Abstract
Over the last decades, the scientific interest in chemistry and pharmacology of cannabinoids has increased. Most attention has focused on ∆9-tetrahydrocannabinol (∆9-THC) as it is the psychoactive constituent of Cannabis sativa (C. sativa). However, in previous years, the focus of interest in the second plant constituent with non-psychotropic properties, cannabidiol (CBD) has been enhanced. Recently, several groups have investigated the pharmacological properties of CBD with significant findings; furthermore, this compound has raised promising pharmacological properties as a wake-inducing drug. In the current review, we will provide experimental evidence regarding the potential role of CBD as a wake-inducing drug.
Keywords: Dopamine, hypothalamus, marijuana, sleep, sleepiness.
INTRODUCTION
Molecular Structure of Marijuana Derivates
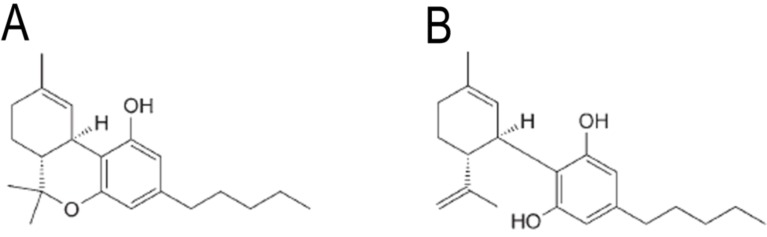
Marijuana, hashish, bhang amongst others are different names given to derived-products from the plant C. sativa [1]. Among the molecules that constitute marijuana, Δ9-THC and CBD are the most significant compounds (Fig. 1) [2]. Whereas Δ9-THCis the psychoactive molecule that binds to the CB1cannabinoid receptor and induces behavioral, neurochemical and molecular effects [3]; CBD does not promote psychotropic disturbances [4]. Moreover, recent evidence suggests that CBD is a compound with potential medical uses.
Fig. (1).
Molecular structures of delta-9-tetrahydrocannabinol (Panel A) and cannabidiol (Panel B).
Biological Effects of Cannabidiol
Despite the fact that CBD does not bind to the CB1 cannabinoid receptor and lacks the psychotomimetic and other psychotropic effects, this cannabinoid modulates several biological functions. Current experimental data suggests that CBD is a potential candidate to be used as a therapeutic in specific medical treatments. For instance, it has been previously described that CBD acts as an anti-inflammatory and anxiolytic compound and modulates psychiatric disorders [5]. Furthermore, Martín-Santos et al., (2012) showed that when given orally CBD (600mg) no signs of anxiety, dysphoria, or physiological effects were observed in healthy male subjects [6]. Moreover, Das et al., (2013) reported that CBD can enhance consolidation of extinction learning in humans suggesting that this cannabinoid may have potential as an adjunct to extinction-based therapies for anxiety disorders [7]. Several studies provide more evidence that CBD possesses pharmacological potential, including anxiolytic, antipsychotic, antiemetic and anti-inflammatory properties [4b, 8]. In the following sections, this review will discuss the hypothesis that CBD modulates the sleep-wake cycle.
MODULATION OF SLEEP-WAKE CYCLE BY CANNABIDIOL
Whereas it has been well established that Δ9-THC promotes sleep [9], contradictory results on the effect of CBD on sleep were reported. For instance, Monti (1977) found a diminution in sleep after systemic administration of CBD [10], whereas Carlini and Cunha (1981) showed an improvement in sleep in insomniacs after using CBD [11]. Moreover, systemic administration of CBD (10 or 40 mg/kg) in male Wistar rats during the light period, enhanced the total percentage of sleep [12]. Further complexity has been added to the understanding of CBD pharmacology since Nicholson et al. (2004) found that 15mg of CBD administered to young adults increased wakefulness (W) during sleeping time [13]. In addition, our laboratory provided further evidence supporting the wake-inducing properties of CBD, as i.c.v. administrations of CBD (10µg/5µL) in rats during the lights-on period increased W, but decreased rapid eye movement (REM) sleep. The alertness was observed after the first hour post-injection [14]. A similar effect on sleep was observed when CBD was injected into the lateral hypothalamus [15] suggesting that CBD behaves as a wake-promoting compound. Supporting this observation, it was also found that this cannabinoid increased c-Fos expression in wake-related brain areas, such as hypothalamic nuclei as well as dorsal raphe nuclei (DRN). These findings were in concordance with previous reports [16]. Even though the mechanism of sleep modulation by CBD remains unclear, our group hypothesized that the dopamine (DA) system could be involved since it has been demonstrated that microinjections of CBD (10µg/1 or 5µL) in rats promotes an enhancement in the extracellular levels of DA [14,17].
Despite the wake-inducing effects caused by CBD, it remained the contradictory data reported by others. One possible explanation could lie in the differences described in the methodological procedures (route of administration, vehicle used, doses, subjects, etc). For example, in some reports, CBD was given systemically whereas others administered it centrally (either i.c.v or perfused directly into lateral hypothalamus). Moreover, some authors reported doses of 40mg/kg whereas others used a maximal dose of CBD 20µg/1µL. Despite the methodological differences reported, further experiments will be needed to clarify the potential mechanism of action of CBD on sleep modulation.
Putative Mechanisms of Action of Cannabidiol on Waking
Despite the lack of solid evidence concerning the role of CBD on sleep modulation, we can draw the following putative mechanism of action:
Neuroanatomical Basis
Hypothalamus.- Central administration of CBD enhances c-Fos expression in the hypothalamus [14]. Several reports suggest a key role of hypothalamus in the modulation of alertness [18].
Dorsal raphe nuclei.- Central administration of CBD enhances c-Fos expression in DRN [14]. It is known that electrophysiological activity of DRN is higher in the waking state and decreases during sleep, being virtually absent in REM sleep [19].
Neurochemical Basis
Using a microdialysis approach, DA contents collected from AcbC have been found enhanced after central administration of CBD [14, 17]. This result was further confirmed by using other experimental techniques [20].
Integrative Perspective
It is known that lesions of DA neurons reduce arousal in animal models [21], as well as in Parkinson’s disease patients that present sleep dysfunction such as excessive daytime sleepiness [22]. Therefore, we postulate that alertness induced by CBD may be associated with the increase in DA release. If CBD induces alertness via activation of neurons in the hypothalamus and promotes the enhancement in DA levels, it would suggest that the enzymatic process involved in the formation of catecholaminesmay be under the influence of CBD. Complementary experiments testing the role of CBD on the biosynthesis of catecholamines would provide us a better understanding of the phenomena. Although this hypothesis would provide scope for speculation on the mechanism of action of CBD on sleep modulation, it might give new insights regarding the role of CBD on the sleep-wake cycle mechanisms (Fig. 2).
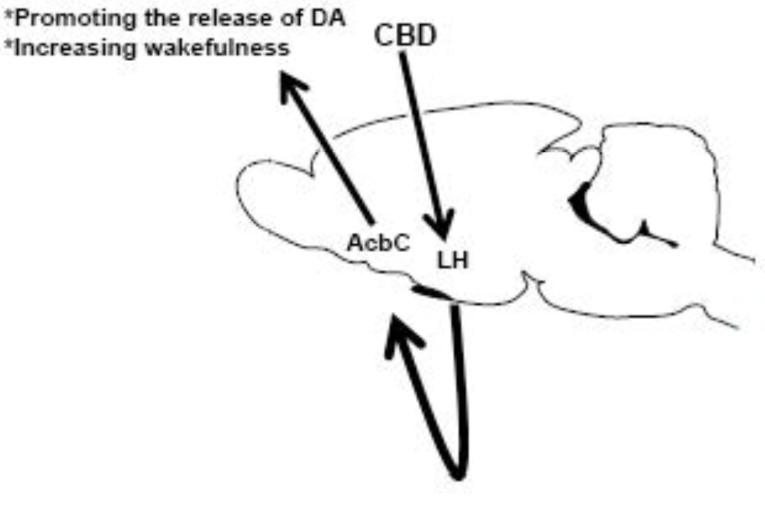
Fig. (2).
Microinjections of cannabidiol (CBD) into lateral hypothalamus (LH) would activate neurons placed into nucleus accumbens (AcbC) leading to an enhancement in the dopamine (DA) contents as well as an increase in wakefulness. Further studies will be needed to address this putative neurobiological mechanism of action of CBD on the sleep-wake cycle.
The Potential Therapeutic use of Cannabidiol on Sleep Disorders
Sleep disturbances display a range of different polysomnographic features classified as sleep disorders. According to the International Classification of Sleep Disorders (ICSD, 2001), the sleep disorders are classified into four categories [23]:
Dyssomnias: Problems initiating and maintaining sleep, and also excessive sleepiness
Parasomnias: Disorders of arousal, partial arousal, or sleep stage transition
Sleep disorders associated with mental, neurologic, or other medical disorders
Other sleep disorders: Sleep disorders not included in categories I, II or III
Epidemiological data obtained from the National Sleep Foundation (NSF) Poll 2008 (USA) showed that 36% of subjects reported that they had fallen asleep while driving. Moreover, the NSF 2012 Sleep in America® poll showed that pilots are most likely to report sleep-related job performance. For example, pilots (23%) admitted that sleepiness has affected their job performance. These striking data suggest a presence of general sleep disturbances and indicate the importance of exploring new therapeutic approaches to managing sleepiness. Thus, based on experimental evidence, it could be interesting to explore the potential therapeutic properties of the use of CBD to treat and manage sleepiness. The ICSD defines somnolence as sleep episodes that are present during the alertness that require mild to moderate attention. Somnolence might occur as a secondary health condition, such as side effects of medication, illicit substance use, or obstructive sleep apnea [24]. The use of CBD for different medical purposes has been recently suggested due to it improves symptoms of several disorders (for a comprehensive review see [25]).
CONCLUSION AND PERSPECTIVES
Several pieces of evidence have shown that CBD acts as a positive compound in different treatments to manage several health conditions, such as psychiatric and neurodegenerative disorders [5a, b, 8c, 26]. Thus, it could be plausible to consider the use of CBD to explore its medical properties in somnolence. The current review highlights the pharmacological evidence on the effects of CBD on sleep modulation and provides a putative mechanism of action. For a better understanding of the molecular and neuroanatomical mechanism by which CBD regulates sleep, this subject should be further investigated.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Lamarine R J. Marijuana: modern medical chimaera. J. Drug Educ. 2012;42 (1):1–11. doi: 10.2190/DE.42.1.a. [DOI] [PubMed] [Google Scholar]
- 2.(a) Mechoulam R, Shani A, Edery H, Grunfeld Y. Chemical basis of hashish activity. Science. 1970;169 (3945):611–2. doi: 10.1126/science.169.3945.611. [DOI] [PubMed] [Google Scholar]; (b) Mechoulam R. Marihuana chemistry. Science. 1970;168 (3936):1159–66. doi: 10.1126/science.168.3936.1159. [DOI] [PubMed] [Google Scholar]; (c) Sharma P, Murthy P, Bharath M M. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iranian J. Psychiatry. 2012;7 (4):149–56. [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Gertsch J, Pertwee R G, Di Marzo V. Phytocannabinoids beyond the Cannabis plant - do they exist?. Br. J. Pharmacol. 2010;160 (3):523–9. doi: 10.1111/j.1476-5381.2010.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fonseca B M, Costa M A, Almada M, Correia-da-Silva G, Teixeira N A. Endogenous cannabinoids revisited: a biochemistry perspective. Prostaglandins Other Lipid Mediators. 2013;102-103:13–30. doi: 10.1016/j.prostaglandins.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 4.(a) Zuardi A W. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Rev. Bras. Psiquiatr. 2008;30 (3):271–80. doi: 10.1590/s1516-44462008000300015. [DOI] [PubMed] [Google Scholar]; (b) Bergamaschi M M, Queiroz R H, Zuardi A W, Crippa J A. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Safety. 2011;6 (4):237–49. doi: 10.2174/157488611798280924. [DOI] [PubMed] [Google Scholar]
- 5.(a) Campos A C, Moreira F A, Gomes F V, Del Bel E A, Guimaraes F S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philosophical Transactions Royal Society of London. Series B Biol. Sci. 2012;367 (1607):3364–78. doi: 10.1098/rstb.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Leweke F M, Piomelli D, Pahlisch F, Muhl D, Gerth C W, Hoyer C, Klosterkotter J, Hellmich M, Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Translational Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Schier A R, Ribeiro N P, Silva A C, Hallak J E, Crippa J A, Nardi A E, Zuardi A W. Cannabidiol, a Cannabis sativa constituent, as an anxiolytic drug. Rev. Bras. Psiquiatr. 2012;34(Suppl 1 ):S104–10. doi: 10.1590/s1516-44462012000500008. [DOI] [PubMed] [Google Scholar]; (d) Sagredo O, Pazos M R, Valdeolivas S, Fernandez-Ruiz J. Cannabinoids: novel medicines for the treatment of Huntington's disease. Recent Patents on CNS Drug Discov. 2012;7 (1):41–8. doi: 10.2174/157488912798842278. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Santos R, Crippa J A, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, Allen P, Seal M, Langohr K, Farre M, Zuardi A W, McGuire P K. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr. Pharm. Design. 2012;18 (32):4966–79. doi: 10.2174/138161212802884780. [DOI] [PubMed] [Google Scholar]
- 7.Das R K, Kamboj S K, Ramadas M, Yogan K, Gupta V, Redman E, Curran H V, Morgan C J. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psycho- pharmacology. 2013;226 (4):781–92. doi: 10.1007/s00213-012-2955-y. [DOI] [PubMed] [Google Scholar]
- 8.(a) Izzo A A, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009;30 (10):515–27. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]; (b) Scuderi C, Filippis D D, Iuvone T, Blasio A, Steardo A, Esposito G. Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders. Phytother. Res. PTR. 2009;23 (5):597–602. doi: 10.1002/ptr.2625. [DOI] [PubMed] [Google Scholar]; (c) Fernandez-Ruiz J, Sagredo O, Pazos M R, Garcia C, Pertwee R, Mechoulam R, Martinez-Orgado J. Cannabidiol for neurodegenerclinical applications for this phytocannabinoid?. Br. J. Clin. Pharmacol. 2013;75 (2):323–33. doi: 10.1111/j.1365-2125.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Pivik R T, Zarcone V, Dement W C, Hollister L E. Delta-9-tetrahydrocannabinol and synhexl: effects on human sleep patterns. Clin. Pharmacol. Ther. 1972;13 (3):426–35. doi: 10.1002/cpt1972133426. [DOI] [PubMed] [Google Scholar]; (b) Feinberg I, Jones R, Walker J M, Cavness C, March J. Effects of high dosage delta-9-tetrahydrocannabinol on sleep patterns in man. Clin. Pharmacol. Ther. 1975;17 (4):458–66. doi: 10.1002/cpt1975174458. [DOI] [PubMed] [Google Scholar]; (c) Feinberg I, Jones R, Walker J, Cavness C, Floyd T. Effects of marijuana extract and tetrahydrocannabinol on electroencephalographic sleep patterns. Clin. Pharmacol. Ther. 1976;19 (6):782–94. doi: 10.1002/cpt1976196782. [DOI] [PubMed] [Google Scholar]
- 10.Monti J M. Hypnoticlike effects of cannabidiol in the rat. Psycho- pharmacology. 1977;55 (3):263–5. doi: 10.1007/BF00497858. [DOI] [PubMed] [Google Scholar]
- 11.Carlini E A, Cunha J M. Hypnotic and antiepileptic effects of cannabidiol. J. Clin. Pharmacol. 1981;21 (8-9 Suppl):417S–427S. doi: 10.1002/j.1552-4604.1981.tb02622.x. [DOI] [PubMed] [Google Scholar]
- 12.Chagas M H, Crippa J A, Zuardi A W, Hallak J E, Machado-de-Sousa J P, Hirotsu C, Maia L, Tufik S, Andersen M L. Effects of acute systemic administration of cannabidiol on sleep-wake cycle in rats. J. Psychopharmacol. 2013;27 (3):312–6. doi: 10.1177/0269881112474524. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson A N, Turner C, Stone B M, Robson P J. Effect of Delta-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J. Clin. Psycho- pharmacol. 2004;v24 (3):305–13. doi: 10.1097/01.jcp.0000125688.05091.8f. [DOI] [PubMed] [Google Scholar]
- 14.Murillo-Rodriguez E, Millan-Aldaco D, Palomero-Rivero M, Mechoulam R, Drucker-Colin R. Cannabidiol, a constituent of Cannabis sativa, modulates sleep in rats. FEBS lett. 2006;580 (18):4337–45. doi: 10.1016/j.febslet.2006.04.102. [DOI] [PubMed] [Google Scholar]
- 15.Murillo-Rodriguez E, Millan-Aldaco D, Palomero-Rivero M, Mechoulam R, Drucker-Colin R. The nonpsychoactive Cannabis constituent cannabidiol is a wake-inducing agent. Behav. Neurosci. 2008;122(6):1378–82. doi: 10.1037/a0013278. [DOI] [PubMed] [Google Scholar]
- 16.Guimaraes VM, Zuardi AW, Del Bel EA, Guimaraes FS. Cannabidiol increases Fos expression in the nucleus accumbens but not in the dorsal striatum. Life Sci. 2004;75(5):633–8. doi: 10.1016/j.lfs.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Murillo-Rodriguez E, Palomero-Rivero M, Millan-Aldaco D, Mechoulam R, Drucker-Colin R. Effects on sleep and dopamine levels of microdialysis perfusion of cannabidiol into the lateral hypothalamus of rats. Life Sci. 2011;88(11-12):504–11. doi: 10.1016/j.lfs.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 18.(a) Sallanon M, Kitahama K, Buda C, Puymartin M, Luppi P H, Jouvet M. Effects of electrolytic lesion of hypothalamic paraventricular nucleus and its related areas on the sleep waking cycle in the cat. Arch. italiennes de Biologie. 1987;125 (4):305–15 . [PubMed] [Google Scholar]; (b) Suntsova N V, Dergacheva O Y, Burikov A A. The role of the posterior hypothalamus in controlling the paradoxical phase of sleep. Neurosci. Behav. Physiol. 2000;30 (2):161–7. doi: 10.1007/BF02463154. [DOI] [PubMed] [Google Scholar]; (c) Suntsova N V, Dergacheva O Y. Dynamics of neuron activity in the lateral preoptic area of the hypothalamus during the sleep-waking cycle. Neurosci. Behav. Physiol. 2003;33 (7):651–8. doi: 10.1023/a:1024452522100. [DOI] [PubMed] [Google Scholar]; (d) Burt J, Alberto C O, Parsons M P, Hirasawa M. Local network regulation of orexin neurons in the lateral hypothalamus. Am. J. Phyiol.Regul. Integ. Comparative Physiol. 2011; 301 (3):R572–80. doi: 10.1152/ajpregu.00674.2010. [DOI] [PubMed] [Google Scholar]
- 19.(a) McGinty D J, Harper R M. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101 (3):569–75. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]; (b) Trulson M E, Jacobs B L. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163 (1):135–50. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]; (c) Jones B E. Arousal systems. Front. in Biosci. J Virtual Library. 2003;8:s438–51. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]; (d) Urbain N, Creamer K, Debonnel G. Electrophysiological diversity of the dorsal raphe cells across the sleep-wake cycle of the rat. J. Physiol. 2006;573 (Pt 3):679–95. doi: 10.1113/jphysiol.2006.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Murillo-Rodriguez E, Arias-Carrion O, Zavala-Garcia A, Sarro-Ramirez A, Huitron-Resendiz S, Arankowsky-Sandoval G. Basic sleep mechanisms: an integrative review. Central Nervous System Agents In Med. Chem. 2012;12 (1):38. doi: 10.2174/187152412800229107. [DOI] [PubMed] [Google Scholar]
- 20.(a) Pandolfo P, Silveirinha V, dos Santos-Rodrigues A, Venance L, Ledent C, Takahashi R N, Cunha R A, Kofalvi A. Cannabinoids inhibit the synaptic uptake of adenosine and dopamine in the rat and mouse striatum. Eur. J. Pharmacol. 2011;655 (1-3):38–45. doi: 10.1016/j.ejphar.2011.01.013. [DOI] [PubMed] [Google Scholar]; (b) Casarejos M J, Perucho J, Gomez A, Munoz M P, Fernandez-Estevez M, Sagredo O, Fernandez Ruiz J, Guzman M, de Yebenes J G, Mena M A. Natural cannabinoids improve dopamine neuro- transmission and tau and amyloid pathology in a mouse model of tauopathy. J. Alzheimer's Dis. 2013;35 (3):525–39. doi: 10.3233/JAD-130050. [DOI] [PubMed] [Google Scholar]
- 21.(a) Jones B E, Bobillier P, Pin C, Jouvet M. The effect of lesions of catecholamine-containing neurons upon monoamine content of the brain and EEG and behavioral waking in the cat. Brain Res. 1973;58 (1):157–77 90830-5. doi: 10.1016/0006-8993(73)90830-5. [DOI] [PubMed] [Google Scholar]; (b) Cano J, Garcia-Uria J, Machado A, Reinoso-Suarez F. Effect of cerebellar lesions on monoamine levels in various brain areas of the cat. J. Neurochem. 1980;35 (6):1446–8. doi: 10.1111/j.1471-4159.1980.tb09021.x. [DOI] [PubMed] [Google Scholar]
- 22.(a) Rye D B, Jankovic J. Emerging views of dopamine in modulating sleep/wake state from an unlikely source PD. Neurology. 2002;58 (3):341–6. doi: 10.1212/wnl.58.3.341. [DOI] [PubMed] [Google Scholar]; (b) Paus S, Brecht H M, Koster J, Seeger G, Klockgether T, Wullner U. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson's disease. Mov. Disorders : Official J. Mov. Disorder Soc. 2003;18 (6):659–67. doi: 10.1002/mds.10417. [DOI] [PubMed] [Google Scholar]; (c) Feve A P. Current status of tyrosine hydroxylase in management of Parkinson's disease. CNS Neurol. Disorders Drug Targets. 2012;11 (4):450–5. doi: 10.2174/187152712800792910. [DOI] [PubMed] [Google Scholar]; (d) Videnovic A, Golombek D. Circadian and sleep disorders in Parkinson's disease. Exper. Neurol. 2013;243:45–56. doi: 10.1016/j.expneurol.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Maass A, Reichmann H. Sleep and non-motor symptoms in Parkinson's disease. J. Neural Transm. 2013;120 (4):565–9. doi: 10.1007/s00702-013-0966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cartwright R. Sleep-related violence: does the polysomnogram help establish the diagnosis?. Sleep Med. 2000;1 (4):331–335. doi: 10.1016/s1389-9457(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 24.Pagel J F. Excessive daytime sleepiness. Am. Family Phys. 2009;79 (5):391–6. [PubMed] [Google Scholar]
- 25.Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus L O. Cannabidiol--recent advances. Chem. Biodiversity. 2007;4 (8):1678–92. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- 26.(a) Deiana S. Medical use of cannabis. Cannabidiol: a new light for schizophrenia?. Drug Testing Analysis. 2013;5 (1):46–51. doi: 10.1002/dta.1425. [DOI] [PubMed] [Google Scholar]; (b) Flachenecker P. A new multiple sclerosis spasticity treatment option: effect in everyday clinical practice and cost-effectiveness in Germany. Expert Rev. Neurother. 2013;13 (3) Suppl 1 :15–9. doi: 10.1586/ern.13.1. [DOI] [PubMed] [Google Scholar]