Abstract
Objectives
Gastric colonisation with intestinal flora (IF) has been shown to promote Helicobacter pylori (Hp)-associated gastric cancer. However, it is unknown if the mechanism involves colonisation with specific or diverse microbiota secondary to gastric atrophy.
Design
Gastric colonisation with Altered Schaedler’s flora (ASF) and Hp were correlated with pathology, immune responses and mRNA expression for proinflammatory and cancer-related genes in germ-free (GF), Hp monoassociated (mHp), restricted ASF (rASF; 3 species), and specific pathogen-free (complex IF), hypergastrinemic INS-GAS mice 7 months postinfection.
Results
Male mice cocolonised with rASFHp or IFHp developed the most severe pathology. IFHp males had the highest inflammatory responses, and 40% developed invasive gastrointestinal intraepithelial neoplasia (GIN). Notably, rASFHp colonisation was highest in males and 23% developed invasive GIN with elevated expression of inflammatory biomarkers. Lesions were less severe in females and none developed GIN. Gastritis in male rASFHp mice was accompanied by decreased Clostridum species ASF356 and Bacteroides species ASF519 colonisation and an overgrowth of Lactobacillus murinus ASF361, supporting that inflammation-driven atrophy alters the gastric niche for GI commensals. Hp colonisation also elevated expression of IL-11 and cancer-related genes, Ptger4 and Tgf-β, further supporting that Hp infection accelerates gastric cancer development in INS-GAS mice.
Conclusions
rASFHp colonisation was sufficient for GIN development in males, and lower GIN incidence in females was associated with lower inflammatory responses and gastric commensal and Hp colonisation. Colonisation efficiency of commensals appears more important than microbial diversity and lessens the probability that specific gastrointestinal pathogens are contributing to cancer risk.
INTRODUCTION
Gastric adenocarcinoma (GAC) represents the most prevalent form (~95%) of gastric cancer, and is the fourth and fifth most common cancer in men and women, respectively.1 Unlike the poorly differentiated diffuse-type GAC which often arises without precancerous lesions, a more differentiated intestinal-type GAC is the end stage of lesions progressing from gastritis, atrophy, metaplasia and dysplasia to carcinoma in situ.2,3 Among risk factors for intestinal-type GAC, Helicobacter pylori (Hp) infection is one of the most important risk factors.4–6 However, of the 50% of the world’s population infected with Hp, only 1–2% develop gastric tumours,7 while most infected individuals experience asymptomatic pangastritis.8,9 This differential susceptibility to GAC has been attributed, in part, to differences in virulence potential of diverse Hp strains,4,10,11 host genetic polymorphisms involved in immune and inflammatory responses, cellular metabolism, growth and differentiation,12,13 and environmental variables including diet, coinfections and age of Hp acquisition.3,14
Recent studies in humans indicate that gastric colonisation by non-Hp bacteria, such as Actinobacteria, Bacteroides, Firmicutes, Fusobacteria and Proteobacteria, many of which normally colonise the lower bowel, could impact the risk for GAC.15–18 Dyspeptic Hp patients treated with acid-suppressive drugs had a significant increase in non-Hp bacteria colonising the stomach, and inflammatory cytokine levels associated with greater risk of atrophic gastritis, suggesting that non-Hp bacteria colonise the stomach during acid-suppressive therapy and promote gastric carcinogenesis.19,20 This paradigm has been modelled in the Hp-infected gerbil with increased gastric atrophy and promotion of GAC when treated long-term with proton pump inhibitors.21 An altered microbial composition with overgrowth of Streptococcus, Lactobacillus, Veillonella and Prevotella has similarly been found in the stomach of Hp-infected GAC and dyspeptic patients.22 Acinetobacter iwofii, a bacteria causing nosocomial infection in ventilated patients, induced gastritis, hypergastrinemia, inflammatory cytokine and gastrin production comparable with Hp infection in C57BL/6 mice, further suggesting that gastric colonisation by non-Hp pathogenic bacteria could promote GAC.23 As previously shown, normal intestinal flora (IF) hastened the onset and promoted the progression of gastrointestinal intraepithelial neoplasia (GIN),24 while antimicrobial therapies delayed onset of GIN in Hp-infected, and importantly, in uninfected INS-GAS mice.25 Notably, Hp-free INS-GAS mice colonised with IF developed GIN more quickly than germ-free (GF) INS-GAS mice which remained free of GIN through 11 months of age.24 Similar results were observed in the K19-Wnt1/C2mE mouse model of GAC.26 Together, these data suggest that non-Hp bacteria, including those considered as pathogenic or commensal IF, can colonise the stomach and represent an additional GAC risk, particularly in Hp-infected, susceptible individuals.19,22
Although evidence supports a role for normal IF in GAC, it is unknown if specific species of IF are required to promote GAC progression or if the mechanism reflects gastric colonisation with diverse IF secondary to Hp-mediated gastric atrophy. Using the transgenic, hypergastrinemic INS-GAS mouse model of Hp-accelerated gastric carcinogenesis, we evaluated if a restricted microbiota limited to three species of Altered Schaedler’s Flora (restricted ASF or rASF), including ASF356 Clostridium species, ASF361 Lactobacillus murinus and ASF519 Bacteroides species, is sufficient to contribute to the GIN incidence after development of gastric atrophy secondary to Hp infection.27,28 Under gnotobiotic conditions, male and female INS-GAS mice that were GF, monoassociated with Hp (mHp) or colonised with rASF, rASFHp, IF (specific pathogen-free or SPF with undefined IF), or IFHp were compared for gastric pathology, ASF and Hp colonisation dynamics, inflammatory responses in serum and mRNA expression of select proinflammatory and cancer-related genes in gastric tissues.
MATERIALS AND METHODS
Experimental design
Animal use was approved by the MIT Committee on Animal Care. Six experimental groups of INS-GAS mice on a FVB/N background (Tg (Ins1-GAS) 1Sbr) included GF (n=32: 15 males and 17 females), rASF (n=27: 15 males and 12 females), IF (n=19: 10 males and 9 females), mHp (n=12: 6 males and 6 females), rASFHp (n=22: 13 males and 9 females) and IFHp (n=24: 15 males and 9 females). Methods for rASF, IF and Hp colonisation of INS-GAS mice, husbandry, necropsy, histologic scoring, analysis of serum and gastric mRNA expression levels of cytokines and chemokines, quantitative PCR (qPCR) for ASF and Hp colonisation levels, ELISA for serum immunoglobulin responses to Hp, and statistical analysis, are provided in online supplementary materials and methods. Because gastritis and development of GIN in the INS-GAS model is male-predominant, the Results section focuses on data from male mice with relevant comparisons made to data from female mice which are more comprehensively presented in online supplementary results. All p values for significant results at p<0.05 are presented in the Figure and Table legends.
RESULTS
rASF Alone was sufficient to promote gastric pathology in gnotobiotic INS-GAS mice
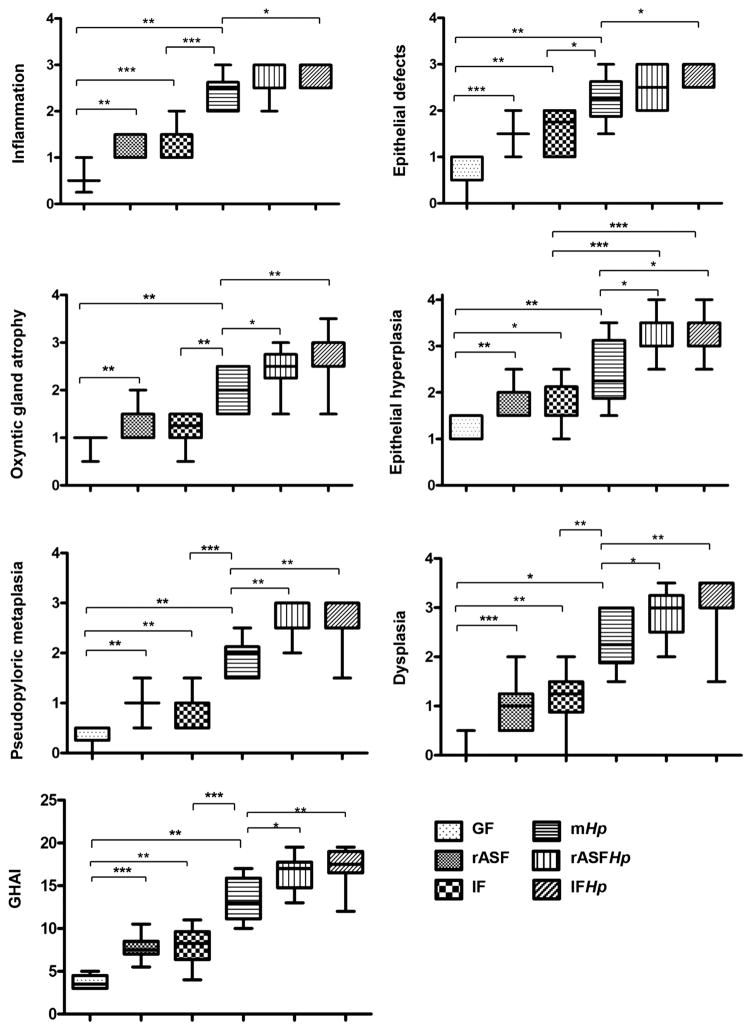
Compared with GF, male INS-GAS mice colonised with rASF or IF had equivalent, higher median Gastric Histologic Activity Index (GHAI) scores, supported by significant differences from GF mice in all GHAI subfeatures, including mild to moderate gastric corpus inflammation, epithelial defects, oxyntic gland atrophy, epithelial hyperplasia, pseudopyloric metaplasia and dysplasia (figures 1 and 2). Although mild to moderate gastric dysplasia developed in rASF and IF INS-GAS mice, none of them developed GIN (table 1) at the age equivalent to 7 months post-infection (mpi) with Hp. GHAI scores for female INS-GAS mice were lower compared with male mice, but differences observed between groups in male mice were also observed between groups of female mice having different colonisation status (see online supplementary figures S1 and S2). These results indicate that rASF alone, similar to IF, can promote gastric pathology in male and female INS-GAS mice independent of Hp infection, however, to a lesser extent in female INS-GAS mice.
Figure 1.
rASF and Hp cocolonisation-promoted gastric pathology in male INS-GAS mice to a similar extent as IF. Gastric Histologic Activity Index scores and subfeature scores for gastric lesions in male GF, rASF, IF, mHp, rASFHp and IFHp INS-GAS mice *p<0.05, **p<0.01, ***p<0.001.
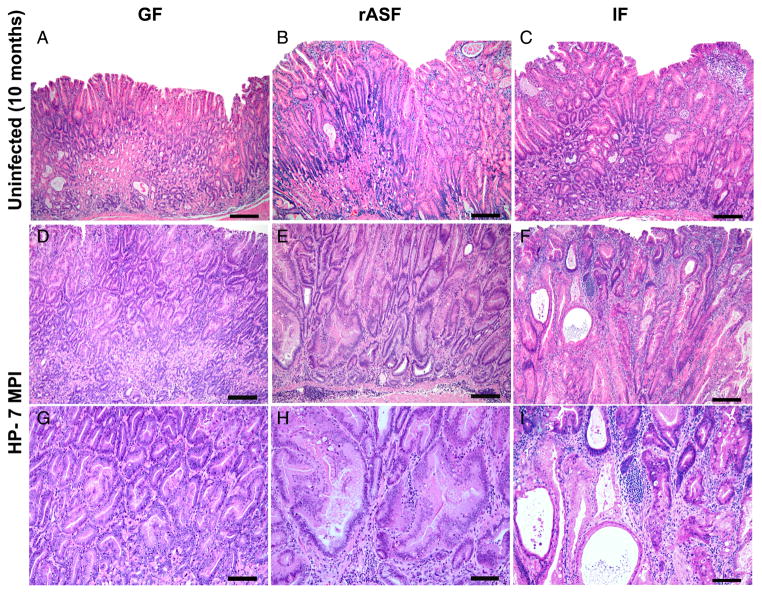
Figure 2.
Representative haematoxylin and eosin stained gastric sections from 10-month old male INS-GAS mice. (A) germfree (GF) mice developed sparse mucosal inflammation, mild oxyntic atrophy and mild foveolar hyperplasia, glandular metaplasia and ectasia with minimal dysplasia. (B) restricted ASF (rASF) mice and (C) mice colonised with normal intestinal flora (IF) developed mildly increased severity of all pathomorphological features included in the Gastric Histologic Activity Index scoring system (see Methods and figure 1) as compared with GF mice. INS-GAS mice monoassociated with Helicobacter pylori (mHp) at 7 months postinfection (mpi) (D) and (G) at low or high magnification, respectively, developed moderate inflammation, diffuse glandular hyperplasia, severe oxyntic atrophy, pseudopyloric metaplasia and moderate to severe dysplasia including cytological atypia and architectural abnormalities. Mice cocolonised as rASFHp (E) and (H) (at low or high magnification) or IFHp (F) and (I) (at low or high magnification) had more severe pathology including high-grade glandular architectural and cytological abnormalities classified as gastrointestinal intraepithelial neoplasia48 with invasion into the lamina propria, that is, intramucosal carcinoma (I). Bar values=a to f: 150 μ; G–I: 75 μ.
Table 1.
Gastrointestinal intraepithelial neoplasia (GIN) incidence in male and female INS-GAS mice at timepoints equivalent to 7 mpi with Helicobacter pylori
| Microbial status | % Of mice with no dysplasia (score=0)
|
% Of mice with non-GIN dysplasia* (score 0.5–2.5)
|
% Of mice with GIN (high-grade dysplasia) (score=3)
|
% Of mice with invasive neoplasms † (score >3)
|
||||
|---|---|---|---|---|---|---|---|---|
| M (%) | F (%) | M (%) | F (%) | M (%) | F (%) | M (%) | F (%) | |
| GF (M=6, F=6) | 17 | 17 | 83 | 83 | 0 | 0 | 0 | 0 |
| rASF (M=10, F=7) | 0 | 14 | 100 | 86 | 0 | 0 | 0 | 0 |
| IF (M=10, F=5) | 10 | 20 | 90 | 80 | 0 | 0 | 0 | 0 |
| mHp (M=6, F=6) | 0 | 66 | 100 | 34 | 0 | 0 | 0 | 0 |
| rASFHp (M=13, F=9) | 0 | 0 | 31 | 100 | 46‡ | 0 | 23‡ | 0 |
| IFHp (M=15, F=9) | 0 | 0 | 7 | 100 | 53‡ | 0 | 40‡ | 0 |
Non-GIN dysplasia defined as low to moderate grade dysplasia (score 0.5–2.5).
Invasive neoplasms defined as either intramucosal carcinomas (extending into the lamina propria or muscularis mucosae) or carcinomas extending into or beyond the submucosal margins.
similar incidence of high-grade dysplasia in 46% and 53%, and similar incidence of invasive neoplasms in 23% and 40% of rASFHp and IFHp mice, respectively, were significantly higher compared with GF, rASF, IF and mHp-colonised male mice (all p<0.05).
F, female INS-GAS mice; GF, germfree; IF, intestinal flora (undefined SPF); M, male; mHp, monoassociated with H pylori; rASF, restricted ASF.
Hp accelerated the onset and progression of GIN in rASF and IF colonised INS-GAS mice
Because our previous studies demonstrated that IF and Hp synergistically promoted invasive GIN in 80% of male SPF INS-GAS mice by 7 mpi,24 we next determined whether rASF could also synergistically promote GIN when combined with Hp infection. Compared with GF, rASF, IF and mHp INS-GAS mice, rASFHp and IFHp-cocolonised male INS-GAS mice developed the most severe gastric pathology, as reflected by higher GHAI scores (figure 1). As evidence that progression of gastric lesions towards GIN was accelerated in Hp-infected INS-GAS mice by cocolonisation with rASF or IF, 69% of male rASFHp and 93% of male IFHp INS-GAS mice developed sufficient dysplasia to be categorised as GIN, whereas GIN was not detected in other groups of mice at 7 mpi (table 1). GIN lesions were characterised by progressive dysplasia including loss of glandular organisation, crowding and atypia with variable globoid dysplasia, a feature characterised by disorderly stratification of proliferating gastric glandular epithelial cells that were expanded by large cytoplasmic mucus vacuoles with nuclear margination (figure 2). Interestingly, both rASFHp and IFHp male INS-GAS mice developed invasive GIN categorised as either intramucosal carcinoma (invasion into the lamina propria or muscularis mucosa) or carcinoma extending into or beyond the submucosal margins. The incidence of invasive GIN was only slightly lower in rASFHp (23%) compared with IFHp (40%)-colonised male INS-GAS mice (p=0.17). Together, these results suggest that colonisation with rASFHp alone was sufficient to promote all GHAI subfeatures comparable with IFHp-colonised male mice. GIN did not develop in any group of female INS-GAS mice (table 1), consistent with prior studies.10,24,29
rASFHp and IFHp-colonised male INS-GAS mice developed robust local gastric tissue and systemic inflammatory responses in serum
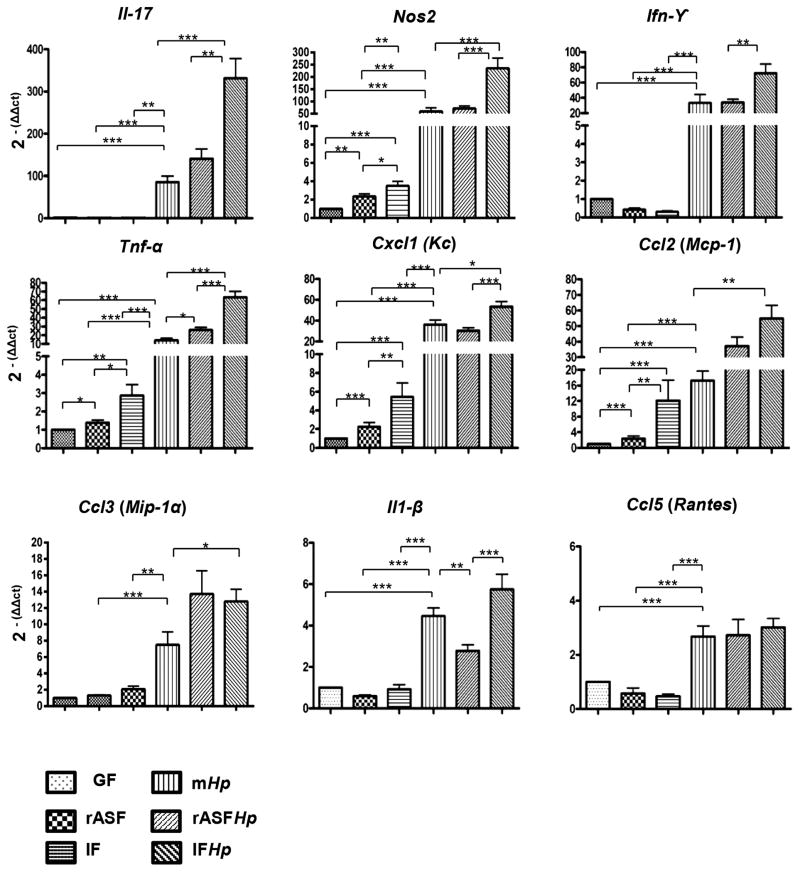
Colonisation of male INS-GAS mice with rASF or IF without Hp infection caused moderate elevation of mRNA for proinflammatory genes in gastric samples, including Nos2, Tnf-α, Cxcl1(Kc) and Ccl2(Mcp-1) (figure 3). Compared with rASF and IF, Hp infection alone induced much higher expression levels for all proinflammatory genes evaluated, suggesting that Hp is a potent stimulator of gastric inflammatory response. Consistent with the synergistic effect of Hp and intestinal micro-biota, expression levels for proinflammatory genes were highest in IFHp-colonised mice, including IL-17, Nos2, Tnf-α and Cxcl1(Kc). rASF cocolonisation with Hp was very similar to IFHp in increasing expression of Tnf-α, IL-17, Ccl2(Mcp-1) and Ccl3(Mip1-α). Similar patterns of mRNA levels were observed in female INS-GAS mice but at lower magnitudes (see online supplementary figure S3).
Figure 3.
mRNA expression levels of proinflammatory genes in the gastric tissue of male GF, rASF, IF, mHp, rASFHp and IFHp INS-GAS mice at 7 mpi. IFHp INS-GAS mice had the highest inflammatory responses. *p<0.05, **p<0.01, ***p<0.001.
Consistent with gastric tissue mRNA expression, serum levels of IL-17, Cxcl1 (Kc), Tnf-α, IL-12 p70 and p40, Ccl2 (Mcp-1), G-CSF, IL-5, CM-CSF and Ccl5 (Rantes) were significantly elevated in response to Hp infection in male INS-GAS mice (see online supplementary figure S4). Except for serum levels of IL-17 which were highest in IFHp-colonised mice, elevated serum levels of all inflammatory mediators evaluated were similar between mHp, rASFHp and IFHp-colonised mice, indicating Hp infection was the main cause of elevated serum inflammatory markers. Similar patterns of increased levels of inflammatory mediators in serum were observed in groups of female INS-GAS (see online supplementary figure S4).
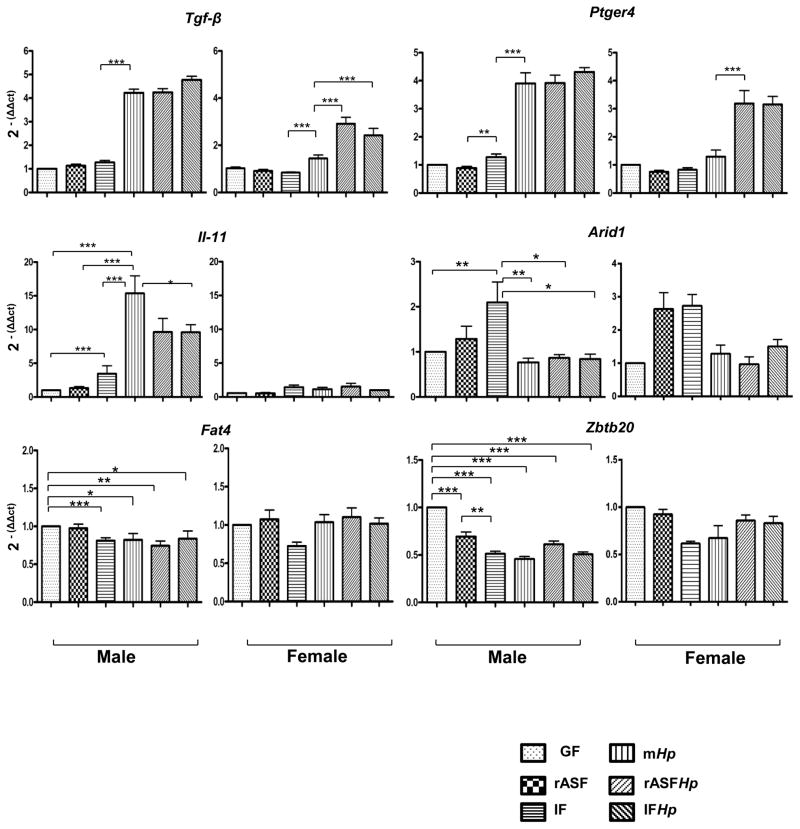
Il-11 expression was positively correlated with Hp infection and gastric atrophy in male INS-GAS mice
Elevated IL-11 expression in parietal cells has been associated with Hp infection and the severity of gastric atrophy in mice.30 IF colonisation in male INS-GAS mice increased gastric IL-11 expression by almost fivefold, and mHp male mice had increased mRNA levels for IL-11 of approximately 15-fold over GF mice (figure 4). IL-11 expression was similarly elevated 10-fold in mice cocolonised with rASFHp and IFHp. By contrast, female INS-GAS mice had no comparable changes in IL-11 expression (figure 4), consistent with greater oxyntic gland atrophy in male INS-GAS mice (see online supplementary figure S1).
Figure 4.
mRNA levels of gastric cancer associated genes in gastric tissue from INS-GAS mice at 7 mpi. Male (left panels) INS-GAS mice had higher expression levels of Il-11, Ptger4 and Tgf-β than female (right panels) INS-GAS mice, particularly when colonised by Hp. Hp colonisation as well as rASFHp and IFHp cocolonisation did not significantly alter the expression of Arid1, Fat4 or Zbtb20 in male or female INS-GAS mice. *p<0.05, **p<0.01, ***p<0.001.
Hp infection caused major shifts in gastric ASF colonisation
Because gastric colonisation with rASF or IF promoted progression of Hp-associated GIN, gastric colonisation with Hp and all eight ASF species was quantified using qPCR as previously reported.31 All three members of rASF (ASF356, ASF361, ASF519) were detected in the stomach of all male and female rASF and rASFHp INS-GAS mice (see online supplementary figure S5) and it was therefore possible to determine total gastric bacterial colonisation levels in the gnotobiotic mice (rASF, mHp and rASFHp) and the potential shift in colonisation as a result of Hp infection between the 3 ASF species. The majority of the 8 ASF species were detected in the IF and IFHp mice (see online supplementary figure S6), but total gastric bacteria were not determined in these mice because the microbiota was undefined.
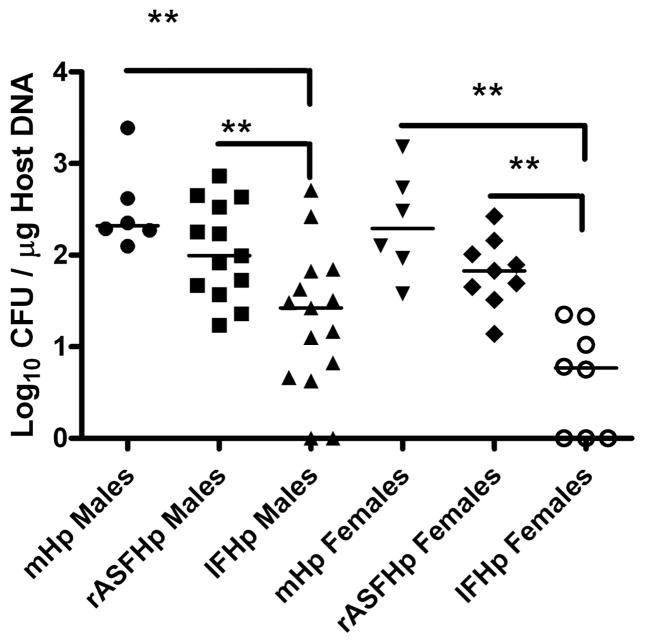
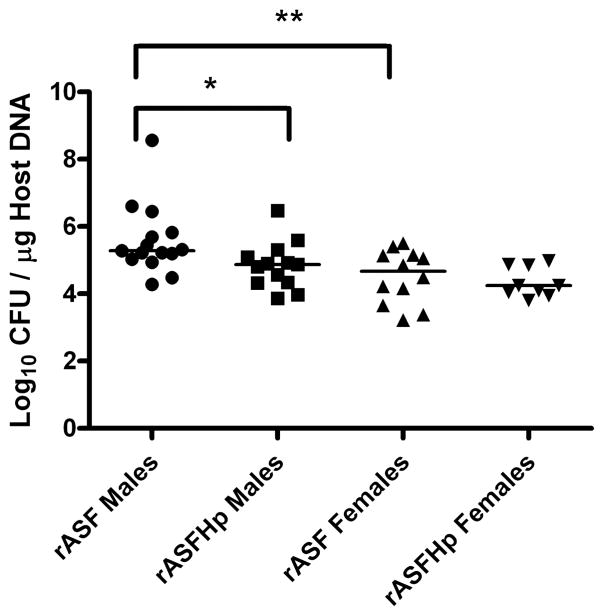
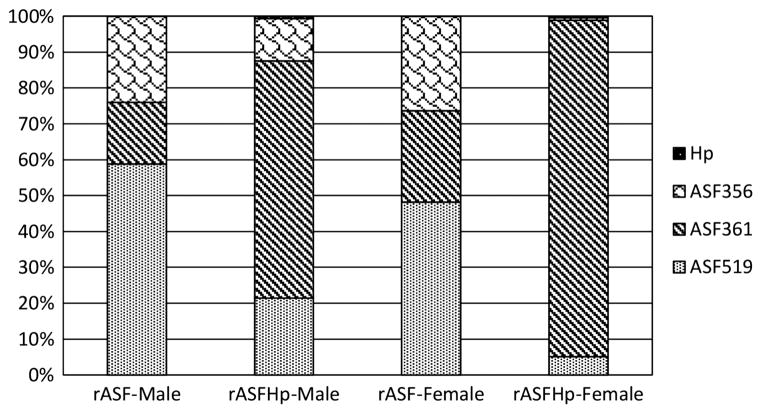
Hp colonisation was similar in male and female mHp and rASFHp mice but was lower in IFHp mice of both genders, particularly in female IFHp mice (figure 5). Two of 15 IFHp males and three of eight IFHp females were PCR negative for Hp by 7 mpi, but remained seropositive to Hp (figure 6) and had gastritis consistent with their experimental cohorts (figure 1; see online supplementary figure S1), indicating Hp had colonised but was cleared late in the infection. Remarkably, total gastric bacteria were significantly higher in male compared with female rASF mice and when cocolonised with Hp, total bacteria significantly decreased only in rASFHp-colonised male mice (figure 7). Hp colonisation was several logs lower than ASF in rASFHp mice, contributing just 0.7% and 0.4% of the total bacterial colonisation levels in male and female rASFHp mice, respectively (figure 8). In male rASF mice, Bacteroides species ASF519 predominated in the stomach followed by smaller, similar percentages of Clostridium species ASF356 and L murinus ASF361. Cocolonisation with Hp significantly increased colonisation of ASF361 with corresponding reductions in ASF356 and ASF519. As a percentage of total gastric bacteria (figure 8), emergence of ASF361 to predominate in the gastric niche was even more dramatic in female rASFHp mice, although females had lower absolute numbers of total gastric bacteria compared with male mice (figure 7).
Figure 5.
Gastric IF colonisation was associated with decreased Hp colonisation in both male and female INS-GAS mice; however, to a greater degree in female INS-GAS mice at 7 mpi. **p<0.01.
Figure 6.
IgG response to Hp in male and female mHp, rASFHp and IFHp INS-GAS mice at 7 mpi. The highest IgG responses were associated with the more diverse microbiota of IFHp mice. *p<0.05, **p<0.01.
Figure 7.
Total gastric bacteria (rASF and Hp) in male and female INS-GAS mice colonised with rASF or rASFHp. Male rASF mice had higher bacterial colonisation in the stomach compared with female mice and Hp infection reduced these levels in male but not female mice. Total gastric bacteria in male rASFHp mice were higher than in female rASFHp mice at p=0.05. *p<0.05, **p<0.01.
Figure 8.
Percentage of total gastric bacteria by bacterial species in rASF and rASFHp male and female INS-GAS mice. Hp levels were less than 1% of the total bacteria. In male rASFHp INS-GAS mice, ASF356 and ASF519 were reduced (p=0.07, p<0.001, respectively). ASF361 colonisation increased significantly as a percentage of total bacteria (p<0.0001). Similar shifts in ASF occurred in female mice with Hp infection.
As the gastric and intestinal microbiota of the IF and IFHp mice were SPF but otherwise undefined, it was not possible to determine the total gastric bacterial colonisation levels, but shifts in the absolute colonisation levels between the eight species of ASF had parallels to observations in rASF mice when cocolonised with Hp (see online supplementary figure S6). Notably, colonisation of Clostridium species ASF356 and Bacteroides species ASF519 were decreased in male IFHp compared with IF mice and Lactobacillus species. ASF360 and L murinus ASF361 increased in response to Hp infection. Additionally, Eubacterium plexicaudatum ASF492 increased when mice were challenged with Hp. Most of these shifts in the balance between ASF observed in male mice were also observed in females (see online supplementary figure S6).
All rASF (see online supplementary figure S7) and eight species of ASF (data not shown) were detected in the caecum of both male and female rASF, rASFHp, IF and IFHp INS-GAS mice, respectively. However, ASF colonisation levels in the cecum were similar between comparable groups of mice with or without Hp infection.
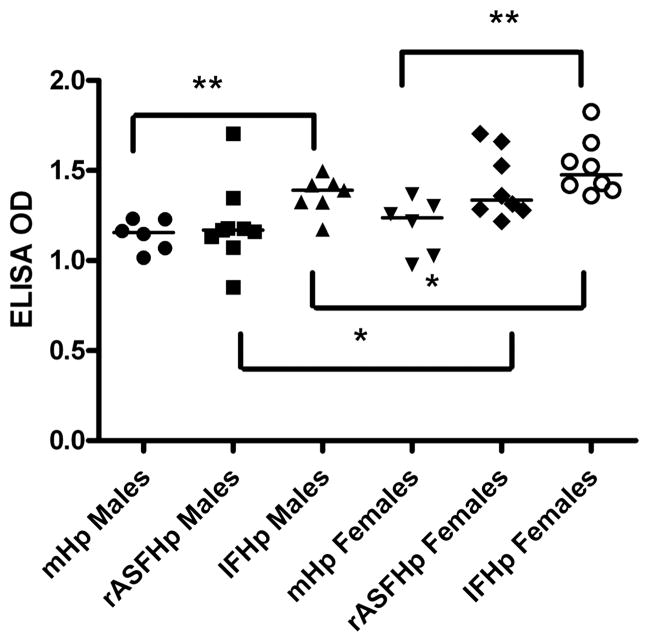
Serologic response to Hp correlated with the diversity of IF colonising the stomach
INS-GAS mice experimentally dosed with Hp developed robust IgG responses to Hp outer membrane antigens (figure 6). IgG responses were highest in IFHp INS-GAS mice, although rASFHp mice had similar promotion of the IgG response compared with mHp mice. Responses were higher in females compared with male IFHp and rASFHp mice, but gender impact was not observed for mHp mice. Th1-associated IgG2a and Th2-associated IgG1 responses followed the same trend as the total IgG response with IgG2a/IgG1 ratios ranging from 1 to 1.7 without differences between groups of mice (data not shown).
Hp infection elevated expression of the prostaglandin Ptger4 and Tgf-β cancer-related genes
Meta-analyses of genome-wide association studies (GWAS) in humans with GAC revealed Prostaglandin E receptor 4 (Ptger4) and Zinc finger and BTB domain containing 20 (Zbtb20) to be highly associated with GAC.12,13 Additionally, FAT tumour suppressor homologue 4 (Fat4) and AT-rich interactive domain-containing protein 1A (Arid1a) were found to be mutated in somatic germ cells of gastric tumours.13,32 Tgf-β has anti-inflammatory and antioncogenic and pro-oncogenic properties33 and altered gene expression may thus be associated with the incidence of GAC in INS-GAS mice. Therefore, mRNA expression levels of these genes were analysed to determine whether Hp, rASF and/or IF are associated with altered gene expression in INS-GAS mice.
Interestingly, male INS-GAS mice had a fourfold higher expression level of Ptger4 when challenged with Hp irrespective of GF, rASF or IF colonisation status, whereas female mice had a threefold higher expression level when cocolonised with rASFHp and IFHp (figure 4). Significant changes (>twofold) in expression of mRNA for zbtb20, Fat4 and Arid1a were not detected between GF, rASF and IF mice (figure 4). Similar to expression levels of Ptger4, male INS-GAS mice that were colonised with mHp, rASFHp or IFHp had similar levels of Tgf-β expression that were fourfold higher than those of male GF, rASF and IF mice. Thus, Tgf-β expression was Hp-dependent in male INS-GAS mice.
DISCUSSION
This study focused on a mechanism of IF promotion of GAC secondary to Hp-mediated gastric atrophy (achlorhydria) as proposed in recent literature in both humans and animal models.22,23 We demonstrated that gastric colonisation with three species of commensal bacteria was comparable with colonisation with diverse intestinal microbiota in promoting gastritis and dysplasia.27 Commensal and Hp cocolonisation were required for peak inflammatory responses and GIN development. rASFHp colonisation was sufficient for GIN development in males. Lower GIN incidence in females was associated with lower inflammatory responses and lower gastric commensal and Hp colonisation. Colonisation efficiency of commensals appears more important than IF diversity and lessens the probability that specific gastrointestinal pathogens are contributing to gastric cancer risk. Consistent with epidemiology studies reporting that the incidence of stomach cancer is approximately twice as high in men compared with women,1 and that oestrogen appears protective in humans,34 the male INS-GAS gnotobiotic model reproduced key features of GAC observed in humans with advanced gastric atrophy secondary to Hp gastritis.
Even without Hp infection, male rASF and IF INS-GAS mice developed gastric pathology similar to the Correa model of progression of chronic gastritis to atrophy and dysplasia in Hp-infected humans.2 Gastric pathology was promoted to a similar extent by rASF and IF colonisation. Although GIN develops spontaneously in older SPF male INS-GAS mice (~20 months),35 in this study, Hp infection in addition to rASF or IF, were required for gastric lesions to progress to GIN within 7 mpi, as has been demonstrated using Hp and Helicobacter felis in SPF INS-GAS mice.10,35 Notably, colonisation with rASFHp was not only sufficient to promote all GHAI subfeatures comparable with IFHp-colonised male mice, the incidence of invasive GIN was only slightly lower in rASFHp compared with IFHp mice. These results indicate that rASF alone, similar to IF, can promote gastric pathology in INS-GAS mice independent of Hp infection; however, when challenged with Hp, time required for progression to GIN is accelerated, and GIN becomes invasive.
By 7 mpi, Hp colonisation was lost in a few IFHp male and female INS-GAS mice, consistent with spontaneous eradication of Hp in humans and with advanced gastric atrophy.36 Interestingly, female IFHp mice had less severe gastritis and atrophy, lower incidence of GIN, and decreased Hp colonisation compared with male IFHp INS-GAS mice. Consistent with less gastric atrophy, female rASF and rASFHp mice had fewer gastric bacteria compared with males. Particularly in mice due to coprophagy, the gastric mucosa is chronically exposed to colonic bacteria, but acidic gastric pH and the relative aerobic environment prevents significant colonisation. As parietal cells are lost due to chronic gastritis, gastric acidity neutralises allowing some aerotolerant colonic bacteria to colonise the stomach. The same phenomenon has been shown in humans with gastric atrophy, presumably from environmental exposure, reflux of intestinal contents into the stomach, and fecal-oral contamination.37 Recent studies highlight the possibility that gender influences the microbiome of mouse models38; it is possible the male predominance of GIN in INS-GAS mice is, in part, related to gender differences in IF and the established protective effect of oestrogen.39,40 Further investigations are needed to decipher the role of sex hormones in shaping the gastrointestinal microbiota and how this may promote male-predominant GIN in INS-GAS mice.
Although GHAI scores for rASFHp and IFHp male INS-GAS mice were similar, IF promoted a greater inflammatory response reflected by higher proinflammatory gene expression levels induced by IFHp compared with mHp or rASFHp microbiota. The inflammatory response also was associated with elevated adaptive immune responses as IgG responses to Hp were highest in IFHp INS-GAS mice. Although the extent of seroconversion to Hp does not correlate with severity of gastritis in humans and only serves as evidence of Hp infection, it is probable that IgG responses to Hp were highest in IFHp INS-GAS mice because recruitment of inflammatory cells to the stomach likely promoted B cell responses to Hp.
Consistent with an inverse relationship between the severity of gastritis and Hp colonisation, there was an inverse association between microbial diversity and Hp colonisation levels. Interestingly, greater microbial diversity of IF was associated with lower Hp colonisation compared with rASF colonisation or Hp monoassociation, a result consistent with studies using H felis-infected gnotobiotic C57BL/6 mice.41 In addition to the inhibitory effects of inflammation on Hp, the microbiota of the IF-colonised mice was SPF, but otherwise undefined, and thus unidentified bacteria may have out-competed and lowered Hp colonisation. L murinus ASF361 increased in rASFHp-colonised mice; additionally in IFHp INS-GAS mice, there was increased gastric colonisation with Lactobacillus species ASF360, L murinus ASF361 and Eubacterium ASF492 in response to Hp infection. These shifts in gastric microbiota were associated with decreased Hp colonisation. It has been shown that Lactobacillus casei inhibits Hp colonisation.41 Recently, investigators using H pylori-infected C57BL mice from two commercial sources noted that mice from one commercial source had significantly higher gastric inflammation scores than the mice from the second source despite similar H pylori colonisation in mice from both sources.42 They attributed these findings, in part, due to differences in gastric colonisation of Lactobacillus species ASF360 and ASF361. The mice with more pronounced H pylori-induced gastritis had significantly more ASF361, whereas those with less pronounced H pylori gastritis had significantly higher numbers of ASF360.42 They also noted that fewer CDT4 T cells infiltrated in the stomach and IFNγ transcript were reduced in H pylori-infected mice pretreated with antibiotics which had altered their gastric colonisation with enteric flora.42 Lactobacillus species present in rASF and IF may have limited Hp colonisation in INS-GAS mice. Furthermore, the negative effect of IF on Hp colonisation may explain why gastric Hp often declines or disappears during progression to atrophic gastritis.36,43,44 These results align with the view that once Hp initiates inflammation and sufficient gastric atrophy develops, eradication of Hp spontaneously or by antimicrobial therapy may not inhibit cancer progression, in part, because IF persistently colonise the stomach and promote inflammation.
Although most of these shifts in the balance between ASF observed in male mice were also observed in females, little is known of the significance of changes in specific ASF species, other than to serve as an indicator that IF commensals may find a preferred niche in the stomach damaged by persistent Hp gastritis. Although ASF appear to be beneficial to mice,31 it is possible they play a role in the formation of nitrosamine, oxygen radicals, other oxidising agents, mutagens or genotoxic compounds, as their colonisation along with Hp induces a greater inflammatory and mutagenic response than Hp alone.11,20,45,46 Importantly, the host is responding to rASF as male rASF INS-GAS mice had more severe gastritis and elevated expression levels for proinflammatory genes compared with GF mice. Alternatively, ASF, which do not normally cause intestinal inflammation, may have novel virulence genes induced in the achlorhydric stomach that promote gastric colonisation and overt inflammation. It is likely that the transition from the anaerobic lower bowel to the relatively aerobic environment of the stomach requires significant biochemical adaptations in bacteria, such as ASF, that vary in aerotolerance by species. Nonetheless, the effects of Hp on ASF colonisation were specific to the gastric environment as cecal ASF colonisation levels were similar between comparable groups of mice with or without Hp infection.
Colonisation of male INS-GAS mice with rASF, IF or Hp alone caused moderate elevation of diverse proinflammatory genes implicated in human gastritis and gastric cancer,3 and the addition of Hp to rASF or IF colonisation caused dramatic increases in select proinflammatory gene expression in gastric tissues. Consistent with gene expression, serum levels of proinflammatory mediators were significantly elevated in response to Hp infection in male INS-GAS mice. Hp was a major driver of mRNA expression and serum levels of IL-17, and IFHp male mice had the highest levels, suggesting that select members of IF, such as segmented filamentous bacteria,47 may have primed TH17 cells to respond to Hp infection. Hp and IF colonisation also had an effect on gastric tissue expression of IL-11, which is expressed by parietal cells and inhibits gastric acid secretion in response to Hp infection in mice.30 IL-11 expression was highest in mHp male mice which had retained more parietal cell mass compared with rASFHp and IFHp INS-GAS male mice which had the highest scores for gastric atrophy. Monitoring IL-11 expression over time may, therefore, represent a potential biomarker for determining the extent of gastric atrophy in Hp-infected INS-GAS mice and humans. Tgf-β expression was Hp-dependent as mHp, rASFHp and IFHp INS-GAS mice had similar expression levels. As Tgf-β signalling has important pleiotropic roles in inflammation and cancer progression,33 the increase in Tgf-β expression levels detected in gastric tissues from mHp, rASFHp and IFHp INS-GAS male mice supports that Tgf-β expression is elevated in response to Hp infection and likely has a role in gastric cancer progression.
Little is known about the role of gastric cancer-associated genes identified in GWAS13 in the INS-GAS mouse model. Interestingly, male and female INS-GAS mice had higher Ptger4 expression level when challenged with Hp irrespective of GF, rASF or IF colonisation. Ptger4 is involved in the synthesis of prostaglandins, which play important roles in immune modulation.26 Thus, Hp-mediated GAC likely involves prostaglandin synthesis induction via Ptger4, consistent with previous observations.26 By contrast, Fat4, involved in cell adhesion, Zbtb20, involved in oncogenesis, and Arid1a, a regulator of cell development, were not significantly changed (>twofold) in expression, indicating these genes do not have an apparent impact in Hp-mediated GAC in INS-GAS mice.
In conclusion, this study highlights the contribution of IF to gastric cancer progression during Hp infection. A key finding was that rASF was comparable with IF in promoting the progression of Hp gastritis to GIN in male INS-GAS mice, indicating colonisation efficiency is more important than IF diversity. Further understanding of the complex interaction between host gender, IF and Hp could lead to more effective treatment and prevention strategies for GAC.
Significance of this study.
What is already known about this subject?
Long-term Helicobacter pylori infection is associated with gastric atrophy, increased gastric cancer risk and overgrowth of gastric bacteria.
Overgrowth of gastric bacteria has also been observed during acid-suppressive therapy in H pylori-infected patients, and is associated with greater gastritis and more severe gastric atrophy.
Human gastric microbiota consists of diverse species of bacteria, some of which are considered commensal, and others have been implicated as pathogens.
What are the new findings?
Colonisation with H pylori and a restricted microbiota consisting of only three species of commensal bacteria promoted gastric cancer in gnotobiotic male INS-GAS mice to a similar extent as mice colonised with complex microbiota.
Gastric pathology induced by the restricted microbiota was associated with robust expression of gastric inflammatory and cancer-associated genes, including TNF-α, Ptger4 and Tgf-β.
Gastric colonisation with complex microbiota lowered gastric H pylori colonisation in both male and female INS-GAS mice. However, males with a restricted microbiota were colonised with higher total numbers of gastric bacteria than female INS-GAS mice, indicating more severe gastric atrophy facilitates gastric colonisation with microbiota normally found in the lower bowel.
How might it impact on clinical practice in the foreseeable future?
Human patients with advanced gastric atrophy should be monitored for histologic progression and opportunistic colonisation of the stomach with bacteria that may promote gastric adenocarcinoma.
Acknowledgments
Funding R01 AI37750 (Fox), R01 CA093405 (Wang, Fox), P30 ES02109 (Fox), P01 CA028842 (Fox), T32 RR07036 (Fox).
Footnotes
Contributors Conception and design, or analysis and interpretation of data: JGF, MTW, KL, TCW, SM, JLL, ERG, YF, ZG. Drafting the article or revising it critically for important intellectual content: JGF, MTW, KL, TCW, SM, JLL, ERG, YF, ZG. Final approval of the version to be published: JGF, MTW, KL, TCW.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 3.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–9. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 5.Krejs GJ. Gastric cancer: epidemiology and risk factors. Digest Dis. 2010;28:600–3. doi: 10.1159/000320277. [DOI] [PubMed] [Google Scholar]
- 6.Nagini S. Carcinoma of the stomach: a review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156–69. doi: 10.4251/wjgo.v4.i7.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–39. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MG, Hold GL, Tahara E, et al. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979–90. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu MS, Chen CJ, Lin JT. Host-environment interactions: their impact on progression from gastric inflammation to carcinogenesis and on development of new approaches to prevent and treat gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1878–82. doi: 10.1158/1055-9965.EPI-04-0792. [DOI] [PubMed] [Google Scholar]
- 10.Fox JG, Wang TC, Rogers AB, et al. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–90. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 11.Toller IM, Neelsen KJ, Steger M, et al. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci USA. 2011;108:14944–9. doi: 10.1073/pnas.1100959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Zhang R, He J, et al. Potentially Functional Variants of PLCE1 Identified by GWASs Contribute to Gastric Adenocarcinoma Susceptibility in an Eastern Chinese Population. PLoS ONE. 2012;7:e31932. doi: 10.1371/journal.pone.0031932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y, Hu Z, Wu C, et al. A genome-wide association study identifies new susceptibility loci for non-cardia gastric cancer at 3q13.31 and 5p13. 1. Nat Genet. 2011;43:1215–8. doi: 10.1038/ng.978. [DOI] [PubMed] [Google Scholar]
- 14.Whary MT, Sundina N, Bravo LE, et al. Intestinal helminthiasis in Colombian children promotes a Th2 response to Helicobacter pylori: possible implications for gastric carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2005;14:1464–9. doi: 10.1158/1055-9965.EPI-05-0095. [DOI] [PubMed] [Google Scholar]
- 15.Stearns JC, Lynch MD, Senadheera DB, et al. Bacterial biogeography of the human digestive tract. Sci Rep. 2011;1:170. doi: 10.1038/srep00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maldonado-Contreras A, Goldfarb KC, Godoy-Vitorino F, et al. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 2011;5:574–9. doi: 10.1038/ismej.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roos S, Engstrand L, Jonsson H. Lactobacillus gastricus sp. nov., Lactobacillus antri sp. nov., Lactobacillus kalixensis sp. nov. and Lactobacillus ultunensis sp. nov. isolated from human stomach mucosa. Int J Syst Evol Microbiol. 2005;55:77–82. doi: 10.1099/ijs.0.63083-0. [DOI] [PubMed] [Google Scholar]
- 18.Ryan KA, Jayaraman T, Daly P, et al. Isolation of lactobacilli with probiotic properties from the human stomach. Lett Appl Microbiol. 2008;47:269–74. doi: 10.1111/j.1472-765x.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 19.Sanduleanu S, Jonkers D, De Bruine A, et al. Non-Helicobacter pylori bacterial flora during acid-suppressive therapy: differential findings in gastric juice and gastric mucosa. Aliment Pharmacol Ther. 2001;15:379–88. doi: 10.1046/j.1365-2036.2001.00888.x. [DOI] [PubMed] [Google Scholar]
- 20.Mowat C, Williams C, Gillen D, et al. Omeprazole, Helicobacter pylori status, and alterations in the intragastric milieu facilitating bacterial N-nitrosation. Gastroenterology. 2000;119:339–47. doi: 10.1053/gast.2000.9367. [DOI] [PubMed] [Google Scholar]
- 21.Fox JG, Kuipers EJ. Long-term proton pump inhibitor administration, H. pylori and gastric cancer: lessons from the gerbil. Gut. 2011;60:567–8. doi: 10.1136/gut.2010.229286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dicksved J, Lindberg M, Rosenquist M, et al. Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. J Med Microbiol. 2009;58:509–16. doi: 10.1099/jmm.0.007302-0. [DOI] [PubMed] [Google Scholar]
- 23.Zavros Y, Rieder G, Ferguson A, et al. Gastritis and hypergastrinemia due to Acinetobacter lwoffii in mice. Infect Immun. 2002;70:2630–9. doi: 10.1128/IAI.70.5.2630-2639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lofgren JL, Whary MT, Ge Z, et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210–20. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CW, Rickman B, Rogers AB, et al. Combination of sulindac and antimicrobial eradication of Helicobacter pylori prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Res. 2009;69:8166–74. doi: 10.1158/0008-5472.CAN-08-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oshima H, Hioki K, Popivanova BK, et al. Prostaglandin E signaling and bacterial infection recruit tumor-promoting macrophages to mouse gastric tumors. Gastroenterology. 2011;140:596–607. e7. doi: 10.1053/j.gastro.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Dewhirst FE, Chien CC, Paster BJ, et al. Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl Environ Microbiol. 1999;65:3287–92. doi: 10.1128/aem.65.8.3287-3292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarma-Rupavtarm RB, Ge Z, Schauer DB, et al. Spatial distribution and stability of the eight microbial species of the altered Schaedler flora in the mouse gastrointestinal tract. Appl Environ Microbiol. 2004;70:2791–800. doi: 10.1128/AEM.70.5.2791-2800.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox JG, Rogers AB, Ihrig M, et al. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 2003;63:942–50. [PubMed] [Google Scholar]
- 30.Howlett M, Chalinor HV, Buzzelli JN, et al. IL-11 is a parietal cell cytokine that induces atrophic gastritis. Gut. 2012;61:1398–409. doi: 10.1136/gutjnl-2011-300539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge Z, Feng Y, Taylor NS, et al. Colonization dynamics of altered Schaedler flora is influenced by gender, aging, and Helicobacter hepaticus infection in the intestines of Swiss Webster mice. Appl Environ Microbiol. 2006;72:5100–3. doi: 10.1128/AEM.01934-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zang ZJ, Cutcutache I, Poon SL, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012 doi: 10.1038/ng.2246. [DOI] [PubMed] [Google Scholar]
- 33.Achyut BR, Yang L. Transforming growth factor-beta in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology. 2011;141:1167–78. doi: 10.1053/j.gastro.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandanos E, Lagergren J. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer. 2008;44:2397–403. doi: 10.1016/j.ejca.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Wang TC, Dangler CA, Chen D, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 36.Kokkola A, Kosunen TU, Puolakkainen P, et al. Spontaneous disappearance of Helicobacter pylori antibodies in patients with advanced atrophic corpus gastritis. APMIS. 2003;111:619–24. doi: 10.1034/j.1600-0463.2003.1110604.x. [DOI] [PubMed] [Google Scholar]
- 37.Bik EM, Eckburg PB, Gill SR, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA. 2006;103:732–7. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–8. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 39.Sheh A, Ge Z, Parry NM, et al. 17 beta-estradiol and tamoxifen prevent gastric cancer by modulating leukocyte recruitment and oncogenic pathways in Helicobacter pylori-infected INS-GAS male mice. Cancer Prev Res (Phila) 2011;4:1426–35. doi: 10.1158/1940-6207.CAPR-11-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohtani M, Garcia A, Rogers AB, et al. Protective role of 17 beta-estradiol against the development of Helicobacter pylori-induced gastric cancer in INS-GAS mice. Carcinogenesis. 2007;28:2597–604. doi: 10.1093/carcin/bgm150. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz JM, Durham CG, Schoeb TR, et al. Helicobacter felis-Associated Gastric Disease in Microbiota-Restricted Mice. J Histochem Cytochem. 2011;59:826–41. doi: 10.1369/0022155411416242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolig AS, Cech C, Ahler E, et al. The degree of Helicobacter pylori-triggered inflammation is manipulated by preinfection host microbiota. Infect Immun. 2013;81:1382–9. doi: 10.1128/IAI.00044-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valle J, Kekki M, Sipponen P, et al. Long-term course and consequences of Helicobacter pylori gastritis. Results of a 32-year follow-up study. Scand J Gastroenterol. 1996;31:546–50. doi: 10.3109/00365529609009126. [DOI] [PubMed] [Google Scholar]
- 44.Niemela S, Karttunen T, Kerola T. Helicobacter pylori-associated gastritis. Evolution of histologic changes over 10 years. Scand J Gastroenterol. 1995;30:542–9. doi: 10.3109/00365529509089787. [DOI] [PubMed] [Google Scholar]
- 45.Ziebarth D, Spiegelhalder B, Bartsch H. N-nitrosation of medicinal drugs catalysed by bacteria from human saliva and gastro-intestinal tract, including Helicobacter pylori. Carcinogenesis. 1997;18:383–9. doi: 10.1093/carcin/18.2.383. [DOI] [PubMed] [Google Scholar]
- 46.Sharma BK, Santana IA, Wood EC, et al. Intragastric bacterial activity and nitrosation before, during, and after treatment with omeprazole. Br Med J (Clin Res Ed) 1984;289:717–9. doi: 10.1136/bmj.289.6447.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reading NC, Kasper DL. The starting lineup: key microbial players in intestinal immunity and homeostasis. Front Microbiol. 2011;2:148. doi: 10.3389/fmicb.2011.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boivin GP, Washington K, Yang K, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–77. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]