Abstract
Obesity is a risk factor for periodontitis, but the pathogenic mechanism involved is unclear. We studied the effects of insulin in periodontal tissues during the state of obesity-induced insulin resistance. Gingival samples were collected from fatty (ZF) and lean (ZL, control) Zucker rats. Endothelial nitric oxide synthase (eNOS) expression was decreased, and activities of protein kinase C (PKC) α, ß2, δ, and ϵ isoforms were significantly increased in the gingiva from ZF rats compared with those from ZL rats. Expression of oxidative stress markers (mRNA) and the p65 subunit of NF-κB was significantly increased in ZF rats. Immunohistochemistry revealed that NF-κB activation was also increased in the gingival endothelial cells from transgenic mice overexpressing NF-κB-dependent enhanced green fluorescent protein (GFP) and on a high-fat vs. normal chow diet. Analysis of the gingiva showed that insulin-induced phosphorylation of IRS-1, Akt, and eNOS was significantly decreased in ZF rats, but Erk1/2 activation was not affected. General PKC inhibitor and an anti-oxidant normalized the action of insulin on Akt and eNOS activation in the gingiva from ZF rats. This provided the first documentation of obesity-induced insulin resistance in the gingiva. Analysis of our data suggested that PKC activation and oxidative stress may selectively inhibit insulin-induced Akt and eNOS activation, causing endothelial dysfunction and inflammation.
Keywords: periodontitis, protein kinase C, oxidative stress, endothelial nitric oxide synthase, periodontal bone loss, NF-kappa B
Introduction
Recent studies have suggested that overweight and obesity are associated with periodontal disease progression (Chaffee and Weston, 2010), independent of glycemic control (Saito and Shimazaki, 2007) or the diagnosis of diabetes (Gorman et al., 2012). Genco et al. (2005) reported that body mass index (BMI) was positively correlated with the severity of periodontal attachment loss and insulin resistance. Insulin resistance, observed in diabetes and obesity, has been associated with increased risk of cardiovascular disease, hypertension, and chronic kidney disease (Rask-Madsen and King, 2007). Thus, it is possible that insulin resistance may also play an important role in the acceleration of periodontitis in patients with metabolic syndrome or diabetes. However, no experimental study has demonstrated either that insulin resistance exists in the gingiva or the mechanism for its induction in obese and diabetic states.
The Zucker fatty (ZF) rat, an established model of obesity-related insulin resistance with pre-diabetes, hyperinsulinemia, hyperlipidemia, and glucose intolerance (Bray, 1977), has been reported to have greater alveolar bone resorption in comparison with normal Sprague-Dawley rats (Perlstein and Bissada, 1977). Pontes Andersen and co-workers experimentally demonstrated the worsening of periodontitis in ZF than Zucker lean (ZL) rats correlated to impaired glucose tolerance (IGT) (Pontes Andersen et al., 2007).
This study characterized insulin signaling and actions and the mechanism of insulin resistance caused by protein kinase C (PKC) activation in the gingiva from obesity-induced insulin-resistant rodent models compared with their lean controls.
Materials & Methods
Animals
Male ZF rats (ZF-fa/fa; n = 12) and their lean matched controls (ZL-fa/+; n = 12) at 12 wk of age were supplied by Charles River Laboratories (Wilmington, MA, USA). After rats fasted for 14 hr, they were anesthetized, and blood was collected for the measurement of glucose, serum insulin, free fatty acids (FFA), and cytokines. Intraperitoneal glucose tolerance test (IGTT) was performed to confirm insulin resistance. To evaluate the nuclear factor-κB (NF-κB) activity in periodontal tissue, we used NF-κB activation-dependent enhanced GFP transgenic mice (cis-NF-κBEGFP). They were produced as described previously (Magness et al., 2004) and generously provided by Drs. Steve Shoelson and Jongsoon Lee at the Joslin Diabetes Center. Eight eight-week-old cis-NF-κBEGFP mice were fed a high-fat diet (HFD) (42% from fat, Harlan Teklad, Indianapolis, IN, USA) or a normal diet for 2 mo. Rodents were sacrificed by intraperitoneal injection of pentobarbital. All protocols were approved by the animal care committee of the Joslin Diabetes Center (2012-06) and complied with the National Institutes of Health (NIH) and Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines.
Plasma Malondialdehyde (MDA) and Plasma C-reactive Protein (CRP)
Plasma MDA and CRP concentrations of Zucker rats were measured with the use of a Lipid Peroxidation Assay Kit (Oxi International, Inc., Foster City, CA, USA) and a Rat CRP Quantitative Kit (Helica, Fullerton, CA, USA), respectively.
Tissue Harvesting and Alveolar Bone Analysis of Zucker Rats
Marginal gingival samples were collected around mandibular molars and stored in liquid N2 or kept in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 0.1% bovine serum albumin (BSA). After the soft tissue was removed, the mandibles were treated with 0.1-N NaOH and stained with 1% methylene blue solution for adequate identification of the cemento-enamel junction (CEJ). The vertical distance from the CEJ to the buccal alveolar bone crest at 9 sites on each side of the mandibular molars was measured by digital stereomicroscope photography. Periodontal bone loss was calculated as the mean of 18 measurements from the left and right mandibles of each animal.
Ex vivo Study of Gingiva from Zucker Rats
Dissected gingiva was kept in DMEM containing 0.1% BSA for 90 min at 37°C and treated with insulin (100 nM) for an additional 30 min. The tissues were frozen and kept at -80°C for analysis. Inhibitors such as a general PKC inhibitor, bisindolylmaleimide I (GF109203X; GFX, 5 μM), or an anti-oxidant, N-acetyl-L-cysteine (NAC, 10 mM), were added 60 min before insulin stimulation.
Immunoprecipitation and Immunoblotting of Gingiva, Liver, and Aorta from Zucker Rats
Tissues were homogenized in T-PER tissue extraction reagent (Pierce, Rockford, IL, USA). Protein content was measured by a Bio-Rad assay kit, separated by SDS-PAGE, transferred to a PVDF membrane, and blocked with 5% BSA. Antigens were detected with anti-rabbit horseradish-peroxidase-conjugated antibody for Western blotting and visualized with enhanced chemiluminescence reagents (Pierce). Lysates from insulin-treated or untreated tissue samples were immunoprecipitated in the presence of protein A-Sepharose with insulin receptor-beta (IR-ß) or IR substrate 1 (IRS-1) antibody (Cell Signaling Technology, Danvers, MA, USA). Immunoblotting was performed with anti-phospho-tyrosine antibodies, total- and phospho-Akt (Ser473) antibodies, total- and phospho-Erk1/2 antibodies, and total- and phospho-eNOS (Ser1177) antibodies (1:1,000 dilution; Cell Signaling Technology). Protein was normalized with a mouse monoclonal anti-ß-actin antibody (1:3,000 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Densitometry quantification was performed with NIH Image J software.
Separation of Cytosol and Membrane Fractions of Gingival Tissue Lysate
Cytosol and membrane fractions of lysate from gingival samples were fractionated by several ultracentrifugation steps, as described previously (Inoguchi et al., 1992). PKC α, ß2, δ, and ϵ isoforms were quantitated by immunoblot analysis (1:1,000 dilution; Santa Cruz Biotechnology, Inc.).
NF-κB Immunoblot Analysis of Gingiva from Zucker Rats
Nuclear fractions of lysate from the gingival samples were fractionated as described above. Immunoblotting was performed with anti-NF-κB (p65) (1:1,000 dilution; Santa Cruz Biotechnology, Inc.) and anti-proliferating cell nuclear antigen (PCNA) antibodies (1:1,000 dilution; Cell Signaling Technology).
Immunohistochemistry in Periodontal Tissue of cis-NF-κBEGFP Mice
Dissected mandibles from obese and control cis-NF-κBEGFP mice were fixed and decalcified with 0.4-M EDTA, containing 1% formaldehyde, for 2 wk. Sections (4-μm thickness) from frozen mandibles were fixed in acetone, blocked with 10% donkey serum, incubated overnight with anti-CD31 antibody (1:50 dilution) (Cell Signaling Technology), and observed by digital fluorescence microscopy.
Real-time Quantitative PCR
Total RNA was extracted from the gingiva by means of an RNeasy® Fibrous Tissue Kit (Qiagen, Valencia, CA, USA). RNA was quantified by NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Reverse transcription of total RNA (1 µg) was performed with a SuperscriptTM III cDNA Synthesis kit (Invitrogen, Carlsbad, CA, USA). Real-time quantitative PCR was run on a LightCycler HT7000 (Roche, Indianapolis, IN, USA) with Power SYBR® Green PCR Master Mix (Applied Biosystems, Grand Island, NY, USA). Specific primers were designed as detailed in Appendix Table 1 and reported previously. Expression levels were normalized to levels of GAPDH.
Statistics
Data are presented as the means ± SD. Comparisons between 2 groups like ZL vs. ZF rats or obese vs. control groups were performed with an unpaired Student’s t test. The p values less than 5% were considered statistically significant.
Results
Physiological Characteristics of Zucker Rats and Mice Fed a High-fat Diet
Compared with ZL rats, body weights were significantly increased in ZF rats by 1.6 ± 0.1-fold (p < .05, Appendix Table 2). Plasma levels of insulin and FFA were increased in ZF rats by 5.3 ± 1.4-fold and 3.0 ± 0.7-fold compared with those in ZL rats, respectively (p < .05, Appendix Table 2). Intraperitoneal glucose tolerance testing showed significant elevations of glucose and insulin levels in ZF vs. ZL rats (Appendix Fig. 2). In cis-NF-κBEGFP mice, the HFD group showed significantly increased body weight and fasting blood glucose levels compared with the normal-diet group (p < .05) (Appendix Table 3).
Systemic Oxidative Stress and Inflammation in Zucker Rats
Oxidative stress markers as measured by plasma MDA and CRP levels were significantly increased in ZF rats by 1.43 ± 0.35-fold and 1.30 ± 0.27-fold, respectively, compared with those in ZL rats (p < .05, Appendix Table 2).
Alveolar Bone Loss in Mandibles of Zucker Rats
After removal of the soft tissue from mandibles, bone loss was observed around lower molars in all mandibles. Morphometrically, the mean distances between the bone crest and the CEJ were 0.82 ± 0.03 mm in ZF rats and 0.79 ± 0.04 mm in ZL rats, which did not differ significantly (Appendix Figure 1).
Phosphorylation of IR-ß, IRS-1, Akt, eNOS, and Erk in Gingiva, Liver, and Aorta of Zucker Rats ex vivo
In the gingival tissue, insulin-induced tyrosine phosphorylation of its receptors, IR-ß and IRS-1, was decreased in ZF rats compared with ZL rats by 80.5% and 63.6%, respectively (p < .05, Appendix Fig. 4). Insulin increased phosphorylation of Akt (p-Akt) by 22.5 ± 3.4-fold in ZL rats, and was decreased by 54.5 ± 8.4% in ZF rats (p < .05, Figs. 1A, B), although Akt protein expression was not significantly different between the 2 groups. Insulin increased phospho-Erk1/2 (p-Erk1/2) levels by more than 10.1 ± 1.1-fold in both ZL and ZF rats (p < .05). Interestingly, basal levels of p-Erk1/2 were increased by 2.2 ± 0.5-fold in ZF compared with ZL rats (p < .05, Fig. 1C).
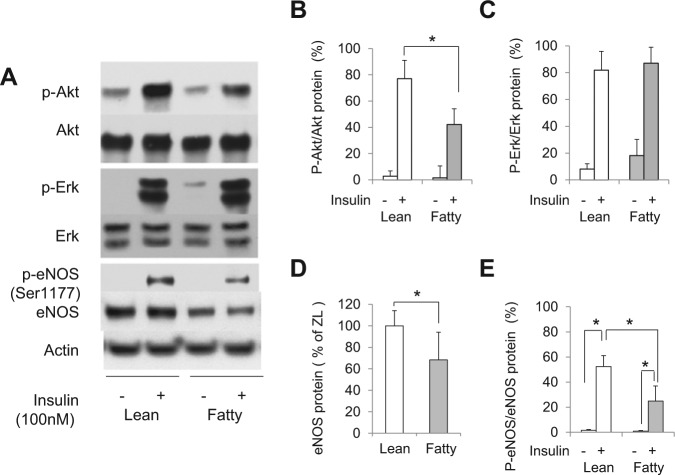
Figure 1.
Insulin’s effect on p-Akt, p-Erk1/2, and p-eNOS in the gingiva from lean and fatty Zucker rats. (A) Representative immunoblots of lysates from insulin-treated or untreated gingival tissues. (B, C, D, E) Data from 3 independent experiments were quantified by densitometry. The p-Akt (B) and p-Erk (C) were quantified by densitometry and expressed as % of that in the gingiva of untreated ZL rats. (D) eNOS protein expression normalized to actin and (E) phosphorylation of eNOS on Ser1177 relative to eNOS expression. Insulin-induced phosphorylation of Akt and eNOS was significantly decreased in ZF rats, but Erk1/2 activation was not affected. These data are expressed as mean ± SD. ZL vs. ZF, n = 6 in each group. *p < .05.
Mean protein expression of eNOS in the gingiva from ZF rats was decreased by 64 ± 14.4% compared with that from ZL rats (p < .05, Fig. 1D). Insulin increased phosphorylation of eNOS on Ser1177 (p-eNOS) in the gingiva from ZL rats by 17.8 ± 3.9-fold, and decreased by 53.6 ± 10.6% in ZF rats (p < .05, Fig. 1E). Insulin activation of p-Akt in the liver and aorta from ZF rats was also inhibited compared with that from ZL rats (Appendix Fig. 3), as previously reported (Naruse et al., 2006).
Activation of PKC Isoforms in the Gingiva of Zucker Rats
Several PKC isoforms are known to induce insulin resistance in obese and diabetic states. Compared with ZL rats, membrane-associated expressions of PKCα, ß2, δ, and ϵ were increased by 137.2 ± 19.7%, 157.0 ± 7.8%, 139.3 ± 22.8%, and 125.9 ± 22.1%, respectively. The cytosol-associated expressions of their PKC isoforms were unchanged (Figs. 2A-2C).
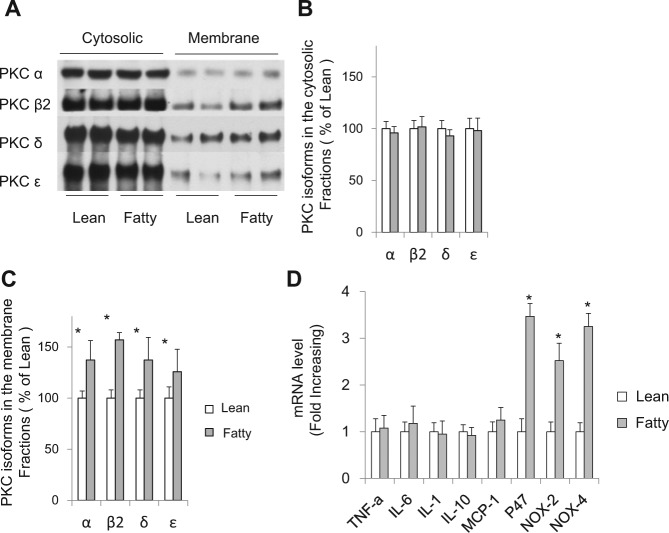
Figure 2.
PKC activation and oxidative stress marker expression in the gingiva from Zucker rats. (A) Representative immunoblot of PKC isoforms in the cytosolic and membrane fractions of gingiva from ZL and ZF rats and (B, C) densitometric quantification. Activities of PKCα, ß2, δ, and ϵ isoforms were significantly increased in the gingiva from ZF rats compared with those from ZL rats. (D) mRNA expression of inflammatory cytokines and oxidative stress markers in gingiva from Zucker rats quantified with real-time quantitative PCR. Expression of oxidative stress markers was significantly increased in ZF rats. These data are expressed as mean ± SD. ZL vs. ZF, n = 6 in each group. *p < .05.
Evaluation of Inflammatory and Oxidative Stress Markers in the Gingiva of Zucker Rats
Inflammatory markers in the gingiva with the induction of insulin resistance were also examined. Expression (mRNA) of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and monocyte chemoattractant protein (MCP)-1 was similar between ZF and ZL rats. In contrast, the mRNA levels of p47phox, NOX2, and NOX4 were significantly increased by 3.47 ± 0.28-fold, 2.52 ± 0.21-fold, and 3.25 ± 0.19-fold in ZF rats, respectively, compared with those in ZL rats (p < .05, Fig. 2D).
Involvement of NF-κB Activation in the Gingival Tissue of Zucker Rats
We evaluated whether the level of the nuclear p65 subunit of NF-κB in the gingiva from ZF rats was increased. Immunoblot analysis showed that the nuclear levels of p65 subunit of NF-κB in the gingival tissue were increased in ZF rats by 4.6 ± 1.9-fold compared with those of ZL rats (p < .05, Figs. 3A, 3B).
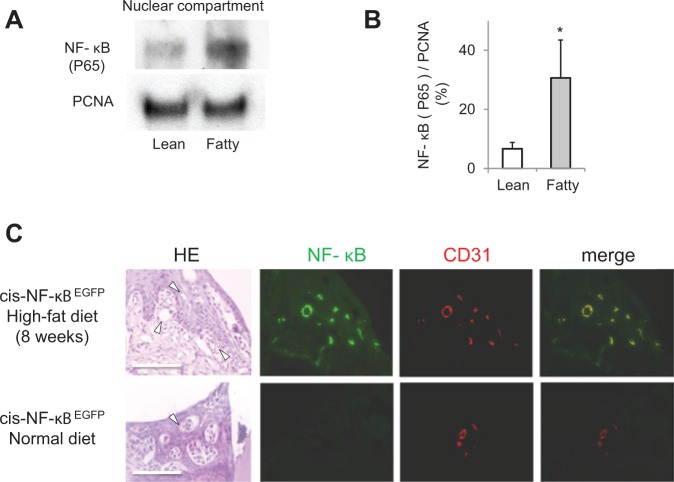
Figure 3.
Activation of NF-κB in the gingiva from insulin-resistant obese rodents. (A) Representative immunoblots of NF-κB (p65) from gingival nuclear proteins from Zucker rats. (B) Data from 3 experiments were normalized with PCNA and actin and quantified by densitometry. The p65 subunit of NF-κB was increased significantly in ZF rats. The data are expressed as mean ± SD. ZL vs. ZF, n = 6.*p < .05. (C) Representative immunohistological images in the mandibular periodontal tissue of cis-NF-κB-EGFP transgenic mice. The increased GFP expression in tissues demonstrated the activated NF-κB after rats consumed a high-fat diet for 2 mos. The GFP-positive areas were few in the normal-diet group. Immunostaining for CD31 and merged images with EGFP fluorescence was assessed by digital fluorescence microscopy. NF-κB activation was increased in the gingival endothelial cells from the mice consuming a HFD. Bar = 100 μm, n = 4 in each group.
Immunohistochemistry of NF-κB Activation in the Periodontal Tissues of Mice Fed a High-fat Diet
NF-κB, when activated, is marked by increased GFP-positive areas in the tissues of cis-NF-κBEGFP mice (Mima et al., 2012). We evaluated NF-κB activation in the periodontal tissues of cis-NF-κBEGFP mice. GFP-positive areas in the periodontal tissue were observed in all mice fed a high-fat diet, while GFP expression was minimally detected in the normal-diet group. The GFP-positive area corresponded with endothelial cells, identified by CD31 immunofluorescence staining (Fig. 3C).
Effects of Anti-oxidant, PKC Inhibitor on Insulin-induced Akt, eNOS, and Erk1/2 Phosphorylation in Gingiva of Zucker Rats ex vivo
PKC activation can inhibit insulin-stimulated p-Akt and p-eNOS (Naruse et al., 2006; Mima et al., 2011). Therefore, we evaluated whether inhibition by GFX, a general PKC inhibitor, can decrease insulin resistance in the gingiva from ZF rats. Addition of GFX reversed the inhibitory effect on insulin-induced p-Akt and p-eNOS detected in the gingiva from ZF rats by 26.4 ± 4.2% and 27.1 ± 6.4%, respectively (p < .05). Similarly, the addition of NAC partially normalized this inhibition on p-Akt and p-eNOS by 23.6 ± 6.8% and 22.3 ± 7.1%, respectively (p < .05, Figs. 4A-4D).
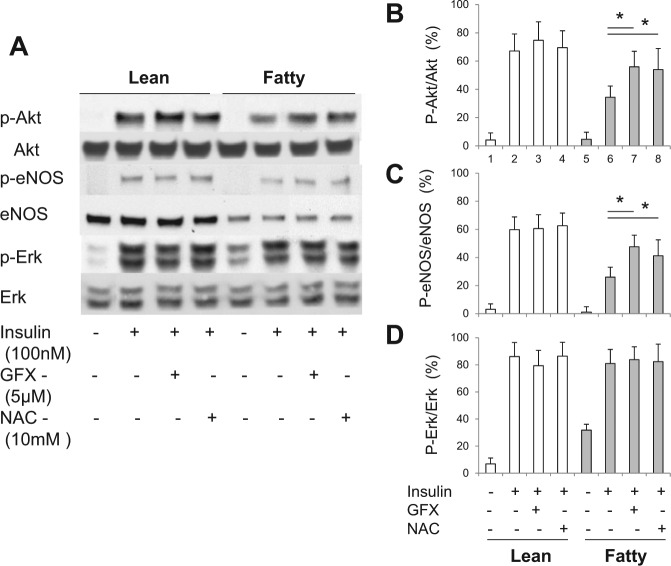
Figure 4.
Effects of GFX and NAC on insulin-induced insulin signaling in the gingiva of Zucker rats. The gingiva were stimulated ex vivo with insulin (100 nmol/L, 30 min) with or without an anti-oxidant, N-acetyl-L-cystein (NAC, 10 mM), or GFX (5 μM). (A) One of 3 independent experiments is shown. Data from 3 experiments on p-Akt (B), p-eNOS (C), and p-Erk1/2 (D) were quantified by densitometry. The addition of a general PKC inhibitor and an anti-oxidant normalized the action of insulin on Akt and eNOS activation in the gingiva from fatty rats. These data are expressed as mean ± SD. ZL vs. ZF, n = 6 in each group. *p < .05.
Discussion
In this study, we provided the first quantitative analysis of insulin-signaling pathways in the gingiva and have demonstrated selective impairment of insulin action on the PI3K/Akt/eNOS pathway in obesity and insulin-resistant states. Further, we showed that the mechanism for insulin resistance in the gingiva is related to oxidative stress and activation of PKC. One potential consequence of insulin action loss is to lower eNOS expression and action, which can increase inflammation and possibly oxidative stress in the gingiva, even when microbiological infection is not present as in this study. Multiple inhibitors of insulin action could be present in the gingiva. It is known that elevated FFA and hyperglycemia, through PKC activation, can decrease eNOS expression via the inhibition of insulin-stimulated PI3K/Akt phosphorylation upstream of eNOS activation. Nitric oxide in the gingiva can regulate cyclooxygenase, osteoblast activities, leukocyte adhesion, and release of superoxide (van’t Hof and Ralston, 2001). Inhibitors of NOS have been shown to increase inflammation and bone resorption in experimental periodontitis (Leitão et al., 2005). Thus, our findings that eNOS expression and activity are decreased in ZF rats could contribute to the acceleration of periodontal tissue breakdown with elevated levels of inflammation and impaired tissue repair, which have been reported in obese and diabetic rodent models without experimentally induced periodontitis (Ohnishi et al., 2009).
Mechanistically, the finding of selective insulin resistance via the IRS/PI3K/Akt cascade, but not the Erk/MAPK pathway, is consistent with similar findings in muscle, liver, kidney, adipose tissues, and vascular tissues from insulin-resistant and diabetic animals and patients (Appendix Figs. 2, 3) (Goodyear et al., 1995; Mima et al., 2011). We have reported that PKC activation can induce specific serine phosphorylation on the insulin receptor and PI3K to inhibit insulin signaling in vascular cells (Park et al., 2013). Further, the PKCβ2 isoform selectively inhibits insulin action in the gingiva consistent with many other tissues in ZF rats (Naruse et al., 2006). Decreased eNOS activity in gingival capillary was especially similar to endothelial cells from kidney or fat tissue (Naruse et al., 2006; Mima et al., 2011). The increase in PKC activation is likely due to the elevation of FFA in ZF rats (Inoguchi et al., 2000) as we have reported to increase synthesis of diacylglycerol (Inoguchi et al., 1992).
It is also possible that PKC can activate oxidases and inflammatory cytokines in the endothelium to attract inflammatory cells (Karima et al., 2005). Thus, we demonstrated that inflammation was increased in the gingiva from ZF rats as described by the high level of NF-κB activation and in mice with the NF-κB promoter-GFP gene on HFD. Interestingly, ROS also appears to be increased in the gingival, as shown by the increased expression of P47, NOX2, and NOX4. Although obesity increased oxidative stress and periodontal inflammation, it did not affect mandibular alveolar bone loss. This is similar to results from previous animal studies, even though the subjected jaw or measured sites (buccal or palatal/lingual) were different. Endo et al. (2010) reported a 2-fold increase in leukocyte infiltration of periodontal tissue in ZF rats compared with ZL rats, but maxillary alveolar bone loss did not develop. Diet-induced obesity showed significantly higher occurrence of spontaneous periodontal breakdown in Wistar rats (Cavagni et al., 2013). However, another study reported maxillary alveolar bone loss in ZF rats when infection is present (Pontes Andersen et al., 2007). Thus, inflammation and oxidative stress alone are not adequate to cause periodontal bone resorption, which requires infection, but may accelerate this process. Impairment of periodontal tissue immune function may explain the underlying mechanism for how obesity affects periodontal tissue breakdown. Impaired immune function can be due to FFA exposure, which attenuates innate responses against P. gingivalis (Zhou et al., 2009; Amar and Leeman, 2013). We propose that gingival insulin resistance can affect periodontal repair and destruction, since insulin signaling regulates survival actions, including cell proliferation and angiogenesis, via the IRS1-Akt pathway (Rask-Madsen and King, 2013).
One potential confounding factor is the leptin receptor mutation, which could affect insulin signaling in the gingiva. However, this is unlikely, since leptin signaling has not affected insulin signaling in peripheral tissues, and full leptin receptors are found mostly in the central nervous system. Further studies will be needed to identify the cell type in ZF rat gingiva, other than endothelial cells, where insulin resistance is occurring in HFD-induced obesity or diabetic models. In summary, PKC activation and oxidative stress selectively inhibit insulin-induced Akt and eNOS activation in gingiva in obesity. This may cause endothelial dysfunction and inflammation, leading to periodontal disease progression and delayed wound-healing.
Supplementary Material
Footnotes
This work was supported by a National Institutes of Health/NIDDK RO1 DK053105-13 grant to G.L.K. K.M. is the recipient of a Research Fellowship (Hiroo Kaneda Scholarship, Sunstar Foundation, Japan) and a Grant-in-Aid for Young Scientists(B) 25862043 from the Japan Society for the Promotion of Science. A.M. is the recipient of a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (24890148) and the Takeda Science Foundation. This project was also supported by a National Institutes of Health/NIDDK 5P30 DK 36836 grant to the Specialized Assay Core of the Joslin Diabetes Center.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Amar S, Leeman S. (2013). Periodontal innate immune mechanisms relevant to obesity. Mol Oral Microbiol 2:331-341. [DOI] [PubMed] [Google Scholar]
- Bray GA. (1977). The Zucker-fatty rat: a review. Fed Proc 36:148-153. [PubMed] [Google Scholar]
- Cavagni J, Wagner TP, Gaio EJ, Rêgo RO, Torres IL, Rösing CK. (2013). Obesity may increase the occurrence of spontaneous periodontal disease in Wistar rats. Arch Oral Biol 58:1034-1039. [DOI] [PubMed] [Google Scholar]
- Chaffee BW, Weston SJ. (2010). Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol 81:1708-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Tomofuji T, Ekuni D, Irie K, Azuma T, Tamaki N, et al. (2010). Experimental periodontitis induces gene expression of proinflammatory cytokines in liver and white adipose tissues in obesity. J Periodontol 81:520-526. [DOI] [PubMed] [Google Scholar]
- Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. (2005). A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol 76(11 Suppl):2075-2084. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL. (1995). Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest 95:2195-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A, Kaye EK, Apovian C, Fung TT, Nunn M, Garcia RI. (2012). Overweight and obesity predict time to periodontal disease progression in men. J Clin Periodontol 39:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. (1992). Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA 89:11059-11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. (2000). High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49:1939-1945. [DOI] [PubMed] [Google Scholar]
- Karima M, Kantarci A, Ohira T, Hasturk H, Jones VL, Nam BH, et al. (2005). Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol 78:862-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão RF, Ribeiro RA, Chaves HV, Rocha FA, Lima V, Brito GA. (2005). Nitric oxide synthase inhibition prevents alveolar bone resorption in experimental periodontitis in rats. J Periodontol 76:956-963. [DOI] [PubMed] [Google Scholar]
- Magness ST, Jijon H, Van Houten Fisher N, Sharpless NE, Brenner DA, Jobin C. (2004). In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-kappa B activation using a novel gene-targeted enhanced GFP reporter gene mouse. J Immunol 173:1561-1570. [DOI] [PubMed] [Google Scholar]
- Mima A, Ohshiro Y, Kitada M, Matsumoto M, Geraldes P, Li C, et al. (2011). Glomerular-specific protein kinase C-β-induced insulin receptor substrate-1 dysfunction and insulin resistance in rat models of diabetes and obesity. Kidney Int 79:883-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima A, Qi W, Hiraoka-Yamomoto J, Park K, Matsumoto M, Kitada M, et al. (2012). Retinal not systemic oxidative and inflammatory stress correlated with VEGF expression in rodent models of insulin resistance and diabetes. Invest Ophthalmol Vis Sci 53:8424-8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, et al. (2006). Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes 55:691-698. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Bandow K, Kakimoto K, Machigashira M, Matsuyama T, Matsuguchi T. (2009). Oxidative stress causes alveolar bone loss in metabolic syndrome model mice with type 2 diabetes. J Periodontal Res 44:43-51. [DOI] [PubMed] [Google Scholar]
- Park K, Li Q, Rask-Madsen C, Mima A, Mizutani K, Winnay J, et al. (2013). Serine phosphorylation sites on IRS2 activated by angiotensin II and protein kinase C to induce selective insulin resistance in endothelial cells. Mol Cell Biol 33:3227-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein MI, Bissada NF. (1977). Influence of obesity and hypertension on the severity of periodontitis in rats. Oral Surg Oral Med Oral Pathol 43:707-719. [DOI] [PubMed] [Google Scholar]
- Pontes Andersen CC, Flyvbjerg A, Buschard K, Holmstrup P. (2007). Periodontitis is associated with aggravation of prediabetes in Zucker fatty rats. J Periodontol 78:559-565. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen C, King GL. (2007). Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab 3:46-56. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen C, King GL. (2013). Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab 17:20-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Shimazaki Y. (2007). Metabolic disorders related to obesity and periodontal disease. Periodontol 2000 43:254-266. [DOI] [PubMed] [Google Scholar]
- van’t Hof RJ, Ralston SH. (2001). Nitric oxide and bone. Immunology 103:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Leeman SE, Amar S. (2009). Signaling mechanisms involved in altered function of macrophages from diet-induced obese mice affect immune responses. Proc Natl Acad Sci USA 106:10740-10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.