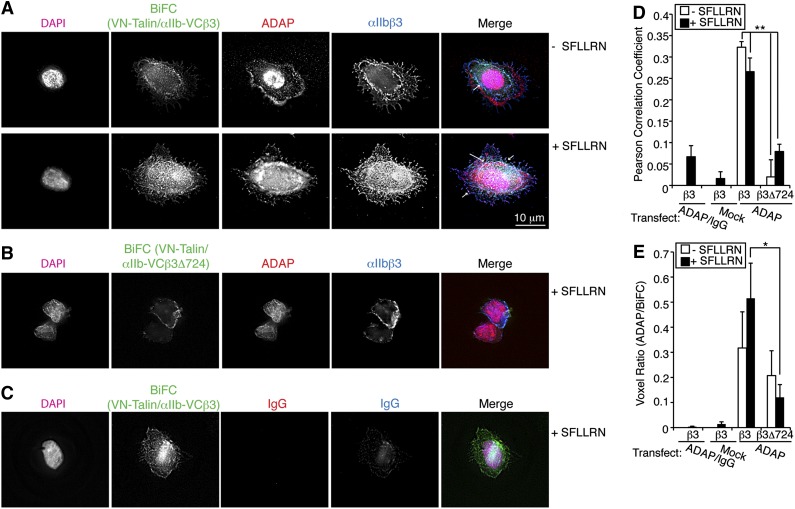
ADAP association with talin and kindlin also occurs in proximity to αIIbβ3 in nonhematopoietic (CHO) cells and promotes αIIbβ3 activation. To address whether ADAP associates with talin in proximity to αIIbβ3, we used a CHO cell model system in which protein-protein interactions involving αIIbβ3 can be detected by BiFC.26 The carboxy-terminal (VC) and amino-terminal (VN) halves of Venus were fused to αIIb and talin, respectively, producing αIIb-VC and VN-talin fusion proteins. These fusion proteins were inducibly expressed along with PAR1 in CHO cells co-expressing wild-type β3 or negative control β3Δ724, which does not bind talin.26 Cells expressing wild-type β3, but not β3Δ724, and stimulated with SFLLRN exhibited BiFC at the cell periphery and in focal adhesions, indicative of a close association between αIIb-VCβ3 and VN-talin. When ADAP was co-expressed in cells expressing wild-type β3, the BiFC signal co-localized with ADAP (Figure 4A, arrows). No such co-localization was observed in cells expressing β3Δ724 (Figure 4B). Two quantitative measures of co-localization, the Pearson correlation coefficient and the voxel ratio (R) of BiFC fluorescence to ADAP fluorescence per voxel in the image field, both indicated significantly higher ADAP co-localization with BiFC VN-talin/αIIb-VCβ3 vs VN-talin/αIIb-VCβ3Δ724 (Figure 4D; P < .01 and P < .05, respectively, n = 4). In the absence of stimulation, cells showed a slight reduction in BiFC signal and spreading (Figure 4A). In separate experiments, co-transfection of ADAP with kindlin-3 (or kindlin-2) in αIIbβ3-CHO cells showed co-localization of ADAP and kindlins at the cell periphery and in focal adhesions (supplemental Materials and supplemental Figure 5). These results provide further evidence that ADAP can co-localize with talin and kindlins in proximity to αIIbβ3 in cells.
Figure 4.
ADAP associates with talin-αIIb/β3 complexes in nonhematopoietic (CHO) cells. (A-B) Chimeric proteins VN-talin and αIIb-VC26 that yield BiFC signals in the 488 channel (green) when the proteins are close were inducibly expressed in conjunction with wild-type β3 (VN-talin/αIIb-VC β3) (A) or the truncated β3Δ724 (VN-talin/αIIb-VC β3Δ724) (B) lacking talin-binding sites. After transient transfection of ADAP, harvested cells were plated on fibrinogen without (top) or with (bottom) SFLLRN stimulation, processed as in Figure 2, and stained with antibodies against ADAP (red) and αIIbβ3 (blue) and with Hoechst (magenta) for nuclei. Arrows indicate co-localization of ADAP and BiFC signals to regions of αIIbβ3 staining at the cell periphery and in focal adhesion structures (arrows). Little BiFC signal was observed in VN-talin/αIIb-VC β3Δ724 cells (B). Neither ADAP nor αIIbβ3 staining was observed when control IgGs were used as secondary reagents (C). Results are representative of 3 experiments. (D-E) Quantification of co-localization of ADAP with BiFC signal. (D) Average Pearson correlation coefficient. (E) Quantification of the voxel ratio, an index of the relative fluorescence (eg, red/green) in a unit voxel (n = 4; *P < .05, **P < .01). Results in (D) and (E) represent means ± SEM.
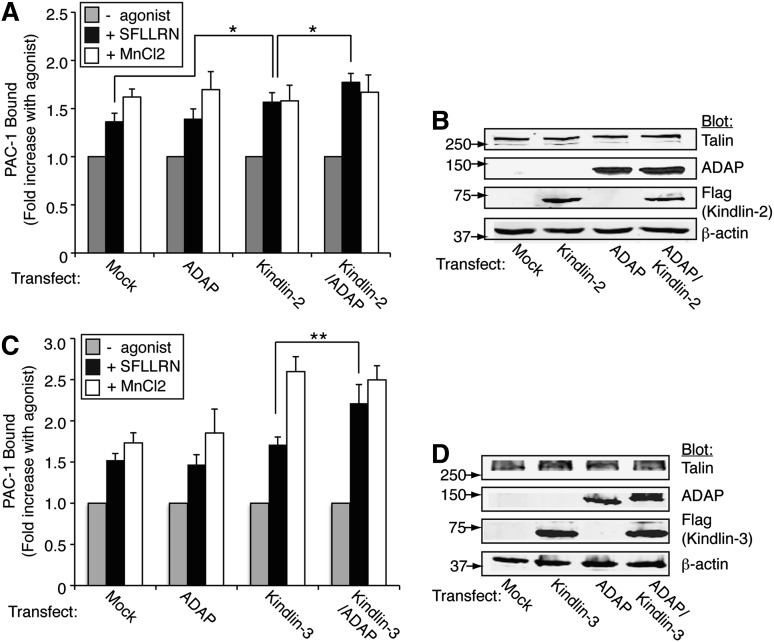
Although hematopoietic-specific kindlin-3 promotes agonist- and talin-dependent αIIbβ3 activation in platelets,8-10 a heretofore unexplained observation is that kindlin-2, but not kindlin-3, promotes αIIbβ3 activation when heterologously expressed in CHO cells along with the talin head domain.34 Given the observed interactions between ADAP and kindlins in platelets and CHO cells, we asked whether the agonist-dependent αIIbβ3 activating function of kindlin-3 could be promoted by co-expression of ADAP with full-length talin in CHO cells. When αIIbβ3-CHO cells engineered to co-express PAR1 and full-length talin were stimulated with SFLLRN, they exhibited a 1.4- to 1.6-fold increase in PAC-1 binding, which was 80% to 110% of that obtained with MnCl2, an extrinsic activator of αIIbβ3. Overexpression of kindlin-2 caused a further increase in PAC-1 binding in response to SFLLRN (P < .05, n = 8), and co-expression of ADAP enhanced binding even further (P < .05, n = 8) (Figure 5 A-B and supplemental Figure 6). In contrast to kindlin-2, expression of kindlin-3 alone failed to enhance SFLLRN-mediated PAC-1 binding, but co-expression of ADAP and kindlin-3 resulted in a significant increase in binding (P < .01, n = 6; Figure 5C-D). No differences in αIIbβ3 or ADAP expression were found among the different transfectants (supplemental Figure 7). Thus ADAP appears to enable kindlin-3 to enhance agonist- and talin-dependent activation of αIIbβ3 in CHO cells.
Figure 5.
ADAP enables kindlin-3 enhancement of αIIbβ3 activation in nonhematopoietic (CHO) cells. CHO cells expressing PAR1, talin, and αIIbβ3 were transiently transfected with mock DNA, ADAP, and/or kindlin-2 (A-B) or kindlin-3 (C-D) along with green fluorescent protein as a transfection marker. Harvested cells were unstimulated or stimulated with 50 μM SFLLRN or 1 mM MnCl2, and specific binding of PAC-1 to green fluorescent protein positive cells, normalized to αIIbβ3 expression, was determined using flow cytometry. (A) Effect of kindlin-2 and ADAP expression on PAC-1 binding (*P < .05). (B) Western blot of lysate from cells in (A) showing expression levels of transfected proteins. (C) Effect of kindlin-3 and ADAP expression on PAC-1 binding (**P < .01). (D) Western blot of lysate from cells in (C) showing expression levels of transfected proteins. Results in (A) and (C) represent the mean ± SEM of 5 experiments.
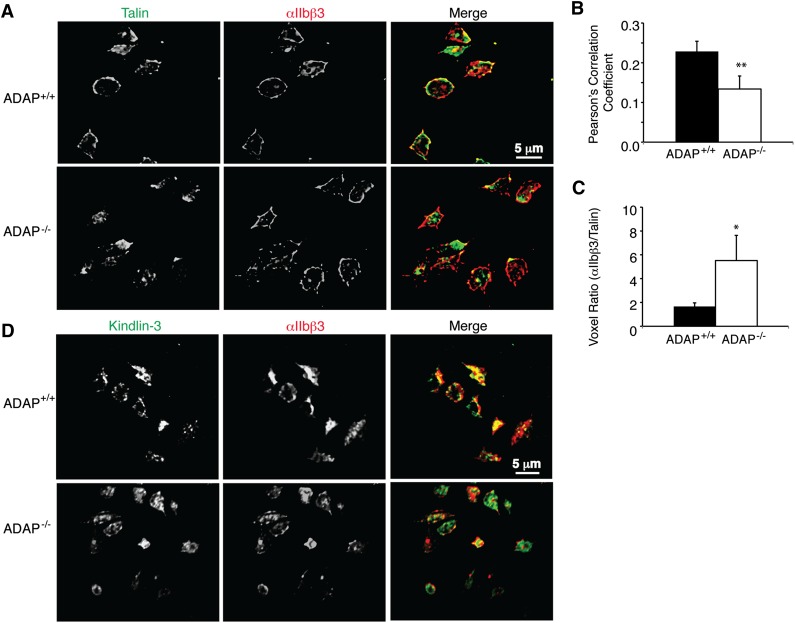
Recruitment of talin and kindlin-3 to αIIbβ3 is compromised in ADAP−/− mouse platelets. Having established that ADAP can physically and functionally associate with talin and kindlin-3, we asked whether the absence of ADAP in platelets would affect the association of talin and/or kindlin-3 with αIIbβ3. In fibrinogen-adherent ADAP+/+ mouse platelets stimulated with the PAR4 agonist peptide, AYPGKF, there was predominant, circumferential talin staining that appeared to co-localize with αIIbβ3 (Figure 6A, top). In contrast, in ADAP−/− platelets, talin staining was either diffuse or asymmetrically clumped, rather than peripherally distributed like αIIbβ3 (Figure 6A, bottom). Quantification of co-localization between αIIbβ3 and talin at the platelet periphery yielded a 71% higher Pearson correlation coefficient for ADAP+/+ platelets (0.229 ± 0.03) than for ADAP−/− platelets (0.134 ± 0.03; P < .02, n = 8) (Figure 6B). The voxel ratio (R) was close to the unity (R = 1.66 ± 0.3) for ADAP+/+ platelets but was significantly higher for ADAP−/− platelets (R = 5.52 ± 2) (P < .05, n = 8), suggesting that αIIbβ3 was more often free of detectable talin in the latter platelets (Figure 6C). We also noted reduced talin and β3 co-immunoprecipitation in AYPGKF-stimulated ADAP−/− platelets in suspension (supplemental Figure 8). In fibrinogen-adherent ADAP+/+ platelets, kindlin-3 was variably distributed between the cytoplasm and the periphery, and in ADAP−/− platelets, the peripheral localization for kindlin-3 was slightly reduced (Figure 6D); however, signal-to-noise ratios were not sufficiently robust to permit quantification. Thus a loss of ADAP in mouse platelets reduces αIIbβ3/talin and potentially αIIbβ3/kindlin-3 co-localization at the plasma membrane.
Figure 6.
αIIbβ3 co-localization with talin and kindlin-3 in ADAP+/+ and ADAP−/− mouse platelets. ADAP+/+ and ADAP−/− mouse platelets stimulated with AYPGKF and processed as in Figure 2 were stained for αIIbβ3 and talin (A-C) or αIIbβ3 and kindlin-3 (D), detected by Alexa-conjugated anti-mouse and anti-rabbit secondary antibodies, respectively. Eight 0.1-μm confocal optical slices were imaged starting at the coverslip and a reconstructed 3-dimensional opacity image was obtained using Volocity software. No signal was seen when IgG control primary antibodies were used. (B-C) Quantification of αIIbβ3 and talin co-localization. (B) Average Pearson correlation coefficient. (C) Quantification of the red/green voxel ratio (n = 8; *P < .05, **P < .02). Results in (B) and (C) represent means ± SEM.
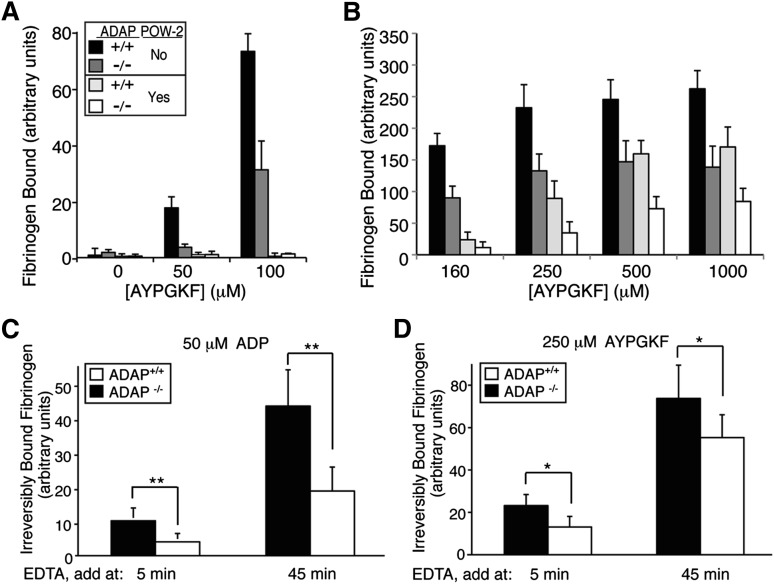
ADAP promotes affinity modulation of αIIbβ3 and stabilization of fibrinogen binding to platelets. To further explore mechanisms by which ADAP promotes ligand binding to αIIbβ3 in platelets, we used POW-2 Fab, a monovalent ligand-mimetic that selectively recognizes high-affinity murine αIIbβ3,29 in competition assays with fibrinogen. ADAP+/+ platelets stimulated with increasing concentrations of AYPGKF showed concentration-dependent specific binding of fibrinogen (Figure 7A-B) that was significantly higher at all AYPGKF concentrations than it was for ADAP−/− platelets, which showed a 50% to 80% reduction. The addition of POW-2 Fab at AYPGKF concentrations ≤100 μM (Figure 7A) reduced fibrinogen binding to baseline levels (P values in supplemental Table 1), suggesting that decreases in fibrinogen binding to ADAP−/− platelets observed under these conditions were caused mostly by reduced affinity modulation. However, in the presence of AYPGKF at concentrations ≥160 µM, POW-2 Fab inhibited fibrinogen binding only partially (Figure 7B), suggesting an additional component of avidity modulation (eg, αIIbβ3 clustering)35 under conditions of strong platelet stimulation, consistent with a recent report showing that kindlin-3 primarily affects αIIbβ3 clustering.36 Nonetheless, even here, ADAP−/− platelets still showed less fibrinogen binding than ADAP+/+ platelets (Figure 7B).
Figure 7.
Role of ADAP in fibrinogen binding to platelets. (A-B) Effect of monovalent POW-2 Fab on binding of multivalent fibrinogen. ADAP+/+ and ADAP−/− platelets were stimulated with AYPGKF in the presence or absence of POW-2 Fab and binding of FITC-fibrinogen was quantified by flow cytometry. Results are presented as specific FITC-fibrinogen binding, as defined in Materials and methods. Note the complete inhibition of FITC-fibrinogen binding by POW-2 Fab at relatively low (≤100 μM) (A) but not high (≥160 µM) (B) concentrations of AYPGKF. Also note the reduced fibrinogen binding to ADAP−/− platelets compared with ADAP+/+ platelets, with or without POW-2 Fab. Results represent the mean ± SEM of at least 3 experiments. Statistical analysis results are presented in supplemental Table 1. (C-D) Irreversible fibrinogen binding. ADAP+/+ and ADAP−/− platelets were incubated with ADP (C) or AYPGKF (D) together with FITC-fibrinogen for 5 or 45 minutes before the addition of EDTA or buffer as a control. Platelets were then incubated for an additional 10 minutes, and irreversible FITC-fibrinogen binding was quantified. Results represent the mean ± SEM of 6 experiments (*P < .05, **P < .01).
In platelets, fibrinogen binding to αIIbβ3 becomes increasingly irreversible (EDTA resistant) over time.30,31 To assess whether ADAP affects this aspect of fibrinogen binding, mouse platelets were stimulated with ADP or AYPGKF for 5 or 45 minutes in the presence of fibrinogen, followed by quantification of irreversible fibrinogen binding. In ADAP+/+ platelets stimulated with ADP, irreversible fibrinogen binding increased fourfold at 45 minutes compared with that seen at 5 minutes (Figure 7B), attaining 40% of total specific binding. In sharp contrast, irreversible fibrinogen binding to ADAP−/− platelets never reached >25% of total binding, and at 45 minutes it was 60% lower than that for ADAP+/+ platelets (P < .01, n = 6). ADAP+/+ and ADAP−/− platelets attained higher levels of irreversible fibrinogen binding upon stimulation with AYPGKF compared with ADP. Nonetheless, ADAP−/− platelets still exhibited 28% less irreversible fibrinogen binding than ADAP+/+ platelets (P < .05, n = 7). Together, these results suggest that ADAP helps to promote affinity modulation of αIIbβ3 as well as irreversible fibrinogen binding to the integrin.
Discussion
Previous work has shown that deletion of ADAP in mouse platelets decreases, but does not eliminate, agonist-induced αIIbβ3 activation, fibrinogen binding, and platelet aggregation.14 In the present study, we have uncovered the mechanistic underpinnings of the previous work by making the following new observations that position ADAP as a member of a previously unrecognized adapter triad for regulating αIIbβ3: (1) In both human and mouse platelets, ADAP, talin, and kindlins are closely associated, as determined by a variety of techniques, including proximity ligation and co-localization in cells and co-immunoprecipitation and pull-down assays in vitro; (2) ADAP association with talin and kindlins is also detectable in a CHO cell model system engineered for studies of αIIbβ3 signaling, thereby illustrating the independence of this association from other hematopoietic-specific binding partners, such as SKAP1 and SKAP2; (3) GST-kindlin and GST-talin fusion proteins pull down distinct ADAP fragments, mapping potentially relevant interaction residues in ADAP to amino acids 150 to 298 and 565 to 615, respectively; (4) ADAP synergizes with kindlin-3 to promote PAR1-mediated talin-dependent αIIbβ3 activation in CHO cells; and (5) deletion of ADAP in mouse platelets is correlated with decreased association of talin with αIIbβ3, reduced agonist-dependent activation of αIIbβ3, and decreased irreversible fibrinogen binding. Altogether, these results are consistent with a scaffolding role for ADAP that promotes both αIIbβ3 activation and irreversible fibrinogen binding through interactions with the integrin-regulatory adapters talin and kindlin-3.
Our results do not definitely establish the direct binding of ADAP to talin or kindlin-3 in cells; consequently, the physical associations we have uncovered could include additional linker proteins. Of ADAP’s known binding partners, only SH2-containing leukocyte protein of 76 kDa (SLP-76)37 and HIP-5538 have been implicated in inside-out αIIbβ3 signaling in platelets, and neither of these proteins is known to associate directly with integrins, talin, or kindlin-3. Regardless, the proximal and functional associations involving ADAP, talin, and kindlin-3 implicate ADAP as a component of the biological circuitry that regulates αIIbβ3 function. Although speculative, a small pool of ADAP that is in proximity to β3 in resting platelets, perhaps through its binding partner, Fyn,12,39 might preassemble and scaffold talin and kindlin in a primed but inactive complex with αIIbβ3, as is found in resting platelets. This pool would be rapidly released from ADAP upon agonist stimulation to serve as “first responders” that bind αIIbβ3 and induce its activation. Talin itself has been shown to associate with integrin in 2 waves, controlling inside-out integrin activation and subsequent outside-in signaling,40 and ADAP may parallel these dynamics.
CHO cell experiments using BiFC and immunofluorescence microscopy demonstrated that ADAP can assemble into complexes with talin and kindlins at the plasma membrane and in focal adhesions, without additional hematopoietic proteins (Figure 4). Of the previously identified protein linkers of ADAP to talin and integrin in T lymphocytes,19,20 SKAP1 and RapL are not expressed in CHO cells, and a role for RIAM appears unlikely based on observations with RIAM knockdown CHO cells. Interestingly, the ubiquitous cytoskeletal protein VASP (vasodilator-stimulated phosphoprotein) binds ADAP, RIAM, and the kindlin-binding protein migfilin,41 and thus may potentially link ADAP with kindlin-3 in platelets. Similarly, the putative talin association region in ADAP, residues 565 to 615, encompasses sequences known to mediate ADAP interaction with NCK and SLP-76.13,42,43 Additional studies will be required to determine whether ADAP interacts with kindlin-3 or talin directly or through such intermediaries.
Overexpression of kindlin-3 alone in CHO cells failed to augment agonist- and talin-dependent PAC-1 binding to αIIbβ3 (Figure 5C), consistent with previous studies of recombinant kindlin-3 and talin head domain in CHO cells.34,44 This is in contrast to observations in hematopoietic cells, where forced expression of kindlin-3 in megakaryocytic cell lines enhances or, in kindlin-3-null lymphocytes from patients with, leukocyte adhesion deficiency syndrome (LAD-III syndrome), rescues agonist-induced ligand binding to β3 and β2 integrins and cell adhesion.9,44,45 This suggests that factors absent in CHO cells but normally present in hematopoietic cells may be necessary for kindlin-3 regulation of β2 or β3 integrin function. In this context, ADAP may partially satisfy this requirement because it functioned together with kindlin-3 to enhance PAR1-mediated PAC-1 binding to αIIbβ3 in CHO cells (Figure 5C).
The loss of ADAP appeared to reduce the association of talin and kindlin-3 with αIIbβ3 at the periphery of mouse platelets (Figure 6). This apparent decreased stoichiometry, primarily between talin and αIIbβ3 at the cell periphery, may be a factor leading to the reduced affinity modulation of αIIbβ3 and the reduced irreversible fibrinogen binding observed with ADAP−/− platelets (Figure 7). Irreversible fibrinogen binding to αIIbβ3 may depend on structural changes in the integrin, clustering of these receptors within the plane of the plasma membrane, and the establishment of integrin-cytoskeletal linkages.31,46,47 Conceivably, any or all of these adaptations may be regulated by ADAP, talin, and kindlin-3.35,48,49 In this context, ADAP’s enhancement of irreversible fibrinogen binding to αIIbβ3 in platelets may reflect an underlying promotion of integrin clustering by ADAP, as was observed for β2 integrins in lymphocytes.50,51
The signaling networks operating within different cellular environments (eg, platelets and lymphocytes) are likely to use cell context–dependent mechanisms to determine how ADAP, talin, and kindlins access and regulate integrins. For example, mouse studies have detailed in vivo roles for ADAP in platelet-dependent hemostasis and thrombosis22 and in lymphocyte-mediated graft rejection,52 apparently through different mechanisms. An improved understanding of how ADAP regulates integrins in these different cellular contexts raises the potential of selective therapeutic targeting of ADAP’s integrin-activating function in platelets or leukocytes, while leaving integrin function in the other cell type unaffected.
Supplementary Material
Acknowledgments
We thank Asoka Banno (University of California-San Diego, La Jolla, CA), David Calderwood (Yale University, New Haven, CT), and Cary Wu (University of Pittsburgh) for the PGEX4T1-GST/talin, PGEX4T3-GST-kindlin-1, and pCMV/human kindlin-2 constructs, respectively.
This study was supported by the National Institutes of Health (HL56595, HL98208).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.K.-F. designed and performed experiments and wrote the paper; J.K. and B.K. performed experiments; F.Y. and M.H.G. contributed vital reagents; and S.J.S. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ana Kasirer-Friede, Department of Medicine, University of California, San Diego, 9500 Gilman Dr, La Jolla, CA 92037-0726; e-mail: akasirerfriede@ucsd.edu.
References
- 1.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11(4):288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15(5):547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Critchley DR. Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu Rev Biophys. 2009;38:235–254. doi: 10.1146/annurev.biophys.050708.133744. [DOI] [PubMed] [Google Scholar]
- 4.Ye F, Kim C, Ginsberg MH. Reconstruction of integrin activation. Blood. 2012;119(1):26–33. doi: 10.1182/blood-2011-04-292128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leisner TM, Yuan W, DeNofrio JC, Liu J, Parise LV. Tickling the tails: cytoplasmic domain proteins that regulate integrin alphaIIbbeta3 activation. Curr Opin Hematol. 2007;14(3):255–261. doi: 10.1097/MOH.0b013e3280dce543. [DOI] [PubMed] [Google Scholar]
- 6.Das M, Subbayya Ithychanda S, Qin J, Plow EF. Mechanisms of talin-dependent integrin signaling and crosstalk. Biochim Biophys Acta. 2014;1838(2):579–588. doi: 10.1016/j.bbamem.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han J, Lim CJ, Watanabe N, et al. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol. 2006;16(18):1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 8.Moser M, Bauer M, Schmid S, et al. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med. 2009;15(3):300–305. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- 9.Svensson L, Howarth K, McDowall A, et al. Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat Med. 2009;15(3):306–312. doi: 10.1038/nm.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malinin NL, Zhang L, Choi J, et al. A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat Med. 2009;15(3):313–318. doi: 10.1038/nm.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye F, Hu G, Taylor D, et al. Recreation of the terminal events in physiological integrin activation. J Cell Biol. 2010;188(1):157–173. doi: 10.1083/jcb.200908045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Silva AJ, Li Z, de Vera C, Canto E, Findell P, Rudd CE. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production. Proc Natl Acad Sci USA. 1997;94(14):7493–7498. doi: 10.1073/pnas.94.14.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musci MA, Hendricks-Taylor LR, Motto DG, et al. Molecular cloning of SLAP-130, an SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J Biol Chem. 1997;272(18):11674–11677. doi: 10.1074/jbc.272.18.11674. [DOI] [PubMed] [Google Scholar]
- 14.Kasirer-Friede A, Moran B, Nagrampa-Orje J, et al. ADAP is required for normal alphaIIbbeta3 activation by VWF/GP Ib-IX-V and other agonists. Blood. 2007;109(3):1018–1025. doi: 10.1182/blood-2006-05-022301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witte A, Degen J, Baumgart K, et al. Emerging roles of ADAP, SKAP55, and SKAP-HOM for integrin and NF-κB signaling in T cells. J Clin Cell Immunol. 2012. http://www.omicsonline.org/emerging-roles-of-adap-skap-and-skaphom-for-integrin-and-nfb-signaling-in-t-cells-2155-9899.S12-002.pdf.
- 16.Jordan MS, Koretzky GA. Coordination of receptor signaling in multiple hematopoietic cell lineages by the adaptor protein SLP-76. Cold Spring Harb Perspect Biol. 2010;2(4):a002501. doi: 10.1101/cshperspect.a002501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marie-Cardine A, Verhagen AM, Eckerskorn C, Schraven B. SKAP-HOM, a novel adaptor protein homologous to the FYN-associated protein SKAP55. FEBS Lett. 1998;435(1):55–60. doi: 10.1016/s0014-5793(98)01040-0. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Norton DD, Precht P, Martindale JL, Burkhardt JK, Wange RL. Deficiency of ADAP/Fyb/SLAP-130 destabilizes SKAP55 in Jurkat T cells. J Biol Chem. 2005;280(25):23576–23583. doi: 10.1074/jbc.M413201200. [DOI] [PubMed] [Google Scholar]
- 19.Ménasché G, Kliche S, Chen EJ, Stradal TE, Schraven B, Koretzky G. RIAM links the ADAP/SKAP-55 signaling module to Rap1, facilitating T-cell-receptor-mediated integrin activation. Mol Cell Biol. 2007;27(11):4070–4081. doi: 10.1128/MCB.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raab M, Wang H, Lu Y, et al. T cell receptor “inside-out” pathway via signaling module SKAP1-RapL regulates T cell motility and interactions in lymph nodes. Immunity. 2010;32(4):541–556. doi: 10.1016/j.immuni.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kliche S, Worbs T, Wang X, et al. CCR7-mediated LFA-1 functions in T cells are regulated by 2 independent ADAP/SKAP55 modules. Blood. 2012;119(3):777–785. doi: 10.1182/blood-2011-06-362269. [DOI] [PubMed] [Google Scholar]
- 22.Kasirer-Friede A, Ruggeri ZM, Shattil SJ. Role for ADAP in shear flow-induced platelet mechanotransduction. Blood. 2010;115(11):2274–2282. doi: 10.1182/blood-2009-08-238238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasirer-Friede A, Cozzi MR, Mazzucato M, De Marco L, Ruggeri ZM, Shattil SJ. Signaling through GP Ib-IX-V activates alpha IIb beta 3 independently of other receptors. Blood. 2004;103(9):3403–3411. doi: 10.1182/blood-2003-10-3664. [DOI] [PubMed] [Google Scholar]
- 24.Lad Y, Harburger DS, Calderwood DA. Integrin cytoskeletal interactions. Methods Enzymol. 2007;426:69–84. doi: 10.1016/S0076-6879(07)26004-5. [DOI] [PubMed] [Google Scholar]
- 25.Lowder MA, Appelbaum JS, Hobert EM, Schepartz A. Visualizing protein partnerships in living cells and organisms. Curr Opin Chem Biol. 2011;15(6):781–788. doi: 10.1016/j.cbpa.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe N, Bodin L, Pandey M, et al. Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin alphaIIbbeta3. J Cell Biol. 2008;181(7):1211–1222. doi: 10.1083/jcb.200803094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahner BN, Kato H, Banno A, Ginsberg MH, Shattil SJ, Ye F. Kindlins, integrin activation and the regulation of talin recruitment to αIIbβ3. PLoS ONE. 2012;7(3):e34056. doi: 10.1371/journal.pone.0034056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrams C, Deng YJ, Steiner B, O’Toole T, Shattil SJ. Determinants of specificity of a baculovirus-expressed antibody Fab fragment that binds selectively to the activated form of integrin alpha IIb beta 3. J Biol Chem. 1994;269(29):18781–18788. [PubMed] [Google Scholar]
- 29.Bertoni A, Tadokoro S, Eto K, et al. Relationships between Rap1b, affinity modulation of integrin alpha IIbbeta 3, and the actin cytoskeleton. J Biol Chem. 2002;277(28):25715–25721. doi: 10.1074/jbc.M202791200. [DOI] [PubMed] [Google Scholar]
- 30.Peerschke EI, Wainer JA. Examination of irreversible platelet-fibrinogen interactions. Am J Physiol. 1985;248(5 Pt 1):C466–C472. doi: 10.1152/ajpcell.1985.248.5.C466. [DOI] [PubMed] [Google Scholar]
- 31.Fox JE, Shattil SJ, Kinlough-Rathbone RL, Richardson M, Packham MA, Sanan DA. The platelet cytoskeleton stabilizes the interaction between alphaIIbbeta3 and its ligand and induces selective movements of ligand-occupied integrin. J Biol Chem. 1996;271(12):7004–7011. doi: 10.1074/jbc.271.12.7004. [DOI] [PubMed] [Google Scholar]
- 32.Tadokoro S, Shattil SJ, Eto K, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302(5642):103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 33.Malinin NL, Plow EF, Byzova TV. Kindlins in FERM adhesion. Blood. 2010;115(20):4011–4017. doi: 10.1182/blood-2009-10-239269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi X, Ma YQ, Tu Y, et al. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J Biol Chem. 2007;282(28):20455–20466. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- 35.Hato T, Pampori N, Shattil SJ. Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin alphaIIb beta3. J Cell Biol. 1998;141(7):1685–1695. doi: 10.1083/jcb.141.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye F, Petrich BG, Anekal P, et al. The mechanism of kindlin-mediated activation of integrin αIIbβ3. Curr Biol. 2013;23(22):2288–2295. doi: 10.1016/j.cub.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bezman NA, Lian L, Abrams CS, et al. Requirements of SLP76 tyrosines in ITAM and integrin receptor signaling and in platelet function in vivo. J Exp Med. 2008;205(8):1775–1788. doi: 10.1084/jem.20080240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tucker KL, Kaiser WJ, Bergeron AL, et al. Proteomic analysis of resting and thrombin-stimulated platelets reveals the translocation and functional relevance of HIP-55 in platelets. Proteomics. 2009;9(18):4340–4354. doi: 10.1002/pmic.200900024. [DOI] [PubMed] [Google Scholar]
- 39.Reddy KB, Smith DM, Plow EF. Analysis of Fyn function in hemostasis and alphaIIbbeta3-integrin signaling. J Cell Sci. 2008;121(Pt 10):1641–1648. doi: 10.1242/jcs.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen B, Zhao X, O’Brien KA, et al. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature. 2013;503(7474):131–135. doi: 10.1038/nature12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Tu Y, Gkretsi V, Wu C. Migfilin interacts with vasodilator-stimulated phosphoprotein (VASP) and regulates VASP localization to cell-matrix adhesions and migration. J Biol Chem. 2006;281(18):12397–12407. doi: 10.1074/jbc.M512107200. [DOI] [PubMed] [Google Scholar]
- 42.Sylvester M, Kliche S, Lange S, et al. Adhesion and degranulation promoting adapter protein (ADAP) is a central hub for phosphotyrosine-mediated interactions in T cells. PLoS ONE. 2010;5(7):e11708. doi: 10.1371/journal.pone.0011708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boerth NJ, Judd BA, Koretzky GA. Functional association between SLAP-130 and SLP-76 in Jurkat T cells. J Biol Chem. 2000;275(7):5143–5152. doi: 10.1074/jbc.275.7.5143. [DOI] [PubMed] [Google Scholar]
- 44.Nakazawa T, Tadokoro S, Kamae T, et al. Agonist stimulation, talin-1, and kindlin-3 are crucial for alpha(IIb)beta(3) activation in a human megakaryoblastic cell line, CMK. Exp Hematol. 2013;41(1):79–90. doi: 10.1016/j.exphem.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14(3):325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 46.Orlando RA, Cheresh DA. Arginine-glycine-aspartic acid binding leading to molecular stabilization between integrin alpha v beta 3 and its ligand. J Biol Chem. 1991;266(29):19543–19550. [PubMed] [Google Scholar]
- 47.Peerschke EI. Maintenance of GPIIb-IIIa avidity supporting “irreversible” fibrinogen binding is energy-dependent. J Lab Clin Med. 1999;134(4):398–404. doi: 10.1016/s0022-2143(99)90155-5. [DOI] [PubMed] [Google Scholar]
- 48.Robert P, Canault M, Farnarier C, et al. A novel leukocyte adhesion deficiency III variant: kindlin-3 deficiency results in integrin- and nonintegrin-related defects in different steps of leukocyte adhesion. J Immunol. 2011;186(9):5273–5283. doi: 10.4049/jimmunol.1003141. [DOI] [PubMed] [Google Scholar]
- 49.Hyduk SJ, Rullo J, Cano AP, et al. Talin-1 and kindlin-3 regulate alpha4beta1 integrin-mediated adhesion stabilization, but not G protein-coupled receptor-induced affinity upregulation. J Immunol. 2011;187(8):4360–4368. doi: 10.4049/jimmunol.1003725. [DOI] [PubMed] [Google Scholar]
- 50.Griffiths EK, Krawczyk C, Kong YY, et al. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science. 2001;293(5538):2260–2263. doi: 10.1126/science.1063397. [DOI] [PubMed] [Google Scholar]
- 51.Peterson EJ, Woods ML, Dmowski SA, et al. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science. 2001;293(5538):2263–2265. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- 52.Tian J, Pabst O, Römermann D, et al. Inactivation of T-cell receptor-mediated integrin activation prolongs allograft survival in ADAP-deficient mice. Transplantation. 2007;84(3):400–406. doi: 10.1097/01.tp.0000269724.06142.92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.