Abstract
Serine/threonine protein kinase C-related kinase (PKN/PRK) is a family of three isoenzymes (PKN1, PKN2, PKN3), which are widely distributed in eukaryotic organisms and share the same overall domain structure. The Nterminal region encompasses a conserved repeated domain, termed HR1a-c as well as a HR2/C2 domain. The serine/threonine kinase domain is found in the C-terminal region of the protein and shows high sequence homology to other members of the PKC superfamily.
In neurons, PKN1 is the most abundant isoform and has been implicated in a variety of functions including cytoskeletal organization and neuronal differentiation and its deregulation may contribute to neuropathological processes such as amyotrophic lateral sclerosis and Alzheimer’s disease. We have recently identified a candidate role of PKN1 in the regulation of neuroprotective processes during hypoxic stress. Our key findings were that: 1) the activity of PKN1 was significantly increased by hypoxia (1% O2) and neurotrophins (nerve growth factor and purine nucleosides); 2) Neuronal cells, deficient of PKN1 showed a decrease of cell viability and neurite formation along with a disturbance of the F-actinassociated cytoskeleton; 3) Purine nucleoside-mediated neuroprotection during hypoxia was severely hampered in PKN1 deficient neuronal cells, altogether suggesting a potentially critical role of PKN1 in neuroprotective processes.
This review gives an up-to-date overview of the PKN family with a special focus on the neuroprotective role of PKN1 in hypoxia.
Keywords: Hypoxia, neuroprotection, PKN, PRK, protein kinase C-related kinase, purine nucleosides, review.
INTRODUCTION
Protein kinase C-related kinase 1 (PKN1/PRK1) belongs to the protein kinase C (PKC)-related kinase family (PKN/PRK), a recently discovered subfamily of the AGC serine/threonine protein kinases [1-3]. PKNs are widely distributed in eukaryotic organisms, such as starfish, amphibians, insects and mammals [4, 5]. To date three different PKN isoforms have been described: PKN1 (PKN alpha/PRK1/PAK-1), PKN2 (PKN gamma/PRK2/PAK-2) and PKN3 (PKN beta/PRK3) [1, 6, 7]. Although PKN isoforms are intimately related they may not substitute for one another, consistent with the isoform-specific effects and varying tissue expression levels, as reviewed: [8]. The expression of PKN1 in human and rat tissue [6, 9, 10] and PKN2 in mouse tissue [11] is rather ubiquitous. However, PKN3 expression is more restricted to specific tissues including skeletal muscle, heart, liver [12] and human cancer cell lines [7]. In neurons PKN1 is the most abundant isoform and has been implicated in a variety of functions including cytoskeletal organization and neuronal differentiation [5, 13-17]. A role of PKN1 in amyotrophic lateral sclerosis (ALS) [18, 19] and in Alzheimer’s disease [20, 21] has been implicated. Furthermore our own data suggested that PKN1 is a key-signaling element in purine nucleoside- and nerve growth factor (NGF)-mediated protection of hypoxic neurons [22, 23].
BASIC STRUCTURE OF PKNs
The PKN family members PKN1, PKN2, and PKN3 share the same overall domain structure. The N-terminal region encompasses a conserved repeated domain, termed HR1a-c (for homology repeat, also known as ACC1-3) as well as a HR2/C2 domain. The latter one is related to the calcium-dependent membrane-targeting domain in PKC and contains a C-terminal auto-inhibitory region (IR) [24]. The serine/threonine kinase domain is found in the C-terminal region of the protein and shows high sequence homology to other members of the PKC superfamily [4, 5] (Fig. 1).
Fig. (1).
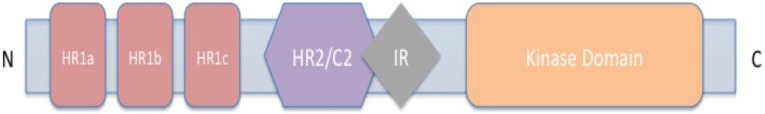
Structure of protein kinase C-related kinase (PKN).
REGULATION OF PKNs
The N-terminal region plays an important role in the regulation of PKNs, for reviews see: [8, 25, 26]. The HR1 region is involved in the interaction with the small GTPases Rho and Rac [2, 4, 5, 25, 27-36]. Rho, binds to PKN and induces a conformational change that allows binding to phosphoinositide-dependent protein kinase 1(PDK1), which phosphorylates PKN in the activation loop and stimulates its protein kinase activity [5, 25, 37]. The HR2/C2-like domain, does not bind Ca2+ as expected, but is potentially involved in the activation of PKNs by lipids or the targeting of PKNs to the membrane [4, 5, 25, 38]. The C-terminal part of the C2-
like region functions as an arachidonic acid-sensitive auto-inhibitory region (IR) [1, 24, 25, 39-42]. N-terminally truncated PKNs e.g. by caspase cleavage [18] apparently behave as constitutively-active isoforms [1, 6, 9, 25, 43-46]. In addition, PKNs were shown to be activated by various fatty acids and phospholipids in vitro, although the in vivo significance is as yet not fully characterized too [1, 6, 7, 9, 43, 44, 47, 48].
CELLULAR PKN UPSTREAM SIGNALS
The individual PKN isoforms have been linked to selective upstream signals [8] and signaling modules like neurotrophins [22, 23] and androgen receptors [49, 50] for PKN1, Platelet-derived growth factor (PDGF) and cell surface molecule CD44 for PKN2 [51] and insulin for PKN3 [52], suggesting that each isoform is associated with different adaptor proteins [11, 53, 54]. PKNs are implicated in signal transduction as effectors of Rho, Rac, PI3K (phosphoinositide 3-kinase) and Rho-like Rho-kinase [51, 52, 55-57] and all three PKN isoforms can support Rho-dependent cell migration [8].
GENERAL FUNCTION OF PKNs
As diverse as the distribution of the PKN family are its functions, which were recently reviewed [26], including regulation of cell cycle [58], receptor trafficking [59], vesicle transport [60] and apoptosis [61]. More than 20 proteins and several peptides were shown to be phosphorylated by PKN1 and PKN2, including the cytoskeletal proteins α-actinin and vimentin, as reviewed [26]. Recently, the same authors also showed that CLIP-170 (cytoplasmic linker protein of 170 kDa) and EGFR (epidermal growth factor receptor) are substrates for PKN1 and PKN3 [26]. Data by us [16] and others [60-63] link PKN1 to several stress induced pathways.
PKN2 is involved in actin cytoskeletal organization [31], mainly through activation by Rho GTPases [5]. PKN2 also plays a role alongside Fyn in controlling cell–cell adhesion in keratinocytes [64] and the maturation of apical junctions [38]. In addition, PKN2 can modulate migration in astrocytes by up-regulating cortactin phosphorylation [51] PKN3 has been identified as an effector required for malignant cell growth, downstream of activated phosphoinositide 3-kinase (PI3K) [52]. More recently, it has been shown that knockdown of PKN3 can decrease the growth of prostate and pancreatic tumors, and prevent lung metastases in mouse models [65, 66].
ROLE OF PKN1 IN NEURODEGENERATIVE DISEASES
In neurons, PKN1 is the most abundant isoform and has been implicated in a variety of functions including cytoskeletal organization and neuronal differentiation [5, 13, 17]. PKN1 was shown to phosphorylate neurofilaments at sites important for neurofilament assembly [14, 15]. Dysfunction of neurofilament metabolism was strongly implicated in amyotrophic lateral sclerosis (ALS) and in some forms of Charcot-Marie-Tooth disease [18, 19]. In ALS, accumulating neurofilaments represent one of the earliest pathological changes seen in several transgenic mouse models of ALS [67-69]. Along this line, it was shown that caspase-mediated processing of PKN1, induced by excitotoxic glutamate release and other disease-associated insults leads to deregulation of PKN1 [18] and subsequently to a disruption of neurofilament organization, axonal transport mechanisms [18, 46] and potentially also to apoptosis [45]. Other results [20, 21], suggested a specific role for PKN in neurofibrillary tangle formation and neurodegeneration in damaged neurons in Alzheimer’s disease. Authors showed that PKN phosphorylated tau protein, potentially playing an important role in the aggregation of tau into helical filaments. However, any clear evidence for the involvement of PKN1 in the pathogenesis of neurodegenerative diseases is as yet missing.
ROLE OF PKN1 IN HYPOXIC NEURONS
Hypoxic stress (1% O2)induces an increase in cell death of PC12 neuronal cells and primary neurons [23, 70, 71]. Targeting apoptotic processes after ischemic stroke has been a key focus of neuroprotective therapeutic interventions. Numerous authors, (see reviews [72-74]) have proposed adenosine and its receptors as targets for therapeutic approaches in stroke and related disorders. We have previously studied neuronal signaling in hypoxia and observed a protective capacity of the purine nucleosides adenosine, guanosine and inosine in both PC12 cells [22, 71, 75, 76] and in primary cerebellar granule neurons [71, 77-79]; see also our latest review: [70], which was inhibited by adenosine receptor (ADORA) antagonists, as reviewed [70].
Furthermore exposure of neuronal cells to low oxygen lead to increased phosphorylation of PKN1, which was augmented by NGF and purine nucleosides [16, 22]. siRNA-mediated knockdown of PKN1 in neuronal PC12 cells leads to an increase of cell death and inhibition of neurite formation accompanied by disturbance of the F-actin-associated cytoskeleton [16]. These results complement a previous report [80], showing the binding of PKN1 to the actin bundling protein alpha-actinin in a phosphatidylinositol-4,5-bisphosphate dependent manner.
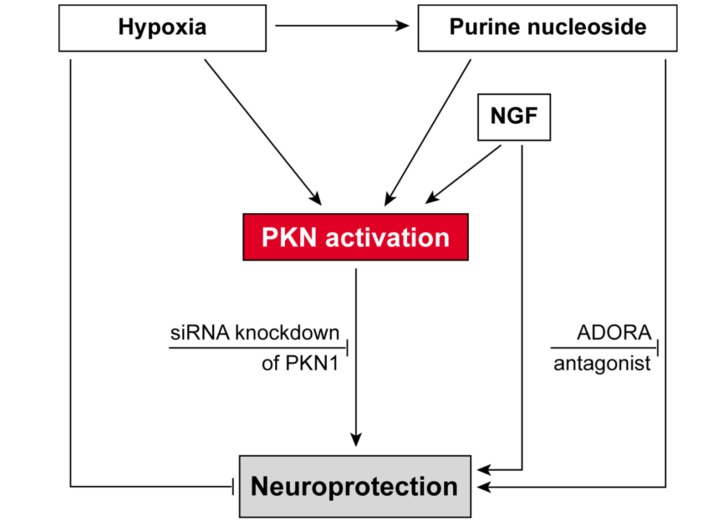
These results indicate that PKN1 may act as a key-signaling element for purine nucleoside- and potentially ADORA-associated protective mechanisms in hypoxic neuronal cells (Fig. 2).
Fig. (2).
Key-role of PKN1 in neurotrophin-mediated rescue of hypoxic neuronal cells.
CONCLUSION
In neurons, PKN1 is the most abundant isoform and has been implicated in a variety of functions including cytoskeletal organization and neuronal differentiation [5, 13, 17]. PKNs are also involved in regulation of stress response of neuronal cells as well as primary neurons and deregulation of PKN1 may contribute to neuropathological processes such as amyotrophic lateral sclerosis [18] and Alzheimer’s disease [20, 21].
Our lab has focused on neuronal stress response during hypoxia. We observed that addition of the neurotrophins NGF and purine nucleosides (PN) resulted in significant neuroprotection [16, 71], whereby the effect associated with PN was inhibited by adenosine receptor (ADORA) antagonists, as reviewed [70]. As observed in our experiments purine nucleosides as well as NGF also lead to the activation of PKN1 and to stabilization of cell viability and neurite formation, whereas knockdown of PKN1 leads to the inhibition of PKN1 and to neurodegeneration [16, 22]. Thus, PKN1 is apparently part of a key-signaling module fostering the response to hypoxic stress and likely indispensable for neurotrophin-mediated protection of hypoxic neuronal cells [16, 22, 70].
ACKNOWLEDGEMENTS
This work was supported by the Austrian Science Fund (FWF): T 421-B18, P 19578-B05 and P 26002-B24. We are grateful to Dr. C. Bandtlow and Dr. G. Baier for helpful discussions.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ABBREVIATIONS
- ADORA
= adenosine receptors
- ALS
= amyotrophic lateral sclerosis
- HR
= homology repeat
- NGF
= nerve growth factor
- PI3K
= phosphoinositide 3-kinase
- PKC
= protein kinase C
- PKN1/PRK1
= Protein kinase C-related kinase 1
REFERENCES
- 1.Mukai H, Ono Y. A novel protein kinase with leucine zipper-like sequences: its catalytic domain is highly homologous to that of protein kinase C. Biochem. Biophys. Res. Comm. 1994;199(2):897–904. doi: 10.1006/bbrc.1994.1313. [DOI] [PubMed] [Google Scholar]
- 2.Palmer RH, Ridden J, Parker PJ. Cloning and expression patterns of two members of a novel protein-kinase-C-related kinase family. Eur. J. Biochem.FEBS. 1995;227(1-2):344–51. doi: 10.1111/j.1432-1033.1995.tb20395.x. [DOI] [PubMed] [Google Scholar]
- 3.Taylor SS, Radzio-Andzelm E. Three protein kinase structures define a common motif. Structure. 1994;2(5):345–55. doi: 10.1016/s0969-2126(00)00036-8. [DOI] [PubMed] [Google Scholar]
- 4.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem. J. 1998;332 ( Pt 2):281–92. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukai H. The structure and function of PKN a protein kinase having a catalytic domain homologous to that of PKC. J Biochem (Tokyo). 2003;133(1):17–27. doi: 10.1093/jb/mvg019. [DOI] [PubMed] [Google Scholar]
- 6.Kitagawa M, Mukai H, Shibata H, Ono Y. Purification and characterization of a fatty acid-activated protein kinase (PKN) from rat testis. Biochem. J. 1995;310 (Pt 2):657–64. doi: 10.1042/bj3100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oishi K, Mukai H, Shibata H, Takahashi M, Ona Y. Identification and characterization of PKNbeta a novel isoform of protein kinase PKN expression and arachidonic acid dependency are different from those of PKNalpha. Biochem Biophys Res Comm. 1999;261(3):808–14. doi: 10.1006/bbrc.1999.1116. [DOI] [PubMed] [Google Scholar]
- 8.Lachmann S, Jevons A, De Rycker M, Casamassima A, Radtke S, Collazos A, Parker PJ. Regulatory domain selectivity in the cell-type specific PKN-dependence of cell migration. PloS one. 2011;6(7):e21732. doi: 10.1371/journal.pone.0021732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukai H, Kitagawa M, Shibata H, Takanaga H, Mori K, Shimakawa M, Miyahara M, Hirao K, Ono Y. Activation of PKN a novel 120-kDa protein kinase with leucine zipper-like sequences, by unsaturated fatty acids and by limited proteolysis. Biochem. Biophys. Res. Comm. 1994;204(1):348–56. doi: 10.1006/bbrc.1994.2466. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto T, Mukai H, Kawamata T, Taniguchi T, Ono Y, Tanaka C. Localization of PKN mRNA in the rat brain. Brain Res. Mol Brain Res. 1998;59(2):143–53. doi: 10.1016/s0169-328x(98)00155-7. [DOI] [PubMed] [Google Scholar]
- 11.Quilliam LA, Lambert QT, Mickelson-Young LA, Westwick JK, Sparks AB, Kay BK, Jenkins NA, Gilbert DJ, Copeland NG, Der CJ. Isolation of a NCK-associated kinase PRK2 an SH3-binding protein and potential effector of Rho protein signaling. J. Biol Chem. 1996;271(46):28772–6. doi: 10.1074/jbc.271.46.28772. [DOI] [PubMed] [Google Scholar]
- 12.Palmer RH, Dekker LV, Woscholski R, Le Good JA, Gigg R, Parker PJ. Activation of PRK1 by phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate.A comparison with protein kinase C isotypes. . J. Biol. Chem. 1995;270(38):22412–6. doi: 10.1074/jbc.270.38.22412. [DOI] [PubMed] [Google Scholar]
- 13.Gudi T, Chen JC, Casteel DE, Seasholtz TM, Boss GR, Pilz RB. cGMP-dependent protein kinase inhibits serum-response element-dependent transcription by inhibiting rho activation and functions. J. Biol. hem. 2002;277(40):37382–93. doi: 10.1074/jbc.M204491200. [DOI] [PubMed] [Google Scholar]
- 14.Mukai H, Miyahara M, Sunakawa H, Shibata H, Toshimori M, Kitagawa M, Shimakawa M, Takanaga H, Ono Y. Translocation of PKN from the cytosol to the nucleus induced by stresses. Proc. Natl. Acad. Sci. U. S.A. 1996;93(19):10195–9. doi: 10.1073/pnas.93.19.10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp. Cell Res. 2007;313(10):2098–109. doi: 10.1016/j.yexcr.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thauerer B, zur Nedden S, Baier-Bitterlich G. Vital role of protein kinase C-related kinase in the formation and stability of neurites during hypoxia. J. Neurochem. 2010;113(2):432–46. doi: 10.1111/j.1471-4159.2010.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volonte C, Greene LA. Nerve growth factor-activated protein kinase N.Characterization and rapid near homogeneity purification by nucleotide affinity-exchange chromatography. J. Biol. Chem. 1992;267(30):21663–70. [PubMed] [Google Scholar]
- 18.Manser C, Stevenson A, Banner S, Davies J, Tudor EL, Ono Y, Leigh PN, McLoughlin DM, Shaw CE, Miller CC. Deregulation of PKN1 activity disrupts neurofilament organisation and axonal transport. FEBS Lett. 2008;582(15):2303–8. doi: 10.1016/j.febslet.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao S, McLean J, Robertson J. Neuronal intermediate filaments and ALS: a new look at an old question. Biochim. et Biophys. Act. 2006;1762(11-12):1001–12. doi: 10.1016/j.bbadis.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Kawamata T, Taniguchi T, Mukai H, Kitagawa M, Hashimoto T, Maeda K, Ono Y, Tanaka C. A protein kinase PKN accumulates in Alzheimer neurofibrillary tangles and associated endoplasmic reticulum-derived vesicles and phosphorylates tau protein. J Neurosci. 1998;18(18):7402–10. doi: 10.1523/JNEUROSCI.18-18-07402.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taniguchi T, Kawamata T, Mukai H, Hasegawa H, Isagawa T, Yasuda M, Hashimoto T, Terashima A, Nakai M, Mori H, Ono Y, Tanaka C. Phosphorylation of tau is regulated by PKN. J. Biol Chem. 2001;276(13):10025–31. doi: 10.1074/jbc.M007427200. [DOI] [PubMed] [Google Scholar]
- 22.Tomaselli B, Podhraski V, Böck G, Baier-Bitterlich G. Early cellular responses of purine nucleoside-mediated protection of hypoxia-induced injuries of neuronal PC12 cells. Am. J Biochem Biotechnol. 2005;2(3):161–167. [Google Scholar]
- 23.Thauerer B, zur Nedden S, Baier-Bitterlich G. Vital role of protein kinase C-related kinase in the formation and stability of neurites during hypoxia. J. Neurochem. 2010;113(2):432–46. doi: 10.1111/j.1471-4159.2010.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshinaga C, Mukai H, Toshimori M, Miyamoto M, Ono Y. Mutational analysis of the regulatory mechanism of PKN: the regulatory region of PKN contains an arachidonic acid-sensitive autoinhibitory domain. J. Biochem. 1999;126(3):475–84. doi: 10.1093/oxfordjournals.jbchem.a022476. [DOI] [PubMed] [Google Scholar]
- 25.Bauer AF, Sonzogni S, Meyer L, Zeuzem S, Piiper A, Biondi RM, Neimanis S. Regulation of protein kinase C-related protein kinase 2 (PRK2): by an intermolecular PRK2-PRK2 interaction mediated by Its N-terminal domain. J. Biol. Chem. 2012;287(24):20590–602. doi: 10.1074/jbc.M111.327437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collazos A, Michael N, Whelan RD, Kelly G, Mellor H, Pang LC, Totty N, Parker PJ. Site recognition and substrate screens for PKN family proteins. Biochem. J. 2011;438(3):535–43. doi: 10.1042/BJ20110521. [DOI] [PubMed] [Google Scholar]
- 27.Maesaki R, Ihara K, Shimizu T, Kuroda S, Kaibuchi K, Hakoshima T. The structural basis of Rho effector recognition revealed by the crystal structure of human RhoA complexed with the effector domain of PKN/PRK1. Mol. Cell. 1999;4(5):793–803. doi: 10.1016/s1097-2765(00)80389-5. [DOI] [PubMed] [Google Scholar]
- 28.Amano M, Chihara K, Nakamura N, Kaneko T, Matsuura Y, Kaibuchi K. Identification of a putative target for Rho as the serine-threonine kinase protein kinase N. Science. 1996;271(5249):648–50. doi: 10.1126/science.271.5249.648. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe G, Saito Y, Madaule P, Ishizaki T, Fujisawa K, Morii N, Mukai H, Ono Y, Kakizuka A, Narumiya S. Protein kinase N (PKN): and PKN-related protein rhophilin as targets of small GTPase Rho. Science. 1996;271(5249):645–8. doi: 10.1126/science.271.5249.645. [DOI] [PubMed] [Google Scholar]
- 30.Flynn P, Mellor H, Palmer R, Panayotou G, Parker PJ. Multiple interactions of PRK1 with RhoA Functional assignment of the Hr1 repeat motif. J. Biol Chem. 1998;273(5):2698–705. doi: 10.1074/jbc.273.5.2698. [DOI] [PubMed] [Google Scholar]
- 31.Vincent S, Settleman J. The PRK2 kinase is a potential effector target of both Rho and Rac GTPases and regulates actin cytoskeletal organization. Mol Cell Biol. 1997;17(4):2247–56. doi: 10.1128/mcb.17.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Y, Settleman J. The Drosophila Pkn protein kinase is a Rho/Rac effector target required for dorsal closure during embryogenesis. Genes Dev. 1999;13(9):1168–80. doi: 10.1101/gad.13.9.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modha R, Campbell LJ, Nietlispach D, Buhecha HR, Owen D, Mott HR. The Rac1 polybasic region is required for interaction with its effector PRK1. J Biol Chem. 2008;283(3):1492–500. doi: 10.1074/jbc.M706760200. [DOI] [PubMed] [Google Scholar]
- 34.Unsal-Kacmaz K, Ragunathan S, Rosfjord E, Dann S, Upeslacis E, Grillo M, Hernandez R, Mack F, Klippel A. The interaction of PKN3 with RhoC promotes malignant growth. Mol. Oncol. 2012;6(3):284–98. doi: 10.1016/j.molonc.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blumenstein L, Ahmadian MR. Models of the cooperative mechanism for Rho effector recognition implications for RhoA-mediated effector activation. J Biol Chem. 2004;279(51):53419–26. doi: 10.1074/jbc.M409551200. [DOI] [PubMed] [Google Scholar]
- 36.Owen D, Lowe PN, Nietlispach D, Brosnan CE, Chirgadze DY, Parker PJ, Blundell TL, Mott HR. Molecular dissection of the interaction between the small G proteins Rac1 and RhoA and protein kinase C-related kinase 1 (PRK1). J. Biol. Chem. 2003;278(50):50578–87. doi: 10.1074/jbc.M304313200. [DOI] [PubMed] [Google Scholar]
- 37.Flynn P, Mellor H, Palmer R, Panayotou G, Parker PJ. Rho GTPase control of protein kinase C-related protein kinase activation by 3-phosphoinositide dependent protein kinase. J. Biol Chem. 2000;275(15):11064–70. doi: 10.1074/jbc.275.15.11064. [DOI] [PubMed] [Google Scholar]
- 38.Wallace SW, Magalhaes A, Hall A. The Rho target PRK2 regulates apical junction formation in human bronchial epithelial cells. Mol. Cell Biol. 2011;31(1):81–91. doi: 10.1128/MCB.01001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrielli B, Wettenhall RE, Kemp BE, Quinn M, Bizonova L. Phosphorylation of ribosomal protein S6 and a peptide analogue of S6 by a protease-activated kinase isolated from rat liver. FEBS Lett. 1984;175(2):219–26. doi: 10.1016/0014-5793(84)80740-1. [DOI] [PubMed] [Google Scholar]
- 40.Shiga K, Takayama K. Futaki S, Hutti J.E. Cantley LC, Ueki K, Ono Y, Mukai H. Development of an intracellularly acting inhibitory peptide selective for PKN. Biochem. J. 2010;425(2):445–543. doi: 10.1042/BJ20090380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibata H, Oishi K, Yamagiwa A, Matsumoto M, Mukai H, Ono Y. Characterization of the interaction between RhoA and the amino-terminal region of PKN. FEBS Lett. 1996;385(3):221–4. doi: 10.1016/0014-5793(96)00385-7. [DOI] [PubMed] [Google Scholar]
- 42.Kitagawa M, Shibata H, Toshimori M, Mukai H, Ono Y. The role of the unique motifs in the amino-terminal region of PKN on its enzymatic activity. Biochem. Biophys Res Comm. 1996;220(3):963–8. doi: 10.1006/bbrc.1996.0515. [DOI] [PubMed] [Google Scholar]
- 43.Morrice NA, Fecondo J, Wettenhall RE. Differential effects of fatty acid and phospholipid activators on the catalytic activities of a structurally novel protein kinase from rat liver. FEBS Lett. 1994;351(2):171–5. doi: 10.1016/0014-5793(94)00854-x. [DOI] [PubMed] [Google Scholar]
- 44.Palmer RH, Parker PJ. Expression purification and characterization of the ubiquitous protein kinase C-related kinase 1. Biochem J. 1995;309 (Pt 1):315–20. doi: 10.1042/bj3090315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi M, Mukai H, Toshimori M, Miyamoto M, Ono Y. Proteolytic activation of PKN by caspase-3 or related protease during apoptosis. Proc. Natl. Acad. Sci. U.S.A. 1998;95(20):11566–71. doi: 10.1073/pnas.95.20.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueyama T, Ren Y, Sakai N, Takahashi M, Ono Y, Kondoh T, Tamaki N, Saito N. Generation of a constitutively active fragment of PKN in microglia/macrophages after middle cerebral artery occlusion in rats. J. Neurochem. 2001;79(4):903–13. doi: 10.1046/j.1471-4159.2001.00624.x. [DOI] [PubMed] [Google Scholar]
- 47.Lim WG, Zhu Y, Wang CH, Tan BJ, Armstrong JS, Dokland T, Yang H, Zhu YZ, Teo TS, Duan W. The last five amino acid residues at the C-terminus of PRK1/PKN is essential for full lipid responsiveness. Cell Signal. 2005;17(9):1084–97. doi: 10.1016/j.cellsig.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Yu W, Liu J, Morrice NA, Wettenhall RE. Isolation and characterization of a structural homologue of human PRK2 from rat liver.Distinguishing substrate and lipid activator specificities. J Biol Chem. 1997;272(15):10030–4. doi: 10.1074/jbc.272.15.10030. [DOI] [PubMed] [Google Scholar]
- 49.Metzger E, Muller JM, Ferrari S, Buettner R, Schule R. A novel inducible transactivation domain in the androgen receptor implications for PRK in prostate cancer. EMBO J. 2003;22(2):270–80. doi: 10.1093/emboj/cdg023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metzger E, Yin N, Wissmann M, Kunowska N, Fischer K, Friedrichs N, Patnaik D, Higgins JM, Potier N, Scheidtmann KH, Buettner R, Schule R. Phosphorylation of histone H3 at threonine 11 establishes a novel chromatin mark for transcriptional regulation. Nat. Cell Biol. 2008;10(1):53–60. doi: 10.1038/ncb1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bourguignon LY, Gilad E, Peyrollier K, Brightman A, Swanson RA. Hyaluronan-CD44 interaction stimulates Rac1 signaling and PKN gamma kinase activation leading to cytoskeleton function and cell migration in astrocytes. J. Neurochem. 2007;101(4):1002–17. doi: 10.1111/j.1471-4159.2007.04485.x. [DOI] [PubMed] [Google Scholar]
- 52.Leenders F, Mopert K, Schmiedeknecht A, Santel A, Czauderna F, Aleku M, Penschuck S, Dames S, Sternberger M, Rohl T, Wellmann A, Arnold W, Giese K, Kaufmann J, Klippel A. PKN3 is required for malignant prostate cell growth downstream of activated PI 3-kinase. EMBO J. 2004;23(16):3303–13. doi: 10.1038/sj.emboj.7600345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gotoh Y, Oishi K, Shibata H, Yamagiwa A, Isagawa T, Nishimura T, Goyama E, Takahashi M, Mukai H, Ono Y. Protein kinase PKN1 associates with TRAF2 and is involved in TRAF2-NF-kappaB signaling pathway. Biochem. Biophys. Res. Comm. 2004;314(3):688–94. doi: 10.1016/j.bbrc.2003.12.148. [DOI] [PubMed] [Google Scholar]
- 54.Shibata H, Oishi K, Yamagiwa A, Matsumoto M, Mukai H, Ono Y. PKNbeta interacts with the SH3 domains of Graf and a novel Graf related protein Graf2 which are GTPase activating proteins for Rho family. J. Biochem. 2001;130(1):23–31. doi: 10.1093/oxfordjournals.jbchem.a002958. [DOI] [PubMed] [Google Scholar]
- 55.Zhu Y, Stolz D.B. Guo F, Ross M.A. Watkins SC, Tan B.J. Qi RZ, Manser E. Li QT, Bay B.H. Teo TS, Duan W. Signaling via a novel integral plasma membrane pool of a serine/threonine protein kinase PRK1 in mammalian cells. FASEB J. 2004;18(14):1722–4. doi: 10.1096/fj.04-1876fje. [DOI] [PubMed] [Google Scholar]
- 56.Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for Rho as the serine-threonine kinase protein kinase N. Science. 1996;271:648–50. doi: 10.1126/science.271.5249.648. [DOI] [PubMed] [Google Scholar]
- 57.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt A, Durgan J, Magalhaes A, Hall A. Rho GTPases regulate PRK2/PKN2 to control entry into mitosis and exit from cytokinesis. EMBO J. 2007;26:1624–36. doi: 10.1038/sj.emboj.7601637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gampel A, Parker PJ, Mellor H. Regulation of epidermal growth factor receptor traffic by the small GTPase rhoB. Curr. Biol. 1999;9(17):955–8. doi: 10.1016/s0960-9822(99)80422-9. [DOI] [PubMed] [Google Scholar]
- 60.Torbett NE, Casamassima A, Parker PJ. Hyperosmotic-induced protein kinase N 1 activation in a vesicular compartment is dependent upon Rac1 and 3-phosphoinositide-dependent kinase 1. J. Biol. Chem. 2003;278(34):32344–51. doi: 10.1074/jbc.M303532200. [DOI] [PubMed] [Google Scholar]
- 61.Takagi H, Hsu CP, Kajimoto K, Shao D, Yang Y, Maejima Y, Zhai P, Yehia G, Yamada C, Zablocki D, Sadoshima J. Activation of PKN mediates survival of cardiac myocytes in the heart during ischemia/reperfusion. Circulation Res. 2010;107(5):642–9. doi: 10.1161/CIRCRESAHA.110.217554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kajimoto K, Shao D, Takagi H, Maceri G, Zablocki D, Mukai H, Ono Y, Sadoshima J. Hypotonic swelling-induced activation of PKN1 mediates cell survival in cardiac myocytes.American journal of physiology. . Heart Circulatory Physiol. 2011;300(1):H191–200. doi: 10.1152/ajpheart.00232.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marinissen MJ, Chiariello M, Gutkind JS. Regulation of gene expression by the small GTPase Rho through the ERK6 (p38 gamma): MAP kinase pathway. Genes Dev. 2001;15(5):535–53. doi: 10.1101/gad.855801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calautti E, Grossi M, Mammucari C, Aoyama Y, Pirro M, Ono Y, Li J, Dotto GP. Fyn tyrosine kinase is a downstream mediator of Rho/PRK2 function in keratinocyte cell-cell adhesion. J Cell Biol. 2002;156(1):137–48. doi: 10.1083/jcb.200105140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aleku M, Schulz P, Keil O, Santel A, Schaeper U, Dieckhoff B, Janke O, Endruschat J, Durieux B, Roder N, Loffler K, Lange C, Fechtner M, Mopert K, Fisch G, Dames S, Arnold W, Jochims K, Giese K, Wiedenmann B, Scholz A, Kaufmann J. Atu027 a liposomal small interfering RNA formulation targeting protein kinase N3 inhibits cancer progression. Cancer Res. 2008;68(23):9788–98. doi: 10.1158/0008-5472.CAN-08-2428. [DOI] [PubMed] [Google Scholar]
- 66.Santel A, Aleku M, Roder N, Mopert K, Durieux B, Janke O, Keil O, Endruschat J, Dames S, Lange C, Eisermann M, Loffler K, Fechtner M, Fisch G, Vank C, Schaeper U, Giese K, Kaufmann J. Atu027 prevents pulmonary metastasis in experimental and spontaneous mouse metastasis models. Clin Cancer Res. 2010;16(22):5469–80. doi: 10.1158/1078-0432.CCR-10-1994. [DOI] [PubMed] [Google Scholar]
- 67.Williamson TL, Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci. 1999;2(1):50–6. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- 68.Collard JF, Cote F, Julien JP. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995;375(6526):61–4. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- 69.Zhang B, Tu P, Abtahian F, Trojanowski JQ, Lee VM. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J. Cell Biol. 1997;139(5):1307–15. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thauerer B, S. zur Nedden S, Baier-Bitterlich G. Purine nucleosides: endogenous neuroprotectants in hypoxic brain. J. Neurochem. 2012;121(3):329–42. doi: 10.1111/j.1471-4159.2012.07692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.zur Nedden S, Tomaselli B, Baier-Bitterlich G. HIF-1 alpha is an essential effector for purine nucleoside-mediated neuroprotection against hypoxia in PC12 cells and primary cerebellar granule neurons. J. Neurochem. 2008;105(5):1901–14. doi: 10.1111/j.1471-4159.2008.05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rudolphi KA, Schubert P, Parkinson F, Fredholm BB. Neuroprotective role of adenosine in cerebral ischaemia. Trends Pharmacol. Sci. 1992;13(12):439–45. doi: 10.1016/0165-6147(92)90141-r. [DOI] [PubMed] [Google Scholar]
- 73.Sweeney MI. Neuroprotective effects of adenosine in cerebral ischemia: window of opportunity. Neurosci. Biobehav. Rev. 1997;21(2):207–17. doi: 10.1016/s0149-7634(96)00011-5. [DOI] [PubMed] [Google Scholar]
- 74.von Lubitz DK. Adenosine and cerebral ischemia: therapeutic future or death of a brave concept?. Eur. J. Pharmacol. 1999;371(1):85–102. doi: 10.1016/s0014-2999(99)00135-1. [DOI] [PubMed] [Google Scholar]
- 75.Tomaselli B, Nedden SZ, Podhraski V, Baier-Bitterlich G. p42/44 MAPK is an essential effector for purine nucleoside-mediated neuroprotection of hypoxic PC12 cells and primary cerebellar granule neurons. Mol. Cell. Neurosci. 2008;38(4):559–68. doi: 10.1016/j.mcn.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Tomaselli B, Podhraski V, Heftberger V, Böck G, Baier-Bitterlich G. Purine nucleoside-mediated protection of chemical hypoxia-induced neuronal injuries involves p42/44 MAPK activation. Neurochem. Int. 2005;46(7):513–21. doi: 10.1016/j.neuint.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 77.Bocklinger K, Tomaselli B, Heftberger V, Podhraski V, Bandtlow C, Baier-Bitterlich G. Purine nucleosides support the neurite outgrowth of primary rat cerebellar granule cells after hypoxia. Eur. J. Cell Biol. 2004;83(2):51–4. doi: 10.1078/0171-9335-00362. [DOI] [PubMed] [Google Scholar]
- 78.Heftberger V, Tomaselli B, Podhraski V, Baier-Bitterlich G. Purine Nucleoside Mediated Protection of Primary Cerebellar Granule Cells after Hypoxic Insult. In: Foc. on Neurochem. Res. Nova Science Publ. 2005:1–19. [Google Scholar]
- 79.Tomaselli B, Nedden SZ, Podhraski V, Baier-Bitterlich G. p42/44 MAPK is an essential effector for purine nucleoside-mediated neuroprotection of hypoxic PC12 cells and primary cerebellar granule neurons. Mol. Cell. Neurosci. 2008;38(4):559–68. doi: 10.1016/j.mcn.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 80.Mukai H, Toshimori M, Shibata H, Takanaga H, Kitagawa M, Miyahara M, Shimakawa M, Ono Y. Interaction of PKN with alpha-actinin. J. Biol. Chem. 1997;272(8):4740–6. doi: 10.1074/jbc.272.8.4740. [DOI] [PubMed] [Google Scholar]