Abstract
Acute endotoxemia is associated with prolonged survival of adherent neutrophils in the lung vasculature. In the present studies, the effects of inflammatory mediators on signaling pathways regulating neutrophil survival were examined. We found that the protein kinase C activator, 12-O-tetradecanoyl-phorbol 13-acetate (TPA), but not interferon-γ (IFN-γ), prolonged the survival of adherent vasculature lung neutrophils from endotoxemic rats, a response that was correlated with reduced apoptosis. Although endotoxin administration to rats induced the expression of the anti-apoptotic protein Mcl-1 in lung neutrophils, TPA had no effect on this response. Endotoxin administration also induced expression of total p38 and p44/42 mitogen activated protein kinases (MAPK) in neutrophils, as well as phosphatidyl inositol 3 kinase (PI3K) and its downstream target protein kinase B (PKB). Treatment of the cells with TPA increased p38 MAPK expression in cells from both control and endotoxin treated animals. Cells from endotoxin treated, but not control animals, were found to exhibit constitutive binding activity of nuclear factor kappa B (NF-κB) which was blocked by TPA. In contrast, constitutive CCAAT/enhancer binding protein (C/EBP) nuclear binding activity evident in neutrophils from control animals was reduced following endotoxin administration. Moreover, this response was independent of TPA. These data suggest that NF-κB plays a role in TPA-induced signaling leading to prolonged survival of adherent vascular neutrophils in the lung during acute endotoxemia.
Under homeostatic conditions, the half-life of neutrophils is approximately 6–10 h after which time they are eliminated by the process of apoptosis. However, during inflammatory responses their survival is prolonged. A number of different inflammatory mediators including lipopolysaccharide (LPS), interferon gamma (IFN-γ), interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), granulocyte monocyte-colony stimulating factor (GM-CSF), and interleukin 3 (IL-3) have been reported to modulate neutrophil apoptosis (Matute-Bello et al., 1997; van den Berg, 2001). However, the effects of these mediators depend on the target cell and/or the microenvironment (Park, 1996). The biochemical signaling pathways initiated by these inflammatory mediators leading to apoptosis also appear to be cell type specific.
The phorbol ester 12-O-tetradecanoyl-phorbol 13-acetate (TPA) is a potent irritant and inflammatory agent (Das, 1991; Fretland et al., 1995). Incubation of neutrophils with TPA has been reported to stimulate oxidative metabolism and nitric oxide production (Larfars and Gyllenhammar, 1998; Lavnikova et al., 1998). Although the initial event in TPA signaling involves activation of protein kinase C, the downstream pathways leading to altered cellular responses are not completely characterized. In previous studies, we analyzed the effects of acute endotoxemia on survival and functional responsiveness of adherent neutrophils recovered from the lung vasculature of rats (Lavnikova et al., 1998; Sunil et al., 2000). Because of their unique location within the tissue, adherent vascular neutrophils are likely to be more relevant than circulating neutrophils or neutrophils recovered by bronchoalveolar lavage, to the pathophysiology of endotoxin-induced lung injury. We found that acute endotoxemia sensitized adherent vascular lung neutrophils to respond to inflammatory mediators (Lavnikova et al., 1998). Thus, cells from endotoxin treated rats produced increased quantities of reactive oxygen and nitrogen intermediates after stimulation with TPA and/or IFN-γ. In the present studies we determined if these cells also exhibited prolonged survival in response to these mediators. Biochemical pathways mediating TPA and IFN-γ induced activity were also investigated. Our studies revealed that TPA, but not IFN-γ, prolonged the survival of adherent vascular lung neutrophils from endotoxemic rats. This was correlated with down regulation of nuclear factor-kappa B (NF-κB) nuclear binding activity. These findings suggest a potential mechanism underlying the effects of protein kinase C activators on neutrophil survival during acute endotoxemia.
MATERIALS AND METHODS
Animals and treatments
Female specific pathogen-free Sprague Dawley rats (200–225 g, 6–8 week) were purchased from Taconic (Germantown, NY). Animals were housed in microisolator cages and maintained on sterile food and pyrogen-free water ad libitum. Acute endotoxemia was induced by intravenous injection of rats with 5 mg/kg Escherichia coli LPS (serotype 0128:B12, Sigma Chemical Co., St. Louis, MO). All experiments were repeated three times using samples from different animals. Data were analyzed using one-way ANOVA.
Cell isolation
Rats were euthanized with Nembutal (125 mg/kg) intraperitoneally. Adherent vascular neutrophils were isolated from the lung as previously described (Lavnikova et al., 1998, 1993). Briefly, the lung was perfused at a rate of 22 ml/min with 50 ml of warm (37°C) Ca+2/Mg+2-free Hank’s balanced salt solution (HBSS, pH 7.4) containing 2.5 mM HEPES and 4.4 M NaHCO3. After lavage with perfusion buffer (5–6 times) to remove alveolar macrophages and loosely adhered neutrophils, the trachea and major bronchi were excised and the lung cut into 500 μm slices (McIlwain mechanical tissue chopper, Brinkmann Instruments, Westbury, NY). Lung slices were washed in ice cold Ca+2/Mg+2-free HBSS with vigorous shaking using a Vortex (Genie 2, Fisher Scientific, Pittsburgh, PA) at speed 7 for 3 min, filtered using a 220 μm mesh, and then incubated in ice cold HBSS for 30 min with periodic shaking. Neutrophils were recovered after digestion of the lung tissue for 25 min with 60 U/ml collagenase D (specific activity 0.22 Wunsch unit = 800 IU, low ancillary protease activity; Boehringer Mannheim, Indianapolis, IN) prepared in HBSS containing 10% fetal bovine serum and 0.01% DNAse 1 (Sigma Chemical Co.) followed by filtering as indicated above. Giemsa staining of cytospin samples showed that the cells were 95% neutrophils. Viability was greater than90% as determined by trypanblue dye exclusion. In previous studies using transmission electron microscopy, we demonstrated that neutrophils recovered using this methodology were adhered to the lung vasculature (Lavnikova et al., 1998).
Measurement of cell survival
Cells were plated in96-well dishes (2 × 105 cells/well) in DMEM containing 10% FBS with and without 10 U/ml rat recombinant IFN-γ (Gibco-BRL, Grand Island, NY), 10 nM TPA (LC Services, Woburn, MA) or IFN-γ + TPA. After 0- and 20-h incubation, the cells were collected by gentle pipetting and the number of viable cells determined by trypan blue dye exclusion using a hemocytometer. The percentage of surviving cells was calculated by dividing the number of viable cells in the cultures after 20-h incubation by the number of cells in the cultures at 0-h incubation and multiplying by 100.
Analysis of DNA fragmentation by agarose gel electrophoresis
Cells were plated in4-well dishes (1 × 106 cells/well) in DMEM containing 10% FBS with and without 10 U/ml IFN-γ, 10 nM TPA, or IFN-γ + TPA. After 20 h incubation, the cells were lysed in 200 μl of buffer (5 mM Tris-Cl, pH 7.4; 2 mM EDTA, 0.5% TritonX-100) onice under DNAse-free conditions. After 30 min, the cell lysates were centrifuged (16,500g, 20 min) and DNA extracted from the supernatants by overnight precipitation in 0.1 × volume 3 M sodium acetate (pH 8.0) and 2 × volume 100% ethanol. The DNA was washed and dissolved in10 μl TBE buffer (45 mM Tris-borate, 1 mM EDTA, pH 8.0) (Chinet al., 1998). DNA samples (1–5 μl) were analyzed on 1.2% agarose gels, stained with ethidium bromide, and visualized under UV light.
TUNEL assay
Neutrophils were incubated in 4-well chamber slides (5 × 105 cells/well) for 20 h in the presence of medium, 10 U/ml IFN-γ, 10 nM TPA, or IFN-γ + TPA. Apoptosis was quantified using a kit from Boehringer Mannheim. Cells were fixed with freshly prepared paraformaldehyde (4% in PBS, pH 7.4) for 30 min at room temperature and then incubated with 0.3% H2O2 in methanol for 30 min. After rinsing with PBS, the cells were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min onice. Positive controls were prepared by treatment of the cells with 1 mg/ml DNAse 1 for 10 min at room temperature. Cells were then rinsed twice with PBS and incubated at 37°C with 100 μl TUNEL reaction mixture (terminal transferase and fluorescin-labeled dUTP). After 60 min, the cells were rinsed three times with PBS and analyzed on a Meridian ACAS 570 Anchored Cell Analysis System (Meridian Instruments, Okemos, MI).
Western blot analysis
Cells were plated in4-well dishes (1 × 106 cells/well) in DMEM containing 10% FBS with and without 10 U/ml IFN-γ, 10 nM TPA, or IFN-γ + TPA. After 1 or 20 h incubation, cells were suspended in buffer containing 50 mM HEPES (pH 7.4), 10 mM KCl, 1 mM EDTA, 1 mM DTT, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, 10 μg/ml soybean trypsin inhibitor, 10 μg/ml aprotinin, and 0.5% NP-40 and mixed periodically (Vortex Genie 2, Fisher Scientific). After 10 min on ice, lysates were centrifuged (4000g, 5 min) and supernatants containing 10 μg of protein fractionated on 10% sodium dodecyl sulfate polyacrylamide gels. The proteins were transferred to nitrocellulose paper, and incubated at room temperature for 3 h or overnight at 4°C with a 1:1000 dilution of mouse monoclonal anti-human Mcl-1 antibody (Transduction Laboratories, San Diego, CA), rabbit monoclonal p44/42 MAPK antibody, (New England Biolabs, Inc., Beverly, MA), rabbit polyclonal dually phosphorylated anti-human Thr180/Tyr182 phospho-p38 MAPK antibody (New England Biolabs, Inc.) or a 1:500 dilution of mouse monoclonal anti-human p38, PI3K (p85 subunit) or PKB-α antibody (Transduction Laboratories). This was followed by incubation with a 1:2000 dilution of goat anti-mouse or sheep anti-rabbit IgG horseradish peroxidase conjugated antibody for 1 h at room temperature. Proteins were detected using an Enhanced Chemi-Luminescence (ECL) detection system (Amersham Life Sciences, Arlington Heights, IL). Protein concentrations were determined using a BCA Protein Assay Kit (Pierce, Rockford, IL) with bovine serum albumin as the standard.
Preparation of nuclear extracts and electrophoretic mobility shift assays
Cells were plated in4-well dishes (1 × 106 cells/well) in DMEM containing 10% FBS with and without 10 U/ml IFN-γ, 10 nM TPA, or IFN-γ + TPA. After 1-h incubation, nuclear extracts were prepared from cells as previously described (Carter et al., 1998). Cells, suspended in buffer (10 mM HEPES pH 7.4, 10 mM KCl, 2 mM MgCl2, and 2 mM EDTA) were incubated on ice for 10 min followed by incubation with 10% NP-40. Five minutes later, the cells were centrifuged (16,500g,5 min) and the pellets resuspended in buffer containing 50 mM HEPES pH 7.4, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, and 10% glycerol. After 20 min on ice, the samples were centrifuged (16,500g, 5 min, 4°C) and supernatants containing nuclear extracts collected. Binding reactions were carried out at room temperature for 30 min in a total volume of 15 μl containing 2–5 μg of nuclear extracts, 5 μl of 5 × gel shift binding buffer (20% glycerol, 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl, 50 mM Tris-HCl, pH 7.5), 2 μg poly (dI-dC) and 3 × 104 cpm/μl of [32P] labeled consensus NF-κB(5′-AGT TGA GGG GAC TTT CCC AGG C-3′) (Promega Gel Shift Assay Systems) or C/EBP (5′-TGC AGA TTG CGC AAT CTG CA-3′) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) oligonucleotides. Probes were labeled using γ[32P]ATP (3000 Ci/mmol, NEN, Boston, MA). Protein-DNA complexes were separated on 5 or 7% non-denaturing polyacrylamide gels run at 250 V in 0.5 × TBE and visualized after the gels were dried and autoradiographed.
RESULTS
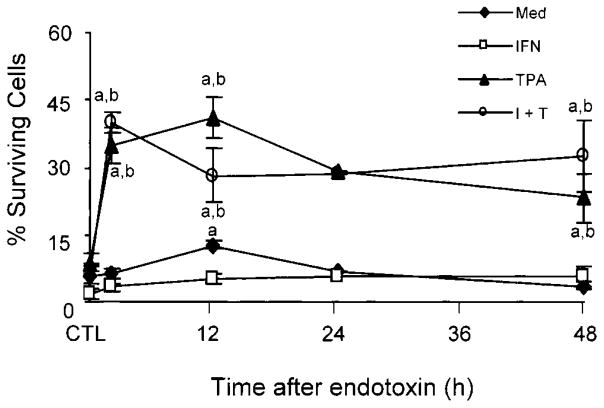
In initial studies, we analyzed the effects of acute endotoxemia on the survival of adherent vascular lung neutrophils. Unstimulated cells from endotoxin treated rats exhibited increased survival in culture when compared to cells from control animals (Fig. 1). However, this was only observed in cells isolated 12 h post treatment. Whereas the presence of IFN-γ in the culture medium reduced or had no effect on the percentage of surviving neutrophils, TPA prolonged the longevity of cells isolated from endotoxin treated rats 3–8 fold, and was able to overcome the inhibitory effects of IFN-γ. No major differences were noted in the response of cells isolated from animals at different times after endotoxin administration to TPA.
Fig. 1.
Effects of endotoxin administration to rats on survival of adherent lung neutrophils. Cells isolated from control (CTL) animals or 2, 12, 24, and 48 h after endotoxin administration to rats were cultured in the presence of medium control, IFN-γ (I) and/or TPA (T). The percentage of surviving cells was calculated 20 h later as described in the Materials and Methods. Values represent the mean ± SE (n = 3–9). aSignificantly different from CTL animals. bSignificantly different from cells cultured with medium.
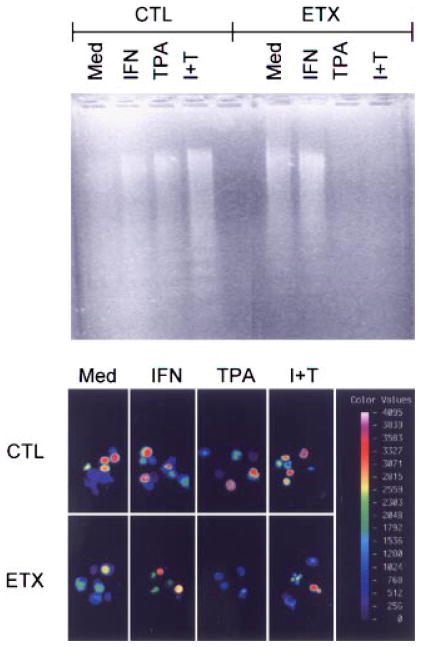
To determine if increased survival of adherent vascular lung neutrophils was due to decreased apoptosis, cytoplasmic DNA fragmentation was analyzed. Neutrophils from both control and endotoxin treated animals showed significant evidence of apoptosis (Fig. 2, upper panel). TPA, which prolonged neutrophil survival was found to prevent apoptosis, but only in cells from rats treated with endotoxin (Fig. 2, upper panel). Fluorescence image analysis using a TUNEL assay confirmed these findings. Thus, fluorescence staining, which is indicative of apoptosis, was apparent in cells from control animals even in the presence of TPA (Fig. 2, lower panel). Endotoxin administration resulted in a decrease (15–33%) in fluorescence staining which was most pronounced in TPA treated cultures (Fig. 2, lower panel). In cells from both control and endotoxin treated animals, IFN-γ by itself either had no effect or increased apoptosis as measured by DNA fragmentation and by fluorescence staining. The addition of IFN-γ to cultures containing TPA had no additional effect on apoptosis. These results are generally consistent with our cell survival data.
Fig. 2.
Effects of TPA and IFN-γ on DNA fragmentation. Neutrophils isolated from control (CTL) animals or 2 h after endotoxin (ETX) administration were cultured for 20 h with medium (Med), IFN-γ (I) and/or TPA (T). Upper panel: Soluble DNA extracts were analyzed on agarose gels and visualized using ethidium bromide. One representative gel from three separate experiments is shown. Lower panel: Cellular fluorescence intensity indicative of apoptosis was assessed using a Meridian ACAS 570. The color bar represents fluorescence on a four decade log scale. The color version of this figure is available online at www.interscience.wiley.com
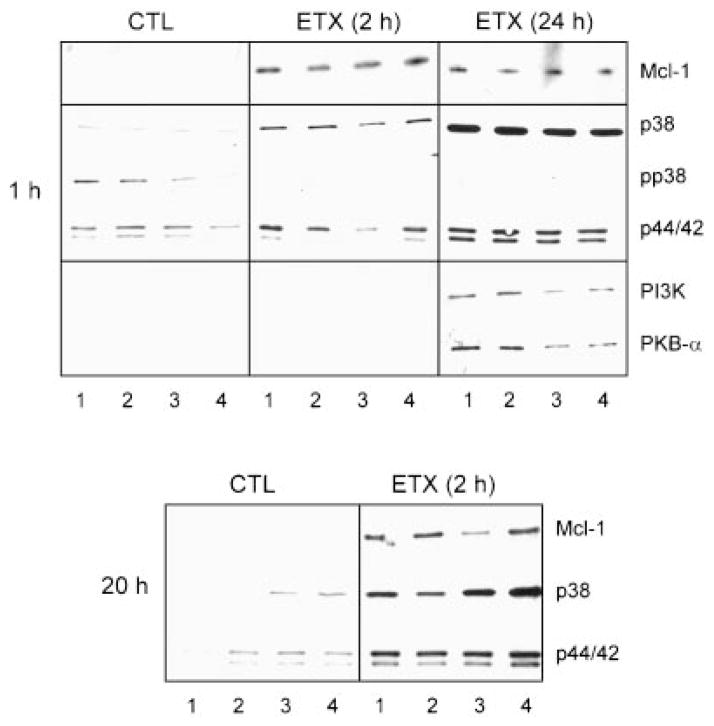
In further studies we determined if increased neutrophil survival following endotoxin administration was associated with altered expression of Mcl-1, an anti-apoptotic member of the Bcl-2 family (Craig, 1995; Moulding et al., 1998; Leuenroth et al., 2000). Mcl-1 was not detected in cells from control animals even after culturing with TPA and/or IFN-γ for up to 20 h (Fig. 3). Treatment of the animals with endotoxin induced expression of this anti-apoptotic protein. This was observed within2 h and persisted for at least 24 h post treatment. The addition of TPA and/or IFN-γ to the cultures did not significantly alter Mcl-1 expression by the cells cultured for 20 h.
Fig. 3.
Effects of TPA and IFN-γ on Mcl-1, MAPK, PI3K, and PKB-α expression. Neutrophils isolated from control (CTL) animals or 2 or 24 h after endotoxin (ETX) administration were treated for 1 h (upper panel) or 20 h (lower panel) with medium control (lane 1), IFN-γ (lane 2), TPA (lane 3), or TPA + IFN-γ (lane 4). Extracts were analyzed by Western blotting as described in the Materials and Methods. One representative gel from three separate experiments is shown.
We next analyzed biochemical signaling pathways known to be involved in regulating neutrophil survival and apoptosis. Initially we focused on p38 and p44/42 MAPK proteins (Pillinger et al., 1998; Abe and Saito, 2000a,b). Cells from control animals were found to constitutively express low levels of p38 MAPK (Fig. 3). This activity decreased with time in culture. Endotoxin administration caused a time-related increase in expression of p38 MAPK. Thus, neutrophils isolated 24 h after endotoxin treatment of the animals expressed 2–3-fold greater quantities of this protein than cells isolated 2 h post treatment (Fig. 3, upper panel). Incubation of neutrophils with TPA alone or in combination with IFN-γ for 20 h, increased expression of p38 MAPK in cells from both control and endotoxin treated animals (Fig. 3, lower panel). Neutrophils from control animals were also found to express phospho-p38 MAPK (Fig. 3, upper panel). Expression of this protein decreased in the cells after endotoxin administration to the rats. The addition of TPA and/or IFN-γ to neutrophil cultures had no effect on phospho-p38 MAPK expression.
As observed with p38 MAPK, relatively low levels of p44/42 MAPK were detected in unstimulated cells from control animals (Fig. 3). After endotoxin administration, p44/42 MAPK expression increased, a response that persisted for at least 24 h post treatment. Incubation of the cells with TPA and/or IFN-γ did not significantly alter expression of p44/42 MAPK (Fig. 3). Phospho-p44/42 MAPK was not detectable in adherent vascular neutrophils (data not shown). Moreover, in accord with previous studies in human peripheral blood neutrophils (Nolan et al., 1999), SAPK/JNK was not observed in lung neutrophils from either control or endotoxin treated rats (data not shown).
We next determined if expression of PI3K and its downstream target PKB-α was altered in lung neutrophils after endotoxin administration. Neither of these proteins was detectable in cells from control animals even after 20 h in culture (Fig. 3, data not shown). Whereas PI3K and PKB-α were not observed in cells isolated 2 h after endotoxin administration to rats, both proteins were identified in cells isolated 24 h post treatment. For both proteins, expression decreased with time in culture (data not shown). TPA and/or IFN-γ had no significant effect on expression of PI3K or PKB-α in cells from control or endotoxin treated animals (Fig. 3, data not shown).
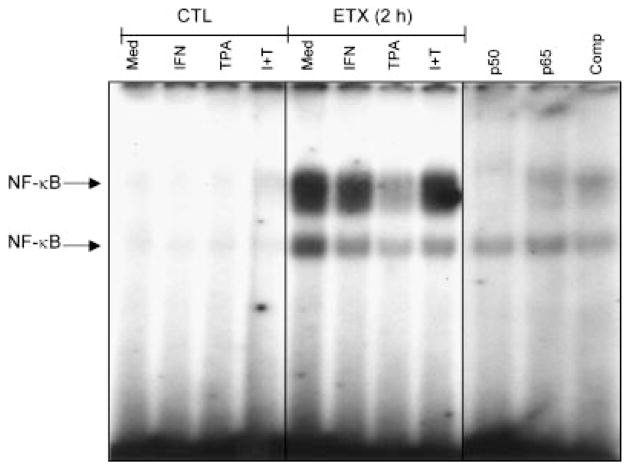
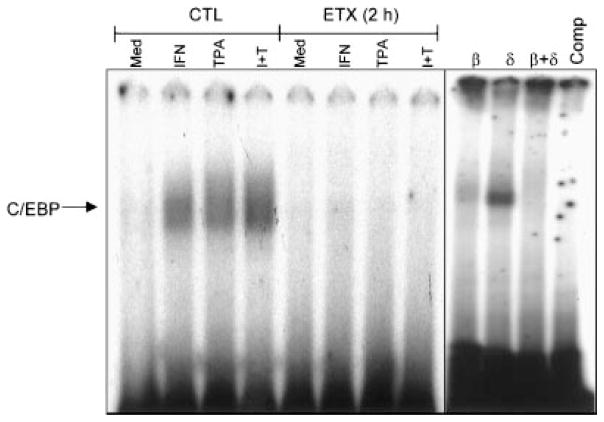
In further studies we analyzed NF-κB and C/EBP nuclear binding activities in adherent vascular lung neutrophils. These transcription factors have also been shown to regulate cell survival (Williams et al., 1999). NF-κB binding activity was not observed in cells from control animals (Fig. 4). Induction of acute endotoxemia was associated with NF-κB nuclear binding activity. Two NF-κB complexes were apparent in the gels. Whereas antibody to p50 completely blocked the migration of the slower moving complex, anti-p65 antibody partially blocked migration of this complex. Binding was also decreased in the presence of 40-fold excess unlabeled NF-κB, demonstrating the specificity of the probe. TPA was found to markedly reduce NF-κB nuclear binding in cells from endotoxin treated animals. As expected, IFN-γ had no effect on NF-κB activity. In contrast to NF-κB, C/EBP nuclear binding activity was not evident in unstimulated neutophils from endotoxin treated animals (Fig. 5). However, in cells from control, but not endotoxin treated rats, TPA and/or IFN-γ caused a marked induction of C/EBP nuclear binding activity. Antibodies to C/EBP-β, but not C/EBP-δ blocked C/EBP activity. Moreover, binding was abolished in the presence of excess unlabeled probe.
Fig. 4.
Effects of acute endotoxemia on NF-κB nuclear binding activity. Neutrophils isolated from control (CTL) animals or 2 h after endotoxin (ETX) administration were treated with medium control (Med), IFN-γ, TPA, or TPA + IFN-γ (I + T) for 1 h. Nuclear extracts were analyzed for NF-κB binding activity using a gel retardation assay. Extracts prepared from medium treated cells isolated 2 h post endotoxin were incubated on ice for 15 min with antibodies to the p50 or p65 subunits of NF-κB (1 μg), or 40-fold excess of unlabeled cold competitor (Comp) prior to the labeled probe. One representative gel from three separate experiments is shown.
Fig. 5.
Effects of acute endotoxemia on C/EBP nuclear binding activity in adherent vascular lung neutrophils. Cells isolated from control (CTL) animals or 2 h after endotoxin (ETX) administration were treated with medium control (Med), IFN-γ (IFN), TPA, or TPA + IFN-γ (I + T) for 1 h. Nuclear extracts were analyzed for C/EBP binding activity by a gel retardation assay. Extracts prepared from IFN-γ treated cells were incubated on ice for 15 min with antibodies to C/EBP-β and/or C/EBP-δ (1 mg), or 40-fold excess of unlabeled cold competitor (Comp) prior to the labeled probe. One representative gel from three separate experiments is shown.
DISCUSSION
Neutrophils constitute the first line of host defense. They rapidly appear at inflammatory sites and generate mediators to destroy pathogens, dead cells, and debris. To exert their biological activity however, neutrophils must remain at these sites and their survival must be prolonged. In this regard, a number of studies have demonstrated that apoptosis is reduced in neutrophils accumulating at sites of injury and that this process is regulated by inflammatory mediators (Chilvers et al., 1998; Fossati et al., 1998; Whyte et al., 1999; Droemann et al., 2000). In the present studies we analyzed signaling pathways induced by inflammatory mediators that may regulate neutrophil survival in the lung vasculature during endotoxin-induced tissue injury.
We found that adherent vascular neutrophils from the lungs of endotoxin treated animals exhibited prolonged survival and decreased apoptosis in culture when compared to cells from control animals. Moreover, these responses were increased in the presence of TPA. These findings suggest that protein kinase C plays a role in regulating survival and apoptosis in neutrophils. This is supported by the observation that GM-CSF, a potent inducer of neutrophil survival, also mediates its action via protein kinase C (Deshpande et al., 1997; Coxon et al., 1999; Robertson et al., 1999; Matute-Bello et al., 2000). Our findings are consistent with previous studies demonstrating that TPA blocks apoptosis in peripheral blood monocytes and neutrophils (Gabrielson et al., 1992; Pae et al., 2000). In contrast to TPA, IFN-γ reduced or had no effect on the survival of adherent vascular lung neutrophils in culture. IFN-γ has previously been reported to increase survival of human peripheral blood neutrophils (Colotta et al., 1992). Differences between our results may be due to distinct species and tissue origin of the two neutrophil populations, as well as differences in the treatment times (20 h vs. 48–96 h).
Mcl-1 is thought to play a role in delaying the death of myeloid cells (Nolan et al., 1999; Abe and Saito, 2000a,b). We found that acute endotoxemia resulted in a rapid and persistent induction of Mcl-1 in adherent vascular lung neutrophils, suggesting that prolonged survival is mediated, at least in part, by this protein. This is supported by findings that LPS treatment increases Mcl-1 in human peripheral blood neutrophils (Nolan et al., 1999). Interestingly, despite its ability to promote the survival of cultured neutrophils from endotoxin treated rats, TPA had no effect on Mcl-1 expression by the cells. TPA has been reported to induce Mcl-1 in ML-1 myeloid leukemia cells (Kozopas et al., 1993) and to phosphorylate and activate this protein in BL4 Burkitt lymphoma cells (Domina et al., 2000). Inhibition of spontaneous apoptosis in peripheral blood lymphocytes by TPA has also been correlated with induction of Mcl-1 (Lomo et al., 1996). It is likely that TPA exerts distinct actions on normal and transformed cells and/or cells of the myeloid and lymphoid lineage which could account for differences in our findings. The fact that TPA did not alter levels of Mcl-1 in adherent vascular lung neutrophils suggests that expression of this protein was not sufficient to prolong the survival of these cells and that additional signaling events are required. IFN-γ, at relatively high concentrations (1000 U/ml) has been reported to induce Mcl-1 expression and promote survival of human umbilical cord-blood derived eosinophils (Druilhe et al., 1998). Our findings that IFN-γ did not induce Mcl-1 expression in adherent vascular lung neutrophil are in accord with its lack of effect on survival of these cells and suggest that the response to this cytokine may be cell type or species specific and/or related to concentrations utilized.
The MAPK are a family of enzymes that participate in downstream signaling initiated by inflammatory mediators such as TPA and IFN-γ (Stadheim and Kucera, 1998; Zhuang et al., 1998). Activation of the MAPK pathway has been shown to delay apoptosis in a number of cell types (Perkins et al., 1996). Acute endotoxemia was associated with a rapid and prolonged increase in total p38 and p44/42 MAPK expression in adherent vascular neutrophils. These findings suggest that these proteins may contribute to neutrophil survival. A similar increase in MAPK has been reported previously in LPS treated macrophages (Valledor et al., 2000). TPA was found to increase p38 MAPK expression in adherent vascular lung neutrophils but only after relatively prolonged incubation times (20 h) in culture. These findings demonstrate that these cells remain primed to respond to inflammatory signals (Pae et al., 2000) and are consistent with the time course of activation of MAPK by TPA in RAW 264.7 cells (Shiraishi et al., 1999). The fact that this was observed in cells from both control and endotoxin treated rats suggests that p38 MAPK does not play a major role in TPA-induced survival. We also found that lung neutrophils from control animals constitutively expressed activated phospho-p38 MAPK. p38 MAPK has been shown to be constitutively phosphorylated and activated during spontaneous apoptosis of neutrophils (Aoshiba et al., 1999) and a similar activity may occur in adherent vascular lung neutrophils from control animals. In contrast, expression of phospho-p38 MAPK was decreased in neutrophils from endotoxin treated rats, suggesting that this protein is not required for neutrophil survival. In rat adherent vascular lung neutrophils, TPA had no effect on phospho-p38 MAPK expression, which is distinct from the response of human peripheral blood leukocytes (Zu et al., 1998). Differences in these results may be attributed to unique actions of TPA on circulating and adherent cells and provide additional support for the idea that these neutrophil subpopulations are distinct. Our inability to detect phospho-p44/42 MAPK in these cells may be due to proteases which inactivate phospho-p44/42 MAPK in lung neutrophils (Mynott et al., 1999). As observed in SV40-transformed mouse macrophages (Kovarik et al., 1999), IFN-γ had no effect on expression of either p38 or p44/42 MAPK in neutrophils.
Inositol lipids generated via PI3K have been implicated in the regulation of cell proliferation, survival, and differentiation, as well as in various inflammatory responses (Vanhaesebroeck and Alessi, 2000). Induction of acute endotoxemia caused a dramatic increase in expression of PI3K, as well as PKB-α in adherent vascular lung neutrophils isolated 24 h post exposure. These results are consistent with reports of induction of PI3K and PKB by LPS in cultured macrophages (Salh et al., 1998). Our findings that TPA had no effect on expression of these proteins suggest that in adherent vascular lung neutrophils, TPA does not utilize the PI3K/PKB-α pathway to promote survival. IFN-γ also had no effect on expression of PI3K or PKB-α in lung neutrophils. This is in accord with findings in human peripheral blood neutrophils (Aas et al., 1999) and B cells (Su and David, 1999).
A number of genes involved in cell survival are regulated by transcription factors such as NF-κB (Wang et al., 1999). However, whether NF-κB promotes or prevents apoptosis appears to depend on the cell type (Barkett and Gilmore, 1999). Adherent vascular lung neutrophils from endotoxin treated, but not control rats, were found to exhibit constitutive NF-κB nuclear binding activity. Interestingly, this activity was inhibited by TPA. Previous studies have demonstrated a direct correlation between levels of NF-κB and apoptosis in developing chick embryos (Abbadie et al., 1993). Similarly, apoptosis induced by serum starvation in 293 embryonic kidney cells was prevented by a dominant-negative RelA mutant (Grimm et al., 1996). These studies suggest that NF-κB promotes programmed cell death. The ability of TPA to prolong survival of cells from endotoxin treated rats may be directly related to its suppressive effects on NF-κB. In contrast TPA was found to induce C/EBP nuclear binding activity, but only in cells from control animals. C/EBP transcription factor activity has been reported to be required for TPA responsiveness in keratinocytes (Agarwal et al., 1999) and may similarly control lung neutrophil responses to TPA. IFN-γ was also found to induce C/EBP activity in these cells. These data suggest that upregulation of C/EBP may be a general response to inflammatory mediators under homeostatic conditions. Our studies are in accord with previous reports that IFN-γ induces C/EBP in J774.2 macrophages (Tengku-Muhammad et al., 2000). Induction of acute endotoxemia resulted in decreased C/EBP nuclear binding activity in cells isolated 2 h post treatment. Neither TPA nor IFN-γ altered this activity suggesting that C/EBP does not mediate the effects of these mediators on survival of adherent vascular lung neutrophils during endotoxemia.
The present studies demonstrate that TPA prolongs the survival of neutrophils in the lung vasculature during acute endotoxemia. This was correlated with decreased NF-κB nuclear binding activity. Further studies using pharmacological inhibitors of NF-κB are needed to determine its precise role in regulating survival of neutrophils in the lung during acute endotoxemia.
Acknowledgments
Contract grant sponsor: NIH; Contract grant numbers: ES04738, GM34310, ES06897.
LITERATURE CITED
- Aas V, Larsen K, Iversen JG. Interferon-gamma elicits a G-protein-dependent Ca2+ signal in human neutrophils after depletion of intracellular Ca2+stores. Cell Signal. 1999;11:101–110. doi: 10.1016/s0898-6568(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Kabrun N, Bouali F, Smardova J, Stehelin D, Vanden-bunder B, Enrietto PJ. High levels of c-rel expression are associated with programmed cell death in the developing avian embryo and in bone marrow cells in vitro. Cell. 1993;75:899–912. doi: 10.1016/0092-8674(93)90534-w. [DOI] [PubMed] [Google Scholar]
- Abe K, Saito H. Neurotrophic effect of basic fibroblast growth factor is mediated by the p42/p44 mitogen-activated protein kinase cascade in cultured rat cortical neurons. Brain Res Dev Brain Res. 2000a;122:81–85. doi: 10.1016/s0165-3806(00)00054-7. [DOI] [PubMed] [Google Scholar]
- Abe K, Saito H. The mitogen-activated protein kinase cascade mediates neurotrophic effect of epidermal growth factor in cultured rat hippocampal neurons. Neurosci Lett. 2000b;282:89–92. doi: 10.1016/s0304-3940(00)00867-3. [DOI] [PubMed] [Google Scholar]
- Agarwal C, Efimova T, Welter JF, Crish JF, Eckert RL. CCAAT/enhancer-binding proteins. A role in regulation of human involucrin promoter response to phorbol ester. J Biol Chem. 1999;274:6190–6194. doi: 10.1074/jbc.274.10.6190. [DOI] [PubMed] [Google Scholar]
- Aoshiba K, Yasui S, Hayashi M, Tamaoki J, Nagai A. Role of p38-mitogen-activated protein kinase in spontaneous apoptosis of human neutrophils. J Immunol. 1999;162:1692–1700. [PubMed] [Google Scholar]
- Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- Carter AB, Monick MM, Hunninghake GW. Lipopolysaccharide-induced NF-kappaB activation and cytokine release in human alveolar macrophages is PKC-independent and TK- and PC-PLC-dependent. Am J Respir Cell Mol Biol. 1998;18:384–391. doi: 10.1165/ajrcmb.18.3.2972. [DOI] [PubMed] [Google Scholar]
- Chilvers ER, Rossi AG, Murray J, Haslett C. Regulation of granulocyte apoptosis and implications for anti-inflammatory therapy. Thorax. 1998;53:533–534. doi: 10.1136/thx.53.7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin BY, Choi ME, Burdick MD, Strieter RM, Risby TH, Choi AM. Induction of apoptosis by particulate matter: Role of TNF-alpha and MAPK. Am J Physiol. 1998;275:L942–L949. doi: 10.1152/ajplung.1998.275.5.L942. [DOI] [PubMed] [Google Scholar]
- Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- Coxon A, Tang T, Mayadas TN. Cytokine-activated endothelial cells delay neutrophil apoptosis in vitro and in vivo. A role for granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:923–934. doi: 10.1084/jem.190.7.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig RW. The bcl-2 gene family. Semin Cancer Biol. 1995;6:35–43. doi: 10.1006/scbi.1995.0005. [DOI] [PubMed] [Google Scholar]
- Das UN. Arachidonic acid as a mediator of some of the actions of phorbolmyristate acetate, a tumor promoter and inducer of differentiation. Prostaglandins Leukot Essent Fatty Acids. 1991;42:241–244. doi: 10.1016/0952-3278(91)90089-n. [DOI] [PubMed] [Google Scholar]
- Deshpande RV, Peterson RH, Moore MA. Granulocyte colony-stimulating factor-induced activation of protein kinase-C in myeloid cells. J Cell Biochem. 1997;66:286–296. doi: 10.1002/(sici)1097-4644(19970901)66:3<286::aid-jcb2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Domina AM, Smith JH, Craig RW. Myeloid cell leukemia 1 is phosphorylated through two distinct pathways, one associated with extracellular signal-regulated kinase activation and the other with G2/M accumulation or protein phosphatase 1/2A inhibition. J Biol Chem. 2000;275:21688–21694. doi: 10.1074/jbc.M000915200. [DOI] [PubMed] [Google Scholar]
- Droemann D, Aries SP, Hansen F, Moellers M, Braun J, Katus HA, Dalhoff K. Decreased apoptosis and increased activation of alveolar neutrophils in bacterial pneumonia. Chest. 2000;117:1679–1684. doi: 10.1378/chest.117.6.1679. [DOI] [PubMed] [Google Scholar]
- Druilhe A, Arock M, Le Goff L, Pretolani M. Human eosinophils express bcl-2 family proteins: Modulation of Mcl-1 expression by IFN-gamma. Am J Respir Cell Mol Biol. 1998;18:315–322. doi: 10.1165/ajrcmb.18.3.3019. [DOI] [PubMed] [Google Scholar]
- Fossati G, Mazzucchelli I, Gritti D, Ricevuti G, Edwards SW, Moulding DA, Rossi ML. In vitro effects of GM-CSF on mature peripheral blood neutrophils. Int J Mol Med. 1998;1:943–951. doi: 10.3892/ijmm.1.6.943. [DOI] [PubMed] [Google Scholar]
- Fretland DJ, Gokhale R, Mathur L, Baron DA, Paulson SK, Stolzenbach J. Dermal inflammation in primates, mice, and guinea pigs: Attenuation by second-generation leukotriene B4 receptor antagonist, SC-53228. Inflammation. 1995;19:333–346. doi: 10.1007/BF01534391. [DOI] [PubMed] [Google Scholar]
- Gabrielson EW, Kuppusamy P, Povey AC, Zweier JL, Harris CC. Measurement of neutrophil activation and epidermal cell toxicity by palytoxin and 12-O-tetradecanoyl-phorbol-13-acetate. Carcinogenesis. 1992;13:1671–1674. doi: 10.1093/carcin/13.9.1671. [DOI] [PubMed] [Google Scholar]
- Grimm S, Bauer MK, Baeuerle PA, Schulze-Osthoff K. Bcl-2 down-regulates the activity of transcription factor NF-kappaB induced upon apoptosis. J Cell Biol. 1996;134:13–23. doi: 10.1083/jcb.134.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovarik P, Stoiber D, Eyers PA, Menghini R, Neininger A, Gaestel M, Cohen P, Decker T. Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated protein kinase whereas IFN-gamma uses a different signaling pathway. Proc Natl Acad Sci U S A. 1999;96:13956–13961. doi: 10.1073/pnas.96.24.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larfars G, Gyllenhammar H. Stimulus-dependent transduction mechanisms for nitric oxide release in human polymorphonuclear neutrophil leukocytes. J Lab Clin Med. 1998;132:54–60. doi: 10.1016/s0022-2143(98)90025-7. [DOI] [PubMed] [Google Scholar]
- Lavnikova N, Prokhorova S, Helyar L, Laskin DL. Isolation and partial characterization of subpopulations of alveolar macrophages, granulocytes, and highly enriched interstitial macrophages from rat lung. Am J Respir Cell Mol Biol. 1993;8:384–392. doi: 10.1165/ajrcmb/8.4.384. [DOI] [PubMed] [Google Scholar]
- Lavnikova N, Prokhorova S, Lakhotia AV, Gordon R, Laskin DL. Distinct inflammatory responses of adherent vascular lung neutrophils to pulmonary irritants. J Inflamm. 1998;48:56–66. [PubMed] [Google Scholar]
- Leuenroth SJ, Grutkoski PS, Ayala A, Simms HH. The loss of Mcl-1 expression in human polymorphonuclear leukocytes promotes apoptosis. J Leukoc Biol. 2000;68:158–166. [PubMed] [Google Scholar]
- Lomo J, Smeland EB, Krajewski S, Reed JC, Blomhoff HK. Expression of the Bcl-2 homologue Mcl-1 correlates with survival of peripheral blood B lymphocytes. Cancer Res. 1996;56:40–43. [PubMed] [Google Scholar]
- Matute-Bello G, Liles WC, Radella F, 2nd, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156:1969–1977. doi: 10.1164/ajrccm.156.6.96-12081. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Liles WC, Radella F, 2nd, Steinberg KP, Ruzinski JT, Hudson LD, Martin TR. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med. 2000;28:1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- Moulding DA, Quayle JA, Hart CA, Edwards SW. Mcl-1 expression in human neutrophils: Regulation by cytokines and correlation with cell survival. Blood. 1998;92:2495–2502. [PubMed] [Google Scholar]
- Mynott TL, Ladhams A, Scarmato P, Engwerda CR. Bromelain, from pineapple stems, proteolytically blocks activation of extracellular regulated kinase-2 in T cells. J Immunol. 1999;163:2568–2575. [PubMed] [Google Scholar]
- Nolan B, Duffy A, Paquin L, De M, Collette H, Graziano CM, Bankey P. Mitogen-activated protein kinases signal inhibition of apoptosis in lipopolysaccharide-stimulated neutrophils. Surgery. 1999;126:406–412. [PubMed] [Google Scholar]
- Pae HO, Yoo JC, Choi BM, Lee EJ, Song YS, Chung HT. 12-O-tetradecanoyl-phorbol 13-acetate, protein kinase C (PKC) activator, protects human leukemia HL-60 cells from taxol-induced apoptosis: Possible role for extracellular signal-regulated kinase. Immunopharmacol Immunotoxicol. 2000;22:61–73. doi: 10.3109/08923970009016406. [DOI] [PubMed] [Google Scholar]
- Park JR. Cytokine regulation of apoptosis in hematopoietic precursor cells. Curr Opin Hematol. 1996;3:191–196. doi: 10.1097/00062752-199603030-00005. [DOI] [PubMed] [Google Scholar]
- Perkins GR, Marshall CJ, Collins MK. The role of MAP kinase kinase in interleukin-3 stimulation of proliferation. Blood. 1996;87:3669–3675. [PubMed] [Google Scholar]
- Pillinger MH, Capodici C, Rosenthal P, Kheterpal N, Hanft S, Philips MR, Weissmann G. Modes of action of aspirin-like drugs: Salicylates inhibit erk activation and integrin-dependent neutrophil adhesion. Proc Natl Acad Sci U S A. 1998;95:14540–14545. doi: 10.1073/pnas.95.24.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Roberts CT, Farr KL, Dunn AR, Seamark RF. Fertility impairment in granulocyte-macrophage colony-stimulating factor-deficient mice. Biol Reprod. 1999;60:251–261. doi: 10.1095/biolreprod60.2.251. [DOI] [PubMed] [Google Scholar]
- Salh B, Wagey R, Marotta A, Tao JS, Pelech S. Activation of phosphatidyl inositol 3-kinase, protein kinase B, and p70 S6 kinases in lipopolysaccharide-stimulated Raw 264.7 cells: Differential effects of rapamycin, Ly294002, and wortmannin on nitric oxide production. J Immunol. 1998;161:6947–6954. [PubMed] [Google Scholar]
- Shiraishi M, Hirasawa N, Kobayashi Y, Oikawa S, Murakami A, Ohuchi K. Participation of mitogen-activated protein kinase in thapsigargin- and TPA-induced histamine production in murine macrophage RAW 264.7 cells. Br J Pharmacol. 1999;129:515–524. doi: 10.1038/sj.bjp.0703085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadheim TA, Kucera GL. Extracellular signal-regulated kinase (ERK) activity is required for TPA-mediated inhibition of drug-induced apoptosis. Biochem Biophys Res Commun. 1998;245:266–271. doi: 10.1006/bbrc.1998.8410. [DOI] [PubMed] [Google Scholar]
- Su L, David M. Inhibition of B cell receptor-mediated apoptosis by IFN. J Immunol. 1999;162:6317–6321. [PubMed] [Google Scholar]
- Sunil VR, Gardner CR, Laskin JD, Laskin DL. Prolonged survival of adherent vascular lung neutrophils in the lung during acute endotoxemia is mediated by NF-κB. Am J Respir Crit Care Med (Abstract) 2000;161:A513. [Google Scholar]
- Tengku-Muhammad TS, Hughes TR, Ranki H, Cryer A, Ramji DP. Differential regulation of macrophage CCAAT-enhancer binding protein isoforms by lipopolysaccharide and cytokines. Cytokine. 2000;12:1430–1436. doi: 10.1006/cyto.2000.0711. [DOI] [PubMed] [Google Scholar]
- Valledor AF, Comalada M, Xaus J, Celada A. The differential time-course of extracellular-regulated kinase activity correlates with the macrophage response toward proliferation or activation. J Biol Chem. 2000;275:7403–7409. doi: 10.1074/jbc.275.10.7403. [DOI] [PubMed] [Google Scholar]
- van den Berg JM. Divergent effects of tumor necrosis factor alpha on apoptosis of human neutrophils. J Leukoc Biol. 2001;69:467–473. [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: More than just a road to PKB. Biochem J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ, Yang-Yen HF. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidyl inositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19:6195–6206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte M, Renshaw S, Lawson R, Bingle C. Apoptosis and the regulation of neutrophil lifespan. Biochem Soc Trans. 1999;27:802–807. doi: 10.1042/bst0270802. [DOI] [PubMed] [Google Scholar]
- Williams DL, Ha T, Li C, Kalbfleisch JH, Ferguson DA., Jr Early activation of hepatic NFkappaB and NF-IL6 inpolymicrobial sepsis correlates with bacteremia, cytokine expression, and mortality. Ann Surg. 1999;230:95–104. doi: 10.1097/00000658-199907000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Lynch MC, Kochevar IE. Activation of protein kinase C is required for protection of cells against apoptosis induced by singlet oxygen. FEBS Lett. 1998;437:158–162. doi: 10.1016/s0014-5793(98)01222-8. [DOI] [PubMed] [Google Scholar]
- Zu YL, Qi J, Gilchrist A, Fernandez GA, Vazquez-Abad D, Kreutzer DL, Huang CK, Sha’afi RI. p38 mitogen-activated protein kinase activation is required for human neutrophil function triggered by TNF-alpha or FMLP stimulation. J Immunol. 1998;160:1982–1989. [PubMed] [Google Scholar]