Abstract
Sulfur mustard (SM) is a chemical weapon that targets the skin, eyes, and lung. It was first employed during World War I and it remains a significant military and civilian threat. As a bifunctional alkylating agent, SM reacts with a variety of macromolecules in target tissues including nucleic acids, proteins and lipids, as well as small molecular weight metabolites such as glutathione. By alkylating subcellular components, SM disrupts metabolism, a process that can lead to oxidative stress. Evidence for oxidative stress in tissues exposed to SM or its analogs include increased formation of reactive oxygen species, the presence of lipid peroxidation products and oxidized proteins, and increases in antioxidant enzymes such as superoxide dismutase, catalase, and glutathione-S-transferase. Inhibition of antioxidant enzymes including thioredoxin reductase by SM can also disrupt cellular redox homeostasis. Consistent with these findings, SM-induced toxicity has been shown to be reduced by antioxidants in both in vitro and in vivo models. These data indicate that drugs that target oxidative stress pathways may represent important candidates for reducing SM-induced tissue injury.
Keywords: oxidative stress, reactive oxygen species, nitric oxide, vesicants, dermatotoxicity
Introduction
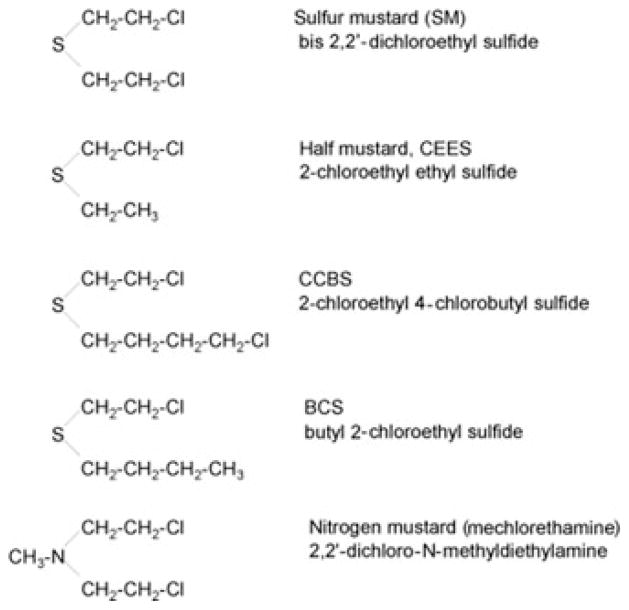
Sulfur mustard (SM), or mustard gas (bis[2-chloroethyl] sulfide), is a nonspecific alkylating agent that primarily targets the skin, cornea, and respiratory tissues (see Fig. 1 for structure of SM and several related analogs that have been used to investigate its mechanism of action). Although responses to SM are tissue specific and dependent on dose, inflammation is an early sign of toxicity.1,2 In the skin, an initial delay in toxicity is followed by inflammation and the formation of vesicles, which can coalesce to form pendulous blisters. Vesicle formation or blistering is due to the separation of the epidermis from the dermis.2,3 In the eye, frank corneal epithelial damage is apparent. SM can also cause corneal edema and neovascularization.2 In the lung, SM can induce bronchial mucosal injury, inflammation, fibrosis, and pneumonia.2 It is generally thought that the effects of SM are primarily due to its ability to form both monofunctional and bifunctional adducts with a variety of cellular components including nucleic acids, lipids and protein. SM initially forms a cyclic ethylene sulfonium ion intermediate followed by electrophilic attack on target molecules. This can result in inhibition of nucleic acid and protein biosynthesis, as well as ATP biosynthesis. Modification of extracellular matrix or critical structural elements in cells by SM may disrupt intracellular dissipative structures and compromise cellular functioning including energy metabolism. SM-induced DNA damage has been linked to the development of cancer.1
Figure 1.
Structures of sulfur mustard and related vesicants.
It is well recognized that chemical-induced cellular damage can lead to oxidative stress. In this process, an imbalance develops in tissues between the generation of reactive oxygen species (ROS) and/or their detoxification. This can result from a variety of changes in cells including altered ROS production, decreases in antioxidants, and alterations in repair processes. ROS are derived from the partial reduction of oxygen and exert cytotoxic effects by directly modifying cellular and extracellular components and/or by altering redox active factors in cells that control metabolism including cell signal transduction pathways. ROS-induced damage to macromolecules is well characterized and includes DNA base oxidation, which can interfere with replication and repair processes, lipid peroxidation, which can generate highly reactive electrophilic lipid peroxidation end products, and protein oxidation, which can modify the functional activity of enzymes and structural proteins. Thus, toxicity from SM may be the result of the direct damage induced by alkylating cellular components, and/or SM-induced ROS production. In addition to directly measuring ROS in cells and tissues, biomarkers of ROS exposure provide evidence for their formation following exposure to SM. For example, application of the half mustard 2-chloroethyl ethyl sulfide (CEES) to mouse skin increases dermal protein oxidation, the formation of the DNA oxidation product, 8-oxo-2-deoxyguanosine, and adducts of 4-hydroxynonenal, a marker of lipid peroxidation,4 whereas in the lung, CEES increases lipid peroxidation, as measured by the formation of malondialdehyde.5–7 Importantly, the use of inhibitors of ROS formation or antioxidants to reverse or ameliorate tissue injury provides indirect support for the idea that ROS mediate the cytotoxic actions of SM. Some prototypical antioxidants effective in blocking vesicant-induced injury include glutathione (GSH) (see further below), vitamin E, flavonoids, or various preparations of antioxidant enzymes including superoxide dismutase and catalase. Evidence for the formation of ROS and alterations in ROS metabolism in cells and tissues following exposure to SM and related vesicants in the skin, eye, lungs, and other tissues are summarized in Tables 1–3.
Table 1.
Vesicant-induced oxidative stress in dermal and ocular tissues
| Vesicant | System | Effects | References |
|---|---|---|---|
| In vitro studies | |||
| CEES | PAM212 mouse keratinocytes | ↑ H2O2 production, ↑ protein oxidation, ↑ CuZn-SOD, catalase, GST, thioredoxin reductase | 30 |
| SM | Human HaCaT keratinocytes | ↑ nitric oxide, nitrotyrosine protein, 8-isoprostane formation, iNOS and eNOS activity | 34 |
| In vivo studies | |||
| CEES | Mice (IP) | ↓ GST, ↓ glyceraldehyde 3-phosphate dehydrogenase | 28 |
| CEES | SKH-1 mice (topical) | ↑ 4-hydroxynonenal and 8-hydroxy-2′-deoxyguanosine (8-OHdG) formation, protein oxidation, protein adduct formation | 4 |
| CEES | SKH-1 mice (topical) | ↑ myeloperoxidase activity | 11 |
| SM | Guinea pigs (topical-shaved) | ↑ inducible nitric oxide synthase (iNOS) protein | 33 |
| Antioxidant treatments | |||
| SM | Guinea pigs (topical-shaved and depilated) | ↓ skin lesions with CuZn-SOD, Mn-SOD No effect if given post-SM exposure |
37 |
| SM | Normal human keratinocytes | ↑ cell survival with sulforaphane pretreatment | 38 |
| SM | SVK-14 human keratinocytes | ↑ cell survival with GSH and methenamine pretreatment | 39 |
| SM | Isolated perfused pig skin | ↓ number of dark basal keratinocytes, no change in vesication with thiosulfate, cysteine, niacinamide, or indomethacin pretreatment | 40 |
| NM | Guinea pigs (topical-shaved and depilated) | ↓ erythema, necrosis, ulceration, edema, inflammation, and dermo-epidermal separation with antioxidants zinc oxide + zinc chloride + dimethylpolysiloxane pretreatment | 41 |
| NM | A431 human epidermoid cells | ↓ lipid peroxidation with butylated hydroxyanisole (BHA), ↓ cytotoxicity with BHA and ebselen | 42 |
| NM | Guinea pigs (topical-shaved and depilated) | ↓ reduced ulceration, fibrosis, nuclear pyknosis, hyperkeratosis with zinc chloride and desferrioxamine post-treatment | 43 |
| SM | Normal human keratinocytes and hairless guinea pig keratinocytes Hairless guinea pigs (vapor cup) |
↓ cytotoxicity with pretreatment of cells with L-thiocitrulline (L-TC) or L-nitroarginine methyl ester decreased cytotoxicity, no effect post-treatment No effects with pre or post treatment observed in vivo |
36 |
| NM | Rabbit cornea (eye drops) | ↓ corneal damage and ↑ corneal re-epithelialization with dexamethasone + zinc desferrioxamine post-treatment | 44 |
SM, sulfur mustard; NM, nitrogen mustard.
Table 3.
Vesicant-induced oxidative stress and systemic toxicity
| Vesicant | System | Effects | References |
|---|---|---|---|
| In vitro studies | |||
| CEES | Murine macrophages, stimulated with lipopolysaccharide | ↓ NO production, ↑ iNOS protein | 35 |
| NM | Human HL-60 cells | ↑ GSTA2 mRNA, ↑ total GST activity | 46 |
| NM | Human Colo 320HSR cells | ↓ apoptosis and cell cycle arrest with GSTA2 overexpression | |
| In vivo studies | |||
| CEES | Mice (IP) | ↓ GAPDH, ↑ GST activity in spleen No change in GST activity in liver |
28 |
| BCS | Mice (SQ) | ↑ glutathione peroxidase and GST activity, ↓ total GSH, ↑ lipid peroxidation in brain | 16 |
| SM | Rats (topical) | ↓ SOD, catalase, glutathione peroxidase activity in red and white blood cells, platelets, liver, kidney, spleen, brain | 47 |
| SM | Mice (topical) | ↓ GSH in liver and blood, ↑ lipid peroxidation in liver | 19 |
| SM | Humans (field exposure) | metabolites detected: thiodiglycol, thiodiglycol sulfoxide, bis-mercapturate of mustard sulfone, glutathione conjugates | 21, 22 |
| SM | Rats (IP) | metabolites detected : thiodiglycol sulfoxide, glutathione conjugates | 48 |
| NM | Rats (IT) | ↑ urine nitrite-nitrate levels | 12 |
| Antioxidant treatments | |||
| SM | Mice (inhalation) | ↓ GSH – restored by Trolox, Quercetin, GSH in liver ↑ lipid peroxidation – reduced by antioxidants in liver |
5 |
| SM | Bovine pulmonary artery endothelial cells | ↓ GSH level – restored with NAc treatment | 15 |
| CEES | Human Jurkat cells Human lymphocytes |
↓ GSH, ↑ ROS production, ↓ mitochondrial membrane potential ↑ cell death – restored with NAc, GSH ethyl ester pretreatment |
17 |
| NM | Isolated rat hepatocytes | ↓ GSH and ↑ lipid peroxidation, GSH depletion, lipid peroxidation, and cytotoxicity – reduced by butylated hydroxyanisole, 3-tocopherol, desferoxamine post-treatment | 18 |
Sources of ROS in tissues exposed to SM and related analogs
A question arises as to the sources of ROS in tissues following exposure to SM. As indicated above, SM is a potent irritant and one of the hallmarks of its cyto-toxic actions is accumulation of inflammatory cells including neutrophils and macrophages at sites of tissue injury. As important effector cells in nonspecific host defense, both of these cell types are capable of generating ROS in a respiratory burst. Localized production of ROS by these cells has been shown to be important in mediating chemical-induced toxicity in many tissues. Neutrophil and macrophage infiltration has been described in both the skin and lung following exposure to SM.8–10 Increased myeloperoxidase in mouse skin following exposure to CEES is thought to be due to infiltration of neutrophils.11 Neutrophils and macrophages are activated to release ROS, as well as nitric oxide (see further below), in tissues following exposure to many toxicants, and inhibition of the migration and activation of these cells in tissues has been shown to be an effective strategy to mitigate chemical-induced tissue injury. In this regard, neutrophil depletion by intraperitoneal injection of antiserum to rat neutrophils has been reported to decrease acute lung injury in rats following exposure to CEES (Fig. 1).12
Mitochondria are also a major intracellular source of ROS. Formed as a byproduct of mitochondrial electron transport, ROS produced by mitochondria are known to be important in regulating cell death processes including apoptosis, as well as autophagy, a process by which cells rid themselves of damaged organelles. Mitochondrial components can be directly modified by SM analogs. In human small airway and bronchial epithelial cells, CEES has been shown to induce mitochondrial dysfunction, a process associated with increased ROS production, DNA oxidation and decreases in intracellular GSH.13 The importance of alterations in mitochondria as a mechanism of toxicity was demonstrated by the finding that a catalytic antioxidant, metalloporphyrin, which possesses high superoxide dismutase (SOD) and catalase activities can rescue airway cells from CEES-induced toxicity and correct, at least in part, CEES-induced mitochondrial dysfunction.13
In addition to mitochondria, a number of enzymes in cells are known to generate ROS including xanthine oxidase and NADPH oxidases. One of the best characterized group of NADPH oxidases capable of producing ROS are enzymes of the cytochrome P450 system including NADPH cytochrome P450 reductase and various cytochrome P450’s. The formation of ROS by this system is thought to be due to autoxidation of NADPH-cytochrome P450 reductase and the nonproductive decay of oxygen-bound cytochrome P450 intermediates. We found that CEES is an effective inhibitor of NADPH cytochrome P450 reductase.14 Interestingly, at the same time, CEES stimulates ROS formation from the enzyme and this, can directly contribute to oxidative stress.14
SM targeting of antioxidants and the potential for antioxidants as therapeutics
An important route by which SM and its analogs can increase oxidative stress is by modulating intracellular antioxidants or enzymes that regenerate antioxidants. GSH is tripeptide nucleophilic antioxidant that readily reacts with reactive SM intermediates. Treatment of cells and tissues with SM and related analogs has been shown to markedly reduce levels of GSH.2,5,6,15–20 Further evidence for the reaction of SM with GSH comes from humans and animal studies where SM-GSH metabolites were detected in the urine.21,22 Depleting cells of GSH increases intracellular ROS as well as markers of oxidative stress, including formation of DNA oxidation products.2 Several studies have shown that GSH or the GSH prodrug, N-acetylcysteine (NAC), can reduce oxidative stress and toxicity induced by SM or its analogs. For example, GSH has been shown to increase the survival time of mice following inhalation of SM5 and NAC has been shown to protect against acute lung injury induced by CEES.10,13 In a rat model, liposomes containing NAC have also been shown to protect against lung toxicity induced by CEES.24 In humans exposed to SM, NAC has also been reported to improve clinical outcomes.25
SM or its analogs can also target enzymes important in the control of cellular antioxidant balance. Decreases in enzyme activity can occur as a result of changes in expression of the enzyme protein and/or SM-induced alkylation, which can inhibit enzyme activity. SM and its derivatives are known to react with cysteine residues in proteins, as well as histidine, glutamic acid, and aspartic acid.2 In recent studies we have shown that thioredoxin reductase can be modified by CEES, as well as nitrogen mustard.26 As a homodimeric flavoprotein, mammalian thioredoxin reductase is an essential antioxidant enzyme catalyzing the reduction of oxidized thioredoxin, redox-active proteins including protein disulfide isomerase and glutaredoxin 2, as well as hydrogen peroxide. It is a selenoprotein containing a C-terminus cysteine-selenocysteine redox pair that is critical for enzyme activity. We found that treatment of lung epithelial cells with CEES inhibits thioredoxin reductase. Using purified rat liver enzyme, inhibition was found to be irreversible and only evident when the enzyme was reduced with NADPH. LC-MS/MS analysis demonstrated that CEES covalently modified selenocysteine in the enzyme, a finding consistent with its inhibitory effects on thioredoxin reductase enzyme activity. Inhibition of thioredoxin reductase has been demonstrated to deplete cells of reduced thioredoxin, a key player in cellular redox regulation. Both thioredoxin reductase and thioredoxin function as antioxidants and inhibition by CEES can lead to oxidative stress. Our data also suggest that other selenocysteine-containing proteins may be inhibited by mustards; several of these proteins such as glutathione peroxidase function as antioxidants and their inhibition may contribute to cellular oxidative stress.
Interestingly, thioredoxin reductase is also known to mediate redox cycling, an NADPH-dependent process whereby the enzyme mediates the one-electron reduction of a variety of quinones, curcumin, flavonoids and the herbicide paraquat into anion radicals.26 Reactions of these radicals with molecular oxygen leads to the formation of ROS, a process that regenerates the parent compounds. Our data showed that although CEES inhibits thioredoxin reductase, it stimulates its redox cycling activity.26 This presents an additional mechanism by which mustards can initiate oxidative stress. Thus, redox cycling of both endogenous and exogenous compounds by thioredoxin reductase can generate ROS, a process that contributes to the disruption of cellular redox homeostasis.
Increases in antioxidant enzymes can occur as a result of compensatory responses to oxidative stress. For example, in mouse and guinea pig lungs following intratracheal, intraperitoneal, or subcutaneous administration of SM analogs, activities of superoxide dismutase, catalase, and glutathione peroxidase are upregulated.20,27–29 In a skin construct model, we have also shown that CEES increases Cu, Zn-superoxide dismutase, catalase, thioredoxin reductase, and the glutathione-S-transferases GSTA1-2 and GSTP1.30 The glutathione-S-transferases function to conjugate glutathione to oxidized cellular macromolecules to facilitate their elimination and limit tissue injury. GSTA1-2 and GSTP1 are also important in breaking lipid peroxidation chain reactions through removal of hydrogen peroxide and aldehydes generated during oxidative stress.
Potential role for nitric oxide in tissue injury induced by SM and related analogs
It is well established that nitric oxide produced endogenously is an important mediator of numerous physiological processes including neuronal activity, the regulation of vascular tone, macrophage-mediated cytotoxicity, and wound healing. However, when produced in excessive amounts and/or at inappropriate times or places, nitric oxide can contribute to toxicity. Indeed, a role for nitric oxide in the action of a variety of chemical toxicants has been described including ozone and silica in the lung, acetaminophen and carbon tetrachloride in the liver, and UVB light in the skin.31,32 Its ability to damage cells is dependent on local concentrations of enzymes that produce nitric oxide, metabolism into reactive intermediates, as well as its detoxification in target tissues. Nitric oxide is synthesized from arginine and oxygen in a two-step reaction mediated by nitric oxide synthase. Three forms of the enzyme have been characterized including endothelial and brain nitric oxide synthases, which are low output isoforms of the enzyme, and a macrophage or inducible high output form of the enzyme. The inducible form of the enzyme can be expressed in both epithelial cells as well as activated neutrophils and macrophages. As a molecule containing a single unpaired electron, nitric oxide can react with many cellular targets that can lead to toxicity. Reactive nitric oxide products include nitrite, nitrogen dioxide, nitronium and nitrosonium cations, nitroxyl, nitrosoperoxycarbonate anion, and nitryl chloride. Of particular importance is the reaction of nitric oxide with the ROS superoxide anion forming peroxynitrite. Peroxynitrite is a strong oxidant and nitrating agent and is known to trigger oxidative injury.
In several cell types in culture including keratinocytes and lung-derived epithelial cells, SM or its analogs have been reported to modulate expression and/or activity of nitric oxide synthases and nitric oxide production.7,12,33–35 Moreover, inhibitors of these enzymes have been reported to protect or rescue cells in vitro from SM-induced toxicity.36 Although promising as a therapeutic target in the skin, nitric oxide synthase inhibitors that have been tested in hairless guinea pigs have been reported to be ineffective against topical SM vapor challenge.36 In contrast, topical iodine preparations that reduce SM-induced skin toxicity in a haired guinea pig model have been shown to suppress inducible nitric oxide synthase expression in infiltrating polymorphonuclear cells and macrophages.33 It may be that different formulations or doses of nitric oxide synthase inhibitors are needed to directly inhibit the enzyme in the guinea pig skin model. In a rat model of lung toxicity, intratracheal administration of nitrogen mustard increased lung inducible nitric oxide synthase and urinary nitrite/nitrate, a marker for nitric oxide production. Aminoguanidine, an inhibitor of nitric oxide synthase, was found to reduce nitric oxide synthase activity, as well as urinary nitrite/nitrate, and this was associated with reduced lung toxicity. Interestingly, ebelson, a peroxynitrite scavenger that did not affect nitric oxide synthase activity, was an effective inhibitor of nitrogen mustard–induced toxicity. These data support the idea that nitric oxide mediates lung toxicity of nitrogen mustard and that peroxynitrite may mediate this process. Further studies are needed to characterize expression of the nitric oxide synthases in different animal models following exposure to SM and evaluating their roles in tissue injury.
Summary
Oxidative stress is an important mechanism by which SM contributes to toxicity. Arising by a variety of mechanisms including disruption of mitochondria, increases in activity of enzymes producing ROS and capable of redox cycling, decreases in small molecular weight intracellular antioxidants including GSH and various antioxidant enzymes, SM-induced oxidative stress is a result of imbalances in the production and/or detoxification of ROS. Nitric oxide, which has been shown to participate in SM toxicity, likely by reacting with ROS and forming highly toxic peroxynitrite, also plays a role in oxidative stress. Increases in a variety of oxidative stress markers have been detected in tissues exposed to SM or its analogs including lipid peroxidation products, as well as protein and DNA oxidation products. Antioxidants and nitric oxide synthase inhibitors have shown varying degrees of protection against SM-induced tissue injury. Successful therapy for SM toxicity may depend on the development of new antioxidants effective against SM-induced ROS and their improved delivery to target tissues.
Table 2.
Vesicant-induced oxidative stress in pulmonary toxicity
| Vesicant | System | Effects | Ref |
|---|---|---|---|
| In vitro studies | |||
| CEES | A549 type II human alveolar epithelial cells | ↓ thioredoxin reductase activity | 26 |
| CEES | Human lung epithelial cells and bronchial epithelial cells | ↑ mitochondrial ROS, ↑ total GSH, ↑ DNA oxidation (8-OHdG), ↓ mitochondrial membrane potential | 13 |
| In vivo studies | |||
| CEES | Guinea pigs (IT) | ↑ CuZn-SOD, n.c. in Mn-SOD, ↓ EC-SOD activity | 29 |
| CEES | Mice (IP) | ↑ GST activity in lung | 28 |
| BCS | Mice (SQ) | ↑ GAPDH, GST activity, ↑ lipid peroxidation, ↑ oxidized GSH | 27 |
| CCBS | Mice (SQ) | ↑ SOD, catalase, glutathione peroxidase, glutathione reductase, GST, GAPDH, ↓ reduced GSH, ↑ oxidized GSH, ↑ lipid peroxidation | 20 |
| SM | Humans (field exposure) | ↓ GSH, ↑ lipid peroxidation, abnormal lung function | 6 |
| NM | Rats (IT) | ↑ lipid peroxidation, ↑ iNOS activation | 12 |
| NM | Rats (IT) | ↓ glutathione peroxidase, ↑ iNOS, ↑ lipid peroxidation | 7 |
| Antioxidant treatments | |||
| SM | Mice (inhalation) | ↓ GSH –restored by Trolox, Quercetin, GSH ↑ lipid peroxidation-reduced by antioxidants |
5 |
| CEES | Guinea pigs (IT) | ↓ AP-1, c-fos, c-jun, cyclin D1/PCNA, ↓ inflammation and neutrophil infiltration with liposomes containing tocopherols + N-acetylcysteine (NAc) | 45 |
| CEES | Guinea pigs (IT) | ↓ lung injury by NAc pretreatment | 23 |
| CEES | Rats (intrapulmonary) | ↓ lung injury with NAc, catalase, resveratrol, DMSO, dimethyl urea pretreatment ↓ lung injury with NAc post-treatment | 24 |
| CEES | Rats (intrapulmonary) | ↓ lung injury with liposomes containing SOD, catalase or NAc, GSH, α-tocopherol, resveratrol post-treatment instillation | 10 |
| CEES | Guinea pigs (intrapulmonary) | ↓ lipid peroxidation and hydroxyproline levels with liposomes containing NAc and α-tocopherol post-treatment instillation | 9 |
| NM | Rats (IT) | ↓ iNOS activation and lung damage with iNOS inhibitor and peroxynitrite scavenger | 12 |
| NM | Rats (IT) | ↑ CuZn-SOD, glutathione peroxidase, and iNOS activity, ↓ lipid peroxidation with melatonin pre- and post-treatment | 7 |
| SM | Humans (field exposure) | ↓ dyspnea, cough sputum and improved spirometry readings with oral NAc treatment | 25 |
BCS, butyl 2-chloroethyl sulfide; CCBS, 2-chloroethyl 4-chlorobutyl sulfide; n.c., no change.
Acknowledgments
This work was supported in part by National Institutes of Health grants CA100994 (J.D.L.), CA093798 (D.E.H.), ES005022 (J.D.L., D.L.L.), ES004738 (D.L.L., J.D.L.), CA132624 (D.L.L., J.D.L.), ES017389 (Y.J.), AR055073 (J.D.L., D.L.L., D.E.H., P.S., N.D.H.), AI051214 (P.S.), AI084137 (P.S.), and GM034310 (D.L.L., J.D.L.). This work was also funded in part by the National Institutes of Health CounterACT Program through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (award #U54AR055073 to J.D.L.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Dacre JC, Goldman M. Toxicology and pharmacology of the chemical warfare agent sulfur mustard. Pharmacol Rev. 1996;48:289–326. [PubMed] [Google Scholar]
- 2.Papirmeister B, et al. Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. CRC Press, Inc; Boca Raton, FL: 1991. [Google Scholar]
- 3.Shakarjian MP, et al. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol Sci. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pal A, et al. Sulfur mustard analog induces oxidative stress and activates signaling cascades in the skin of SKH-1 hairless mice. Free Radic Biol Med. 2009;47s:1640–1651. doi: 10.1016/j.freeradbiomed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar O, Sugendran K, Vijayaraghavan R. Protective effect of various antioxidants on the toxicity of sulphur mustard administered to mice by inhalation or percutaneous routes. Chem Biol Interact. 2001;134:1–12. doi: 10.1016/s0009-2797(00)00209-x. [DOI] [PubMed] [Google Scholar]
- 6.Shohrati M, et al. Glutathione and malondialdehyde levels in late pulmonary complications of sulfur mustard intoxication. Lung. 2010;188:77–83. doi: 10.1007/s00408-009-9178-y. [DOI] [PubMed] [Google Scholar]
- 7.Ucar M, et al. Melatonin alleviates lung damage induced by the chemical warfare agent nitrogen mustard. Toxicol Lett. 2007;173:124–131. doi: 10.1016/j.toxlet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Dannenberg AM, Jr, et al. Inflammatory mediators and modulators released in organ culture from rabbit skin lesions produced in vivo by sulfur mustard. I. Quantitative histopathology; PMN, basophil, and mononuclear cell survival; and unbound (serum) protein content. Am J Pathol. 1985;121:15–27. [PMC free article] [PubMed] [Google Scholar]
- 9.Mukherjee S, et al. Protection of half sulfur mustard gas-induced lung injury in guinea pigs by antioxidant liposomes. J Biochem Mol Toxicol. 2009;23:143–153. doi: 10.1002/jbt.20279. [DOI] [PubMed] [Google Scholar]
- 10.McClintock SD, et al. Attenuation of half sulfur mustard gas-induced acute lung injury in rats. J Appl Toxicol. 2006;26:126–131. doi: 10.1002/jat.1115. [DOI] [PubMed] [Google Scholar]
- 11.Tewari-Singh N, et al. Inflammatory biomarkers of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced skin injury in SKH-1 hairless mice. Toxicol Sci. 2009;108:194–206. doi: 10.1093/toxsci/kfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaren H, et al. Lung toxicity of nitrogen mustard may be mediated by nitric oxide and peroxynitrite in rats. Res Vet Sci. 2007;83:116–122. doi: 10.1016/j.rvsc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Gould NS, White CW, Day BJ. A role for mitochondrial oxidative stress in sulfur mustard analog 2-chloroethyl ethyl sulfide-induced lung cell injury and an-tioxidant protection. J Pharmacol Exp Ther. 2009;328:732–739. doi: 10.1124/jpet.108.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray JP, et al. Inhibition of NADPH-cytochrome P450 reductase by 2-chloroethyl ethyl sulfide, a model sulfur mustard vesicant, is associated with increaed production of reactive oxygen intermediates. Toxicol Appl Pharmacol. 2010 doi: 10.1016/j.taap.2010.05.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkins KB, et al. N-acetylcysteine and endothelial cell injury by sulfur mustard. J Appl Toxicol. 2000;20(Suppl 1):S125–S128. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat671>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Elsayed NM, et al. Response of mouse brain to a single subcutaneous injection of the monofunctional sulfur mustard, butyl 2-chloroethyl sulfide (BCS) Toxicology. 1989;58:11–20. doi: 10.1016/0300-483x(89)90100-5. [DOI] [PubMed] [Google Scholar]
- 17.Han S, et al. Protection by antioxidants against toxicity and apoptosis induced by the sulphur mustard analog 2-chloroethylethyl sulphide (CEES) in Jurkat T cells and normal human lymphocytes. Br J Pharmacol. 2004;141:795–802. doi: 10.1038/sj.bjp.0705591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan S, Ramwani JJ, O’Brien PJ. Hepatocyte toxicity of mechlorethamine and other alkylating anticancer drugs. Role of lipid peroxidation. Biochem Pharmacol. 1992;43:1963–1967. doi: 10.1016/0006-2952(92)90639-z. [DOI] [PubMed] [Google Scholar]
- 19.Vijayaraghavan R, et al. Dermal intoxication of mice with bis(2-chloroethyl)sulphide and the protective effect of flavonoids. Toxicology. 1991;69:35–42. doi: 10.1016/0300-483x(91)90151-p. [DOI] [PubMed] [Google Scholar]
- 20.Elsayed NM, Omaye ST. Biochemical changes in mouse lung after subcutaneous injection of the sulfur mustard 2-chloroethyl 4-chlorobutyl sulfide. Toxicology. 2004;199:195–206. doi: 10.1016/j.tox.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Barr JR, et al. Analysis of urinary metabolites of sulfur mustard in two individuals after accidental exposure. J Anal Toxicol. 2008;32:10–16. [PubMed] [Google Scholar]
- 22.Black RM, Read RW. Biological fate of sulphur mustard, 1,1′-thiobis(2-chloroethane): identification of beta-lyase metabolites and hydrolysis products in human urine. Xenobiotica. 1995;25:167–173. doi: 10.3109/00498259509061842. [DOI] [PubMed] [Google Scholar]
- 23.Das SK, et al. Prophylactic protection by N-acetylcysteine against the pulmonary injury induced by 2-chloroethyl ethyl sulfide, a mustard analogue. J Biochem Mol Toxicol. 2003;17:177–184. doi: 10.1002/jbt.10076. [DOI] [PubMed] [Google Scholar]
- 24.McClintock SD, et al. Protection from half-mustard-gas-induced acute lung injury in the rat. J Appl Toxicol. 2002;22:257–262. doi: 10.1002/jat.856. [DOI] [PubMed] [Google Scholar]
- 25.Ghanei M, et al. N-acetylcysteine improves the clinical conditions of mustard gas-exposed patients with normal pulmonary function test. Basic Clin Pharmacol Toxicol. 2008;103:428–432. doi: 10.1111/j.1742-7843.2008.00318.x. [DOI] [PubMed] [Google Scholar]
- 26.Jan YJ, et al. Selective targeting of selenocysteine in thioredoxin reductase by the half mustard 2-chloroethyl ethyl sulfide in lung epithelial cells. Chem Res Toxicol. 2010 Mar 29; doi: 10.1021/tx100040k. Epub ahead of print In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elsayed NM, et al. Free radical-mediated lung response to the monofunctional sulfur mustard butyl 2-chloroethyl sulfide after subcutaneous injection. Toxicology. 1992;72:153–165. doi: 10.1016/0300-483x(92)90109-r. [DOI] [PubMed] [Google Scholar]
- 28.Kim YB, et al. Change in glutathione S-transferase and glyceraldehyde-3-phosphate dehydrogenase activities in the organs of mice treated with 2-chloroethyl ethyl sulfide or its oxidation products. Food Chem Toxicol. 1996;34:259–265. doi: 10.1016/0278-6915(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 29.Mukhopadhyay S, et al. Modulation of the expression of superoxide dismutase gene in lung injury by 2-chloroethyl ethyl sulfide, a mustard analog. J Biochem Mol Toxicol. 2006;20:142–149. doi: 10.1002/jbt.20128. [DOI] [PubMed] [Google Scholar]
- 30.Black AT, et al. Role of MAP kinases in regulating expression of antioxidants and inflammatory mediators in mouse keratinocytes following exposure to the half mustard, 2-chloroethyl ethyl sulfide. Toxicol Appl Pharmacol. 2010 doi: 10.1016/j.taap.2010.04.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laskin DL, Fakhrzadeh L, Laskin JD. Nitric oxide and peroxynitrite in ozone-induced lung injury. Adv Exp Med Biol. 2001;500:183–190. doi: 10.1007/978-1-4615-0667-6_24. [DOI] [PubMed] [Google Scholar]
- 32.Laskin JD, et al. Prooxidant and antioxidant functions of nitric oxide in liver toxicity. Antioxid Redox Signal. 2001;3:261–271. doi: 10.1089/152308601300185214. [DOI] [PubMed] [Google Scholar]
- 33.Nyska A, et al. Effects of iodine on inducible nitric oxide synthase and cyclooxygenase-2 expression in sulfur mustard-induced skin. Arch Toxicol. 2001;74:768–774. doi: 10.1007/s002040000199. [DOI] [PubMed] [Google Scholar]
- 34.Steinritz D, et al. Sulphur mustard induces time- and concentration-dependent regulation of NO-synthesizing enzymes. Toxicol Lett. 2009;188:263–269. doi: 10.1016/j.toxlet.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Qui M, et al. Inhibition of inducible nitric oxide synthase by a mustard gas analog in murine macrophages. BMC Cell Biol. 2006;7:39. doi: 10.1186/1471-2121-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawyer TW, Risk D. Effects of selected arginine analogues on sulphur mustard toxicity in human and hairless guinea pig skin keratinocytes. Toxicol Appl Pharmacol. 2000;163:75–85. doi: 10.1006/taap.1999.8837. [DOI] [PubMed] [Google Scholar]
- 37.Eldad A, et al. Superoxide dismutase (SOD) for mustard gas burns. Burns. 1998;24:114–119. doi: 10.1016/s0305-4179(97)00085-5. [DOI] [PubMed] [Google Scholar]
- 38.Gross CL, et al. Pretreatment of human epidermal keratinocytes with D,L-sulforaphane protects against sulfur mustard cytotoxicity. Cutan Ocul Toxicol. 2006;25:155–163. doi: 10.1080/15569520600859985. [DOI] [PubMed] [Google Scholar]
- 39.Smith CN, Lindsay CD, Upshall DG. Presence of methenamine/glutathione mixtures reduces the cytotoxic effect of sulphur mustard on cultured SVK-14 human keratinocytes in vitro. Hum Exp Toxicol. 1997;16:247–253. doi: 10.1177/096032719701600502. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Riviere JE, Monteiro-Riviere NA. Evaluation of protective effects of sodium thiosulfate, cysteine, niacinamide and indomethacin on sulfur mustard-treated isolated perfused porcine skin. Chem Biol Interact. 1995;96:249–262. doi: 10.1016/0009-2797(94)03596-z. [DOI] [PubMed] [Google Scholar]
- 41.Kenar L, et al. Evaluation of protective ointments used against dermal effects of nitrogen mustard, a vesicant warfare agent. Mil Med. 2005;170:1–6. doi: 10.7205/milmed.170.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Pino MA, Billack B. Reduction of vesicant toxicity by butylated hydroxyanisole in A-431 skin cells. Cutan Ocul Toxicol. 2008;27:161–172. doi: 10.1080/15569520802092070. [DOI] [PubMed] [Google Scholar]
- 43.Karayilanoglu T, et al. The protective and therapeutic effects of zinc chloride and desferrioxamine on skin exposed to nitrogen mustard. Mil Med. 2003;168:614–617. [PubMed] [Google Scholar]
- 44.Morad Y, et al. Treatment of ocular tissues exposed to nitrogen mustard: beneficial effect of zinc desferrioxamine combined with steroids. Invest Ophthalmol Vis Sci. 2005;46:1640–1646. doi: 10.1167/iovs.04-1165. [DOI] [PubMed] [Google Scholar]
- 45.Mukhopadhyay S, et al. Role of MAPK/AP-1 signaling pathway in the protection of CEES-induced lung injury by antioxidant liposome. Toxicology. 2009;261:143–151. doi: 10.1016/j.tox.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Xie J, et al. Overexpression of GSTA2 protects against cell cycle arrest and apoptosis induced by the DNA inter-strand crosslinking nitrogen mustard, mechlorethamine. J Cell Biochem. 2005;95:339–351. doi: 10.1002/jcb.20440. [DOI] [PubMed] [Google Scholar]
- 47.Husain K, et al. Effect of topically applied sulphur mustard on antioxidant enzymes in blood cells and body tissues of rats. J Appl Toxicol. 1996;16:245–248. doi: 10.1002/(SICI)1099-1263(199605)16:3<245::AID-JAT339>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Black RM, et al. Biological fate of sulfur mustard, 1,1′-thiobis(2-chloroethane). Urinary excretion profiles of hydrolysis products and beta-lyase metabolites of sulfur mustard after cutaneous application in rats. J Anal Toxicol. 1992;16:79–84. doi: 10.1093/jat/16.2.79. [DOI] [PubMed] [Google Scholar]