Abstract
Objective:
Recent evidence has associated immune and inflammatory changes to cognitive performance in many diseases, including schizophrenia. Since this is a new research field where concepts are not yet solid and new questions and hypothesis are still arising, the present study aimed at summarizing the available clinical data associating schizophrenia, cognition and inflammation/immune function.
Methods:
A systematic review of the literature was made by searching the following terms in Medline: “schizophrenia or psychosis or psychotic” AND “inflamm* or immun* or cytokine or IL-* or TNF-* or kynureni* or KYNA”, AND “cognit* or attention or memory or executive function”.
Results:
Seventy five papers were identified using the selected terms, and seven papers were included in the review. Papers excluded focused mainly on basic research or other neuropsychiatric disorders.
Conclusions:
Recent findings link inflammatory markers to cognition in schizophrenia, suggesting that inflammation is associated with worst cognitive performance. Microglial activation, monoaminergic imbalance, brain abnormalities and the kynurenine pathway are possible mechanisms underlying cognitive impairment in schizophrenia. Clinical trials with addition of immunomodulatory drugs have shown promising results, opening new windows to tackle cognition in schizophrenia.
Keywords: Cognition, immunology, inflammation, schizophrenia.
INTRODUCTION
Schizophrenia is the most debilitating neuropsychiatric disorder. Patients with schizophrenia present major impairment in social functioning, independent living and work status, compared to healthy subjects [1, 2].
Besides the well-established positive and negative symptoms, recently it has been largely recognized that a generalized cognitive deficit is at the core of schizophrenia, and it significantly affects patient's social functioning [2-5]. Cognitive impairment in schizophrenia ranges from sensory and perceptual dysfunctions to higher order cognitive dysfunctions like working and episodic memory, attention, problem solving, processing speed [6-8]. The course of cognitive impairment in schizophrenia is characterized by deterioration close to and during the first psychotic episode and a relative stability afterwards. Nevertheless, patients with schizophrenia tend to present lower cognitive performance than the general population even in the premorbid phase [9, 10]. Antipsychotic medications have shown modest positive effects on different cognitive domains, without a preferential effect over a specific function [11-14]. Better treatment approaches to cognition could be achieved with a greater understanding on the pathogenesis of cognitive impairment in schizophrenia, which remains poorly understood. Evidence from studies with other neuropsychiatric disorders indicates that immuno-inflammatory processes may play a central role in cognitive deficits. For instance, it has been shown that inflammation may be an important neuropathological mechanism underlying cognitive decline and dementia in elderly population [15]. Executive dysfunction was associated to inflammatory parameters in bipolar patients, with inhibitory control being positively correlated to TNF-α levels [16]. Levels of inflammatory markers have also been linked to cognitive function in major depressive disorder. For instance, high IL-6 levels were associated to low performance in immediate and delayed verbal recall tests in recurrent depressed women [17].
In schizophrenia, there is considerable evidence of changes in the immune system. Previous studies have shown an imbalance between type-1 and type-2 immune responses with a predominant type-2 response [18]. However, a recent meta-analysis showed dominant pro-inflammatory changes in schizophrenia, but not type-2 or type-1 predominant immune response. After controlling for antipsychotic use as a confounding factor, only IL-1RA and IL-6 levels were elevated in schizophrenia [19]. These data also indicate that antipsychotic medication alters immune parameters [20]. Another meta-analysis, that considered clinical status and antipsychotic effects, did not find an increase in type-2 cytokines either [21]. Instead, the authors suggested that some cytokines are state markers of acute exacerbations (IL-1β, IL-6 and TGF-β), while others may be trait markers (IL-12, IFN-γ, TNF-α, sIL-2R). Th1 derived cytokines IL-12 and IFN-γ were elevated only in acute relapses and first episode psychotic patients, as well as macrophage derived cytokines IL-6, TNF-α and IL-1β [21].
Prenatal and perinatal infections could disrupt fetal neurodevelopmental processes, leading to brain long-lasting changes and increasing the risk of psychotic disturbances in early adulthood [22]. Prenatal and perinatal infections could also act on the priming of immune activation in this early period of life [23]. In this scenario, altered levels of IL-1 and IL-6 could influence the release of hormones by the hypothalamic-pituitary-adrenal axis, contributing to changes in monoamine neurotransmission [24]. Therefore, infection and inflammation may trigger pathological mechanisms, resulting in proneness to psychosis and, possibly, cognitive dysfunction.
Given the relevance of the cognitive impairment and the immune changes in schizophrenia as well as the putative role of the immune system in cognitive dysfunction, it turns relevant to investigate the association between cognitive and immune variables in schizophrenia. Therefore, the aim of the present study was to systematically review all the papers published concerning schizophrenia, cognition and immunity. We focused on studies that assessed cognitive and immune variables directly on the patients. By excluding studies that used other populations (e.g. Alzheimer, Bipolar Disorder) or basic research we tried to reduce complexity, homogenize results and to emphasize on studies with direct clinical implications. Nevertheless, articles not included in our selection, but whose content is relevant to our results, were included in the discussion.
METHODS
A search was conducted in the Medline database, up to April 2013, comprising studies written in English, with the following terms in the abstract and/or title: “schizophrenia or psychosis or psychotic” AND “inflamm* or immun* or cytokine or IL-* or TNF-* or kynureni* or KYNA”, AND “cognit* or attention or memory or executive function”. Only original papers were included.
RESULTS
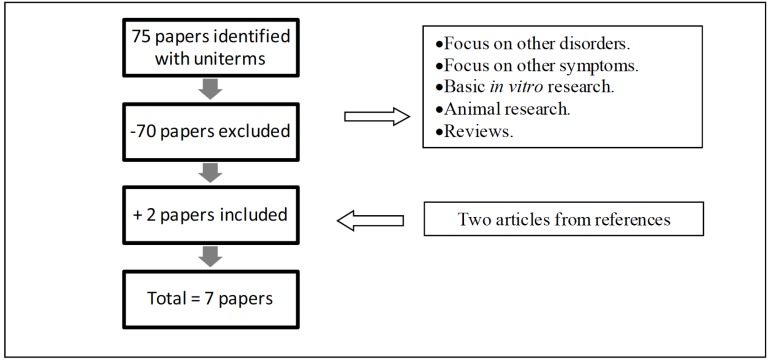
Seventy five papers were identified using the selected terms. After a critical analysis of the abstracts, seventy papers were excluded, due to diverging focus (Fig. 1). The papers excluded were either too basic, concerning chemical or biological in vitro research or animal studies with murine models with little contribution for clinical practice; or too broad, revising immunological aspects in other neuro-psychiatric disorders or concerning different symptomatological domains. Papers that only inferred that immune changes could affect cognition in schizophrenia, without actual tests, were also excluded to homogenize the sample and focus on clinical and practical aspects.
Fig. (1).
Flow chart of the selection of studies on the role of immune system in cognitive changes in schizophrenia.
Two articles from references of the selected papers were also included. In sum, seven original papers were included in the present study. Papers that assessed cognition and immune and/or inflammatory markers in patients with schizophrenia are displayed on Table 1. Clinical trials that added immuno-modulatory drugs to antipsychotic regimen are shown in Table 2.
Table 1.
Studies assessing inflammatory/immune markers and cognition in schizophrenia.
| Authors | Year | N | Control type | Cognitive Functions and Instruments | Inflammatory Markers | Results | Conclusions |
|---|---|---|---|---|---|---|---|
| Dickerson et al. | 2007 | 413 SZ patients. | Patients divided by CRP levels (high or low). No healthy controls. | RBANS (based on 5 scales: immediate and delayed memory, visuo-constructional, language and attention) | CRP levels (˂ 5.0 mg/μl or ≥ 5.0 mg/μl) |
p=0.0047 (PCR negatively associated to RBANS) | CRP levels related to cognitive impairment measured by RBANS composite score in SZ. |
| Dickerson et al. | 2012 | 588 SZ patients. | Patients divided in four groups, according to HSV-1 serological status and CRP levels. No healthy controls. | RBANS | CRP levels (˂ 5.0 mg/μl or ≥ 5.0 mg/μl (and HSV1 status) |
Effect sizes on RBANS were .10 for HSV1+, .10 for high CRP, .14 for HSV1+ and high CRP together. | Additive effects of elevated CRP and exposure to HSV-1 in cognitive impairment in SZ. |
| MartÍnez-Cengotitabengoa et al. | 2012 | 28 First Episode Psychosis patients. | 28 healthy controls. | Fluency Assessment Scale, WAIS III, TMTA, TMTB Stroop Color and Word Test, WMS III, WCST. | MCP-1 (and oxidative stress markers). | Negative association between MCP-1 levels and learning and memory (p=0.009). | Learning, verbal and working memory correlates inversely with chemokine levels and executive function directly to antioxidant markers in first psychotic episodes. |
| Zhang et al. | 2013 | 77 First Episode and Drug NaÏve SZ patients | 75 healthy, gender, education and age-matched controls. | RBANS | IL-18 serum levels. | Positive association between IL-18 levels and RBANS visuospatial/constructional index (p=0.03). | IL-18 may be associated to cognitive deficits in SZ. Association was the opposite than expected, suggesting neuroprotection (hypothesis of antiviral activity). |
Abbreviations: CRP: C reactive protein, HSV1: Herpes Simplex Virus type 1, IL-18: interleukin 18, MCP-1: monocyte chemoattractant protein 1.RBANS: Repeatable Battery for the Assessment of Neuropsychological status, Th1/Th2: T helper cells type 1 and 2, SZ: schizophrenia, TMTA/B: Trail Making Test A and B, WAIS III: Wechsler Adult Intelligence Scale, WCST: Winconsin Card Sorting Test, WMS III: Wechsler Memory Scale
Table 2.
Clinical trials with immunomodulatory drugs augmentation to antipsychotics.
| Authors | Year | Trial Design | Cognitive Functions and Instruments | Inflammatory Markers | Results | Conclusions |
|---|---|---|---|---|---|---|
| MÜller et al. |
2005 | 25 SZ patients with risperidone + celecoxib, 25 patients with risperidone + placebo, for 5 weeks. | PANSS cognition subscale – items conceptualization and abstract thinking. | NA | p=0.06 (improvement under treatment) | Celecoxib add-on to risperidone shows trends of improvement on PANSS total score and cognition subscale. |
| Levkovitz et al. | 2010 | 36 early-phase SZ patients assigned to minocycline as add-on, 18 patiens to placebo (6 months follow up). |
CANTAB (Cambridge Neuropsychological Test Automated Battery), SANS (Scale for the Assessment of Negative Symptoms). | NA | Improvement in executive functions, mainly working memory, cognitive shifting and planning. | Minocycline ameliorates negative symptoms and cognitive deficits in early-phase SZ. |
| Laan et al. |
2010 | 27 patients assigned to Aspirin 1000mg as add-on for 3 months, and 31 patients assigned to the placebo group; groups were stratified by Th1/Th2 balance. | Rey Auditory Verbal Learning, HQ Continuous Performance Test, Purdue Pegboard Test, Trail Making Test. | IFN-γ and IL-4 (Th1/Th2 ratio). |
No significant effects on cognitive function. | Aspirin add-on reduces symptomsof SZ spectrum disorders (PANSS positive, subscale and total score), but does not affect cognition. Greater symptomatic reduction in those with more altered immune function. |
Abbreviations: CANTAB: Cambridge Neuropsychological Test Automated Battery, IFN-γ: interferon gamma, IL-4: interleukin 4, NA: not available, PANSS: Positive and Negative Syndrome Scale, RBANS: Repeatable Battery for the Assessment of Neuropsychological status, Th1/Th2: T helper cells type 1 and 2, SANS: Scale for the Assessment of Negative Symptoms, SZ: schizophrenia, TMTA/B: Trail Making Test A and B.
Four studies addressed associations between immune/inflammatory markers and cognitive functions in schizophrenia or first-episode psychosis. Elevated serum C-reactive protein (CRP) levels were associated with the severity of cognitive impairment, but not with positive or negative symptoms. Patients with CRP levels above 5 mg/μl (high level group) had a lower score on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), which is based on scales for immediate and delayed memory, visuo-constructional, language and attention [25]. A recent study of the same group showed that the effects of elevated CPR levels and Herpes Simplex Virus type 1 (HSV-1) seropositivity on cognitive impairment as assessed by RBANS in patients with schizophrenia were additive and statistically independent. The largest cognitive impairment was found in the group with both factors, i.e. high levels of CRP and HSV-1 antibodies, reinforcing the hypothesis that inflammation and infection may play a role in cognition [26].
Another study correlated levels of either the chemokine monocyte chemoattractant protein 1 (MCP-1/CCL-2) or oxidative stress markers (nitrites and glutathione) with performance on cognitive tasks in a group of first-episode psychosis patients. MCP-1 levels were negatively correlated to learning and memory performance, while oxidative stress markers were associated with poorer executive functioning [27]. A recent Chinese study found a positive association between interleukin 18 (IL-18) levels and RBANS visuospatial and constructional indexes in first episode and drug naïve psychotic patients [28].
Three studies yielded data about the effect of anti-inflammatory or immunomodulatory drugs as add-on therapy to the antipsychotic regimen on cognition of patients with schizophrenia. Using celecoxib (cyclooxygenase type 2 (COX-2) inhibitor) 400mg/day or placebo as add-on to risperidone, Müller et al. [29] assigned 25 patients to each group during a five week trial. Patients using celecoxib had a greater reduction of total score in the Positive and Negative Symptoms Scale (PANSS) when compared to the placebo group. Specific effects on cognition were evidenced after re-evaluation of data that showed that the effect on the PANSS cognition factor (items ‘difficulty in abstract thinking’ and ‘conceptual disorganization’) was the most pronounced. The effect seems to be stronger in cases with more recent onset and shorter disease duration [30].
Another trial added Aspirin (1000mg) to antipsychotic treatment in a group of 70 patients with schizophrenia for a three month period. It was argued that aspirin has a cardioprotective profile and that its unselective inhibition of COX-1 and COX-2 enzymes would allow a wider range of action. Statistically significant effects on the total score and positive subscale of PANSS were observed. Interferon-γ and inteleukin-4 levels were also analyzed before, during and after treatment. The effect was larger in patients with a lower type-1/type-2 cytokine balance (defined by the median interferon-γ/ interleukin-4 ratio), suggesting that the reduction of positive symptoms was larger in patients with an immune profile tending to the type-2 response. Although Aspirin seemed to improve psychiatric symptoms, cognition was not affected [31].
A double-blind, randomized, placebo-controlled study with minocycline, an antibiotic which also exerts immuno-modulatory activity, as augmentation to antipsychotics found that it may alleviate negative symptoms and improve cognitive functioning in early-phase schizophrenia. Patients using minocycline showed improvement in executive functions (working memory, cognitive shifting and planning) in comparison to the placebo group measured by the Cambridge Neurospychological Automated Battery (CANTAB) [32].
DISCUSSION
Although the research linking cognition and inflammation in schizophrenia is still scarce, initial results are promising. MCP-1 and IL-18 levels, as well as CRP levels and HSV-1 seropositivity, were linked to cognition in schizophrenia. Interventional studies that added immunomodulatory drugs to antipsychotic regimen also yielded interesting results, opening new possibilities to improve cognition and other psychiatric symptoms in schizophrenia.
CRP has been used for years as a biomarker for cardiovascular risk. Elevated high sensitivity CRP may be also a marker of memory and visuospatial impairment in the elderly, being associated with dementia. CRP is known to be elevated in those with risk factors common to stroke and dementia, such as diabetes, obesity and smoking [33]. Recent studies suggested that assessment of low grade inflammation by high sensitivity CRP can be related to cerebral microstructural disintegration, affecting frontal lobe pathways and leading to executive dysfunction [34]. Frontal dysfunction is a common feature in schizophrenia and these findings may help explain how inflammation may undermine cognitive functions. CRP levels have been negatively associated to cognitive performance in patients with schizophrenia [25]. High CRP levels may be a candidate biomarker for worse prognosis in schizophrenia. CRP assessment could also help identify groups of patients with schizophrenia that would benefit from immunomodulatory drugs, improving cognitive function or even psychiatric symptoms.
As well as inflammatory markers such as CRP, it appears that immune activators such as viruses could be related to cognition in schizophrenia. The concept of “early-life programming of adult disease” postulates that specific environmental factors acting during sensitive prenatal or early postnatal developmental periods can induce persistent changes in physiological, emotional and behavioral functions throughout life [35]. These factors could prime not only the immune system and its responses in adult life, but cause neurodevelopmental changes and increase proneness to psychosis. Prenatal maternal immune activation, inflammation, viral infections, as well as psychological stress and malnutrition appear to affect offspring development, increasing the risk of psychotic disorders later on, impairing sensorimotor gating, information processing, cognition, social function and leading to subcortical hyperdopaminergia [22, 36]. Prenatal influenza infections are associated with altered neuronal migration in cortical and hippocampal neurons [37], which are essential for broad functioning of many cognitive domains. Different kinds of viruses are known to alter the monoaminergic balance within the brain after experimental infection, particularly serotonin and noradrenaline-mediated systems [38]. HSV-1 lifelong cycles in the brain may cause neuronal damage and dysfunction and may also be associated with longitudinal gray matter loss in the posterior cingulate gyrus and decline in executive functioning among subjects with schizophrenia [39]. Significant association between the neurocognitive summary score, verbal memory, vigilance and processing speed; and antibodies to HSV-1 was described in the CATIE trial (Clinical Antipsychotic Trial of Intervention Effectiveness) [40]. These findings may help explain the association of HSV-1 and cognition in schizophrenia described in the results by Dickerson [25, 26] and also possible underlying pathological changes. We could also hypothesize that microglial activation may occur in early neurodevelopment in these cases and be linked to first episode psychosis as well, converging previous neuro-structural abnormalities and monoaminergic imbalance to behavioral and cognitive impairment.
MCP-1/CCL-2 was found to be associated to cognitive deficit in first episode psychosis. MCP-1 is a chemokine that recruits monocytes, dendritic and T cells to the inflammation site. It is also associated to microglia activation in neuro-inflammatory diseases. It was hypothesized that in psychotic patients the systemic oxidative-inflammatory status may influence cognitive performance through inflammatory mediators in cerebral vasculature or increase in blood-brain permeability [27]. MCP-1 has already been linked with cognitive deficits associated with HIV dementia and Alzheimer’s disease [41, 42]. Therefore, inflammatory processes initiated by MCP-1, like microglia activation and leucocyte migration, appear to be associated to cognition in schizophrenia as shown by the negative association between MCP-1 levels and learning and memory (p=0.009) [27]. This is in line with the microglia hypothesis of schizophrenia, which postulates that pro-inflammatory cytokines and free radicals produced by an activated microglia may decrease neurogenesis, result in white matter abnormalities and favor neurodegeneration, contributing to the neuropathology of the disorder [43]. Animal experiments have shown that IL-6 can increase dopaminergic neurotransmission in the hippocampus, and IL-2 also increases serotonin and noradrenaline-mediated neurotransmission [44]. This could be another possible mechanism linking cognitive dysfunction to MCP-1 levels.
Although no differences were found between IL-18 levels in first episode patients with schizophrenia and healthy controls, there was a positive association between IL-18 levels and RBANS visuospatial/constructional cognitive index. These findings seem paradoxal since there are studies associating IL-18 levels to worse cognitive function in Alzheimer’s disease and multiple sclerosis. However, IL-18 may contribute differently to acute neuroinflammation and chronic neurodegeneration [45]. IL-18 might display neuro-protective effects, besides its pro-inflammatory actions. Due to the association between viral infections and schizophrenia, it is proposed that IL-18 release after viral infection would lead to microglial activation with interferon gamma (IFN-γ) release in the brain parenchyma and viral clearance. Another explanation is that the visuospatial index may be more resilient to be impaired by IL-18 than other cognitive functions measured by RBANS [28]. It is still uncertain how each immune marker affects neurodevelopment, and therefore also influences cognition. IL-18 could be associated to ‘damage control’, for example, following viral infection in pre or perinatal period, avoiding or minimizing structural abnormalities associated to schizophrenia and cognitive impairment.
Most clinical trials that used anti-inflammatory or immunomodulatory drugs in addition to antipsychotics in order to verify cognitive effects yielded positive results.
Minocycline add-on to antipsychotics showed improvement in cognition. It is an antibiotic with anti-inflammatory, anti-oxidative and anti-apoptotic properties. Its neuroprotective properties may arise from astrocytic and microglial caspase 1 inhibition, as well as nitric oxide synthase inhibition, which also affects the glutamatergic system. The dopaminergic system may also be affected, since minocycline attenuates dopamine elevation following NMDA agonist administration. Preclinical inflammation models also showed impact on inflammatory markers, such as TNF-α, IL-1β, PGE2 and COX-2 [32, 46, 47]. Pro-inflammatory cytokines and free radicals produced by microglia can contribute to neuron degeneration. There have been reports of inhibitory effects of typical and atypical antipsychotics in inflammatory and oxidative stress mediated by the microglia; factors which have been recently associated with reduced neurogenesis and white matter abnormalities [43]. Minocycline may exert its beneficial effects on cognition by controlling microglial activation. Since patients were described as having early-phase schizophrenia, authors suggest that intervention with immunomodulatory drugs may display better results in initial stages of the disease and improve clinical course [32].
Celecoxib also seems to improve cognition, although the observed improvement derived from PANSS cognitive factors and not neurocognitive tests per se. Celecoxib is a selective anti-inflammatory drug that inhibits COX-2, but not COX-1. Contrasting with its isoform COX-1, COX-2 is induced by stimulation in most tissues, but it is constitutively expressed in the central nervous system structures such as frontal cortex, amygdala and hippocampus which are critically involved in cognitive functioning [38, 48]. COX-2 activity can be stimulated by tissue damage and also by glutamate excitation, showing its high sensitivity to neuronal stress [49]. Cytokines such as IL-2, IL-6 and IL-10 activate COX-2, and result in inflammatory response also in the central nervous system. Hence, COX-2 inhibition seems to balance type 1/ type 2 immune responses by inhibition of PGE2 and stimulation of type 1 immune response, and also by inhibition of KYNA (kynurenic acid) production [50]. KYNA is an antagonist at the glycine site of the N-methyl-D-aspartic acid receptor (NMDAR) and at the α7 nicotinic acetylcholine receptor (α7nAChR), both of which implicated in the cognitive impairment of schizophrenia [51, 52]. Increased immune function, primarily in the blood, would raise most kynurenine metabolites and in result, elevate also KYNA levels in the brain. This would impair glutamatergic neurotransmission and possibly affect dopaminergic neurotransmission as well. Since the kynurenine pathway of tryptophan metabolism is induced by immunological activation and stress, it can mediate the effects of environmental factors in cognition and behavior. Therefore, it may be a promising metabolic pathway for developing new pharmacological interventions to treat and prevent cognitive dysfunction in schizophrenia and other neuro-psychiatric disorders [53].
The beneficial effects of COX-2 inhibition may also be due to alteration in glutamate neurotransmission, with inhibition of the NMDA receptors and activation of kainate receptors [29]. Müller et al. [30] also proposed that COX-2 inhibitors may have a role in learning and memory by affecting long-term potentiation and long-term depression, as well as attenuating cholinergic dysfunction. Therefore, many mechanisms could explain the positive effects of COX-2 inhibitors on cognition in schizophrenia. As argued by Müller and Schwarz [20] when evaluating the effect of anti-inflammatory drugs on cognition in schizophrenia, one should take into account the disease stage. Therapeutic studies have shown beneficial effects of anti-inflammatory drugs mostly in early stages, with little effect in late stages. Müller et al. stated that patients with a shorter duration of disease improved more in the aforementioned clinical trial [30]. These findings could reflect that an early anti-inflammatory therapy could prevent neuronal damage and structural changes caused by chronic inflammation in schizophrenic patients over many years of disease evolution [20].
Aspirin (acetylsalicylic acid) is a widely used anti-inflammatory agent that inhibits inflammatory cyclo-oxygenase pathways and suppresses prostaglandins and tromboxane. Aspirin also exerts positive effects through modulation of oxidative and nitrosative stresses. Laan et al. [31] found that the group of patients with lower Th1/Th2 balance benefited more from the use of Aspirin than the others. This finding is in line with other studies and the hypothesis of an imbalance between pro and anti-inflammatory forces. Cognition was not affected, but authors argued that it was a stable domain over time in these patients, and that a longer time could be necessary to observe changes. Laan et al. [31] also hypothesized that Aspirin may act by antagonizing NMDA dysfunction and that it exerts its effects by interacting with antipsychotics.
As only a few studies that addressed simultaneously cognition and immunological changes in schizophrenia are available to date, the results must be interpreted cautiously. The exclusion of research or animal models may have narrowed the discussion, excluding other hypothetical mechanisms linking immune changes and cognition in schizophrenia that have not been transposed into clinical studies yet. Regarding immunomodulatory drugs, many parameters are still undefined, such as duration, dose and time of initiation of a possible immunomodulatory drug. Moreover, it appears that positive effects are more evident in the initial stages of schizophrenia. One of the clinical trials revised did not use standard neuropsychological assessment battery in schizophrenia (PANSS cognition factor) [30]. It is advised a careful selection of the neuropsychological battery used to obtain more reliable results in future trials, in order to compare drug efficacy and cognitive improvement.
CONCLUSIONS
Schizophrenia is a heterogeneous syndrome and immune changes may be another neuropathogenic mechanism among many already described. Cognition is one of the main compromised domains in schizophrenia and accounts for a great part of the patient’s functioning and quality of life. Recent discoveries link inflammation to the cognitive deficits seen in the disease. Clinical trials have shown improvement on cognitive parameters and other symptoms with the addition of immunomodulatory drugs. It seems very likely, though, that inflammation and immune dysfunction affect neurodevelopment, increase risk of psychotic illnesses and continue to affect cognitive processes throughout life. Possible underlying mechanisms include monoaminergic imbalance, microglial activation, structural abnormalities in white matter, frontal lobe, hippocampus; as well as changes in the kynurenine pathway of the tryptophan metabolism affecting glutamatergic and cholinergic neurotransmission. Future clinical trials using either anti-inflammatory drugs, nicotinic or glutamatergic agents or manipulation of brain KYNA levels should address cognition more directly. More basic and clinical studies are needed in order to further enlighten the mechanisms underlying cognitive deficits and pursue new cognitive-enhancing drugs to the treatment of the disorder.
ACKNOWLEDGEMENTS
This work was partly funded by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig, Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia just the facts 4.Clinical features and conceptualization. Schizophr Res. 2009;110:1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363:2063–2072. doi: 10.1016/S0140-6736(04)16458-1. [DOI] [PubMed] [Google Scholar]
- 3.Ross CA, Margolis RL, Reading SAJ, Pletnikov M. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Keefe RS, Poe M, Walker TM, Kang JW, Harvey PD. The Schizophrenia Cognition Rating Scale an interview-based assessment and its relationship to cognition real-world functioning and functional capacity. Am J Psychiatry. 2006;163(3):426–432. doi: 10.1176/appi.ajp.163.3.426. [DOI] [PubMed] [Google Scholar]
- 5.Niitsu T, Shirayama Y, Matsuzawa D, Hasegawa T, Kanahara N, Hashimoto T, Shiraishi T, Shiina A, Fukami G, Fujisaki M, Watanabe H, Nakazato M, Asano M, Kimura S, Hashimoto K, Iyo M. Associations of serum brain-derived neurotrophic factor with cognitive impairments and negative symptoms in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(8):1836–1840. doi: 10.1016/j.pnpbp.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RS, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR. Approaching a consensus cognitive battery for clinical trials in schizophrenia the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56(5):301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia reliability, sensitivity and comparison with a standard neuro- cognitive battery. Schizophr Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Dickinson D, Harvey PD. Systemic hypotheses for generalized cognitive deficits in schizophrenia a new take on an old problem. Schizophr Bull. 2009;35:403–414. doi: 10.1093/schbul/sbn097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilder RM, Reiter G, Bates J, Lencz T, Szeszko P, Goldman RS, Robinson D, Lieberman JA, Kane JMl. Cognitive development in schizophrenia follow-back from the first episode. J. Clin. Exp Neuropsychol. 2006;28:270–282. doi: 10.1080/13803390500360554. [DOI] [PubMed] [Google Scholar]
- 10.Hughes C, Kumari V, Soni W, Das M, Binneman B, Drozd S, O’Neil S, Matthew V, Sharma T. Longitudinal study of symptoms and cognitive function in chronic schizophrenia. Schizophr Res. 2002;59:137–146. doi: 10.1016/s0920-9964(01)00393-0. [DOI] [PubMed] [Google Scholar]
- 11.Mishara AL, Goldberg TE. A meta-analysis and critical review of the effects of conventional neuroleptic treatment on cognition in schizophrenia opening a closed book. Biol. Psychiatry. 2004;55:1013–1022. doi: 10.1016/j.biopsych.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine and risperidone in schizophrenia. International Int. J. Neuropsychopharmacol. 2005;8:457–472. doi: 10.1017/S146114570500516X. [DOI] [PubMed] [Google Scholar]
- 13.Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA. Neurocognitive effects of antipsychotic medications in patients with schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 14.Keshavan MS, Nasrallah HA, Tandon R. Schizophrenia "Just the Facts" 6.Moving ahead with the schizophrenia concept from the elephant to the mouse. Schizophr Res. 2011;127:3–13. doi: 10.1016/j.schres.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorelick PB. Role of inflammation in cognitive impairment results of observational epidemiological studies and clinical trials. Ann. N.Y. Acad Sci. 2010;1207:155–162. doi: 10.1111/j.1749-6632.2010.05726.x. [DOI] [PubMed] [Google Scholar]
- 16.Barbosa IG, Rocha NP, Huguet RB, Ferreira RA, Salgado JV, Carvalho LA, Pariante CM, Teixeira AL. Executive dysfunction in euthymic bipolar disorder patients and its association with plasma biomarkers. J. Affect. Disorders. 2012;137:151–155. doi: 10.1016/j.jad.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Grassi-Oliveira R, Bauer ME, Pezzi JC, Teixeira AL, Brietzke E. Interleukin 6 and verbal memory in recurrent major depressive disorder. Neuroendocrinol Lett. 2011;32(4):540–544. [PubMed] [Google Scholar]
- 18.Schwarz MJ, Müller N, Riedel M, Ackenheil M. The Th2-hypothesis of schizophrenia a strategy to identify a subgroup of schizophrenia caused by immune mechanisms. Med Hypotheses. 2001;56(4):483–486. doi: 10.1054/mehy.2000.1203. [DOI] [PubMed] [Google Scholar]
- 19.Potvin S, Stip E, Sepehery AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia a systematic review. Biol Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Müller N, Schwarz M. Immune system and schizophrenia. Curr Immunol Rev. 2010;6(3):213–220. doi: 10.2174/157339510791823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller N, Schwarz MJ. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006;10(2):131–148. doi: 10.1007/BF03033242. [DOI] [PubMed] [Google Scholar]
- 24.Dantzer R. Cytokine-induced sickness behavior where do we stand?. Brain Behav. Immun. 2001;15:7–27. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 25.Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr. Res. 2007;93:261–5. doi: 10.1016/j.schres.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, olken R. Additive effects of elevated C-reactive protein and exposure to Herpes Simplex Virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr. Res. 2012;134:83–88. doi: 10.1016/j.schres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Cengotitabengoa M, Mac-Dowell KS, Leza JC, Micó JA, Fernandez M, Echevarría E, Sanjuan J, Elorza J, González-Pinto A. Cognitive impairment is related to oxidative stress and chemokine levels in first psychotic episodes. Schizophr. Res. 2012;137:66–72. doi: 10.1016/j.schres.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XY, Tang W, Xiu MH, Chen DC, Yang FD, Tan YL, Wang ZR, Zhang F, Liu J, Liu L, Chen Y, Wen N, Kosten TR. Interleukin 18 and cognitive impairment in first episode and drug naïve schizophrenia versus healthy controls. Brain Behav Immun. 2013;32:105–111. doi: 10.1016/j.bbi.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Müller N, Riedel M, Scheppach C, Brandstätter B, Sokullu S, Krampe K, Ulmschneider M, Engel RR, Möller HJ, Schwarz MJ. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am. J Psych. . 2002;159:1029–1034. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- 30.Müller N, Riedel M, Schwarz MJ, Engel RR. Clinical effects of COX-2 inhibitors on cognition in schizophrenia. Eur Arch Psychiatry Clin. Neurosci. 2005;255(2):149–151. doi: 10.1007/s00406-004-0548-4. [DOI] [PubMed] [Google Scholar]
- 31.Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders results from a randomized double-blind placebo-controlled trial. J. Clin Psychiatry. 2010;71(5):520–527. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- 32.Levkovitz Y, Mendlovic S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G, Fennig S, Treves I, Kron S. A double-blind randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J. Clin. Psychiatry. 2010;71(2):138–149. doi: 10.4088/JCP.08m04666yel. [DOI] [PubMed] [Google Scholar]
- 33.Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-Reactive Protein With Cognitive. Arch. Neurol. 2010;67(1):87–92. doi: 10.1001/archneurol.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, Conty M, Minnerup J, Ringelstein EB, Berger K, Deppe M, Knecht S. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74(13):1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- 35.Meyer U, Schwarz MJ, Müller N. Inflammatory processes in schizophrenia a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol. Ther. 2011;132:96–110. doi: 10.1016/j.pharmthera.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Markham JA, Koenig JI. Prenatal stress role in psychotic and depressive diseases. Psychopharmacology. (Berl). 2011;214(1):89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotter D, Takei N, Farrell M, Sham P, Quinn P, Larkin C, Oxford J, Murray RM, O'Callaghan E. Does prenatal exposure to influenza in mice induce pyramidal cell disarray in the dorsal hippocampus?. Schizophr Res. 1995;16:233–241. doi: 10.1016/0920-9964(94)e0082-i. [DOI] [PubMed] [Google Scholar]
- 38.Müller N, Strassnig M, Schwarz MJ, Ulmschneider M, Riedel M. COX-2 inhibitors as adjunctive therapy in schizophrenia. Expert Opin Investig Drugs. 2004;13(8):1033–1044. doi: 10.1517/13543784.13.8.1033. [DOI] [PubMed] [Google Scholar]
- 39.Prasad KM, Eack SM, Goradia D, Pancholi KM, Keshavan MS, Yolken RH, Nimgaonkar VL. Progressive gray matter loss and changes in cognitive functioning associated with exposure to herpes simplex virus 1 in schizophrenia a longitudinal study. Am J Psychiatry. 2011;68(8):822–830. doi: 10.1176/appi.ajp.2011.10101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yolken RH, Torrey EF, Lieberman JA, Yang S, Dickerson FB. Serological evidence of exposure to Herpes Simplex Virus type 1 is associated with cognitive deficits in the CATIE schizophrenia sample. Schizophr Res. 2011;128(1):61–65. doi: 10.1016/j.schres.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Monteiro de Almeida S, Letendre S, Zimmerman J, Lazzaretto D, McCutchan A, Ellis R. Dynamics of monocyte chemoattractant protein type 1 (MCP-1) and HIV viral load in human cerebrospinal fluid and plasma. J. Neuroimmunol. 2005;169:144–152. doi: 10.1016/j.jneuroim.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corrà B, Scalabrini D, Clerici F, Mariani C, Bresolin N, Scarpini E. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer`s disease. Neurobiol. Aging. 2006;27 (12):1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Monji A, Kato T, Kanba S. Cytokines and schizophrenia Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci. 2009;63(3):257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- 44.Zalcman S, Green-Johnson JM, Murray L, Nance DM, Dyck D, Anisman H, Greenberg AH. Cytokine-specific central monoamine alterations induced by interleukin-1-2 and 6. Brain Res. 1994;643:40–49. doi: 10.1016/0006-8993(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 45.Felderhoff-Mueser U, Schmidt OI, Oberholzer A, Buhrer C, Stahel PF. IL-18 a key player in neuroinflammation and neurodegeneration?. Trends Neurosci. 2005;28 (9):497–493. doi: 10.1016/j.tins.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Miyaoka T, Yasukawa R, Yasuda H, Hayashida M, Inagaki T, Horiguchi J. Minocycline as adjunctive therapy for schizophrenia an open-label study. Clin. Neuropharmacol. 2008;31(5):287–292. doi: 10.1097/WNF.0b013e3181593d45. [DOI] [PubMed] [Google Scholar]
- 47.Dodd S, Maes M, Anderson G, Dean OM, Moylan S, Berk M. Putative neuroprotective agents in neuropsychiatric disorders. Prog. Neuropsychopharmacol. Biol Psychiatry. 2013;42:135–145. doi: 10.1016/j.pnpbp.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Turini ME, DuBois RN. Cyclooxigenase-2 a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 49.Planas AM, Soriana MA, Justicia C , et al. Introduction of cyclooxigenase-2 in the rat brain after a mild episode of focal ischemia without tissue inflammation or neural cell damage. Neurosci. Lett. 1999;275:141–144. doi: 10.1016/s0304-3940(99)00756-9. [DOI] [PubMed] [Google Scholar]
- 50.Müller N, Riedel M, Schwarz MJ. Psychotropic effects of COX-2 inhibitors-a possible new approach for the treatment of psychiatric disorders. Pharmacopsychiatry. 2004;37(6):266–269. doi: 10.1055/s-2004-832682. [DOI] [PubMed] [Google Scholar]
- 51.Müller N, Schwarz MJ. The immunological basis of glutamatergic disturbance in schizophrenia towards an integrated view. J. Neural Transm. Suppl. 2007;72:269–280. doi: 10.1007/978-3-211-73574-9_33. [DOI] [PubMed] [Google Scholar]
- 52.Müller N. Inflammation and the glutamate system in schizophrenia implications for therapeutic targets and drug development. Expert Opin. Ther. Targets. 2008;12(12):1497–1507. doi: 10.1517/14728220802507852. [DOI] [PubMed] [Google Scholar]
- 53.Stone TW, Darlington LG. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br. J. Pharmacol. 2013;169:1211–1227. doi: 10.1111/bph.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]