Abstract
Alcoholism frequently occurs in returning U.S. Veterans, and is often comorbid with Post Traumatic Stress Disorder (PTSD). The goal of this study was to investigate the relationship between white matter changes and neuropsychological alterations in Operation Enduring Freedom, and/or Operation Iraqi Freedom (OEF/OIF) alcoholic Veterans with two primary aims: (1) to examine the relationship of alcoholism to brain structure and function while controlling for the potential effects of comorbid PTSD, and (2) to examine whether the effects of alcoholism are moderated by the quantity of lifetime alcohol consumption. Our sample consisted of 71 deployed OEF/OIF Veterans stratified into four groups: alcoholics without PTSD, alcoholics with PTSD, participants with PTSD without comorbid alcoholism, and control participants without alcoholism or PTSD. Participants were given an extensive neuropsychological and psychiatric assessment battery, as well as Magnetic Resonance Diffusion Tensor Imaging (DT-MRI) scans. Results showed that disruption of executive functioning, and abnormal fractional anisotropy (FA; a measure of axonal integrity) within the frontal subcortical and dorsolateral frontal-parietal regions, occurred independently of the effects of PTSD. Furthermore, these cognitive and neuronal alterations were unique to the most severe subgroup of alcoholics who consumed the greatest amount of alcohol over the course of their lifetime, as compared to the rest of the sample. Axonal integrity within this subgroup, in regions underlying the frontal subcortical area, was shown to be decreased independently of cognitive changes. Integrity of axons underlying the dorsolateral frontal-parietal region, however, was increased. We hypothesized that this is a compensatory mechanism for executive dysfunction.
Keywords: Alcoholism, Brain white matter, Post-traumatic stress disorder, OEF, OIF, Veterans
Introduction
Alcoholism is a devastating disease that affects over eighteen million Americans, with a single episode lasting, on average, three to four years [1]. A recent study sponsored by the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism [1], estimated the annual cost of this disorder to be $148 billion, accounting for 60% of national yearly spending allocated to substance use disorders. These numbers do not incorporate the unquantifiable impact of alcoholism on the quality of life or the magnitude of opportunity costs that are nearly impossible to calculate. Additionally, within the general population, 30% of successful suicides have been carried out by individuals who have been under the influence of a substance [2].
Alcoholism has been particularly problematic for returning U.S. Veterans. It was reported that 27% of the U.S. Veteran population has been diagnosed with alcoholism [3], many of whom used alcohol to self-medicate symptoms of Post Traumatic Stress Disorder (PTSD). According to a survey collected in 2009, this issue has become more pressing, as the number of affected Veterans has increased by 56% since 2003, which marked the start of Operation Iraqi Freedom (OIF) [2]. Alcoholism also has impacted active military personnel.
Magnetic Resonance Diffusion Tensor Imaging (DTI) provides a means with which to investigate the impact of alcoholism on the integrity of the brain’s white matter. DTI is sensitive to the distribution of water diffusion that is impacted by local tissue boundaries. Fractional Anisotropy (FA) is a common DTI-derived measure of the directionality of water diffusion. The value range is 0 to 1; higher values indicate more restriction and values of 0 indicate perfect diffusivity in all directions. Higher FA values reflect anatomical integrity of white matter, including axon caliber, fiber density and myelination [4]. A number of recent findings indicate anatomical white matter changes associated with prolonged alcohol abuse. For instance, smaller white matter volumes have been reported [5-8], particularly within the corpus callosum [9-11]. Researchers also have found white matter abnormalities in the cingulum bundle, superior longitudinal fasciculi, as well as internal and external capsules [12-15].
White matter abnormalities have been associated with cognitive decline in alcoholic participants. A recent study [15] demonstrated that decreased performance on a test sensitive to response inhibition (a modified Stroop test) was correlated with lower white matter integrity in the cingulate and the corpus collosum. Another study [13] reported diminished psychomotor abilities (using balance and response speed measures) that were associated with white matter degradation within the internal and external capsules, inferior cingulate bundle, fornix, as well as frontal and occipital forceps. Additionally, findings have demonstrated that increased radial diffusivity within the occipital forceps and external capsule were associated with decreased visuoconstruction accuracy (measured by the Rey-Osterrieth Complex Figure Test) in alcoholics [14].
PTSD has been found to be associated with many overlapping anatomical and cognitive changes that have been shown to be present in alcoholics. For example, the integrity (measured by FA values), volume and area of the corpus collosum were shown to be decreased in individuals suffering from PTSD [16-18]. PTSD participants also were found to have FA values that were asymmetrically decreased when compared to control participants [19]. Further studies have shown reduced microarchitectural integrity of the posterior cingulum [20]. Superior longitudinal fasciculi also were shown to have decreased FA values in those affected by PTSD [21]. Within cognitive functional domains, altered performance on a modified Stroop task [22], decrease psychomotor abilities [23] and visuocontruction changes [24] were found to be present in individuals who were diagnosed with PTSD.
Given the overlapping effects of PTSD and alcoholism, the interpretation of published findings pertaining to these disorders has been complicated by their frequent co-occurrence in Veterans. Recent studies indicate that OEF/OIF Veterans who were exposed to combat are at higher risk for alcohol abuse than unexposed Veterans [25,26]. Longitudinal studies suggest that these rates increase with traumatic experience, and are most likely a result of “self-medication” for PTSD symptoms [25,27]. Veterans tend to engage in heavy alcohol consumption, in order to numb and alleviate the debilitating symptoms of PTSD. The comorbidity rates of these disorders within the OEF/OIF population are alarmingly high. A recent study showed that 14.3% of U.S. Veterans who engaged in heavy weekly drinking were also diagnosed with PTSD, 59.3% of Veterans who engaged in binge drinking had PTSD, and 22.2% of Veterans who were identified to have more than one drinking-related problem were diagnosed with PTSD [25]. These striking co morbidity rates are reasons to question a large percentage of the published neurological and cognitive findings on the effects of these disorders in isolation. They further complicate the interpretation of the results by introducing the possibility that the effects can be attributed to the synergistic impact of the disorders’ co-occurrence.
In the present study, we examined the association of alcoholism with abnormalities in brain structure (white matter) and function (neuropsychological status), within the OEF/OIF Veteran population by: (1) controlling for an important, common comorbidity in this population (i.e. PTSD); and (2) examining whether the effects of alcohol use are moderated by the quantity of consumption. Although, it is widely aknowledged that PTSD is one of the most common comorbidities with alcoholism, particularly in war Veterans [28-33], and that PTSD effects white matter microstructure and neuropsychological function [34-39], no study to our knowledge has reported on the effects of alcoholism, while independently examining the synergism with comorbid PTSD.
To accomplish the first goal, we selected comparison groups of participants with a disorder of alcoholism only (no PTSD), alcoholism with comorbid PTSD, PTSD only (without alcoholism), and a control group (without alcoholism or PTSD). This stratification allowed us to investigate the effects of alcoholism independently from the potential synergistic effects of both disorders, as well as from PTSD alone. The second aim was to differentiate among subgroups of alcoholics according to their lifetime drinking severity and its relation to PTSD. These subgroups were created based upon the quartile distribution of a lifetime drinking measure and served as a secondary classification of participants as follows: (1) low drinkers, (2) moderate drinkers, (3) high drinkers and (4) excessive drinkers. This approach allowed us to account for the likely heterogeneity among alcoholics by examining the moderating effects of lifetime drinking quantity.
Since prior research has identified cognitive changes in specific domains that are associated with structural alterations in localized regions of white matter, we employed a factor analysis technique in order to examine large scale whole-brain effects of alcoholism. Our primary motivation was to identify associations between whole-brain white matter changes and neuropsychological decline among alcoholics, examine whether these are consistent with prior findings, and specify which brain regions remain associated with cognitive dysfunction when controlling for the heterogeneity of drinking and comorbid PTSD.
Materials and Methods
Participants
Seventy-one participants were selected from among 226 service members consecutively enrolled in the Translational Research Center for Traumatic Brain Injury and Stress Related Disorders (TRACTS), a U.S. Department of Veterans Affairs Rehabilitation Research and Development TBI Center of Excellence at the VA Boston Healthcare System. Participants were recruited from the Boston metropolitan area through outreach at Yellow Ribbon and other military-associated events. Individuals were not enrolled in TRACTS if they had a history of the following illnesses: seizures; cerebrovascular accident; myocardial infarction; diabetes; current active suicidal, and/or homicidal ideation, intent or plan requiring crisis intervention, current DSM-IV-TR [40] diagnosis of bipolar disorder, schizophrenia or any other psychotic disorder (except psychosis NOS due to trauma-related hallucinations) or cognitive disorder due to general medical condition other than traumatic brain injury (TBI).
In order to best isolate the effects of alcoholism, PTSD, as well as their synergistic impact, we classified participants who passed the exclusion criteria (see below) into four diagnostic groups (N=71):
Alcoholism only (ALC)
17 participants diagnosed with current, and/or lifetime alcohol abuse or dependence without comorbid PTSD.
PTSD only (PTSD)
16 participants suffering from current PTSD without comorbid alcoholism.
Comorbid (COMO)
20 participants diagnosed with comorbid alcoholism and PTSD.
Controlparticipants (CTL)
18 participants not diagnosed with either PTSD or alcohol abuse/ dependence.
Exclusion criteria
155 participants excluded in total.
Substance use
Based on SCID-IV [40] diagnostic criteria, 38 participants with a history of substance abuse other than alcohol and cannabis (current or lifetime; abuse or dependence) were excluded.
Incomplete data
There were 44 subjects who did not complete the MRI scan, Lifetime Drinking History (LDH) questionnaire or had other classes of data missing, and thus were excluded from the sample.
Deployment status
In order to limit the heterogeneity of factors within our analysis, we excluded 15 military personnel without prior history of deployment on at least one OEF/OIF tour.
Diagnostic group matching
The diagnostic groups were matched for PTSD severity, according to current scores on the Clinician-Administered PTSD Scale (CAPS) [41] (COMO matched with PTSD and ALC matched with CTL), weight adjusted lifetime drinking quantity (COMO matched with ALC, and PTSD matched with CTL), years of education, age and premorbid IQ (matched for all groups). Thirty-four participants were excluded during the group matching process, which involved removing participants from diagnostic groups, until the measures described above were no longer significantly different from one another. Table 1 provides details of these characteristics. Since chronic depression has been shown to impact the brain [42], we identified participants with a diagnosis of a recurrent Major Depressive Disorder who were “in episode” during the time of their assessment session. The percentage of affected participants with this diagnosis was low (10% being the maximum, within the COMO group), and chi-square tests did not reveal any significant differences between the diagnostic groups on this measure.
Table 1.
Participants’ demographic and clinical characteristics.
| Alcoholism (n=17) | PTSD (n=16) | Comorbid (n=20) | Control (n=18) | Interaction of Having Alcoholism and PTSD | Main Effect of Having PTSD | Main Effect of Having Alcoholism | |
|---|---|---|---|---|---|---|---|
| χ 2 | |||||||
| Race (% Caucasian) | 71% | 56% | 95% | 61% | 6.55** | 0.08 | 0.35 |
| Gender (% Male) | 100% | 75% | 100% | 89% | 2.35 | 1.12 | 2 |
| Current Depressive Episode (% R-MDD) | 0% | 6% | 10% | 0% | 1.9 | 1.16 | None |
| Mild TBI: % with history of mTBI | 59% | 63% | 80% | 28% | 10.45** | 3.44 | 4.14* |
| % with 1 mTBI | 35% | 44% | 75% | 28% | 8.47** | 0.95 | 0.23 |
| % with 2 mTBI | 24% | 19% | 5% | 0% | 0.92 | 3.7* | 4.8* |
| % with 3 mTBI | 0% | 6% | 0% | 0% | None | 1.16 | None |
| % Excessive Drinkers | 47% | 0% | 50% | 0% | 18.22*** | None | 18.6*** |
| F | |||||||
| Mean Age (years) | 34.06 (8.14) | 34.62 (10.56) | 31.85 (7.82) | 31.22 (8.18) | 0.06 | 1.18 | 1.06 |
| Mean Estimated Premorbid Verbal IQ (WTAR) | 101.06 (11.45) | 105.06 (10.53) | 98.75 (11.38) | 102.94 (9.66) | 1.48 | 0.34 | 0.28 |
| Mean Education (years) | 13.06 (1.56) | 13.81 (1.8) | 13.6 (1.79) | 14 (1.41) | 0.58 | 0.12 | 3.5 |
| Mean Total Alcohol Consumed (LDH) | 1,708.24 (1,494.64) | 278.87 (279.14) | 1,443.5 (1,068.56) | 423.39 (309.78) | 15.21*** | 2.02 | 12.74** |
| SMAST Total Score: Mean Lifetime | 2 (1.8) | 0.25 (0.58) | 3.56 (4.09) | 0.78 (1.77) | 7* | 1.3 | 3.71 |
| Mean 12 Month | 0.88 (1.73) | 0.2 (0.41) | 2.37 (2.77) | 0.33 (0.84) | 8.91** | 0.31 | 1.46 |
| CAPS Mean Total Score (Current) | 26.41 (20.48) | 68.06 (22.53) | 72.45 (15.65) | 16.89 (8.45) | 179.5*** | 80.44*** | 3.3 |
Alcoholism group=participants diagnosed with current or lifetime alcohol abuse or dependence who do not have PTSD; PTSD group=participants diagnosed with a current Post Traumatic Stress Disorder; Comorbid group=Participants diagnosed with both, Alcoholism and PTSD; R-MDD=Recursive Major Depressive Disorder, current diagnosis; mTBI=Mild Traumatic Brain Injury; Excessive Drinkers=percent within the fourth quartile of the LDH total amount of alcohol consumed, weight corrected Measure; LDH=Lifetime Drinking History Questionnaire, weight corrected total score (ml); WTAR=Wechsler Test of Adult Reading; SMAST=Short Michigan Alcohol Screening Test, Lifetime and 12 months scores; CAPS=Clinician Administered PTSD Scale, current score.
Standard deviations are in parentheses.
Chi-squared tests confirm that there is no significant difference of excessive drinkers between the Alcohol and Comorbid groups.
p<.05
p<.01
p<.001
TBI
Since TBI commonly occurs in this population [43], it is difficult to completely eradicate its effects when studying the Veteran sample. We attempted to control for its effects by excluding all participants with a history of moderate or severe TBI; only mild TBI (mTBI) was allowed (four participants were excluded). Most mTBI cases have been shown to have good recovery, with symptoms clearing within the first few weeks or months after injury [44]. A particularly good prognosis is given to patients under age 40 [45], which falls above our sample’s mean age. Since frequency of TBI occurrences has been shown to affect recovery [46], in our sample, we limited the maximum number of mTBI occurrences to three, thereby excluding 12 participants whose TBI history exceeded this number. This was based on prior findings indicating that patients with more than three mTBIs were shown to be the most affected [46].
Effort
According to Slick et al. [47], probable malingering can be defined by a response bias on two or more objective measures from neuropsychological tests. In accordance with these recommendations, we chose to define poor effort by failure of the Medical Symptom Validity Test (MSVT) [48] and any other embedded measure (i.e. California Verbal Learning Test (CVLT): Forced Choice Recognition (FCR); Reliable Digit Span and Test of Variable Attention [49] Symptom Exaggeration Index [50]). If MSVT scores were not available poor effort was determined by poor performance on two of the three embedded measures. A failing MSVT subtest score was considered to be 85. Participants were considered to have failed each of the embedded measures, if they received a score of 15 or less on CVLT Forced Choice, a score of 3 on the T.O.V.A. Symptom Exaggeration Index, a score of 6 or less on the Reliable Digit Span. Twelve participants who screened positive for malingering, according to these criteria were excluded from our sample.
Clinical assessments
All participants went through a comprehensive clinical assessment consisting of self-report questionnaires and diagnostic interviews conducted by clinical psychologists. The assessments centered upon diagnoses of PTSD, TBI and alcoholism.
PTSD
The presence and severity of PTSD were assessed using the CAPS. The CAPS is a semi-structured clinical interview used to evaluate the DSM-IV-TR Criteria for PTSD. A total score of PTSD symptom severity is also derived from the CAPS (min score=0, max=136). The reliability and validity of this assessment tool are well documented [41]. Participants were diagnosed with PTSD if they met DSM-IV-TR criteria [40]. Diagnoses were made using a standard scoring rule (Rule of 3), such that for a given symptom to count toward diagnosis, the participant must score at least a 1 for frequency and a 2 for intensity. PTSD diagnosis ultimately was confirmed via consensus conference with at least three doctoral-level psychologists.
TBI
The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) [51] is an assessment of blast and blunt force TBI across the lifetime. Criteria are evaluated through a structured clinical interview using open-ended questions of symptoms. Injuries are graded (mild stage I, II, III; moderate; severe), according to a hybrid classification system that takes loss of consciousness, altered mental state and peri-traumatic amnesia into account. This measure is based on self-report. Information on the number of TBIs, the nature by which they were incurred, and their severity was collected for three time periods: pre-military, post-military and during military service. Note that these events do not necessarily correspond to the psychologically traumatic events collected during the CAPS interview. There was a good correspondence between the BAT-L assessment scores and the validated Ohio State TBI Assessment Method [52]. TBI diagnosis was confirmed via diagnostic consensus conference.
Alcohol use
The presence and severity of alcohol abuse and dependence was determined using a combination of three clinical measures. The DSM-IV-TR structured clinical interview for alcohol use disorders was used as the primary diagnostic tool for alcoholism. The semi-structured clinical interview LDH questionnaire was used to measure the severity and duration of alcohol consumption. The LDH yields an estimate of total lifetime exposure to alcohol using standard drink conversions (grams absolute alcohol) via two methods: (1) total lifetime drinks and (2) and total lifetime drinks controlling for weight (body weight in kg). This index frequently has been used to examine the pathological effects of alcoholism [53]. Through a semi-structured interview, the LDH provides quantitative indices of alcohol consumption patterns, including: quantity consumed frequency of use, variability in consumption, types of beverages, change in drinking patterns over lifetime and time of day when alcohol was consumed. The Short Michigan Alcohol Screening Test (SMAST) [54] is a self-reported measure that was used to quantify alcoholic behavior. It has been shown to provide a consistent, quantifiable method of detecting alcoholism with adequate reliability and validity in clinical and research settings [55]. Weighted reliability estimates centered on .80 suggesting that the SMAST generally produces scores of similar and adequate reliability for most research purposes [56]. Internal consistency is not as high, but is adequate for this sample [56]. Seltzer et al. [54] suggested that a score of 0-1 on the SMAST represents a nonalcoholic profile, a score of 2 indicates a possible alcoholic profile and a score of 3 or higher represents an alcoholic profile. In the present study, this measure was administered twice to assess general drinking behaviors over the course of the participant’s lifetime, as well as a more up-to-date assessment by focusing on the 12 months prior to the participant’s session. Participants were diagnosed with a current or lifetime alcohol abuse or dependence if they met the DSM-IV-TR diagnostic criteria, and if the outcomes of the supporting clinical assessment measures were consistent with this diagnosis. This diagnosis was confirmed via a consensus conference.
Neuropsychological assessment
Simple and divided attention
To assess simple and divided attention, participants were administered the Digit Span Forward and Backward, Wechsler Adult Intelligence Scale, Fourth Edition (WAISIV) [57], and the Test of Variables of Attention (TOVA) [49]. During the Digit Span Forward, participants were asked to repeat a series of numbers read by the examiner that increase in length during each trial. This test is a widely-used assessment of basic attention and its reliability, validity, and test-retest reliability are well-established [57]. The Digit Span Backward provided an assessment of more complex attention, by requiring participants to repeat a series of numbers in reverse order.
The TOVA is an objective measure of attention commonly known as a Continuous Performance Task (CPT). It is a 20-minute long computerized, non-language based fixed interval visual performance test. The TOVA is presented in a game-like format making it more acceptable to subjects than traditional CPT tasks. The test is comprised of two phases: infrequent target presentation and frequent target presentation. The TOVA was used to assess inattentiveness, activation/ arousal problems and difficulties maintaining vigilance and sustaining attention.
Information processing speed
Information processing speed was assessed using the Cambridge Neuropsychological Test Automated Battery (CANTAB) [58] Simple Reaction Time (SRT) test. The SRT test measures simple reaction time through delivery of a known stimulus to a known location to elicit a known response. The only uncertainty is with regard to when the stimulus will occur, by having a variable interval between the trial response and the onset of the stimulus for the next trial. It is useful for testing general alertness and motor speed, and it is sensitive to medication effects. The reliability, validity, and test-retest reliability are well established [59,60].
Executive functions
Executive functioning was assessed with tasks from the Delis Kaplan Executive Function Scale (D-KEFS) [61] and from the CANTAB [58]. The ability to focus attention was assessed using two conditions of the Trail Making Test from the D-KEFS [61], i.e. Letter Sequencing (Condition 2) and Number Sequencing (Condition 3). The combined score was used as a measure of focused attention. Attention shifting was assessed using Number-Letter switching (Condition 4) from the same task. The reliability and validity of these tasks are well established [61]. Internal consistency coefficients ranged from moderate to high and the total score reliability coefficients for the examined groups (ages 20-49) ranged from 0.74 to 0.78.
The D-KEFS Color-Word Interference Test modeled on the Stroop test [61], was used to assess the ability to inhibit a pre-potent response. During this task, participants were asked to perform two baseline conditions, one in which they were asked to name color patches (Condition 1), and one in which they were asked to read color-words printed in black ink (Condition 2). In a third condition, participants were asked to name the incongruent colors in which color-words were printed. The contrast measure evaluating time to completion in Condition 3 compared to Condition 1 was the dependent variable of interest. The reliability and validity of these tasks are well established [61]. Internal consistency values ranged from moderate to high for the composite score (comprised of color naming and word reading). For the examined age groups (20-49), internal consistency coefficients ranged from 0.72 to 0.82. Test-retest reliability coefficients were also in the moderate to high range (0.49 to 0.86) for the four conditions of the Stroop task.
The D-KEFS Verbal Fluency Test (phonemic fluency only) was used to evaluate generative ability and verbal fluency; we administered the letter fluency condition of this test. The dependent measure is the number of acceptable responses generated within each 60-second trial. There is no evidence for practice effects in this task [62]. This test has appropriate internal consistency and test-retest reliability [61]. Internal consistency for the letter fluency condition was high with internal consistency coefficients ranging from 0.77 to 0.90 across the three age groups (20-29, 30-39, 40-49). The test-retest reliability coefficient (age range of 20-49) for letter fluency was 0.76.
The CANTAB is a widely-used battery for assessing brain-behavior relations in adults, and its reliability and validity has been supported by numerous studies of healthy controls, as well as patients with brain lesions, degenerative disorders and psychiatric illnesses [59,60,63-66]. Temporal stability in a large group of younger, healthy controls (mean age=44, SD=16) was moderate to high with test-retest reliability coefficients ranging from 0.40 to 0.87 across all CANTAB subtests. We used the Intra-Extra Dimensional Set Shift and the Affective Go/No-Go subtests. The former is a computerized analogue of the Wisconsin Card Sorting test [60] that is sensitive primarily to changes to the frontostriatal areas of the brain [67]. It is a test of rule acquisition and reversal, using a visual discrimination task to assess intentional set formation, set maintenance, set shifting and flexibility of attention. The CANTAB Affective Go/No-Go subtest is sensitive to functions of ventral and medial-prefrontal cortical brain regions (having strong limbic connections). It assesses information processing biases for positive and negative stimuli, and as such, the test represents a powerful research assessment tool for current studies on the neural substrates of PTSD, since this disorder has been shown to be associated with information processing biases [22].
Memory
Memory was assessed with the California Verbal Learning Test-II (CVLT-II) [68], and the Green Nonverbal Medical Symptom Validity Tests (NV-MVST) [69]. CVLT-II is a list-learning task consisting of 16 words belonging to four distinct semantic categories forming a “shopping list.” The test includes five learning trials, a second interference list, an immediate and delay recall trial and a yes/no recognition task. The scores of interest to us were the total immediate recall correct (sums of trials 1-5) the number of correct delayed recall and recognition items. Forced choice recognition was used as an embedded effort measure [70]. Reliability and validity have been reported [68]. A recent study in patients with TBI supports the test’s construct validity in this population [71]. There is strong temporal stability for the primary and alternate forms of the test [72]. The verbal and nonverbal MSVT is a brief computerized screening test for verbal and nonverbal memory impairment that includes built-in symptom validity testing. It contains two primary symptom validity subtests, a “consistency” subtest and two memory subtests. The available data suggest that, of those groups assumed to be trying their best to do well on testing, only patients with advanced dementia score below the specified cut-off on the primary symptom validity subtests [69]. A recent study by Henry et al. [73] demonstrated that the NV-MSVT has high specificity (98.5% for neurological patients; 100% for control participants). Data also have demonstrated that the V-MSVT and NVMSVT have high inter correlations (0.70-0.80) with the longer Word Memory Test, which has well-established psychometric properties [69]. Preliminary data showed high specificity of the MSVT in the OIF/OEF population [48].
Premorbid function
Premorbid functioning or estimated verbal intelligence was assessed with the Wechsler Test of Adult Reading (WTAR) [74]. This is a measure of word reading that estimates premorbid verbal IQ. The test contains 50 words that increase in difficulty of pronunciation. Reliability, validity and test-retest reliability are well established [74]. The WTAR shows high correlations with other measures of reading [75], but moderate correlations with measures of language production [57], and internal consistency coefficients ranged from 0.90 to 0.97 in U.S. standardization samples.
Psychomotor function
This component was assessed with the Grooved Pegboard [76] and an ataxia measure. Grooved Pegboard is a test of manual dexterity and psychomotor speed that requires participants to put pegs in small holes. The dependent variable is time to completion [77]. The reliability and validity have been reported elsewhere [78]. Temporal stability is high (0.67-0.86) [78]. Ataxia was assessed by the stand-on-one-foot balance test from the Fregly Graybiel ataxia battery [79], performed with eyes open, maximum of 30 seconds on each foot. A mean measure was taken of the two trials.
MRI and DTI acquisition
Two whole-brain high-resolution T1-weighted MP-RAGE image (TI=1000 ms, TR=2.53 ms, TE=3.32 ms, flip angle=7°, slice thickness=1.0 mm, 256 slices, FOV=256×256 mm) were acquired for each individual on a Siemens 3 T TIM Trio scanner with a 12-channel head coil at the VA Boston Healthcare facility. The two images were averaged for each participant to create a single image volume with high contrast-to-noise. Total imaging time was approximately six minutes for each MP-RAGE scan. DTI acquisition used a 2 mm isotropic, 60 direction single shot echo planar sequence with a twice-refocused spin echo pulse sequence, optimized to minimize eddy current-induced image distortions [80] (10 T2 {b=0}+60 diffusion directions; b=700, TR=7920ms, TE=83ms; bandwidth=1396 Hz/px, slice thickness=2.0 mm, FOV=256×256 mm; 128×128 matrix; 64 slices; 2 mm3 voxel dimensions with 0 gap; automatically acquired in the AC-PC plane; 2x GRAPPA; total acquisition time 9:40). The 60 diffusion-weighted directions were obtained using the electrostatic shell method [81], resulting in a high signal-to-noise volume. The diffusion tensor was calculated on a voxel by voxel basis using conventional reconstruction methods [82].
MRI image processing
MRI images were processed to create individualized models of each participant’s cortical mantle through the cortical reconstruction procedure in the FreeSurfer image analysis suite, which is documented and freely available for download online (http:// surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications [50,83-88]. Briefly, this processing first involves motion correction and averaging of the two volumetric T1-weighted images, removal of non-brain tissue using a hybrid watershed/surface deformations procedure [89], automated Talairach transformation, intensity normalization [90], tessellation of the gray matter white matter boundary, automated topology correction [86,91] and surface deformation, following intensity gradients to optimally place the gray/white and gray/CSF borders at the location where the greatest shift in intensity defines the transition to the other tissue class [84,92]. Surface inflation and spherical registration procedures based on the matching of cortical folding patterns across individuals were used for group-based analyses [84,85]. Cortical thickness was computed as the closest distance from the gray matter/white matter boundary to the gray matter/Cerebro Spinal Fluid (CSF) boundary at each vertex on the tessellated surface [92]. In Free Surfer, the cortical surface boundaries were occasionally slightly misplaced, with regard to the boundary of the gray matter/white matter, and/or gray matter/CSF border, and occasionally need to be manually edited for very small adjustments. Due to this, cortical models were checked slice-by-slice throughout the entire brain image to assure that surfaces were appropriately placed at the gray/white and gray/CSF border. Thickness data were smoothed, using a circularly symmetric Gaussian kernel across the surface with a standard deviation of 20 mm. The reliability of obtaining cortical thickness measurements from MRI scans has been well documented in several instances [93-95].
Diffusion data were processed using a multistep procedure involving the Free Surfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/) and FSL (http://www.fmrib.ox.ac.uk.fsl/) processing streams. A T2-weighted structural volume, collected using identical sequence parameters as the directional volumes with no diffusion-weighting, and thus, in register with the final diffusion maps, was used for all registration and motion correction, using a 12-parameter affine mutual information procedure in FMRIB’s Linear Image Registration Tool (FLIRT) [96-98]. The diffusion tensor (including the six components; three eigenvectors and three eigen values) was calculated for each voxel using a least-square fit to the diffusion signal [99], and brain-extracted using BET [100]. FA was derived from the 3D maps of the three eigen values in the diffusion tensor, as described previously [99]. Data were then prepared for statistical analysis using TBSS Tract-Based Spatial Statistics [101], distributed as part of the FSL package. TBSS was used for the analyses with FA. First, all participants’ FA data were aligned into a common space using the nonlinear registration tool FNIRT [102], which uses a b-spline representation of the registration warp field [103]. Next, the mean FA image was created and thinned to generate a mean FA skeleton that represents the centers of all fiber tracts common to the entire sample. The mean skeleton was masked to only include voxels with FA values greater than 0.2, so as to avoid inclusion of regions that could be composed of multiple tissue types or fiber orientations. Each subject’s aligned, common-space FA data were then projected onto this skeleton to create a 4D skeletonized volume; this was then fed into voxel wise group statistics. Data along the skeleton were smoothed utilizing an anatomical constraint to limit the smoothing to neighboring data within adjacent voxels along the skeleton. Statistical maps were dilated from the TBSS skeleton for visualization purposes.
Regions-of-Interest (ROIs) were determined from a combination of two separate atlases. First, the Johns Hopkins White Matter Atlas was used to segment white matter deep within the brain. Then, the FreeSurfer white matter segmentation atlas was used to segment the white matter that lies directly below the cortical surface. Following the segmentation of the skeletal white matter, the skeleton was deprojected back to native space. All ROI values were then taken from native space.
We selected FA as a primary measure of white matter integrity based on its common use in previous literature examining white matter in alcoholism [104]. FA values have been calculated with a formula that takes into account all eigen values, ranging from 0 to 1. The variables included in the data set all were native-space mean FA values from the deprojected skeletal ROI’s.
Data Analysis
All statistical analyses were completed using R version 3.0.1 on a x86_64-apple-darwin10.8.0 (64-bit) platform [105]. In order to perform factor analyses with clinical and neuropsychological scores and avoid list or pair-wise deletion of data, we employed the recommended procedure [106] of multiple imputations using Fully Conditional Specification implemented by the multiple imputation using chained equations algorithm [107]. Gibbs sampling and predictive mean matching was used to replace missing data on continuous variables, while logistic and polytomous logistic regression was used for dichotomous data. Final values were generated, taking into account the uncertainty introduced by the missing variables and remaining statistically close to the original variance.
The Procedures for Personality and Psychological Research software package [108] was used to conduct Principle Axis Factor analysis. Five principal factors were obtained from with a Varimax (orthogonal) rotation of 20 neuropsychological test components (Table 2). The five cognitive factors were labeled: (1) Inhibition and Switching Speed, (2) Memory, (3) Verbal Memory, (4) Emotional Valence Processing and (5) Psychomotor Factor. The same statistical package was utilized to obtain four principal factors based on 61 FA ROIs using orthogonal rotation. The DTI factors consisted of the following: F1 (Dorsolateral Frontal-Parietal Factor), F2 (Frontal Subcortical Factor), F3 (Limbic Nucleus Accumbens Factor), and F4 (Subcortical Limbic Cerebellar Factor) (Table 3).
Table 2.
Obliquely rotated component loadings for 20 neuropsychological test sub-scores.*
| Component | Inhibition & Switching Speed | Memory | Verbal Memory | Emotional Valence Processing | Psychomotor |
|---|---|---|---|---|---|
| Stroop: Inhibition/Switching Scaled Score | 0.71 | 0.05 | 0.2 | −0.2 | −0.18 |
| Trails: Visual Scanning Scaled Scores | 0.7 | 0.12 | 0.16 | 0.06 | 0.15 |
| Trails: Motor Speed Scaled Score | 0.64 | 0.32 | −0.31 | 0.08 | −0.2 |
| Stroop: Inhibition/Switching Total Time | −0.73 | −0.12 | −0.22 | 0.16 | 0.18 |
| Trails: Motor Speed Total Time | −0.75 | −0.26 | 0.09 | −0.11 | 0.11 |
| FAS: Category Fluency Scaled Score | 0.04 | 0.79 | 0.03 | −0.07 | −0.09 |
| FAS: Letter Fluency Scaled Score | 0.19 | 0.72 | −0.05 | −0.1 | −0.16 |
| Digit Span Total | 0.38 | 0.7 | 0.25 | −0.08 | 0.4 |
| Digit Span Total Scaled Score | 0.23 | 0.68 | 0.27 | 0.03 | 0.38 |
| Digit Span Sequencing | 0.27 | 0.66 | 0.3 | 0 | 0.36 |
| CVLT: Total Learning T-Score | 0.01 | 0.3 | 0.84 | −0.07 | −0.15 |
| CVLT Total Trials 1-5 | 0.05 | 0.3 | 0.82 | −0.09 | −0.15 |
| CVLT: Recognition Hits Z-Score | 0.12 | −0.05 | 0.73 | −0.12 | −0.02 |
| Affective Go/No-go: Total Commissions (negative) | −0.22 | 0.04 | 0.02 | 0.82 | 0.03 |
| Affective Go/No-go: Total Omissions (positive) | 0.22 | −0.19 | −0.15 | 0.8 | −0.04 |
| Affective Go/No-go: Total Commissions (positive) | −0.27 | 0.12 | 0.03 | 0.79 | −0.09 |
| Affective Go/No-go: Total Omissions (negative) | 0.24 | −0.23 | −0.2 | 0.73 | 0.03 |
| Grooved Peg. Non- Dom. Z Score | −0.17 | 0.08 | −0.09 | −0.06 | 0.77 |
| Grooved Peg. Dom. Z Score | −0.23 | 0.05 | −0.18 | −0.01 | 0.65 |
| Ataia Mean: Standing on Left and Right Foot (Eyes Open) | 0.19 | −0.25 | 0.6 | 0.09 | 0.53 |
| Eigenvalues | 2.39 | 3.29 | 3 | 2.51 | 2.26 |
| Percent of Total Varience | 12% | 16% | 15% | 13% | 11% |
| Number of Test Measures | 5 | 5 | 3 | 4 | 3 |
Loadings =>.01. When loadings less than 0.51 were excluded, the analysis yielded a five-factor solution with a simple structure (factor loadings => .51)
Table 3.
Obliquely rotated component loadings for 61 fractional anisotropy regions of interest*.
| Component | F1 (Dorsolater Frontal-Parietal Factor) | F2 (Frontal Subcortical Factor) | F3 (Limbic Nucleus Accumbens Factor) | F4 (Subcortical Limbic Cerebellar) |
|---|---|---|---|---|
| Right rostral middle frontal cortex | 0.77 | 0.34 | 0.09 | 0.04 |
| Right paracentral gyrus | 0.77 | −0.01 | −0.01 | 0.3 |
| Right inferior parietal cortex | 0.76 | 0.19 | 0.28 | 0.03 |
| Left inferior parietal cortex | 0.76 | 0.2 | 0.26 | 0.15 |
| Left superior parietal cortex | 0.75 | 0.17 | 0.29 | 0.35 |
| Left rostral middle frontal cortex | 0.73 | 0.35 | 0.32 | −0.03 |
| Left precentral gyrus | 0.73 | 0.04 | 0.3 | 0.24 |
| Right superior frontal gyrus | 0.72 | 0.46 | 0.11 | 0.12 |
| Right superior parietal cortex | 0.72 | 0.26 | 0.27 | 0.33 |
| Left superior frontal gyrus | 0.7 | 0.39 | 0.23 | 0.13 |
| Right superior temporal gyrus | 0.7 | 0.21 | 0.4 | 0.27 |
| Right caudal middle frontal cortex | 0.69 | 0.32 | 0.09 | 0.08 |
| Left lateral occipital cortex | 0.69 | 0.2 | 0.28 | 0.17 |
| Right inferior temporal cortex | 0.68 | 0.28 | 0.23 | 0.05 |
| Left caudal middle frontal cortex | 0.68 | 0.21 | 0.34 | 0.07 |
| Left precuneus | 0.67 | 0.23 | 0.39 | 0.19 |
| Left postcentral gyrus | 0.67 | −0.08 | 0.27 | 0.37 |
| Left supramarginal cortex | 0.66 | 0.36 | 0.34 | 0.11 |
| Right precentral gyrus | 0.66 | 0.23 | 0.11 | 0.34 |
| Right precuneus | 0.66 | 0.17 | 0.38 | 0.36 |
| Right pars opercularis | 0.65 | 0.28 | 0.18 | −0.09 |
| Right middle temporal gyrus | 0.65 | 0.27 | 0.32 | 0.21 |
| Left posterior cingulate gyrus | 0.63 | 0.21 | 0.4 | −0.08 |
| Right supramarginal cortex | 0.63 | 0.28 | 0.25 | 0.29 |
| Right lateral occipital cortex | 0.63 | 0.23 | 0.24 | 0.42 |
| Right pars triangularis | 0.61 | 0.23 | 0.28 | 0.11 |
| Left superior temporal gyrus | 0.61 | 0.32 | 0.44 | 0.28 |
| Genu of the corpus callosum | 0.6 | 0.39 | 0.28 | −0.1 |
| Right banks of the superior temporal sulcus | 0.59 | 0.19 | 0.05 | 0.16 |
| Left banks of the superior temporal sulcus | 0.57 | 0.26 | 0.29 | −0.1 |
| Right postcentral gyrus | 0.54 | 0.14 | 0.02 | 0.48 |
| Left pars triangularis | 0.51 | 0.29 | 0.36 | 0.12 |
| Left retrolenticular part of the internal capsule | 0.11 | 0.8 | 0.16 | 0.23 |
| Left superior corona radiata | 0.24 | 0.79 | 0.03 | 0 |
| Right posterior limb of the internal capsule | 0.12 | 0.79 | 0.04 | 0.23 |
| Right retrolenticular part of the internal capsule | 0.16 | 0.78 | −0.07 | 0.21 |
| Right posterior corona radiata | 0.36 | 0.76 | 0.04 | 0.18 |
| Right superior corona radiata | 0.25 | 0.75 | 0.06 | 0.02 |
| Left posterior limb of the internal capsule | 0.05 | 0.73 | 0.12 | 0.13 |
| Left posterior corona radiata | 0.22 | 0.72 | 0.05 | 0.2 |
| Left anterior corona radiata | 0.39 | 0.63 | 0.29 | −0.11 |
| Right superior longitudinal fasciculus | 0.44 | 0.63 | 0.19 | 0.2 |
| Left external capsule | 0.24 | 0.6 | 0.27 | 0.12 |
| Right anterior corona radiata | 0.46 | 0.59 | 0.31 | −0.21 |
| Left superior longitudinal fasciculus | 0.44 | 0.58 | 0.32 | 0.18 |
| Left anterior limb of the internal capsule | 0.27 | 0.54 | 0.44 | 0.09 |
| Right anterior limb of the internal capsule | 0.28 | 0.53 | 0.44 | 0.13 |
| Left thalamus | 0.31 | 0.07 | 0.73 | 0.25 |
| Left fornix and stria terminalis | 0.22 | 0.09 | 0.71 | −0.02 |
| Right thalamus | 0.34 | 0.09 | 0.71 | 0.3 |
| Right cingulum bundle connecting the cingulate gyrus | 0.29 | 0.37 | 0.64 | −0.02 |
| Left ventral diencephalon | 0.14 | 0.04 | 0.64 | 0.33 |
| Right caudal anterior cingulate cortex | 0.36 | −0.05 | 0.62 | −0.21 |
| Body of the corpus callosum | 0.55 | 0.15 | 0.59 | −0.12 |
| Left caudate | 0.27 | 0.14 | 0.58 | 0.37 |
| Right putamen | 0.17 | 0.14 | 0.53 | 0.18 |
| Right hemisphere of the cerebellum | 0.36 | 0.4 | 0.52 | 0.24 |
| Right cingulum bundle connecting the hippocampus | 0.23 | 0.15 | 0.02 | 0.75 |
| Left cingulum bundle connecting the hippocampus | 0.16 | 0.32 | 0.11 | 0.64 |
| Brain stem | 0.14 | 0.34 | 0.36 | 0.59 |
| Right superior cerebellar peduncle | 0.15 | 0.16 | 0.3 | 0.56 |
| Eigenvalues | 16.59 | 9.53 | 7.98 | 4.28 |
| Percent of Total Varience | 27% | 16% | 13% | 7% |
| Number of Test Measures | 32 | 15 | 10 | 4 |
Loadings => .01. When loadings less than 0.51 were excluded, the analysis yielded a four-factor solution with a simple structure (factor loadings => .51)
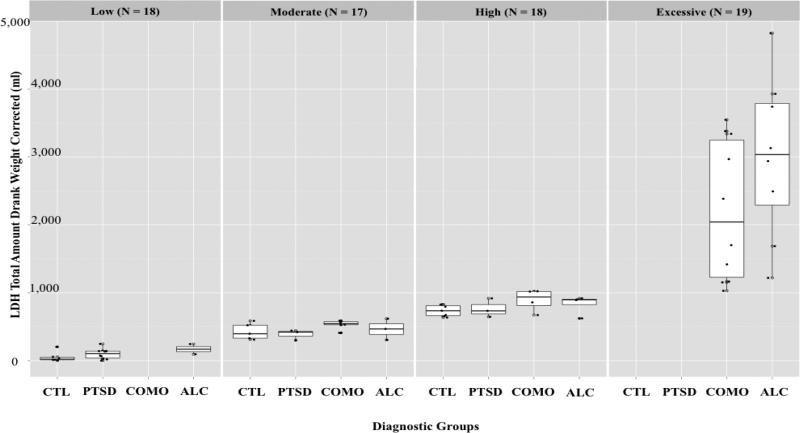
Multiple linear regression models were employed to investigate the impact of alcohol use and PTSD on the relationship between the brain and cognitive factors. Twenty regression analyses were performed, using each of the DTI factors as independent variables and the behavioral factors, along with the diagnostic groups, as dependent measures. Whenever results yielded a main effect of Alcoholism or PTSD, follow-up regression analyses were conducted in order to examine whether the main effect was driven by drinking quantity (using LDH) or PTSD severity (using CAPS). The LDH measure used indicated the total weight-corrected amount of alcohol consumed over the course of each participant’s lifetime. Aggregate results from all participants have been broken up into quartiles based on this measure of lifetime drinking quantity: low, moderate, high, and excessive drinkers. See Figure 1 for results of a detailed breakdown of these categories, as well as the primary group distribution within the quartiles. As expected, low and moderate drinkers consisted primarily of CTL and PTSD participants, while the high and excessive drinkers consisted mainly of COMO and ALC groups. The secondary regression for drinking quantity was set up with the LDH variable as a dependent measure; current CAPS score was included as a covariate in order to control for the effects of PTSD. Since none of the primary regression analyses revealed a main effect of PTSD, the secondary regression for PTSD severity was not applied, and thus, is not discussed in further detail. The CTL group was used as a reference. Table 4 summarizes the results of the significant regression models.
Figure 1.
LDH Drinking Severity Quartile Distribution.
Table 4.
Multiple linear regression summary.
| Effect of Alcoholism/Effect of Excessive Drinking | |||
|---|---|---|---|
| Alcoholism | Inhibition Switching Speed Factor | ||
| F1 | t value | −0.29 | −2* / 2.9** |
| (Dorsolateral Frontal-Parietal Factor) | R2 | 0.03 | 0.15 / 0.17 |
| Adjusted R2 | 0.01 | 0.06 / 0.06 | |
| F2 | t value | −2.04* / −2.6** | ------------ |
| (Frontal Subcortical Factor) | R2 | 0.07 / 0.17 | ------------ |
| Adjusted R2 | 0.03 / 0.1 | ------------ | |
| Observations: 71 | |||
Note: The post-hoc tests for high drinking were conducted only if the initial regression result yielded at least a marginally significant p-value (p<.1) for the main effect of Alcohol, PTSD, or synergistic interaction of the two disorders
p<0.07
p<.05
p<.01
Results
Demographics
Chi-Square tests were conducted to assess primary group differences in their composition of race (percent Caucasian), gender (percent male), current depressive episodes (percent “in episode” of current recurrent Major Depressive Disorder), percentage of participants with a history of mTBIs and percentage of excessive drinkers (based on the LDH quartile distribution described below). The COMO group was significantly different in its minority composition (p<0.01), consisting of 95% Caucasians. There were no significant group differences in gender distribution or percentage of participants diagnosed with current recurrent depression. The COMO and ALC groups contained the most participants with a history of at least one mTBI (80%, p<0.01 and 59%, p<0.05, respectively), however, there were no group differences in participants who had more than three mTBIs over the course of their lifetime, with 6% being the highest percentage (within the PTSD group) of affected participants. The PTSD and CTL groups did not contain any excessive drinkers, while the COMO (50% excessive drinkers) and ALC (47% excessive drinkers) were equivalent on this distribution.
After performing the factor analysis, one participant was identified as an outlier due to two FA values that deviated significantly from the rest of their corresponding group and impacted the regression models by adding significance to otherwise insignificant equations. Cook’s Distance test confirmed the high influence of this variable. Therefore, two of the participant’s FA values were replaced with the closest values from another participant.
ANOVA tests were conducted to examine group differences on age, years of education, estimated pre-morbid verbal IQ, weight corrected total amount of alcohol consumed over the course of a lifetime, current CAPS score and SMAST total scores (lifetime cumulative and recent year scores). There were no group differences on age, education or premorbid verbal IQ. As expected, COMO (mean=11,443.5) and ALC (mean=1,708.24) groups drank significantly more over the course of their lifetime than the CTL and PTSD groups (p<.001 and p<.01, respectively), but did not differ significantly from one another. The CTL (mean=278.87) and PTSD (mean=423.39) groups did not differ in their drinking levels (p>.05). The comorbid group had significantly higher SMAST scores (current mean=7 and lifetime mean= 8.91) than the CTL and PTSD groups (mean score<1), indicating a higher level of behavioral disruptions due to the presence of both disorders. Although lower than comorbid group, the ALC group did have clinically significant SMAST lifetime scores (mean>3), indicating alcoholic behavior. The ALC group’s SMAST twelve-month scores were below one, confirming abstinence. The comorbid group showed significantly higher drinking activity that the CTL group within the past year (Table 1).
Main effects of PTSD and comorbid diagnostic groups
Regression analyses did not reveal any significant effects of PTSD or comorbid diagnostic groups on the FA factors. That is, the dorsolateral frontal-parietal factor, frontal subcortical factor, limbic accumbens factor and the subcortical limbic cerebellar factor were not significantly associated with any of the cognitive changes (p>.05) within the PTSD or comorbid diagnostic groups (p>.05).
Main effects of excessive drinking
Regression analyses did not reveal any significant effects of the ALC group on limbic nucleus accumbens or the subcortical limbic cerebellar factors. Multiple linear regression model revealed significantly (p<.05) reduced FA values within the frontal subcortical factor region within the ALC group, as compared to CTL participants (t=-2.04, R2=0.07, adjusted R2=0.03). Follow-up regression analyses revealed that this effect has been driven by excessive drinkers (p<.01, t=-2.6, R2=0.17, adjusted R2=0.1), collapsed across all diagnostic groups. Regression analyses also revealed a significant (p<.05) association between a decrease of the inhibition and switching speed cognitive factor scores and an increased of the dorsolateral frontal-parietal factor scores (t=-2, R 2=0.15, adjusted R2=0.06). This relationship was driven by excessive drinkers (p<.01) and collapsed across all diagnostic groups (t=-2.9, R 2=0.17, adjusted R2=0.06). See Figure 1 for a distribution of drinking quartiles and Table 4 for regression summaries and results.
Discussion
Primary findings of this study suggested that sheer quantity of alcohol consumption in OEF/OIF Veterans account for the greatest amount of variance in FA measures within the frontal subcortical region, whereas the presence of an alcohol- or stress-related diagnosis (PTSD) per se was not predictive. The decrease of FA values within this region occurred as a function of excessive drinking and was independent of cognitive dysfunction. These data indicated that FA values underlying the dorsolateral frontal-parietal regions increased within the ALC group as a function of decreasing inhibition and switching speed abilities. FA values within this region were also increased within excessive drinkers independently of PTSD. The increase of FA values within the dorsolateral frontal-parietal regions is consistent with prior findings, indicating a compensatory involvement of this region [109]. We did not have sufficient statistical power to conclude whether this relationship is the same within excessive drinkers. Given the previously reported compensatory role of this region in alcoholics and the increase of FA values within excessive drinkers, it is likely that this relationship remains consistent within the heaviest drinking category and the entire ALC group.
Effects of the diagnostic groups
Results of the present study indicated that neither the PTSD diagnosis nor the comorbid diagnosis of PTSD and alcoholism had a measurable effect on alcoholism-related brain structure or cognitive function. This allows us to conclude that white matter underlying the frontal subcortical and dorsolateral frontal and parietal regions are sensitive to pathological alcohol consumption, and is not affected by the presence or absence of the frequently comorbid PTSD. The sensitivity of these regions to alcohol consumption is consistent with prior findings that have reported alcohol susceptibility within the frontocerebellar and mesocorticolimbic circuitry [109].
Disregulation of different areas within these circuits has been found to be associated with various behavioral changes that, when combined together, constitute what has been coined as “alcoholic behavior” [109,110]. Such behavioral patterns are often marked by impaired judgement and decreased ability to avoid negative actions pertaining to the downward spiral of addiction [111]. The frontocerebellar system is functionally characterized with verbal abilities, attention, decision making, and planning capabilities, all of which can be compromised when the frontocerebellar circuit is impaired [112].
The mesocorticolimbic circuit is a dopaminergic pathway in the brain that is involved in processing incentives and rewards associated with various pleasurable activities, and it plays a key role in addiction [113]. Main structures within this pathway include the orbitofrontal cortex, medial prefrontal cortex, anterior cingulate cortex, amygdala, hippocampus, thalamus, ventral and dorsal striatum, as well as the globus pallidus [109]. The salient involvement of this pathway in addictive behavior is evident from studies that demonstrate its activation with visual [114] and taste [113] alcohol cues, without actual consumption of the substance. In addition to playing a role in dopaminergic mediation of reward (alcohol related, in this case), the mesocorticolimbic pathway is also believed to play a role in aversive stressful stimuli [115]. Although the exact mechanisms of its role are still unknown, it is clear that this pathway is involved and abnormally activated in alcoholics [109].
The frontal subcortical factor consists of bilateral retrolenticular, posterior and anterior limb of the internal capsule, bilateral superior longitudinal fasciculus, anterior, posterior and superior componenets of the bilateral corona radiata, as well as the left external capsule. The anterior limb of the internal capsule contains frontopontine fibers that project from the frontal portions of the cerebral cortex to the pons; the pons plays a cruicial role in the intermediate steps of the feed-forward loop of the cerebrocerebellar circuit [116], and has been found to be adversely affected by alcohol abuse [117]. The retrolenticular portion of the internal capsule contains crucial fibers that project from the lateral geniculate nucleus of the thalamus, a part of the optic system and a key portion in the feed-forward loop. The posterior limb of the internal capsule contains sensory and corticobulbar fibers, both of which are involved in communication with the thalamus and crucial for the feedback loop described above [118].
Part of a bidirectional bundle of fibers that radiate from the frontal lobes through the occipital lobes, connecting the anterior and posterior portions of the cerebellum [119], is a key component of the feedforward loop. Another key component of this loop is the corona radiata, which contains ascending and descending fibers that pass through the internal capsule, connecting most parts of the cerebral cortex with ventrally located structures. Previous studies have reported the deliterious effect of alcohol on these fibers [120]. These data indicated that there are long-term effects of high alcohol consumption over the course of a lifetime on the internal capsule. Finally, the left external capsule contains fibers that project from the basal forebrain to the cerebral coretex, and it too is a crucial component of the feedback loop. It was previously shown to be affected in men [13], which is consistent with our male-dominated sample.
Given that the initial regression models revealed significant results for ALC, but not COMO groups, it is possible that the synergistic effect of the comorbidity of alcoholism and PTSD might be playing a role in modifying drinking behavior. This is a possibility since the frontal subcortical factor and dorsolateral frontal-parietal factor regions were affected only by alcoholism within the COMO group when the lifetime drinking quantity fell within the highest quartile of all participants. Since both the ALC and COMO groups were matched on lifetime drinking quantity, one possible explanantion is that individuals in the low, moderate, and high drinking ranges who suffer from PTSD and alcoholism, consume alcohol in different patterns than alcoholics who do not have PTSD. Specific drinking patterns that have been reported in individuals who suffer from PTSD include binge drinking [121] and morning drinking [122]; these patterns might affect brain structure and function in different ways from chronic alcoholism. Furthemore, genetic predispositions and alcohol exposure during fetal periods might be important in accounting for the differences of alcoholic profiles. For example, Goodlett demonstrated using a nonhuman animal model, that even a single day of alcohol exposure during the brain’s growth spurt within fetal development accounted for brain weight restriction and Purkinje cell loss [123]. It is thus possible that the ALC group without PTSD suffered from certain environmental and genetic predispositons for the disorder, which contributed to the frontal subcortical region alterations. The COMO group, on the other hand, might have engaged in alcoholism as a coping mechanisms for their PTSD symptomotology [124], thus lacking the additional factors that were present within noncomorbid alcoholics.
Heterogeneity of alcohol consumption
The highest drinking category (excessive drinkers), based on the weight-corrected lifetime alcohol consumption measure, was shown to be predictive of all alcoholism-related changes. Thus, the commonly reported alterations within the frontal subcortical and the dorsolateral frontal-parietal regions were not characteristic of our entire alcoholic sample, but specific only to the highest drinking subgroup of alcoholics. Particular findings included lower FA values within the frontal subcortical regions, independent of cognitive impairment and increased FA values within the dorsolateral frontal-parietal regions within the excessive drinking subgroup who also had impaired executive function.
The latter finding is consistent with prior studies that indicated a compensatory role of the dorsolateral frontal-parietal areas in individuals who suffer from executive impairments [109]. Axons within this region underlie areas that have been shown to engage in higher activity as executive function decreased. Gilman et al. [125] demonstrated that the left inferior and right middle frontal gyri activated within alcoholics to a larger extent than in healthy controls during the same task requiring executive function. Desmond et al. [126] found that although there were no performance differences in abstinent alcoholics, they exhibited greater fMRI activation than a control group in the left frontal and right superior cerebellar regions during the same task. Additional studies have reported that increased activity in certain brain regions [7,126] appeared to serve as compensatory mechanisms for the damaged circuits, thereby allowing alcoholics to maintain the same level of performance as healthy controls [9]. Within our sample, the dorsolateral frontal-parietal factor involves two affected circuits (described above) that were associated with decreased performance on the corresponding executive tasks. Although hypothetical (due to the cross-sectional design), these data support the notion that the integrity of white matter bundles underlying the compensatory regions have changed from their premorbid state, and in this manner, facilitated functional compensation for the corresponding gray matter.
It is important to address our previous finding [127], indicating that PTSD was associated with reduced cortical thickness within gray matter regions, located superior to certain subcortical regions within dorsolateral frontal-parietal factor. Specifically, gray matter within the left pre- and post-central gyri, right dorsal pre-central gyrus and superior frontal gyrus were all found to be decreased in indivudals affected by PTSD. Although analyzing the gray/white matter relationship between the two disorders is outside of the scope of this study, these data raise several questions for future research. First, it would be important to examine whether the decreased cortical thickness of the noted gyri is associated with a change in executive functioning. Since FA value increase underlying these gyri seems to serve a compensatory role for decreased executive function, it would be useful to examine whether cortical thinning is related to the same change in cognition. Furthermore, since the methods of the current study utilized a factor analysis technique, conclusions about specific ROIs cannot be made. It is, therefore, possible that a certain amount of variability exists within each of the factor regions. Another direction for future research would thus be to examine whether there are white matter fluctuations within each of the areas that were shown to be affected in alcoholics, and if so, it would be important to examine the nature of their association with each of the overlaying cortical areas.
Limitations
Although this is the first study to examine the associations of white matter and cognitive changes in alcoholics, while controlling for PTSD and heterogenuity of drinking, there are several important limitations that need to be taken into account. First, these results can be applied only to men, as female Veterans were less available in our sample than their male counterparts, and were difficult to stratify within the diagnostic groups. Given that recent findings have shown clear gender differences in alcoholism-related brain and behavioral functioning [9,109], further research needs to explore these variations as the number of deployed women Veterans continues to increase. A second limitation comes from the retrospective cohort design of this study, which did not allow for the addition of measurement tools, such as those that would allow us to control for the length of abstinence or indentify different drinking patterns (i.e. morning drinking or binge drinking). Functional recovery and neurogenesis are important factors to consider in relation to the length of abstinence and might shed further light on these findings. Collecting more detailed data on various drinking patterns would help identify the unique cognitive and anatomical signatures of additional drinking profiles, which might help differentiate them from the “excessive drinkers” discussed in this research. Another limitation of this design is that we did not control for alcohol consumption prior to testing. However, it is unlikely that alcohol was consumed immediately before testing for two reasons. First, participants were asked to fast (including not consuming alcohol) for 12 hours prior to their first session, in order to accommodate a blood draw. If an alcohol abuse issue was revealed at the time of scheduling, the first session was delayed by one month, during which participants were requested to abstain from consuming alcohol. Second, as mentioned earlier, the ALC group’s SMAST scores for the past year before the session were below 1, indicating abstinence. We did not use a breathalyzer test that would indicate whether participants had abstained from consuming alcohol beyond the 12 hours prior to their appointment. Therefore, acute effects of alcohol cannot be ruled out with certainty.
Another limitation involves the use of the factor analysis approach that was employed in this study. Given the high number of ROI studies that have been published in the past examining the effects of alcohol, the factor-analysis approach was used as a data reduction technique. This provided a way to examine larger whole-brain brain regions and allowed us to study any alterations within entire axon bundles that might span across networks and predefined brain areas, while decreasing the chance of a Type II error. A notable limitation of this approach is decreased sensitivity; factor-analysis techniques sacrifice a more sensitive measurement approach that might help delineate subtle changes within the factor’s components. Finally, as discussed earlier, we did not have the sufficient statistical power to measure whether the cognitive and white matter changes relating to the dorsolateral frontal-parietal regions were driven by excessive drinkers or independent of the amount of lifetime alcohol consumption. It is likely that the relationship of these changes is the same as it is for the ALC group, but statistically derived conclusions cannot be made due to the standard limitations of a cross-sectional design.
Conclusions
Results of the present study showed that several of the previously identified associations between brain structure and function were (a) unique to those who fell within the highest quartile of lifetime alcohol consumption, and (b) independent of the effects of the frequently comorbid PTSD. Alterations within the white matter regions underlying the frontal portions of the frontocerebellar and the mesocorticolimbic pathways were independent of cognitive dysfunction. Changes within the white matter regions, underlying the dorsolateral frontal-parietal areas, showed higher axon integrity, that likely served a compensatory role for decreased executive functioning. When this sample was examined in relation to the diagnostic groups, white matter and cognitive changes occurred only within the ALC participants, without comorbid PTSD. These data open the possibility that individuals who suffer from both disorders might engage in different drinking patterns that are less deleterious to this region. These differences, however, do not apply to the highest drinking category of individuals, whose white matter and cognitive changes were associated directly with pathological alcohol consumption.
Acknowledgments
This research was support by the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research and Development Traumatic Brain Injury Center of Excellence (B6796-C). In addition, support for this work was provided by VA CSR&D Merit Review Award to Regina McGlinchey, NIH NIA K01AG024898 awarded to Catherine Brawn Fortier, as well as funds from the National Institute on Alcohol Abuse and Alcoholism [1] grants R01-AA007112 and K05-AA000219, and from the Department of Veterans Affairs Medical Research Service awarded to Dr. Marlene Oscar Berman. The authors would like to thank the members of the Core B TRACTS team for their contributions to the data presented in this manuscript, in particular: Melissa Amick, PhD, Alexandra Kenna, PhD, Ann Rasmusson, MD, Erica Scoli, PhD, and David Zade for clinical assessment, and Lindsay Morra, BA, Roxana Moayer, MS, Andrea Levine, MA for neuropsychological assessment. We are very grateful to Walter Musto for his extraordinary recruitment efforts and to Emily Lindemer for her help with imaging data analysis.
Footnotes
Citation: Maksimovskiy L, McGlinchey RE, Fortier CB, Salat DH, Milberg WP (2013) White Matter and Cognitive Changes in Veterans Diagnosed with Alcoholism and PTSD. J Alcoholism Drug Depend 2: 144. doi:10.4172/2329-6488.1000144
References
- 1.NIAAA recognizes alcohol awareness month. National Institute on Alcohol Abuse Alcoholism (NIAAA) 2013.
- 2. http://www.ncadd.org/
- 3. http://www.drugabuse.gov/
- 4.Concha L, Beaulieu C, Collins DL, Gross DW. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:312–319. doi: 10.1136/jnnp.2007.139287. [DOI] [PubMed] [Google Scholar]
- 5.Chen CH, Walker J, Momenan R, Rawlings R, Heilig M, et al. Relationship between liver function and brain shrinkage in patients with alcohol dependence. Alcoholism: Clin Experiment Res. 2012;36:625–632. doi: 10.1111/j.1530-0277.2011.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harper CG, Kril JJ. Neuropathology of alcoholism. Alcohol Alcoholism. 1990;25:207–216. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- 7.Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- 8.Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, et al. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz SM, Oscar-Berman M, Sawyer KS, Valmas MM, Urban T, et al. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcohol Clin Exp Res. 2013;37:110–122. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: A magnetic resonance imaging study. Alcohol Clin Exp Res. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 11.Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- 12.Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, et al. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol Clin Exp Res. 2008;32:1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenbloom MJ, Sassoon SA, Pfefferbaum A, Sullivan EV. Contribution of regional white matter integrity to visuospatial construction accuracy, organizational strategy, and memory for a complex figure in abstinent alcoholics. Brain Imaging Behav. 2009;3:379–390. doi: 10.1007/s11682-009-9080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulte T, Muller-Oehring EM, Sullivan EV, Pfefferbaum A. White matter fiber compromise contributes differentially to attention and emotion processing impairment in alcoholism, HIV-infection, and their comorbidity. Neuropsychologia. 2012;50:2812–222. doi: 10.1016/j.neuropsychologia.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, et al. Corpus callosum in maltreated children with posttraumatic stress disorder: A diffusion tensor imaging study. Psychiatry Res. 2008;162:256–261. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villarreal G, Hamilton DA, Graham DP, Driscoll I, Qualls C, et al. Reduced area of the corpus callosum in posttraumatic stress disorder. Psychiatry Res. 2004;131:227–235. doi: 10.1016/j.pscychresns.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, et al. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Jeong DU, Sim ME, Bae SC, Chung A, et al. Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology. 2006;54:120–125. doi: 10.1159/000098262. [DOI] [PubMed] [Google Scholar]
- 20.Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, et al. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med. 2012;42:533–543. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels JK, Lamke JP, Gaebler M, Walter H, Scheel M. White matter integrity and its relationship to PTSD and childhood trauma--A systematic review and meta-analysis. Depress Anxiety. 2013;30:207–216. doi: 10.1002/da.22044. [DOI] [PubMed] [Google Scholar]
- 22.Constans JI, McCloskey MS, Vasterling JJ, Brailey K, Mathews A. Suppression of attentional bias in PTSD. J Abnorm Psychol. 2004;113:315–323. doi: 10.1037/0021-843X.113.2.315. [DOI] [PubMed] [Google Scholar]
- 23.Hart J, Jr, Kimbrell T, Fauver P, Cherry BJ, Pitcock J, et al. Cognitive dysfunctions associated with PTSD: Evidence from World War II prisoners of war. J Neuropsychiatry Clin Neurosci. 2008;20:309–316. doi: 10.1176/jnp.2008.20.3.309. [DOI] [PubMed] [Google Scholar]
- 24.Emdad R, Sondergaard HP. Visuoconstructional ability in PTSD patients compared to a control group with the same ethnic background. Stress Health. 2006;22:35–43. [Google Scholar]
- 25.Jacobson IG, Ryan MA, Hooper TI, Smith TC, Amoroso PJ, et al. Alcohol use and alcohol-related problems before and after military combat deployment. JAMA. 2008;300:663–675. doi: 10.1001/jama.300.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milliken CS, Auchterlonie JL, Hoge CW. Longitudinal assessment of mental health problems among active and reserve component soldiers returning from the Iraq war. JAMA. 2007;298:2141–2148. doi: 10.1001/jama.298.18.2141. [DOI] [PubMed] [Google Scholar]
- 27.Hooper R, Rona RJ, Jones M, Fear NT, Hull L, et al. Cigarette and alcohol use in the UK Armed Forces, and their association with combat exposures: A prospective study. Addict Behav. 2008;33:1067–1071. doi: 10.1016/j.addbeh.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 28.McFarlane AC. Epidemiological evidence about the relationship between PTSD and alcohol abuse: The nature of the association. Addict Behav. 1998;23:813–825. doi: 10.1016/s0306-4603(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 29.Epstein JN, Saunders BE, Kilpatrick DG, Resnick HS. PTSD as a mediator between childhood rape and alcohol use in adult women. Child Abuse Negl. 1998;22:223–234. doi: 10.1016/s0145-2134(97)00133-6. [DOI] [PubMed] [Google Scholar]
- 30.Brady KT, Waldrop AE, McRae AL, Back SE, Saladin ME, et al. The impact of alcohol dependence and posttraumatic stress disorder on cold pressor task response. J Stud Alcohol. 2006;67:700–706. doi: 10.15288/jsa.2006.67.700. [DOI] [PubMed] [Google Scholar]
- 31.Back SE, Brady KT, Sonne SC, Verduin ML. Symptom improvement in co-occurring PTSD and alcohol dependence. J Nerv Ment Dis. 2006;194:690–696. doi: 10.1097/01.nmd.0000235794.12794.8a. [DOI] [PubMed] [Google Scholar]
- 32.Shipherd JC, Stafford J, Tanner LR. Predicting alcohol and drug abuse in Persian Gulf War veterans: what role do PTSD symptoms play? Addict Behav. 2005;30:595–599. doi: 10.1016/j.addbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Brady KT, Sonne SC, Roberts JM. Sertraline treatment of comorbid posttraumatic stress disorder and alcohol dependence. J Clin Psychiatry. 1995;56:502–505. [PubMed] [Google Scholar]
- 34.Villarreal G, Petropoulos H, Hamilton DA, Rowland LM, Horan WP, et al. Proton magnetic resonance spectroscopy of the hippocampus and occipital white matter in PTSD: Preliminary results. Can J Psychiatry. 2002;47:666–670. doi: 10.1177/070674370204700709. [DOI] [PubMed] [Google Scholar]
- 35.Canive JM, Lewine JD, Orrison WW, Jr, Edgar CJ, Provencal SL, et al. MRI reveals gross structural abnormalities in PTSD. Ann N Y Acad Sci. 1997;821:512–515. doi: 10.1111/j.1749-6632.1997.tb48318.x. [DOI] [PubMed] [Google Scholar]
- 36.Pitman RK. Hippocampal diminution in PTSD: more (or less?) than meets the eye. Hippocampus. 2001;11:73–74. doi: 10.1002/hipo.1022. [DOI] [PubMed] [Google Scholar]
- 37.Vasterling JJ, Brailey K. Neuropsychological findings in adults with PTSD. Neuropsychology of PTSD: Biological, cognitive, and clinical perspectives. Guilford Pres; New York, USA: 2005. pp. 178–207. [Google Scholar]
- 38.Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr, et al. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- 39.Uddo M, Vasterling JJ, Brailey K, Sutker PB. Memory and attention in combat-related post-traumatic stress disorder (PTSD). J Psychopathol Behav Assess. 1993;15:43–52. [Google Scholar]
- 40.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th Edn DSM-IV-TR, American Psychiatric Publishing Inc.; Arlington, USA: 2000. [Google Scholar]
- 41.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthews SC, Strigo IA, Simmons AN, O'Connell RM, Reinhardt LE, et al. A multimodal imaging study in U.S. veterans of Operations Iraqi and Enduring Freedom with and without major depression after blast-related concussion. Neuroimage. 2011;54:S69–S75. doi: 10.1016/j.neuroimage.2010.04.269. [DOI] [PubMed] [Google Scholar]
- 44.Crooks CY, Zumsteg JM, Bell KR. Traumatic brain injury: A review of practice management and recent advances. Phys Med Rehabil Clin N Am. 2007;18:681–710. doi: 10.1016/j.pmr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Katz DI, Alexander MP. Traumatic brain injury. Predicting course of recovery and outcome for patients admitted to rehabilitation. Arch Neurol. 1994;51:661–670. doi: 10.1001/archneur.1994.00540190041013. [DOI] [PubMed] [Google Scholar]
- 46.Iverson GL, Lovell MR, Collins MW. Validity of ImPACT for measuring processing speed following sports-related concussion. J Clin Exp Neuropsychol. 2005;27:683–689. doi: 10.1081/13803390490918435. [DOI] [PubMed] [Google Scholar]
- 47.Slick DJ, Sherman EM, Iverson GL. Diagnostic criteria for malingered neurocognitive dysfunction: proposed standards for clinical practice and research. Clin Neuropsychol. 1999;13:545–561. doi: 10.1076/1385-4046(199911)13:04;1-Y;FT545. [DOI] [PubMed] [Google Scholar]
- 48.Whitney KA, Shepard PH, Williams AL, Davis JJ, Adams KM. The Medical Symptom Validity Test in the evaluation of Operation Iraqi Freedom/Operation Enduring Freedom soldiers: A preliminary study. Arch Clin Neuropsychol. 2009;24:145–152. doi: 10.1093/arclin/acp020. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg LM, Waldman ID. Developmental normative data on the test of variables of attention (T.O.V.A.). J Child Psychol Psychiatry. 1993;34:1019–1030. doi: 10.1111/j.1469-7610.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 50.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 51.Fortier CB, Amick MM, Grande L, McGlynn S, Kenna A, et al. The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) Semistructured Interview: Evidence of Research Utility and Validity. J Head Trauma Rehabil. 2013 doi: 10.1097/HTR.0b013e3182865859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morra L, Fortier CB, Amick AM, Grande LJ, Milberg WP, et al. Assessment of mild traumatic brain injury in OEF/OIF service members: Blast and lifetime TBI semi-structured interview (BLT).. International Neuropsychological Society Annual Meeting, and Federal Interagency Conference on Traumatic Brain Injury; Boston, MA, USA. 2011. [Google Scholar]
- 53.Charness ME. Clinical and pathological overview of the brain disorders in alcoholics. In: Hunt Nixon., editor. Alcohol-induced brain damage. NIH, NIAAA; Rockville, USA: 1993. pp. 15–38. [Google Scholar]
- 54.Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST). J Stud Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- 55.Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST). J Stud Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- 56.Shields AL, Howell RT, Potter JS, Weiss RD. The Michigan Alcoholism Screening Test and its shortened form: A meta-analytic inquiry into score reliability. Subst Use Misuse. 2007;42:1783–1800. doi: 10.1080/10826080701212295. [DOI] [PubMed] [Google Scholar]
- 57.Wechsler D. Manual for the Wechsler adult intelligence scale-III. Psychological Corporation; San Antonio, TX, USA: 1997. [Google Scholar]
- 58.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes LL, et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 59.Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J Int Neuropsychol Soc. 1998;4:474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- 60.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 61.Delis DC, Kaplan E, Kramer JH. D-KEFs examiners and technical manual. Pearson Education; San Antonio, Texas, USA: 2001. [Google Scholar]
- 62.Basso MR, Bornstein RA, Lang JM. Practice effects on commonly used measures of executive function across twelve months. Clin Neuropsychol. 1999;13:283–292. doi: 10.1076/clin.13.3.283.1743. [DOI] [PubMed] [Google Scholar]
- 63.Chase HW, Clark L, Sahakian BJ, Bullmore ET, Robbins TW. Dissociable roles of prefrontal subregions in self-ordered working memory performance. Neuropsychologia. 2008;46:2650–2661. doi: 10.1016/j.neuropsychologia.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 64.Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, et al. Detecting dementia: Novel neuropsychological markers of preclinical Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;17:42–48. doi: 10.1159/000074081. [DOI] [PubMed] [Google Scholar]
- 65.Jazbec S, Pantelis C, Robbins T, Weickert T, Weinberger DR, et al. Intra-dimensional/extra-dimensional set-shifting performance in schizophrenia: Impact of distractors. Schizophr Res. 2007;89:339–349. doi: 10.1016/j.schres.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 66.Luciana M. Practitioner review: Computerized assessment of neuropsychological function in children: Clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB). J Child Psychol Psychiatry. 2003;44:649–663. doi: 10.1111/1469-7610.00152. [DOI] [PubMed] [Google Scholar]
- 67.Rogers RD, Sahakian BJ, Hodges JR, Polkey CE, Kennard C, et al. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson's disease. Brain. 1998;121:815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- 68.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test®. 2nd Edn The Psychological Corporation; San Antonio, TX, USA: 2000. [Google Scholar]
- 69.Green P. Green's medical symptom validity test (MSVT) for windows: User's manual. Green's Publishing; Edmonton, Canada: 2004. [Google Scholar]
- 70.Moore BA, Donders J. Predictors of invalid neuropsychological test performance after traumatic brain injury. Brain Inj. 2004;18:975–984. doi: 10.1080/02699050410001672350. [DOI] [PubMed] [Google Scholar]
- 71.DeJong J, Donders J. A confirmatory factor analysis of the California Verbal Learning Test--Second Edition (CVLT-II) in a traumatic brain injury sample. Assessment. 2009;16:328–336. doi: 10.1177/1073191109336989. [DOI] [PubMed] [Google Scholar]
- 72.Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test--Second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropsychol. 2006;21:413–420. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Henry M, Merten T, Wolf SA, Harth S. Nonverbal medical symptom validity test performance of elderly healthy adults and clinical neurology patients. J Clin Exp Neuropsychol. 2010;32:19–27. doi: 10.1080/13803390902791653. [DOI] [PubMed] [Google Scholar]
- 74.Wechsler D. Wechsler test of adult reading. Psychological Corporation; San Antonio, TX, USA: 2001. [Google Scholar]
- 75.Holdnack HA. Wechsler test of adult reading: WTAR. Psychological Corporation; San Antonio, TX, USA: [Google Scholar]
- 76.Bryden PJ, Roy EA. A new method of administering the Grooved Pegboard Test: Performance as a function of handedness and sex. Brain Cogn. 2005;58:258–268. doi: 10.1016/j.bandc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Tiffen J. Purdue pegboard examiner's manual. London House; Rosemont, IL, USA: 1968. [Google Scholar]
- 78.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: Administration, Norms, and Commentary. 3rd Edn Oxford University Press; New York, USA: 2006. [Google Scholar]
- 79.Fregly AR, Graybiel A, Smith MJ. Walk on floor eyes closed (WOFEC): A new addition to an ataxia test battery. Aerosp Med. 1972;43:395–399. [PubMed] [Google Scholar]
- 80.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 81.Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42:515–525. [PubMed] [Google Scholar]
- 82.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brands AM, Kessels RP, Hoogma RP, Henselmans JM, van der Beek Boter JW, et al. Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes. 2006;55:1800–1806. doi: 10.2337/db05-1226. [DOI] [PubMed] [Google Scholar]
- 84.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 85.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fischl B, Liu A, Dale AM. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 87.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 88.Fischl B, Salat DH, van der Kouwe AJ, Makris N, Ségonne F, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 89.Ségonne F, Dale AM, Busa E, Glessner M, Salat D, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 90.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 91.Ségonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 92.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, et al. Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners and field strengths. Neuroimage. 2008;39:10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 95.Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- 97.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 98.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 99.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 100.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 102.Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB Technical Report TR07JA1. FMRIB Centre; Oxford, United Kingdom: 2007. [Google Scholar]
- 103.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, et al. Nonrigid registration using free-form deformations: Application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 104.Oouchi H, Yamada K, Sakai K, Kizu O, Kubota T, et al. Diffusion anisotropy measurement of brain white matter is affected by voxel size: Underestimation occurs in areas with crossing fibers. AJNR Am J Neuroradiol. 2007;28:1102–1106. doi: 10.3174/ajnr.A0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dean CB, Nielsen JD. Generalized linear mixed models: A review and some extensions. Lifetime Data Anal. 2007;13:497–512. doi: 10.1007/s10985-007-9065-x. [DOI] [PubMed] [Google Scholar]
- 106.Royston P. Multiple imputation of missing values. The Stata Journal. 2004;4:227–241. [Google Scholar]
- 107.Van Buuren S, Groothuis-Oudshoorn K. Mice: Multivariate imputation by chained equations in R. J Statist Softw. 2011;45:1–67. [Google Scholar]
- 108.Revelle W. psych: Procedures for psychological, psychometric, and personality research. Northwestern University; Evanston, Illinois, USA: 2013. [Google Scholar]
- 109.Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz MS, Luhar RB, et al. Profiles of impaired, spared, and recovered neuropsychological processes in alcoholism. In: Pfefferbaum A, Sullivan EV, editors. Handbook of clinical neurology. Elsevier; Edinburgh, Scotland: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fortier CB, Leritz EC, Salat DH, Venne JR, Maksimovskiy AL, et al. Reduced cortical thickness in abstinent alcoholics and association with alcoholic behavior. Alcohol Clin Exp Res. 2011;35:2193–2201. doi: 10.1111/j.1530-0277.2011.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- 112.Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, et al. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res. 2003;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- 113.Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Braus DF, Wrase J, Grüsser S, Hermann D, Ruf M, et al. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm. 2001;108:887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- 115.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schmahmann JD, Pandya DN. The cerebrocerebellar system. Int Rev Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 117.Adams A. Studies on the flat electroencephalogram in man. Electroencephalogr Clin Neurophysiol. 1959;11:35–41. doi: 10.1016/0013-4694(59)90005-7. [DOI] [PubMed] [Google Scholar]
- 118.Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci. 1997;17:438–458. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 120.McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, et al. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cisler JM, Amstadter AB, Begle AM, Resnick HS, Danielson CK, et al. PTSD symptoms, potentially traumatic event exposure, and binge drinking:A prospective study with a national sample of adolescents. J Anxiety Disord. 2011;25:978–987. doi: 10.1016/j.janxdis.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bradley KA, Bush KR, Davis TM, Dobie DJ, Burman ML, et al. Binge drinking among female Veterans Affairs patients: Prevalence and associated risks. Psychol Addict Behav. 2001;15:297–305. doi: 10.1037//0893-164x.15.4.297. [DOI] [PubMed] [Google Scholar]
- 123.Goodlett CR, Gilliam DM, Nichols JM, West JR. Genetic influences on brain growth restriction induced by development exposure to alcohol. Neurotoxicology. 1989;10:321–334. [PubMed] [Google Scholar]
- 124.Pfefferbaum B, Doughty DE. Increased alcohol use in a treatment sample of Oklahoma City bombing victims. Psychiatry. 2001;64:296–303. doi: 10.1521/psyc.64.4.296.18598. [DOI] [PubMed] [Google Scholar]
- 125.Gilman JM, Davis MB, Hommer DW. Greater activation in left hemisphere language-related regions during simple judgment tasks among substance-dependent patients in treatment for alcoholism. Alcohol Clin Exp Res. 2010;34:331–341. doi: 10.1111/j.1530-0277.2009.01095.x. [DOI] [PubMed] [Google Scholar]
- 126.Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, et al. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 127.Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. Neuroimage Clin. 2013;2:601–611. doi: 10.1016/j.nicl.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]