Abstract
Background:
Dimethyl fumarate (BG-12, Tecfidera®) is a new oral drug approved by FDA and EMA in March 2013 for relapsing – remitting multiple sclerosis (RRMS). The drug was much anticipated because of its possible superiority over currently available medications: fingolimod and teriflunomide as the only MS treatments currently available in oral form.
Objective:
The aim of this systematic review with meta-analysis was to assess the efficacy and safety of BG-12 in the treatment of RRMS.
Methods:
A systematic literature search was conducted in Medline/PubMed, EMBASE, and Cochrane Library up till 3rd November, 2013. We sought all published randomized clinical trials evaluating the use of dimethyl fumarate for the treatment of patients with RRMS. All included studies were critically appraised and analyzed with the use of Review Manager 5.1.0. software according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement protocol.
Results:
Two trials, DEFINE and CONFIRM involved 2 651 patients and compared dimethyl fumarate taken either two or three times daily with placebo in patients with RRMS. Additionally in CONFIRM trial third group of patients received glatiramer acetate. The overall results of the meta-analysis showed that BG-12 (at both dosages) given to patients with RRMS is safe and statistically significantly more effective than placebo in reducing the proportion of patients who had a relapse by 2 years, the rate of disability progression and the mean number of gadolinium-enhancing lesions at 2 years. The comparison between BG-12 and glatiramer acetate revealed that the analyzed agent could potentially be more effective in the treatment of RRMS.
Conclusions:
Despite limited RCTs data available, both analyzed BG-12 regimens showed their efficacy on clinical disease parameters and other measures of disease activity in RRMS. The safety profile of the study agent was acceptable.
Keywords: Autoimmune disorder, dimethyl fumarate, disease-modifying therapies, relapsing-remitting multiple sclerosis.
INTRODUCTION
Multiple sclerosis (MS) is a inflammatory and chronic autoimmune disorder of the central nervous system with unknown etiology and the onset usually during young adulthood [1, 2]. Like many other autoimmune diseases (such as: autoimmune thyroid disease, type 1 diabetes mellitus, inflammatory bowel disease, psoriasis, systemic lupus erythematosus and rheumatoid arthritis), the risk of developing MS appears to be influenced by genetics, environment and infectious factors [1]. This debilitating disease affects about 2.5 million people worldwide [3] and about 85-87% of patients with MS are initially diagnosed with relapsing-remitting MS (RRMS) [1, 2]. Early studies of MS established that the incidence of this disease increased with the distance from the equator and was about twice higher in women compared to men. However, these classic tenets of the epidemiology of MS may be questionable [2, 3].
Currently available treatments for MS are only partially effective [4]. MS remains an incurable condition, however a definitive treatment can reduce worsening of symptoms and postpone the development of long-term disability [1, 4, 5]. Beginning in 1993, disease-modifying therapies (DMTs) for RRMS were introduced [4, 5]. The most common treatments for patients with MS include therapy with interferon (IFN)-beta or glatiramer acetate, which are considered to be first-line drugs for relapsing-remitting MS (RRMS) [4-7]. Many of oral DMTs have demonstrated satisfactory safety and tolerability profiles in clinical trials, but important concern of this agent is the long-term safety [8]. Even though patients are treated with this kind of therapy, relapses and disability progression may still occur. Thus, there is an unmet need for the development of new treatment alternatives which potentially could improve outcomes in patients with RRMS [4, 5].
Over the last few years several oral drugs have been developed and many of them have shown benefit for treatment of RRMS [7, 8]. Dimethyl fumarate (BG-12, Tecfidera® – Biogen Idec, Germany), a simple, second-generation molecule derived from naturally occurring fumaric acid, is one of the potentially beneficial drugs [3, 9, 10]. The exact mechanisms of action of BG-12 are still under investigation, but experimental studies suggest immunomodulatory and neuroprotective properties [10].
The aim of this systematic review with meta-analysis was to assess the efficacy and safety of BG-12 in the treatment of RRMS. By systematically combining results from the identified studies, our goal is to provide precise estimate of a treatment effect and therefore strengthen the currently available evidence of BG-12 effectiveness.
METHOD
Data Sources
Primary studies were identified by searching the electronic databases, such as PubMed, EMBASE and Cochrane Central Register of Controlled Trials. PubMed, EMBASE, Cochrane Database of Systematic Reviews, CRD databases and Trip database were also searched for review articles. We also hand searched the reference lists of all included studies and any related review articles to identify relevant studies missed in the initial search. The search was conducted up till 3rd November, 2013. Searches for the primary studies were based on appropriate MeSH and EMTREE terms that met the requirement: RRMS and BG-12, combined with Boole's logical operators (Table 1).
Table 1.
MeSH subject headings and EMTREE keywords used in constructed search strategy for the primary studies (last updated: 03.11.2013).
| Keywords (Combined with Boole's Logical Operator, OR/ AND) | |
|---|---|
| Medical condition | (Multiple Sclerosis OR Disseminated Sclerosis OR MS OR Multiple Sclerosis, Acute Fulminating OR Chariot disease OR Insular sclerosis OR Sclerosis multiplex) AND (Relapsing Remitting OR Remitting-Relapsing OR Acute Relapsing OR Acute Relapsing) |
| Intervention | (dimethyl fumarate OR dimethylfumarate OR Fumaderm OR FAG 201 OR FAG201 OR FAG-201 OR BG 12 OR BG12 OR BG-12 OR BG 00012 OR BG00012 OR BG-00012 OR Tecfidera OR Panaclar OR trans butenedioic acid dimethyl ester) |
| Methodological limits | PubMed: No limits applied; EMBASE: No limits applied, Embase only; Cochrane: Cochrane Central Register of Controlled Trials: No limits applied, word variations have been searched |
| Language limits | No limits applied |
Eligibility Criteria and Study Selection
We sought all parallel, randomized controlled trials (RCTs) evaluating the use of a BG-12 monotherapy in direct comparison with placebo or active comparator for the treatment of RRMS. The included studies had to be performed with a control group and blinding. The full-text articles were preferable because of an opportunity to verify the reliability of potentially useful studies (availability of full text of clinical trials allows for an accurate assessment of their quality and also provide essential information about study population, applied treatment regimens and specific data to extract), therefore unpublished works were not taken into consideration (e.g. conference abstracts and posters). Adult patients (≥18 age) with RRMS as defined according to the McDonald criteria [11]participated in these studies. Two BG-12 dosage regimens were included in the analysis: 240 mg twice daily or 240 mg three times daily (on the basis of preclinical results), and the follow-up period should be adequately long in order to determinate the efficacy and safety outcomes accurately. To be included in our analysis, trials were required to have similar: study design, baseline characteristics of patients, follow-up period and identically defined outcomes.
The search and eligibility assessments were performed independently by two authors (P.K., AM.), and disagreements were resolved by consensus, and when needed a third author acted as an adjudicator (N.W.). One author (P.K.) read the titles, abstracts, and if necessary, full text of studies to identify potentially relevant trials, resolving any uncertainties with the second author (A.M.). Three authors (P.K., N.W., A.M.) read all full-text articles selected as potentially relevant. Studies fulfilling the eligibility criteria were included in the meta-analysis.
Outcomes
Efficacy evaluations were based on the annualized relapse rate (ARR) at 2 years, the proportion of patients who relapsed or had confirmed progression of disability by 2 years. We also assessed the change in the mean number of gadolinium-enhancing lesions on MRI at 2 years. The safety profile was evaluated on the basis of the proportion of patients who experienced any adverse events (AEs), any serious adverse events (SAEs) or discontinued the treatment due to adverse events or died from any cause.
Data Extraction
Authors (P.K. and N.W.) independently extracted the published data from each study using a standard data extraction form, and any disagreements were discussed or resolved with a fourth author (A.P.). From each selected study, we extracted information on the author, year of publication, characteristics of participants, population size, study design, intervention and dosing regimen, duration of treatment and clinical outcomes (Table 2). The methodological quality of the included randomized studies was evaluated using the Jadad scale [12].
Table 2.
Characteristics of the randomized clinical trials of BG-12 monotherapy in relapsing-remitting multiple sclerosis (RRMS).
| Study [References] |
Participants | Study Size/ No. (ITT/ PP) |
Study Design | Dosage, Follow up Period |
Clinical Endpoints | Jadad Quality Scorea |
|---|---|---|---|---|---|---|
| Gold R., 2012 [15] (DEFINE Study [15-19]) |
Adults (age 18-55 years) with a diagnosis of RRMS as defined according to the McDonald criteria, a baseline score of 0 to 5.0 on the EDSS, and disease activity as evidenced by at least one clinically documented relapse within 12 months before randomization or a brain MRI scan, obtained within 6 weeks before randomization, that showed at least one gadolinium-enhancing lesion | 1 234/ ITT | RCT (randomization performed centrally and was stratified according to site), double-blind, placebo-controlled, PG, phase III | BG-12: 480 mg or 720 mg/day, Placebo 96 weeks |
Primary end point: the proportion of patients who had a relapse by 2 years. Secondary end points: number of gadolinium-enhancing lesions and of new or enlarging hyperintense lesions on T2-weighted images, the annualized relapse rate, the time to disability progression, progression of disability, safety profile. |
4 |
| Fox R.J., 2012 [20] (CONFIRM Study [20-24]) |
1 417/ ITT | RCT, double-blind, placebo-controlled, PG, phase III | BG-12: 480 mg or 720 mg/day, GA: 20 mg/day, Placebo 96 weeks |
Primary end point: the annualized relapse rate at 2 years. Secondary end points: the number of new or enlarging hyperintense lesions on T2-weighted images, the number of new hypointense lesions on T1-weighted images, the proportion of patients with a relapse, the time to disability progression, progression of disability, safety profile. |
3 | |
| Kappos L., 2008 [38, 39] |
Adults (age 18-55 years) with a diagnosis of RRMS as defined according to the McDonald criteria, a baseline score of 0 to 5.0 on the EDSS, and disease activity as evidenced by at least one clinically documented relapse within 12 months before randomization or a brain MRI scan, obtained within 6 weeks before randomization, that showed at least one gadolinium-enhancing lesion | 256/ ITT | RCT, double-blind, placebo-controlled, PG, phase IIb | BG-12: 120 mg or 360 mg or 720 mg/day, Placebo 24 weeks; part I (placebo-controlled): 24 weeks; part II (dose-blinded safety assessment) |
Primary end point: the total number of new gadolinium-enhancing lesions over four scans. Secondary end points: the cumulative number of new gadolinium-enhancing lesions, the numbers of new or enlarging T2-hyperintense lesions and new T1 hyperintense lesions, annualised relapse rate, and disability progression, safety profile. |
4 |
EDSS- Expanded Disability Status Scale; GA- Glatiramer Acetate; ITT- intention-to-treat; MRI- magnetic resonance imaging; PG- parallel group; PP- per protocol; RCTrandomized clinical trial; RRMS- relapsing–remitting multiple sclerosis. a Jadad Quality Score range: 1- lowest, 5- highest.
Statistical Analysis
We performed meta-analyses and all statistical tests or created forest plots using Review Manager (RevMan) version 5.1.0. software. For dichotomous data, the impact of the intervention was expressed as relative risk (RR) or odds ratio (OR) when in one group any event has not occurred, with 95% confidence intervals (CI). The continuous data were expressed as weighted mean difference (WMD) with 95% confidence intervals (CI). The results obtained from separate trials were combined using appropriate statistical methods of meta-analysis. The inverse variance in Mantel-Haenszel or Der Simonian-Laird effects model was used according to the data input and heterogeneity of test results. The results for sufficiently similar outcomes and homo-genous data (determined by the degree of clinical and statistical heterogeneity) were pooled. The clinical hetero-geneity was assessed by examining the characteristics of the featured studies, whereas the statistical heterogeneity was assessed using the chi-square test, with the significance set at P value =0.10. Relative parameters were calculated using the fixed effects model when the statistical heterogeneity was not detected, and the random effects model was used when heterogeneity was present (p<0.10). This systematic review was performed according to the methods and recommendations from the Cochrane handbook [13], and the meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement protocol, as described elsewhere [14].
RESULTS
Description and Quality of Included Studies
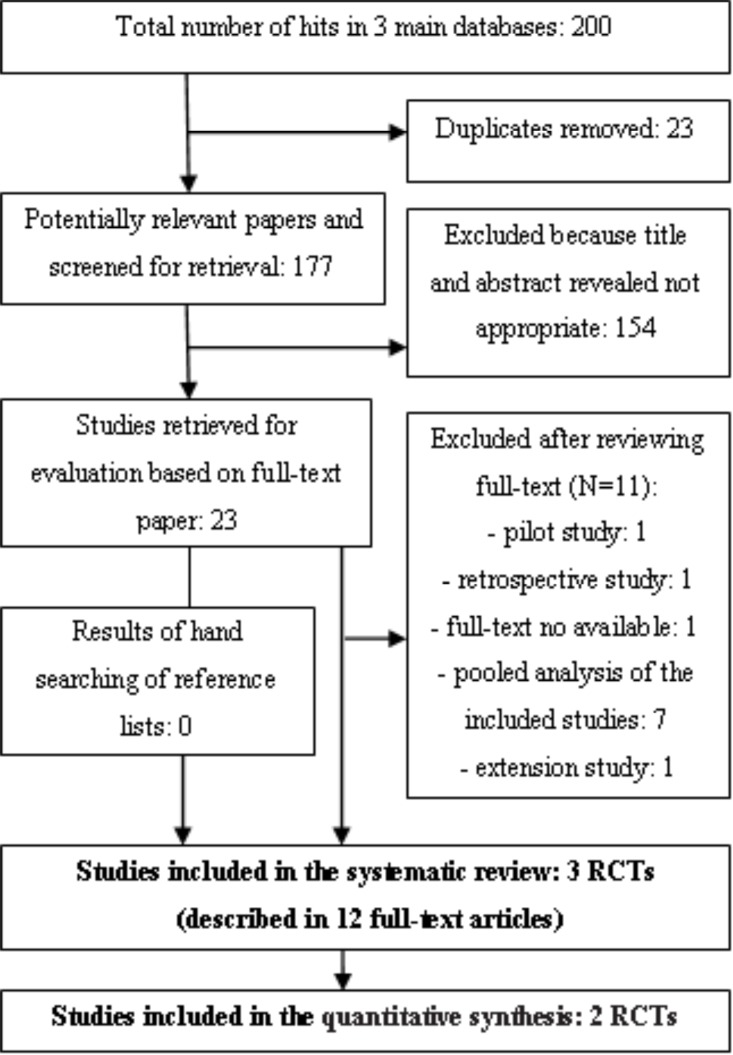
The initial search yielded 200 citations, of which 154 were excluded after examining the titles and abstracts. A further performed selection resulted in the identification of 23 potentially eligible reference papers. A total of 11 references were excluded for various reasons, and 3 RCTs described in 12 reference papers were included in qualitative synthesis, but only 2 RCTs (described in 10 full text articles) fulfilled the inclusion criteria for meta-analysis (Fig. 1).
Fig. (1).
PRISMA flow diagram for identification and selection of primary studies included in the systematic review and meta-analysis.
Two phase III RCTs (DEFINE; Determination of the Efficacy and Safety of Oral Fumarate in Relapsing–Remitting MS [15-19] and CONFIRM; Comparator and an Oral Fumarate in Relapsing–Remitting MS [20-24]) evaluated the effectiveness of BG-12 monotherapy in adult patients with RRMS, in comparison with placebo. The starting dose was 120 mg two or three times daily for the first 7 days, followed by 240 mg two or three times daily. Additionally, in CONFIRM trial [20-24] third group of patients received subcutaneous daily injections of glatiramer acetate at a dose of 20 mg. Duration of treatment in DEFINE[15-19] and CONFIRM [20-24] trials was 96 weeks. In phase IIb RCT [38, 39] adult patients with RRMS were given BG-12 at a dose of 120 mg once daily, 120 mg three times daily or 240 mg three times daily or placebo during 24 weeks of treatment, followed by additional 24 weeks for dose-blinded safety assessment. In extension period patients who received placebo were transitioned to 240 mg three times daily of BG-12. All studies were published in English as peer-reviewed articles and were randomized and double-blind trials with parallel group design. The included studies scored ≥3 points on the Jadad scale indicating good methodological quality. The characteristics and the methodological quality of the trials included are described in Table 2.
We performed a meta-analysis of the results from DEFINE[15-19] and CONFIRM [20-24] trials that compared BG-12 separately for both dosages (240 mg twice daily or three times daily) with a placebo. We also performed a direct comparison between BG-12 (separately for 240 mg twice daily or three times daily) with glatiramer acetate. The efficacy endpoints assessed in both trials were: the annualized relapse rate at 2 years, the proportion of patients who had a relapse by 2 years, the rate of disability progression and the mean change in gadolinium-enhancing lesions at 2 years. We also analyzed the incidence of any AEs and SAEs, the overall incidence of AEs leading to discontinuation of the study drug and the incidence of death from any cause. Results concerning comparison between BG-12 at a dose of 240 mg three times daily and placebo from Kappos et al. study [38, 39] were excluded from the meta-analysis because the reported outcomes were extracted at week 24, whereas results in studies DEFINE [15-19]and CONFIRM [20-24] were obtained at 96 weeks of treatment period; therefore, they should not be aggregated together. Individual results from study [38, 39] will be presented below the particular results of meta-analysis.
Outcomes
Annualized Relapse Rate at 2 Years
As presented below (Table 3) the annualized relapse rate (ARR) at 2 years was significantly reduced with each BG-12 regimen as compared to placebo (p<0.001). In this paper we were not be able to calculate the pooled ARR because the publications did not provide enough detailed and extractable data on this outcome defined as total number of relapses divided by patient-years in the study. However, the integrated analysis [25] showed that ARR in the treatment groups was reduced by 50% (BG-12 twice daily vs. placebo) and 53% (BG-12 three times daily vs. placebo) in patients with ≤1 relapse in the year before study entry and by 47% and 41% in patients with ≥2 relapses.
Table 3.
Comparison of the annualized relapse rate at 2 years.
| Study, Year | Annualized Relapse Rate [95% CI] | Relative Rate Reduction or Rate Ratio [95% CI], p value | |
|---|---|---|---|
| BG-12 Twice Daily | Placebo | BG-12 vs. Placebo | |
| Gold R., 2012 [15] (DEFINE Study) | ARR=0.17 [0.14-0.21] | ARR=0.36 [0.30-0.44] | RRR=53%; RR=0.47 [0.37-0.61], p<0.001 |
| Fox R.J., 2012 [20] (CONFIRM Study) | ARR=0.22 [0.18-0.28] | ARR=0.40 [0.33-0.49] | RRR = 44.0% [26.0-57.7], p<0.001 |
| Study, year | BG-12 twice daily | Glatiramer acetate | BG-12 vs. glatiramer acetate |
| Fox R.J., 2012 [20] (CONFIRM Study) | ARR=0.22 [0.18- 0.28] | ARR=0.29 [0.23-0.35] | RR=0.78 [0.59-1.05]; p>0.05 |
| Study, year | BG-12 three times daily | Placebo | BG-12 vs. placebo |
| Gold R., 2012 [15] (DEFINE Study) | ARR=0.19 [0.15-0.23] | ARR=0.36 [0.30-0.44] | RRR=48%; RR=0.52 [0.40-0.67], p<0.001, |
| Fox R.J., 2012 [20] (CONFIRM Study) | ARR=0.20 [0.16-0.25] | ARR=0.40 [0.33-0.49] | RRR=50.5% [33.8-63.1], p<0.001 |
| Study, year | BG-12 three times daily | Glatiramer acetate | BG-12 vs. glatiramer acetate |
| Fox R.J., 2012 [20] (CONFIRM Study) | ARR=0.20 [0.16-0.25] | ARR=0.29 [0.23-0.35] | RR=0.69 [0.51-0.94]; p<0.05 |
ARR- annualized relapse rate; CI- confidential interval; RR - rate ratio; RRR- relative rate reduction (percentage ARR reduction vs. placebo).
The results of direct comparison between BG-12 and glatiramer acetate presented in Supplementary Appendix to publication of CONFIRM study [20] showed a significantly greater treatment effect of BG-12 three times daily versus GA as measured by ARR. The difference between BG-12 twice daily and GA did not reach statistical significance.
In phase IIb study [38, 39] the annualized relapse rate in group of patients who received BG-12 at a dose of 240 mg three times daily decreased by 32% during the first 24 weeks of treatment, nonetheless the difference between BG-12 and placebo was not significant.
Proportion of Patients with Relapse and the Rate of Disability Progression by 2 Years
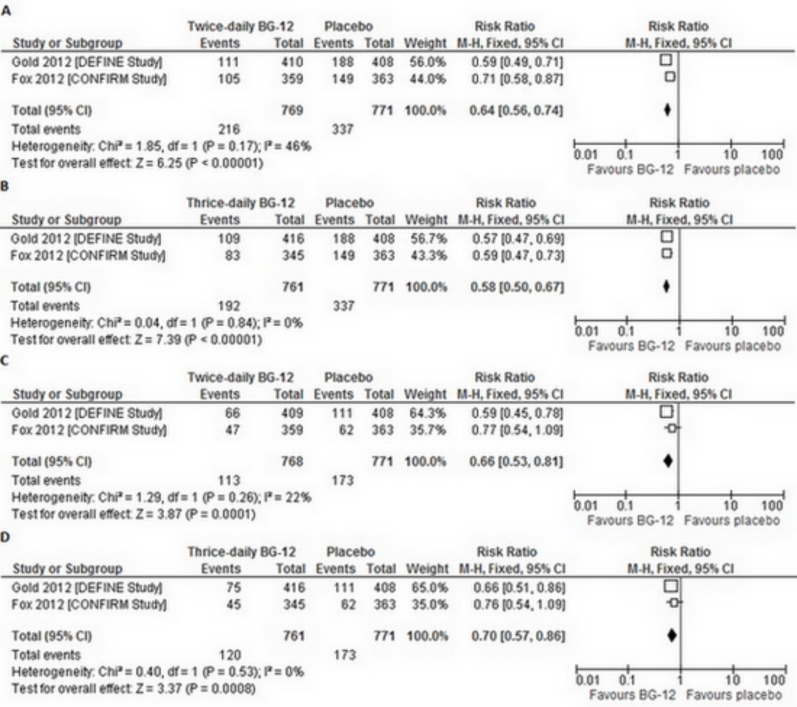
In both included RCTs, the proportion of patients who had at least one relapse of MS by 2 years was significantly reduced with each BG-12 regimen as compared with placebo. Therefore, the overall result of the meta-analysis of BG-12 efficacy (at both dosages) revealed a statistically significant difference in the proportion of patients who had a relapse in favor of the BG-12 over the placebo (for BG-12 twice daily RRfixed=0.64 [95% CI: 0.56–0.74], p<0.00001; for BG-12 three times daily RRfixed=0.58 [95% CI: 0.50–0.67], p<0.00001) (Fig. 2A,b). What is more, both BG-12 regimens prolonged the time to the first relapse, as compared to placebo [15, 20].
Fig. (2).
Forest plot of the proportion of patients who had least one relapse ([a] BG-12 240 mg twice daily vs. placebo [b] BG-12 240 mg three times daily vs. placebo) or confirmed progression of disability ([c] BG-12 240 mg twice daily vs. placebo [d] BG-12 240 mg three times daily vs. placebo) over the 2-year study period.
A comparison between BG-12 three times daily and glatiramer acetate in CONFIRM study revealed a statistically significant difference in the proportion of patients who had a relapse in favour of BG-12 (RR=0.75 [95% CI: 0.59–0.96], p<0.05). However, the result of comparison between lower dosage of BG-12 (240 mg twice daily) and GA showed no statistically significant differences between groups (RR=0.91 [95% CI: 0.72–1.13], p>0.05).
In study [38, 39], there was no statistically significant difference between BG-12 at a dose of 240 mg three times daily and placebo in respect to the relative risk of relapse during 24 weeks of treatment (RR=0.77 [95% CI: 0.40–1.48], p>0.05). The rate of disability progression was not evaluated in this trial.
In the DEFINE trial, BG-12 significantly reduced the risk of confirmed progression of disability over the 2-year study period in the twice-daily and the thrice-daily BG-12 groups when compared to placebo. In the CONFIRM study, the disability progression was not significantly reduced with twice-daily and thrice-daily BG-12, when compared to placebo. However, the overall result of the meta-analysis revealed a statistically significant difference in the proportion of patients who had confirmed progression of disability in favor of the BG-12 (both dosages) over the placebo (for BG-12 twice daily RRfixed=0.66 [95% CI: 0.53–0.81], p=0.0001; for BG-12 three times daily RRfixed=0.70 [95% CI: 0.57–0.86]], p=0.0008) (Fig. 2c,d).
The result of the comparison between each BG-12 regimen and glatiramer acetate did not reveal a statistically significant difference between analyzed groups in the proportion of patients who had confirmed progression of disability (for BG-12 twice daily and BG-12 three times daily RR=0.82 [95% CI: 0.57–1.17], p>0.05).
Gadolinium-Enhancing Lesions at 2 Years
The treatment with BG-12 was also associated with the reduction in the mean number of gadolinium-enhancing lesions on MRI at 2 years in comparison with placebo. The differences between BG-12 and the placebo were statistically significant for both dosages of the study agent (for BG-12 twice daily WMDfixed=-1.64 [95% CI: -2.17 – -1.10], p<0.00001; for BG-12 three times daily WMDfixed=-1.41 [95% CI: -1,96 – -0.85], p<0.00001).
The comparison between each BG-12 regimen and glatiramer acetate in CONFIRM trial did not reveal a statistically significant difference of the reduction in the mean number of gadolinium-enhancing lesions on MRI (for BG-12 twice daily MD=-0.20 [95% CI: -0.59–0.19], p>0,05; for BG-12 three times daily MD=-0.30 [95% CI: -0.64–0.04], p>0,05). However, post hoc evaluation showed a significant treatment effects of BG-12 (both dosages) compared to glatiramer acetate for the number of new or enlarging T2 lesions on MRI [20].
In study [38, 39] excluded from the meta-analysis, treatment with BG-12 at a dose of 240 mg three times daily was associated with significant reduction in the mean number of new gadolinium-enhancing lesions, when compared to placebo at weeks 12-24 (p<0.0001).
Any Adverse Events and Serious Adverse Events
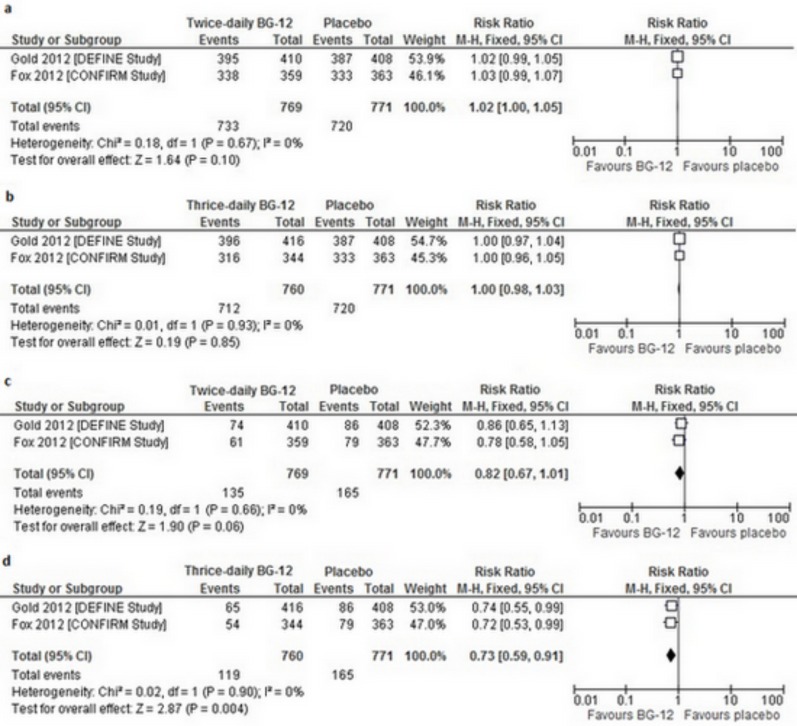
The overall incidence of AEs was similar across the study groups in both trials and the reported events were mild or moderate in severity [15, 20]. In the overall analysis, there was no significant difference in the frequency of any AEs between BG-12 given at both dosages and the placebo (for BG-12 twice daily RRfixed=1.02 [95% CI: 1.00–1.05], p=0.1; for BG-12 three times daily RRfixed=1.00 [95% CI: 0.98–1.03], p=0.85) (Fig. 3A,b). AEs that occurred more frequently in both trials in patients receiving BG-12 than in placebo groups included: flushing and gastrointestinal events (e.g. diarrhea, nausea, upper abdominal pain) [15, 20].
Fig. (3).
Forest plot of the incidence of adverse events ([a] BG-12 240 mg twice daily vs. placebo [b] BG-12 240 mg three times daily vs. placebo) and serious adverse events ([c] BG-12 240 mg twice daily vs. placebo [d] BG-12 240 mg three times daily vs. placebo) over the 2- year study period.
On the other hand, there was a significant difference in the risk of any AEs between BG-12 given at both dosages and glatiramer acetate in favor of glatiramer acetate (for BG-12 twice daily RR=1.09 [95% CI: 1.04–1.14], p<0.05; for BG-12 three times daily RR=1.06 [95% CI: 1.01–1.12], p<0.05).
In the DEFINE trial and the CONFIRM study, the incidence of SAEs was also similar across the study groups.No statistically significant differences in the overall analysis of the incidence of any SAEs were detected in the comparison of the BG-12 240 mg twice daily with the placebo (RRfixed=0.82 [95% CI: 0.67–1.01], p=0.06) (Fig. 3c). However, the overall result of the meta-analysis of BG-12 at a dose of 240 mg three times daily revealed a statistically significant difference in the frequency of SAEs in favor of the BG-12 over the placebo (RRfixed=0.73 [95% CI: 0.59–0.91], p=0,004) (Fig. 3d).
Comparison between each BG-12 regimen and glatiramer acetate did not reveal a statistically significant difference between groups in the risk of any SAEs (for BG-12 twice daily RR=0.99 [95% CI 0.72–1.38], p>0.05; for BG-12 three times daily RR=0.92 [95% CI: 0.66–1.29], p>0.05).
Results from study [38, 39], which was not included in the meta-analysis, demonstrated no statistically significant differences between BG-12 240 mg three times daily and placebo in the frequency of AEs (RR=1.16 [95% CI: 0.98–1.39], p>0.05) as well as in the risk of SAEs (RR=0.90 [95% CI: 0.36–2.26], p>0.05) during 24 weeks of treatment.
Adverse Events Leading to a Discontinuation of the Study Drug
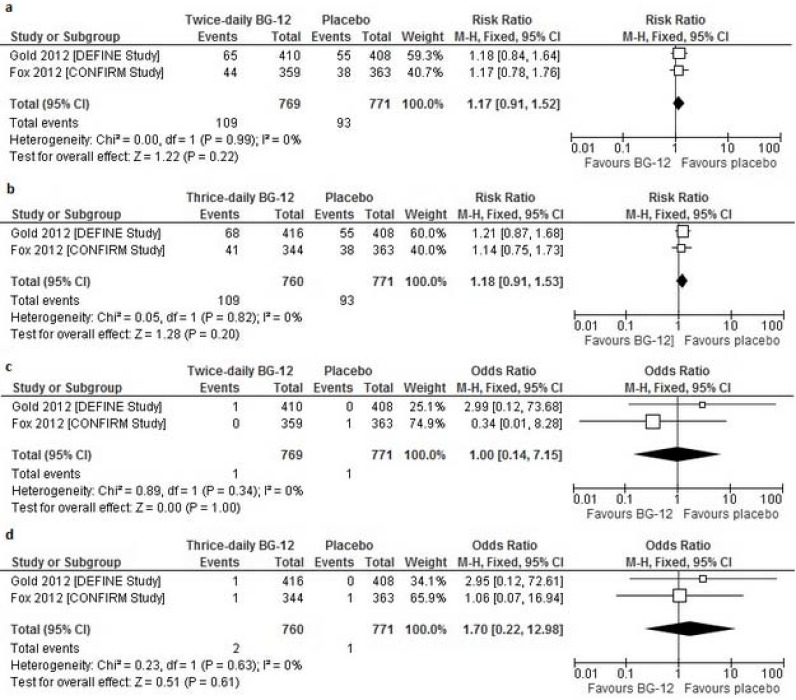
Overall, the incidence of AEs leading to discontinuation of the treatment was similar across the groups in both studies, however, discontinuations due to flushing and overall gastrointestinal events (nausea and diarrhea) occurred more frequently in patients who received BG-12 than in the placebo groups in both trials included in the current meta-analysis [15, 20]. The overall analysis showed no significant difference in the frequency of any AEs leading to discontinuation between BG-12 given at both dosages and the placebo (for BG-12 twice daily RRfixed=1.17 [95% CI: 0.91–1.52], p=0.22; for BG-12 three times daily RRfixed=1.18 [95% CI: 0.91–1.53], p=0.20) (Fig. 4a,b).
Fig. (4).
Forest plot of the incidence of adverse events leading to treatment discontinuation ([a] BG-12 240 mg twice daily vs. placebo [b] BG-12 240 mg three times daily vs. placebo) and deaths of any cause ([c] BG-12 240 mg twice daily vs. placebo [d] BG-12 240 mg three times daily vs. placebo) over the 2-year study period.
Also, the comparison between each BG-12 regimen and glatiramer acetate in CONFIRM trial did not reveal a statistically significant difference in the risk of AEs leading to discontinuation (for BG-12 twice daily RR=1.23 [95% CI: 0.81–1.87], p>0.05; for BG-12 three times daily RR=1.20 [95% CI: 0.78–1.83], p>0.05).
Death from any cause
In the DEFINE trial there were two deaths, both of which were the result of road accidents (a patient in the twice-daily BG-12 group died in a bicycle accident 3 weeks after withdrawing from the study and a patient in the thrice-daily group died in a motor vehicle accident during the study period) [15].The incidents of deaths occurring during the CONFIRM study or within 30 days after study withdrawal were due to stroke in the placebo group and complications after a MS relapse in BG-12 given at a dose of 240 mg three times daily [20]. The overall result of the meta-analysis of BG-12 did not reveal any significant difference in frequency of death from any cause between both doses of BG-12 and the placebo (for BG-12 twice daily ORfixed=1.00 [95% CI: 0.14–7.15], p=1.00; for BG-12 three times daily ORfixed=1.70 [95% CI: 0.22–12.98], p=0.61) (Fig. 4C,d).
In the CONFIRM trial there was one death in glatiramer acetate, which was the result of suicide [20]. The results of the analysis revealed no statistically significant differences between each BG-12 regimen and glatiramer acetate (for BG-12 twice daily OR=0.32 [95% CI: 0.01–8.00], p>0.05; for BG-12 three times daily RR=1.02 [95% CI: 0.06–16.25], p>0.05).
DISCUSSION
The purpose of this review and meta-analysis was to summarize the available evidences on BG-12 in patients with RRMS. The methodology of our systematic review followed the guidelines described in the Cochrane Handbook [13], and the meta-analysis was performed according to the PRISMA statement protocol [14]. The applied search strategy revealed all of the relevant published and unpublished articles. Our systematic review include data from full text studies evaluating the effectiveness of BG-12 in the treatment of RRMS, that have been published in 2013. Nevertheless, during our search we identified some integrated analyses containing the pooled data from phase III DEFINE and CONFIRM studies [25-31], but at the moment they are available only as abstracts. Additionally, there has been recently published a review summarizing results from two above-mentioned studies [32], moreover, we identified systematic review and mixed treatment comparisons between BG-12 and other disease-modifying therapies approved for RRMS [33].
As MS remains an incurable condition and requires lifelong therapy, development of beneficial oral drugs for patients with RRMS is challenging. Before 2010, when the first oral drug for MS was approved, all available DMTs required subcutaneous injection or intravenous infusion [6]. The current DMT therapies used to treat multiple sclerosis exert their effects through anti-inflammatory or generalized immunomodulatory mechanisms and are still moderately effective in reducing relapse rate, prolonging remission and delaying accumulation of disability, and many patients discontinue therapy due to tolerability concerns, fear or inconvenience of frequent injections [4, 5]. The oral therapies have immediate benefits over injectable drugs in terms of the ease of administration. Moreover, oral drugs can lead to increased patient compliance and contribute to longer sustained symptom-free periods, but we must remember that patients will prefer oral administration only if efficacy is not compromised [7].
Advanced MS therapies should not only delay progression but indeed block manifestation of disease development symptoms, which has been defined as freedom from gadolinium-enhancing T1 or new T2 lesions detected by MRI, relapse-free survival, and absence of disability progression [5]. The adverse events associated with the use of immunosuppressive drugs and the requirement for parenteral administration of immunomodulatory agents have prompted researchers to search for new oral drugs that combine equal or preferably higher clinical efficacy with improved long-term safety (no malignancies and no opportunistic infections) and a high degree of tolerability in long-term use [3]. The availability of new therapeutic options for disease modification has the potential to expand options for patients with MS. These new therapies are able to redefine the goals of therapies in the context of appropriate patient selection and management strategies [5].
It can be expected that there may be some similarity in side effects between groups of DMTs considering the mechanism of action of the available drugs (interferon beta [INF beta], GA, and natalizumab) and the new oral therapies (fingolimod, cladribine, terifluonamide, laquinimod, and BG12). Fingolimod and cladribine reduce available circulating lymphocytes by blockade of lymphocyte egress from lymph nodes (fingolimod) or by cytotoxicity and depletion of these cells (cladribine). Natalizumab acts by blockade of adhesion and transmigration across the blood–brain barrier. All three drugs (fingolimod, cladribine, and natalizumab), indirectly (fingolimod and cladribine) or directly (natalizumab), reduce the number of lymphocyte in the CNS [7]. The mechanism of action of terifluonamide, laquinimod and BG12 is quite similar to IFN-beta and GA. IFN-beta and GA shift the balance of lymphocytes and modulate cytokine secretion. Terifluonamide, laquinimod, and BG-12, do not reduce lymphocyte count in the CNS and appear to act in a similar way to IFN-beta and GA by modulating cytokines and lymphocyte activation. None of these injectable (IFN-beta and GA) and oral (terifluonamide, laquinimod, and BG-12) drugs appear to significantly reduce the numbers of circulating lymphocytes, suggesting an immunomodulatory rather than an immunosuppressive profile [7].
As stated above, apart from recently published meta-analysis with mixed treatment comparison [33], during our systematic search we identify some systematic reviews on the topic [34, 35] containing concise data (mechanism of action, indications, efficacy, side effects or safety) for currently available therapeutic agents for MS providing clinicians and patients with more therapeutic choices. These systematic reviews were published in 2011 [34] and 2012 [35] so the fully described, completed results of the DEFINE and CONFIRM studies were not available, yet. However, the available data described in the above-mentioned reviews suggest that BG-12 could be more effective in reducing relapses and slowing the progression of MS than commonly prescribed injectable agents, such as GA, or INF beta. The authors of both articles confirm that oral therapies will create desires for more convenient treatment, which may improve needle-phobic patients’ adherence and may be an option for patients with intolerance to currently recommended medical treatment [34, 35]. As emphasized in the reviews,it is not exactly clearhow therapeutic decisions will be made with respectto the new oral therapies. These decisionswill encompass most likely the points raised above,efficacy, tolerability, safety, potential need for monitoring and adherence, as well asoverall cost effectiveness [34, 35].There is no doubt that appropriate data comparing the new oral agents with existing and well-established MS therapies in patients with the disease at different stages of severity are necessary to make these treatment and formulary decisions clearer [34]. Due to the lack of direct comparative evidence of the efficacy and safety of BG-12 and other disease-modifying treatments for RRMS, Hutchinson and colleagues have recently carried out a systematic review with mixed treatment comparison in order to indirectly compare BG-12 with IFN beta-1a, IFN beta-1b, fingolimod, natalizumab, teriflunomide and glatiramer acetale in adults patients with RRMS. The results of the network meta-analysis based on 27 RCTs showed that treatment with BG-12 given at a dose of 240 mg twice daily was associated with statistically significant reduction in ARR compared to placebo, GA and interferons, however when compared to fingolimod, the observed difference did not reach statistical significance. In reducing ARR BG-12 was significantly less effective than natalizumab. For confirmed disability progression by 2 years, BG-12 demonstrated favourable effect but the differences were significant only when compared to placebo. An overview of safety outcomes suggested that BG-12 may provide additional benefits in reducing the risk of prevalent adverse events associated with existing therapies such as fatigue, flu-like symptoms or injection site reaction [33]. It should be noted that the above-mentioned mixed-treatment comparison considered only annualized relapse rate and disability progression without assessing of brain MRI lesions, and, unlike our direct comparisons, the safety results were not presented as comparative risk ratio but as annual incidence rates with no p values.
The performed mixed treatment comparison [33] has some drawbacks, such as notable heterogeneity between the studies and among enrolled patients, as well as, variability in the definition of outcomes across the studies, that should be taken into consideration when interpreting the obtained results. Nevertheless, in the lack of head-to-head studies comparing the effectiveness of BG-12 and other drugs approved for the treatment of RRMS, an indirect comparison appears to be the most appropriate method for such evaluations.
Notably, the increasing number of established effective therapies for RRMS and emerging consensus for early treatment raise ethical dilemmas and practical concerns for placebo-controlled clinical trials in this disease [36]. In opinion of an international group of experts, it would be ethically acceptable to perform placebo-controlled trials, with some restrictions even if they are difficult to do practically [36]. For patients with RRMS for which established effective therapies exist, the placebo-controlled trials should only be offered with rigorous informed consent if the subjects refuse to use these treatments, have not responded to them, or if these treatments are not available to them for other reasons (e.g., economics). What is more, alternatives to placebo-controlled trials may be attractive but they have been little explored in MS, and each of them presents its own ethical challenges [36].
BG-12 recently approved (March 2013 in USA and UE) for the treatment of RRMS has already been shown to be a promising option in adults patients in an open-label pilot study [37], phase IIb RCT [38, 39] or retrospective study [40] in regard to significant reduction in the number of GdE lesions on brain MRI scans. These studies revealed also a good safety profile of BG-12, without evidence of immuno-suppression [9, 10]. There have been some tolerability issues concerning the appearance of flushing and gastrointestinal symptoms such as nausea and vomiting, however, these events were generally transient and resolved within about 8 weeks after therapy initiation [9, 10]. The newest results from phase III trials clearly demonstrated superior efficacy of BG-12 more extensively than in phase II trials and confirmed its acceptable safety profile [9, 31].
We provided a direct comparison of BG-12 (240 mg twice daily or three times daily) with placebo on the basis of two RCTs (DEFINE trial [15-19] and CONFIRM study [20-24])and with glatiramer acetate on the basis of one RCT (CONFIRM study [20-24]). The results of meta-analysis based on these two phase III studies indicate the superiority of BG-12 (both dosages) over placebo in respect to the proportion of patients who had at least one relapse of multiple sclerosis, proportion of patients who had reduced confirmed progression of disability and reduction in the mean number of gadolinium-enhancing lesions on MRI at 2 years. The improvements in these clinically important outcomes are consistent with beneficial effects of BG-12 on the inflammatory damage associated with multiple sclerosis [15, 32]. Additionally, findings from the recent subgroup analyses of the DEFINE [16] and CONFIRM [21] studies indicate that BG-12 is consistently effective across a broad range of patients with varying baseline demographic and disease characteristics. As multiple sclerosis is associated with health-related quality of life impairment, it is important to emphasize that in both pivotal III phase studies treatment with BG-12 resulted in significant improvements in general well-being as well as in physical and mental aspects of health and functioning in patients with relapsing MS [17, 18, 22, 23]. Oral administration of BG-12 appears to be convenient and may improve adherence to treatment, thus providing better clinical outcomes and improving quality of life in patients with RRMS. It could be hypothesized that treatment with BG-12 would be preferred by the patients over therapy with injectable and infusible drugs, such as natalizumab, interferon and glatiramer acetate.
Although CONFIRM study [20-24] was not designed to test the superiority or noninferiority of BG-12 to GA, the direct comparison between these drugs revealed that the analyzed agent could potentially be more effective in treatment of relapsing-remitting multiple sclerosis, in regard to reducing the annualized relapse rate and in the proportion of patients who had a relapse. It should be noted that the result of mixed treatment comparison conducted by the authors of review [33] confirmed that BG-12 given at a dose of 240 mg twice daily is statistically significantly superior to glatiramer acetate in the respect of ARR.
Both dosages of the study drug BG-12 administered as monotherapy showed also an acceptable safety profile, comparable to placebo in respect to the risk of any adverse events, discontinuations due to adverse events or deaths from any cause. Gastrointestinal disorders were one the most common adverse events during the initial treatment period with BG-12, however they were mild or moderate in severity and infrequently led to discontinuation of therapy [29, 31]. It should be noticed that the overall result of the meta-analysis for the BG-12 at a dose of 240 mg given three times daily revealed a statistically significant difference in the frequency of SAEs in favor of the study agent over the placebo (this difference was not statistically significant with BG-12 given at a dose of 240 mg twice daily). We assume that this result was probably due to the fact that in both studies relapses of multiple sclerosis were reported as SAEs and as such they were the most frequently reported events (which occurred more often in the placebo group than in the other groups). Also the safety profile of both active drugs (BG-12 and glatiramer acetate) can be considered comparable. Remarkably, patients treated with BG-12 did not show an increased risk of serious infections or malignancies, even though neuroprotective action of this agent is probably associated with the depletion of white blood cells [9, 15, 20]. It is worth to emphasize that the relative long-term safety results from 96-week analysis in the DEFINE and CONFIRM studies confirm findings seen previously in phase IIb study with shorter treatment period [38, 39]. Moreover, the interim results from the ongoing 5-year extension of DEFINE and CONFIRM studies did not reveal any new or worsening safety signals in patients continuing BG-12 therapy [41].
We should emphasize that dose finding in the Phase IIb study led to the using the BG-12 at the dosages of 240 mg twice or thrice daily (with a total daily dose of 480 and 720 mg, respectively) in the phase III RCTs, although according to the FDA's approval, the licensed dosage is 120 mg twice daily that should be increased to the maintenance dose of 240 mg twice daily after 7 days. In the meta-analysis we took into consideration both regimens of BG-12 administration evaluated in the III phase studies, however authors of the network meta-analysis [33] evaluated only the twice-daily schedule.
An important limitation of this review is a small number of trials included in the quantitative analysis, as BG-12 is being still under investigation. Currently, there is a lack of head-to-head trials comparing BG-12 to other drugs indicated for the treatment of RRMS and therefore we could aggregate the results from two, phase III RCTs evaluating the effectiveness of BG-12 with placebo serving as a main comparator.
It could be suggested that a network meta-analysis should be performed to determine the efficacy and safety of BG-12 in terms of the other therapies. However, a mixed treatment comparison combining direct and indirect evidence for particular pairwise comparisons, has some limitations. The most importantly, the mixed treatment approach is not a substitute for a large, well-designed RCTs examining relative clinical efficacy and safety and tends only to be valid for very similar studies [42]. As stated above, in meta-analysis with mixed treatment comparison performed by Hutchinson [33] there was a considerable heterogeneity among included studies, nonetheless, this network analysis with its limitations is currently the only study assessing the effectiveness of BG-12 relative to other treatment options in RRMS.
On the other hand, two RCTs included in quantitative analysis in our review (DEFINE and CONFIRM studies) had relatively high methodological and reporting quality and were sufficiently homogenous. As the statistical hetero-geneity was not detected, we calculated the risk ratios using the fixed effects model. However, there was a single comparison for which a moderate heterogeneity was generated (Fig. 2a). As the particular characteristics of the included studies are similar, it can be assumed that the observed heterogeneity between the studies may result from the slightly different methods of the outcome estimation, as “the proportion of patients who had a relapse by 2 years” was a primary end point in DEFINE study and secondary end point in CONFIRM study.
With minimal variability between studies, the performed meta-analysis met the goal of summarizing the available data on the effectiveness of BG-12. Considering design and method of conducting the included studies, outcome data and reporting the results we can assume that the risk of bias across the studies was low. Moreover, both RCTs included in the meta-analysis involved over 1 000 participants each, so the sample size was apparently adequate to test the outcomes, providing more precise effect estimation. It should be also emphasized that pooling of results from phase IIb study [38, 39] and data from DEFINE and CONFIRM studies was found to be contraindicated because of considerable different period of treatment and the minor discrepancy in the dosage schedule and the analyzed outcomes. On the contrary, in the network meta-analysis [33] the duration of treatment periods varied across the included studies, ranging from 13 weeks to 260 weeks.
In summary, the results of the meta-analysis demonstrated that BG-12 therapy has a beneficial effect in the treatment of relapsing-remitting multiple sclerosis patients, when compared with placebo and when compared to glatiramer acetate in one of the trials. Based on the safety analysis, there was no evidence for an increase in the incidence of any adverse events. The risk–benefit ratio of therapy with BG-12 in RRMS is therefore in favor of this oral agent. The established effectiveness in combination with the ease of administration make BG-12 very promising treatment option for patients with RRMS, which may serve as an alternative to other presently available therapies. However, there is still a need for head-to-head studies determining the relative efficacy and safety of BG-12 and others therapies, as well as, long-term, post-marketing studies to define the effectiveness of the analyzed drug, especially in respect to its safety profile in a real-world population of patients suffering from RRMS.
ACKNOWLEDGEMENTS
All listed authors approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest to disclose in relation to this study; none of the authors have financial relationships with commercial companies involved with the products under study. The manuscript is self-financing by the authors.
REFERENCES
- 1.Loma I, Heyman R. Multiple sclerosis: pathogenesis and treatment. Curr. Neuropharmacol. 2011;9(3):409–416. doi: 10.2174/157015911796557911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso A, Hernán MA. Temporal trends in the incidence of multiple sclerosis a systematic review. Neurology. 2008;71:129–135. doi: 10.1212/01.wnl.0000316802.35974.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limmroth V. Multiple sclerosis: oral BG12 for treatment of relapsing-remitting MS. Nat. Rev. Neurol. 2013;9:8–10. doi: 10.1038/nrneurol.2012.231. [DOI] [PubMed] [Google Scholar]
- 4.Tullman M. A Review of current and emerging therapeutic strategies in multiple sclerosis. Am. J. Manag Care. 2013;19:21–27. [PubMed] [Google Scholar]
- 5.Fox EJ, Rhoades RW. New treatments and treatment goals for patients with relapsing-remitting multiple sclerosis. Curr. Opin. Neurol. 2012;25 Suppl:11–19. doi: 10.1097/01.wco.0000413320.94715.e9. [DOI] [PubMed] [Google Scholar]
- 6.Killestein J, Rudick RA, Polman CH. Oral treatment for multiple sclerosis. Lancet Neurol. 2011;10:1026–1034. doi: 10.1016/S1474-4422(11)70228-9. [DOI] [PubMed] [Google Scholar]
- 7.Nicholas R, Giannetti P, Alsanousi A, Friede T, Muraro PA. Development of oral immunomodulatory agents in the management of multiple sclerosis. Drug Des Devel. Ther. 2011;5: 255–274. doi: 10.2147/DDDT.S10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh J, O'Connor PW. Safety, tolerability, and efficacy of oral therapies for relapsing-remitting multiple sclerosis. CNS Drugs. 2013;27(8):591–609. doi: 10.1007/s40263-013-0080-z. [DOI] [PubMed] [Google Scholar]
- 9.Stangel M, Linker RA. Dimethyl fumarate (BG-12) for the treatment of multiple sclerosis. Expert Rev. Clin. Pharmacol. 2013;6(4):355–362. doi: 10.1586/17512433.2013.811826. [DOI] [PubMed] [Google Scholar]
- 10.Moharregh-Khiabani D, Linker RA, Gold R, Stangel M. Fumaric Acid and its esters: an emerging treatment for multiple sclerosis. Curr. Neuropharmacol. 2009;7(1):60–64. doi: 10.2174/157015909787602788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O'Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann. Neurol. 2005;58(6):840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Control Clin. Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S, editors. [updated March 2011. Available from: www.cochrane-handbook.org. Accessed 3 Nov. Cochrane: Collaboration and John Wiley 2011; 2013. (editors) Cochrane handbook for systematic reviews of interventions Version 5..0. [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Med. 2009;151:264–649. [PMC free article] [PubMed] [Google Scholar]
- 15.Gold R, Kappos L, Arnold DL, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT. DEFINE Study Investigators.DEFINE Study Investigators. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 16.Bar-Or A, Gold R, Kappos L, Arnold DL, Giovannoni G, Selmaj K, O'Gorman J, Stephan M, Dawson KT. Clinical efficacy of BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis subgroup analyses of the DEFINE study. J Neurol. 2013;260(9):2297–2305. doi: 10.1007/s00415-013-6954-7. [DOI] [PubMed] [Google Scholar]
- 17.Kappos L, Gold R, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Sarda SP, Agarwal S, Zhang A, Sheikh SI, Seidman E, Dawson KT. Quality of life outcomes with BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis The DEFINE study. Mult. Scler. 2013;22 doi: 10.1177/1352458513507817. [DOI] [PubMed] [Google Scholar]
- 18.Kappos L, Gold R, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, Sarda SP, Agarwal S, Zhang A, Sheikh SI, Dawson KT. Effects of BG-12 on quality of life in relapsing-remitting multiple sclerosis Findings from the phase 3 define study. Value in Health. 2012;15(7):A557. [Google Scholar]
- 19.Bar-Or A, Gold R, Kappos L, Arnold D, Giovannoni G, Selmaj K, O'Gorman J, Stephan M, Dawson K. Effect of BG-12 on magnetic resonance imaging activity in subgroups of patients with relapsing-remitting multiple sclerosis Findings from the DEFINE study. Eur. J. Neurol. 2012;19 (SUPPL1 ):356. [Google Scholar]
- 20.Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, Yang M, Raghupathi K, Novas M, Sweetser MT, Viglietta V, Dawson KT. CONFIRM Study Investigators.Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. . N. Engl. J. Med. 2012;367:1087–1097. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 21.Hutchinson M, Fox RJ, Miller DH, Phillips JT, Phillips JT, Kita M, Havrdova E, O'Gorman J, Zhang R, Novas M, Viglietta V, Dawson KT. Clinical efficacy of BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis subgroup analyses of the CONFIRM study. J. Neurol. 2013;260(9):2286–2296. doi: 10.1007/s00415-013-6968-1. [DOI] [PubMed] [Google Scholar]
- 22.Kita M, Fox RJ, Phillips JT, Hutchinson M, Havrdova E, Sarda SP, Agarwal S, Kong J, Zhang A, Viglietta V, Sheikh SI, Seidman E, Dawson KT. Effects of BG-12 (dimethyl fumarate) on health-related quality of life in patients with relapsing-remitting multiple sclerosis findings from the CONFIRM study. Mult. Scler. 2013;22 doi: 10.1177/1352458513507818. [DOI] [PubMed] [Google Scholar]
- 23.Kita M, Fox RJ, Phillips JT, Hutchinson M, Havrdova E, Sarda SP, Kong J, Viglietta V, Sheikh SI, Dawson KT. Effects of BG-12 on quality of life in relapsing-remitting multiple sclerosis Findings from the phase 3 confirm study. Value Health. 2012;15(7):A556. doi: 10.1177/1352458513507818. [DOI] [PubMed] [Google Scholar]
- 24.Havrdova E, Miller D, Fox RJ, Phillips JT, Kita M, Hutchinson M, MacManus D, Yousry T, Yang M, Zhang R, Novas M, Viglietta V, Dawson KT. Clinical and neuroimaging outcomes with BG-12 treatment in CONFIRM (comparator and an oral fumarate in relapsing-remitting multiple sclerosis) a multicenter randomized placebocontrolled phase-3 study. Eur. J. Neurol. 2012;19(SUPPL187Print ISSN ):1351–5101. [Google Scholar]
- 25.BarOr A, Fox RJ, Gold R, Miller DH, Arnold DL, O'Gorman J, Yang M, Sheikh SI, Viglietta V, Dawson KT, Hutchinson M. Clinical and neuroradiological effect of BG-12 (dimethyl fumarate) in subgroups of patients with relapsing-remitting multiple sclerosis (RRMS) An integrated analysis of the phase 3 define and confirm studies. Value Health. 2013;16(3) [Google Scholar]
- 26.Bar-Or A, Gold R, Fox RJ, Havrdova E, Selmaj K, Kurukulasuriya NC, Yang M, Raghupathi K, Novas M, Sweetser MT, Viglietta V, Dawson KT, Phillips JT. Clinical efficacy and safety of oral BG-12 (dimethyl fumarate) in relapsing-remitting multiple sclerosis (RRMS) An integrated analysis of the phase 3 define and confirm studies. Mult. Scler. 2012;18(12):1828. [Google Scholar]
- 27.Bar-Or A, Arnold DL, Gold R, Fox RJ, MacManus DG, Yousry T, Kurukulasuriya NC, Zhang R, Viglietta V, Stephan M, Dawson KT, Miller DH. Effects of oral BG-12 (Dimethyl Fumarate) on magnetic resonance imaging (Mri) outcomes in relapsing-remitting multiple sclerosis (RRMS) An integrated analysis of the phase 3 define and confirm studies. Mult. Scler. 2012;18(12):1836. [Google Scholar]
- 28.Giovannoni G, Gold R, Fox RJ, Kita M, Yang M, Zhang R, Dawson KT, Viglietta V, Sheikh SI, Havrdova E. An integrated analysis of relapses requiring intravenous steroid use and multiple sclerosis (MS)-related hospitalizations from the BG-12 (dimethyl fumarate) phase 3 define and confirm studies. Value Health. 2013;16(3):A101–A102. [Google Scholar]
- 29.Bar-Or A, Selmaj K, Gold R, Fox RJ, Havrdova E, Kurukulasuriya NC, Pace A, Novas M, Meltzer L, Hotermans C, Dawson KT, Phillips JT. Gastrointestinal tolerability events in relapsing-remitting multiple sclerosis (RRMS) patients treated with oral BG-12 (Dimethyl Fumarate) in define and confirm. Mult. Scler. 2012;18(12):1840–1841. [Google Scholar]
- 30.Havrdova E, Gold R, Fox RJ, Kappos L, Kita M, Sarda SP, Yang M, Zhang R, Dawson KT, Viglietta V, Sheikh SI, Giovannoni G. Relapses requiring intravenous steroids and multiple sclerosis related hospitalizations Findings from the phase 3 define and confirm studies. Value Health. 2012;15(7):A546. [Google Scholar]
- 31.Havrdova E, Phillips JT, Selmaj K, Gold R, Fox RJ, Giovannoni G, Pace A, Novas M, Kurukulasuriya NC, Hotermans C, Meltzer L, Dawson KT. Gastrointestinal tolerability events in relapsing- remitting multiple sclerosis patients treated with BG-12 ( dimethyl fumarate) Integrated analysis of DEFINE and CONFIRM. J. Neurol. Sci. 2013;333(Suppl 1 ):368–369. [Google Scholar]
- 32.Havrdova E, Hutchinson M, Kurukulasuriya NC, Raghupathi K, Sweetser MT, Dawson KT, Gold R, Gold R, Kappos L, Arnold D, Fox RJ, Miller DH, Phillips JTetal. Oral BG-12 (dimethyl fumarate) for relapsing-remitting multiple sclerosis a review of DEFINE and CONFIRM.Evaluation of Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl J Med. 2012 3671098-107 Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N. Engl. J .Med. 2012 367 1087-97. Expert Opin Pharmacother. 2013;14(15):2145–56. doi: 10.1517/14656566.2013.826190. [DOI] [PubMed] [Google Scholar]
- 33.Hutchinson M, Fox RJ, Havrdova E, Kurukulasuriya NC, Sarda SP, Agarwa S, Siddiqui MK, Taneja A, Deniz B. Efficacy and safety of BG-12 (dimethyl fumarate) and other disease-modifying therapies for the treatment of relapsing–remitting multiple sclerosis a systematic review and mixed treatment comparison. Curr. Med. Res. Opin. 2013;26 doi: 10.1185/03007995.2013.863755. [DOI] [PubMed] [Google Scholar]
- 34.Gold R. Oral therapies for multiple sclerosis a review of agents in phase III development or recently approved. CNS Drugs. 2011;25:37–52. doi: 10.2165/11539820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Castro-Borrero W, Graves D, Frohman TC, Flores AB, Hardeman P, Logan D, Orchard M, Greenberg B, Frohman EM. Current and emerging therapies in multiple sclerosis A systematic review. Ther. Adv Neurol Disord. 2012;5(4):205–220. doi: 10.1177/1756285612450936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polman CH, Reingold SC, Barkhof F, Calabresi PA, Clanet M, Cohen JA, Cutter GR, Freedman M.S. Kappos, L. Lublin, F.D. McFarland, H.F. Metz, L.M. Miller, A.E. Montalban, X. O'Connor PW, Panitch H, Richert JR, Petkau J, Schwid SR, Sormani MP, Thompson AJ, Weinshenker BG, Wolinsky JS. Ethics of placebo-controlled clinical trials in multiple sclerosis a reassessment. Neurology. 2008;70:1134–1140. doi: 10.1212/01.wnl.0000306410.84794.4d. [DOI] [PubMed] [Google Scholar]
- 37.Schimrigk S, Brune N, Hellwig K, Lukas C, Bellenberg B, Rieks M, Hoffmann V, Pöhlau D, Przuntek H. Oral fumaric acid esters for the treatment of active multiple sclerosis an open-label, baseline-controlled pilot study. Eur. J. Neurol. 2006;13(6):604–610. doi: 10.1111/j.1468-1331.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 38.Kappos L, Gold R, Miller DH, Havrdova E, Limmroth V, Polman CH, Schmierer K, Yousry TA, Yang M, Eraksoy M, Meluzinova E, Rektor I, Dawson KT, Sandrock AW, O'Neill GN. BG-12 Phase IIb Study Investigators BG-12 Phase IIb Study Investigators.Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis a multicentre randomised double-blind placebo-controlled phase IIb study. Lancet. 2008;372:1463–1472. doi: 10.1016/S0140-6736(08)61619-0. [DOI] [PubMed] [Google Scholar]
- 39.Kappos L, Gold R, Miller DH, MacManus DG, Havrdova E, Limmroth V, Polman CH, Schmierer K, Yousry TA, Eraksoy M, Meluzinova E, Dufek M, Yang M, Dawson K, O'Neill GN. Effect of BG-12 on contrast-enhanced lesions in patients with relapsing--remitting multiple sclerosis subgroup analyses from the phase 2b study. Mult Scler. 2012;18(3):314–321. doi: 10.1177/1352458511421054. [DOI] [PubMed] [Google Scholar]
- 40.MacManus DG, Miller DH, Kappos L, Gold R, Havrdova E, Limmroth V, Polman CH, Schmierer K, Yousry TA, Eraksoy M, Meluzinova E, Dufek M, Yang M, O'Neill GN, Dawson K. BG - 12 reduces evolution of new enhancing lesions to T1-hypointense lesions in patients with multiple sclerosis. J Neurol. 2011;258(3):449–456. doi: 10.1007/s00415-010-5777-z. [DOI] [PubMed] [Google Scholar]
- 41.Selmaj K, Phillips JT, Fox RJ, Raghupathi K, Yuan H, Novas M, Sweetser MT, Kurukulasuriya NC, Viglietta V, Dawson KT, Gold R. Safety and tolerability of BG-12 (dimethyl fumarate) in relapsing- remitting multiple sclerosis: Interim results from the endorse extension study. J. Neurol. Sci. 2013;333(Suppl 1 ):367–368. [Google Scholar]
- 42.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]