Abstract
Since its discovery in 1982, the global importance of H. pylori-induced disease, particularly in developing countries, remains high. The use of rodent models particularly mice, and the unanticipated usefulness of the gerbil to study H. pylori pathogenesis have been used extensively to study the interactions of the host, the pathogen and the environmental conditions influencing the outcome of persistent H. pylori infection. Dietary factors in humans are increasingly recognized as being important factors in modulating progression and severity of H. pylori-induced gastric cancer. Studies using rodent models to verify and help explain mechanisms whereby various dietary ingredients impact disease outcome should continue to be extremely productive.
Keywords: Helicobacter pylori, Helicobacter felis, mice, gerbils, diet
Introduction
Helicobacter pylori, first isolated from inflamed gastric tissues of humans in 1982, is now firmly established as an important human pathogen. Prior to its discovery, despite earlier observations of gastric spiral organisms being present in human and animal gastric tissues (Freedberg and Barron, 1940, Doenges, 1938), an often stated principle, championed particularly by gastroenterologists, was the stomach for all practical purposes was considered to be sterile because of the stomach’s low pH. It is now known that H. pylori infection is persistent and is always associated with inflammation consisting of mononuclear cells and polymorphonuclear cells. When the infected individual is treated with antibiotics known to eradicate H. pylori, the gastritis regresses over time. However, if there is recrudescence of the organism due to ineffective antibiotic therapy or the patient becomes reinfected with another H. pylori strain, the gastritis reappears. It has been determined through rigorous clinical and epidemiologic studies that H. pylori is directly linked to peptic ulcer disease and importantly, the World Health Organization has listed H. pylori as a class I carcinogen (IARC, 1994). H. pylori is also considered to initiate and sustain low grade gastric mucosal-associated lymphoma (Muller, 2009). Prior to the discovery of H. pylori, peptic ulcer disease was linked to excessive gastric acid production and/or stress, whereas gastric cancer was linked to a variety of dietary factors documented by extensive epidemiologic surveys.
Although 50% of the world population is infected with H. pylori, only a small percentage (1–3%) of individuals have the gastric disease progress to gastric adenocarcinoma. Gastric adenocarcinoma (GAC) represents the most commonly diagnosed (~95%) of gastric cancers, and is the fourth and fifth most common cancer in males and females, respectively (Jemal et al., 2011). Worldwide, there were an estimated 989,000 cases of gastric cancer and 738,000 gastric cancer associated deaths in 2008, making gastric cancer the fourth most common cancer and the second leading cause of cancer death, after that recorded for lung cancer (Ferlay et al., 2010).
A strong genetic predisposition to development of H. pylori-associated gastric cancer has been established. Gastric cancer risk is increased up to threefold in individuals with a first-degree relative with gastric cancer, and 10% of gastric cancer cases exhibit familial clustering. While it’s well established that H. pylori can infect multiple family members, family history remains a risk factor even after controlling for H. pylori infection. However, only a small fraction of the familial clustering of gastric cancer is attributable to known family cancer syndromes. It was observed that H. pylori infected patients who developed gastric cancer secreted lower levels of gastric acid compared to H. pylori infected patients with duodenal ulcers. Thus, in a landmark study, the authors chose to study ILβ, a known proinflammatory cytokine with marked acid inhibitory properties in families with precancerous lesions attributable to H. pylori (El-Omar et al., 2000). They demonstrated that individuals with specific genetic polymorphisms in IL1β and IL1RN had a 2 to 3 fold increased risk of developing gastric atrophy and gastric cancer (El-Omar et al., 2000). These findings have been confirmed in some studies, but not others. In a meta-analysis of ILβ and ILR antagonist gene polymorphism and risk of gastric cancer, IL1β-511T and IL1RN*2 were associated with gastric cancer risk in Caucasians, but not in Asians. For IL1β-511T, the association in Caucasians was stronger when intestinal-subtypes and noncardia gastric cancer cases were examined (Camargo et al., 2006). Further genetic polymorphisms in TNFα and IL10, when combined with IL1B polymorphisms, result in higher risk genotype, conferring a 27-fold or greater risk of developing gastric cancer (El-Omar et al., 2003).
The mechanisms involved in the pathogenesis of GAC remains incompletely characterized but clearly the interactions of host, environmental variables, and H. pylori virulence factors, particularly the cag pathogenicity island (PAI) and vacuolating cytotoxin, play a role in cancer initiation and progression (Jones et al., 2010). These virulence factors are polymorphic and affect a multitude of host cellular pathways and thereby contribute to differences in disease severity given that various CagA and VacA alleles differentially target selected pathways (Jones et al., 2010). As reviewed by Jones et al in 2010, cytotoxin-associated Gene A is encoded on the cag PAI, which is a horizontally acquired 40kb DNA segment that encodes for a type IV secretion system (Jones et al., 2010). cagA is the terminal gene on the cag PAI, and encodes for the 120–145kDa immunodominant CagA protein (Covacci et al., 1993, Tummuru et al., 1993). Once injected into host cells, CagA acts directly in an unphosphorylated state to influence cellular tight junction (Oliveira et al., 2009), cellular polarity (Zeaiter et al., 2008), cell proliferation and differentiation (Lee et al., 2010), cell scattering (Mimuro et al., 2002), and induction of the inflammatory response (Brandt et al., 2005). Upon entering the gastric epithelia, CagA localizes to the plasma membrane and can be phosphorylated by either Abl kinase or Src family kinases (Poppe et al., 2007, Tammer et al., 2007). These kinase phosphorylate tyrosine residues are located within the carboxy-terminus of CagA. These repeats are based on the amino acid sequences found within the regions flanking the EPIYA sequence and are classified as four distinct EPIYA motifs: EPIYA-A, -B, -C, and –D (Jones et al., 2010). Once phosphorylated, CagA can form cellular interactions that influence cellular shape and motility (Tammer et al., 2007, Jones et al., 2010, Brandt et al., 2007). The presence of CagA is associated with more severe disease. Cancer patients are at least twice as likely to be infected with an H. pylori strain that is cagA positive than one that is cagA negative (Gwack et al., 2006). The VacA cytotoxin is produced and secreted by the majority of H. pylori strains. Vacuolating cytotoxin A activity was first noted when H. pylori filtrates induced large host cell vacuoles (Leunk et al., 1988). VacA remains on the bacterial surface or is secreted as an approximately 88 kDa toxin (Cover and Blaser, 1992). Exposure to alkaline or acidic conditions amplifies that activity of VacA, where the toxin induces large cytoplasmic vacuoles in host cells that contain the markers for late endosomes and lysosomes. VacA also causes apoptosis, which is dependent on interaction with the mitochondria (Foo et al., 2010). VacA also deregulates multiple cellular pathways as well as inducing inflammation. VacA intoxication of host cells induces production of a variety of inflammatory cytokines that include TNFα, IL-1β, IL-6, IL-10, and IL-13 (Supajatura et al., 2002).
Animal models, particularly rodents, continue to play an invaluable role in dissecting interconnected mechanisms that determine gastric disease outcome.
Rodent Models for Helicobacter-Associated Gastric Cancer
Mice
H. felis infection
Most of the major advances in our understanding of gastric cancer pathogenesis have been derived from studies with mice. While a number of other animal models have been developed for both gastric cancer (e.g., MNNG-induced cancer in rats) and for Helicobacter-mediated gastric infection (ferret, gerbil, gnotobiotic pig, primate, etc.), the mouse model has clear advantages with respect to their small size, cost, ease of infection, reproducibility, and especially the power of genetic manipulation. The first study to ascertain whether gastric helicobacters would colonize the stomach of rodents utilized germ-free Swiss Webster mice or Sprague Dawley rats infected with H. felis (Fox et al., 1991, Lee et al., 1990). Although the rat is the preferred model for gastric carcinogenesis studies, long-term H.felis and H. pylori colonization studies in this species have not been performed. This model therefore requires further development before it can be used in carcinogenesis studies. In the first description of H.felis gastritis in the germ-free mouse, a moderate degree of fundic glandular epithelial cell hyperplasia was evident at 4 weeks in portions of the gastric mucosa (Lee et al., 1990). This was characterized by an overall increase in thickness of the mucosa in areas of inflamed tissue. The changes included lack of epithelial cell maturation and differentiation, increased basophilia and increased nuclear to cytoplasmic ratio. Also of interest was the enlargement of individual glands and the presence of columnar type epithelium vs. cuboidal epithelium. Occasional branching of glands was noted as well as luminal epithelium forming small pseudovillus projections. These lesions persisted for the duration of the 8 week study (Lee et al., 1990).
These studies have been extended by documentation of persistent active, chronic gastritis in germ-free mice and SPF mice, infected with H. felis for 1 year (Fox et al., 1993). Studies of 72–76-week colonization of conventional and SPF mice have provided additional clues on progression of the lesions in the stomach of infected mice. Studying the role of genetic factors in the pathogenesis of Helicobacter-associated gastritis by taking advantage of the wide range of inbred and other types of genetically defined mice was a logical next step in further exploration of Helicobacter pathogenesis using this model. Two different laboratories inoculated three inbred strains of mice with H. felis and tabulated the intensity of inflammation; in BALB/c mice inflammation was minimal, in C3H/He moderate and was most severe in C57BL/6 when examined 2–11 weeks post-infection (Mohammadi et al., 1996, Sakagami et al., 1994). The authors also stated that C57BL/6 mice had mucuous cell hyperplasia and parietal cell loss in gastric fundic glands (Mohammadi et al., 1996). Furthermore, the study demonstrated that by using congenic strains on the BALB/c and C57BL background, both MHC and non-MHC genes contributed to H. felis-associated gastritis (Mohammadi et al., 1996). The H. felis infected mouse model continues to be used frequently with considerable success in studying environmental and host factors centered on Helicobacter spp. pathogenesis (See Table 1 and below) (Wang et al., 2000).
Table 1.
The Helicobacter infected INS/GAS mouse model of Gastric Cancer
| Purpose of analysis | Results | Reference |
|---|---|---|
| H. pylori and H. felis and gastric mucosa | progression to gastric atrophy, metaplasia, dysplasia, and cancer | (Wang et al., 2000, Fox et al., 2003b, Fox et al., 2003a) |
| Gender differences | increased gastric carcinogenesis in males | (Fox et al., 2003a, Fox et al., 2003b) |
| Importance of CagE | loss of cagE temporally retards but does not abrogate cancer progression | (Fox et al., 2003b) |
| Interaction with G-Gly | G-gly synergizes with amidated gastrin to stimulate acid secretion and inhibits atrophy | (Cui et al., 2004) |
| CCK2R and Histamine receptor inhibitors | CCK2R and H2R antagonists synergistically inhibit gastric atrophy and cancer | (Takaishi et al., 2005) |
| Apoptosis of gastric epithelium | gastrin induces apoptosis and contribute to gastric carcinogenesis | (Cui et al., 2006, Przemeck et al., 2008) |
| TFF2 expression | TFF2 expression in the gastric fundus was elevated | (Tu et al., 2007) |
| Swedish variant of moist oral smokeless tobacco | tobacco promotes cancer formation | (Stenstrom et al., 2007) |
| Reg-1 expression | Reg1 is increased in the stomachs of H. felis-infected INS-GAS mice | (Steele et al., 2007) |
| Role of 17-beta-estradiol | 17beta-estradiol has protective effects on gastric cancer development | (Ohtani et al., 2007, Ohtani et al., 2011, Sheh et al., 2011) |
| Eradication of gastric Helicobacter spp. | eradication inhibits mouse gastric carcinogenesis | (Cai et al., 2005, Lee et al., 2008a, Lee et al., 2008b) |
| Commensal bacterial flora in the stomach | SPF mice are more susceptible to gastric cancer than germ-free mice | (Lofgren et al., 2011) |
| ASF restricted flora | restricted Altered Schaedler flora colonizes gastric niche and enhances gastric | (Lertpiriyapong et al., 2013) |
| High salt diet | high salt did not promote carcinogenesis | (Fox et al., 2003a) |
| Antral carcinogenesis | gastrin suppresses antral carcinogenesis | (Tomita et al., 2011, Takaishi et al., 2009) |
| H. felis-induced iron deficiency | marked changes in expression of gastric iron transporters and lowered systemic iron | (Thomson et al., 2012) |
| Folic acid supplementation | prevents gastric cancer | (Gonda et al., 2012) |
In the early investigations by a number of groups, it became clear from studies with inbred mice that progression to preneoplasia was largely determined by the host response. For example, the C57BL/6 inbred mouse strain responded to H. felis infection with a robust Th1 immune response, in contrast to the BALB/c inbred strain which showed a predominant Th2 response (Mohammadi et al., 1996, Sakagami et al., 1994, Wang and Fox, 1998). Consequently, C57BL/6 mice showed rapid premalignant progression to atrophy and metaplasia with declining colonization levels, while BALB/c mice maintained high numbers of organisms with minimal epithelial in jury or atrophy. Additional studies employing RAG and SCID mice and T-cell-deficient mice pointed to the importance of T-cell responses (Roth et al., 1999), while the use of cytokine knockout mice indicated that H. pylori-induced mucosal inflammationis Th1-mediated and exacerbated in IL-4 but not IFN-γ gene-deficient mice (Smythies et al., 2000).
However, while genetic manipulation of the various cytokine genes clarified many aspects of the immunopathogenesis of the disease, the unmanipulated C57BL/6 mouse has proved surprisingly robust as a model for cancer development. Interestingly, many of the early H. felis infection studies included observations periods that extended up to one year but not beyond, and described changes of atrophy and metaplasia, but not more advanced pathology. The presence of dysplasia and invasive carcinoma in the H. felis-infected C57BL/6 mouse was first described by Fox and Wang (Fox et al., 2002), when the observation period was extended to 14 months. More recently, progression to antral carcinomas has been reported in mice in which the model has been extended out to 22 months (Cai et al., 2005).
The first Helicobacter-dependent mouse model of gastric cancer was reported by Wang et al., who described H. felis infection of insulin-gastrin (INS-GAS) transgenic mice in an FVB/N inbred background (Wang et al., 2000). The model has proved ideal as an accelerated model of carcinogenesis. A role for host and dietary factors also has been demonstrated using Helicobacter-infected INS/GAS mice (Table 1).
The gastric hormone gastrin stimulates gastric acid secretion and growth of the acid-secreting part of the stomach. H. pylori infection in humans results in a mild hypergastrinemia (1.5–2-fold elevation compared with uninfected subjects) that occurs early in the course of infection (Mulholland et al., 1993, Levi et al., 1989), precedes the development of atrophic gastritis, and often resolves after eradication of the infection. Chronic atrophic gastritis, a pathological condition characterized by decreased numbers of parietal cells and low levels of acid secretion and occurs as part of progression of Helicobacter spp. associated gastric lesions in the carcinogen cascade. The insulin-gastrin (INS/GAS) transgenic mice have a chimeric transgene in which the human gastrin gene is transcribed from the rat insulin I promoter (Wang et al., 1993). These mice express human heptadecapeptide gastrin (G-17) in the pancreatic beta cells, which is then secreted into the circulation. Changes in gastrin secretion are divided into 2 distinct phases: an early phase (1–4 months) and a later phase (5 months and older). Gastrin levels increased modestly during the early phase, and approximately 2-fold compared with wild-type FVB/N mice (Wang et al., 1996). Slightly more than 50% of the serum gastrin (and all of the increase) in young INS-GAS mice is human gastrin resulting from pancreatic secretion. In contrast to the elevated plasma amidated gastrin levels, glycine-extended gastrin levels were all <20 pmol/L. Serum amidated gastrin levels are increased gradually beginning at 6 months, with levels peaking at 550 pmol/L by 20 months of age. Most of the increase in serum gastrin resulted from increased secretion of endogenous mouse amidated gastrin, with lesser changes in the level of human G-17, which increases 3-fold from 3 months to 20 months. During the late gastrin phase (5 months and older), INS/GAS mice have a gradual change in gastric function with a marked decline in the level of stimulated acid secretion. These decreases in acid production are mirrored by decreases in parietal and ECL cell numbers (Wang et al., 2000).
In the INS/GAS mouse model there is an increased expression of growth factors, heparin-binding epidermal growth factor and TNFα. At 20 months of age, INS/GAS mice don’t have increased enterochromaffin-like cell number, but rather exhibited gastric metaplasia, dysplasia, carcinoma in situ, and gastric cancer with vascular invasion. Experimental H. felis infection of INS-GAS mice led to accelerated (≤ 8 mo) development of intramucosal carcinoma (85%), with submucosal invasion (54%) and intravascular invasion (46%; P ≤ 0.05) (Wang et al., 2000). These findings support the conclusion that chronic hypergastrinemia in mice synergizes with H. felis and H. pylori infections and contributes to eventual parietal cell loss and progression to proximal gastric cancer. The model leads to accelerated gastric cancer in part due to the effect of elevated circulating levels of amidated gastrin which bind to CCK-B receptors in the gastric corpus to activate a histamine pathway, leading to altered growth and immune responses (Takaishi et al., 2005), and to relatively low levels of glycine-extended gastrin which may inhibit progression to preneoplasia (Cui et al., 2003). However, while hypergastrinemia clearly accelerates proximal gastric cancer, it also appears to protect against the more distal, antral gastric cancers (Takaishi et al., 2009, Tomita et al., 2011).
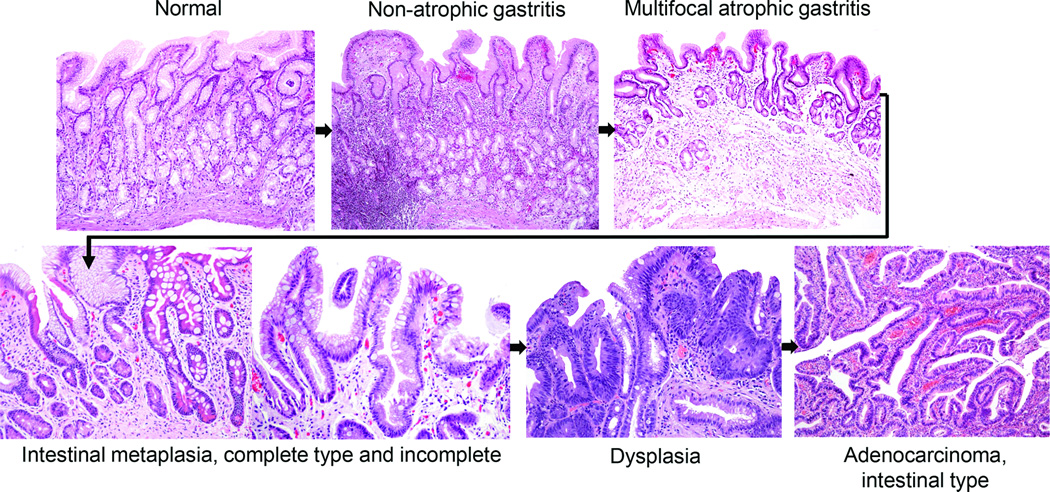
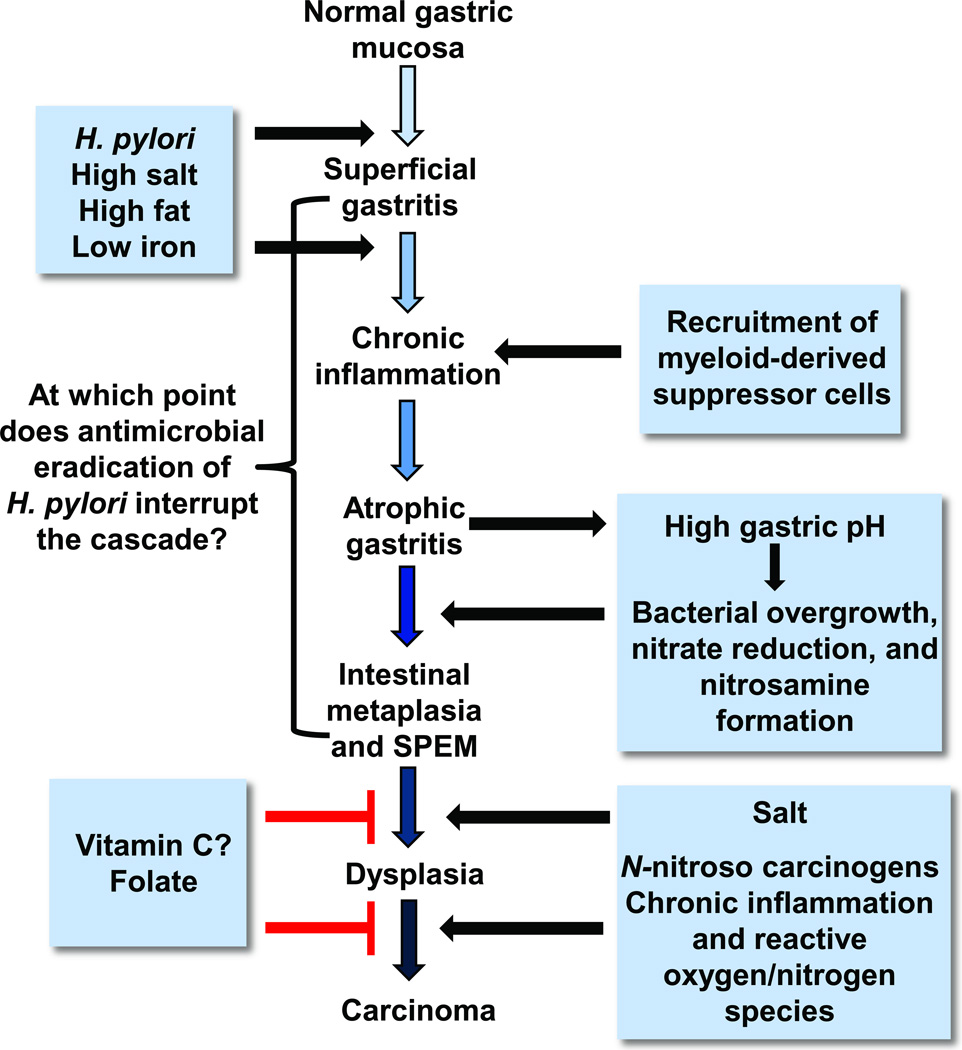
The INS-GAS mouse model has been used to demonstrate that male gender is important in Helicobacter-associated cancer progression (Fox et al., 2003a, Fox et al., 2003b). Further studies demonstrated the protective effects of estrogen (Sheh et al., 2011, Ohtani et al., 2007). Thus, the INS/GAS model mimics the 2:1 ratio of male:female in gastric cancer development of humans. The INS/GAS model infected with either H. felis or H. pylori, consistently reproduces the progression of gastric diseases that is recognized in humans infected with H. pylori, known as Correa’s Model of Gastric Carcinogenesis (Figures 1 and 2) (Fox and Wang, 2007). However, the hypergastrinemic phenotype to date appears most prominent in INS-GAS mice in an FVB/N background, and is less prominent in a C57BL/6 background (Takaishi et al., 2009).
Figure 1.
Correa’s Cascade of Gastric Carcinogenesis. Courtesy of Pelayo Correa
Figure 2.
Proposed Correa pathway of pathological events and dietary influence on in gastric cancer progression. In well-differentiated, intestinal-type gastric cancer, histopathological studies indicated that chronic H. pylori infection progresses over decades through stages of chronic gastritis, atrophy, intestinal metaplasia, dysplasia, and cancer (Correa, 1991). The development of cancer has been attributed to alterations in DNA caused by chronic inflammation, recruitment and engraftment of bone marrow–derived cells, an imbalance between epithelial cell proliferation and apoptosis, and, in a milieu of atrophy and achlorhydria, gastric colonization by enteric bacteria with nitrate reductase activity, which facilitates the formation of carcinogenic nitrosamines. Corpus-predominant atrophy, or the loss of specialized glandular cell types such as parietal and chief cells, appears to be the critical initiating step in the progression toward cancer. (Modified from (Fox and Wang, 2007))
H. pylori infection
As described above, the C57BL/6 mouse model of gastric H. felis infection has been very useful for the study for manipulation of the Th1-Th2 immune response. However, another factor is likely to be the particular H. pylori strain used to infect mice, and this question has been more difficult to address using the wild type C57BL/6 mouse. A preliminary report published in 1991, indicated that fresh clinical isolates of H. pylori, but not stock culture isolates colonized the stomach of athymic and euthymic mice for a short time interval (Karita et al., 1991). The mouse model was further developed to test the ability of H. pylori strains that expressed vascuolating cytotoxin (VacA) and the immunodominant cytotoxin associated antigen (CagA) vs. H. pylori strains that did not express the VacA and CagA antigens (Marchetti et al., 1995). The ability of H. pylori to colonize was enhanced (over that of clinical isolates) by serial passage of H. pylori in mice. Thus ‘mouseified’ H. pylori strains used in the experiments assessing gastric pathology were isolated from mice 2 weeks after inoculation, expanded in vitro and used in subsequent studies. The strains expressing the VacA and CagA experimentally produced gastritis for 8 weeks, whereas the strain lacking these antigens did not produce gastric pathology (Marchetti et al., 1995). In addition, the infected mice had a systemic antibody response demonstrated by immunoblot to several dominant antigens. Interestingly, the CagA antigen was not recognized; perhaps because of the early phase of infection in the mice (Marchetti et al., 1995), or expression of the functionality of CagA was lost after colonization (Rieder et al., 2005). Also of considerable importance was the original description of the Sydney strain of H. pylori which colonizes mice up to 12 months after inoculation, and importantly induces a significant gastritis, particularly in C57BL mice and B6129 mice (Lee et al., 1997, Rogers et al., 2005). However, most H. pylori strains- including the Sydney strain- while showing fairly consistent patterns of colonization, have proven less carcinogenic in C57BL/6 mice (Thompson et al., 2004), although a study published in 2005 demonstrated in situ carcinoma in C57BL/6 X 129SvEv (B6129) mice infected for 15 months with H. pylori SS1 (Rogers et al., 2005). It is now appreciated that use of H. pylori SS1, though successful in generating preneoplastic and neoplastic lesions in specified strains of mice is somewhat limited due to its inability to express CagA in vivo. These results are compatible with H. felis mouse models of gastric cancer given that H. felis does not have Cag genes located on a pathogenicity island (PAI).
The cag PAI encodes an active type IV secretion system (T4SS) and after H. pylori epithelial cell adherence, the secretion system translocates the CagA protein into epithelial cells where the CagA protein undergoes tyrosine phosphorylation (CagAP-tyr) (Jones et al., 2010, Muller, 2012). This process is associated with host-cell morphological changes and phosphorylation of host-cell proteins. Several genes within the cag PAI, but not cagA itself, are required for the induction of proinflammatory cytokines or chemokines, such as IL-8. The production of proinflammatory cytokines by H. pylori is dependent on the nuclear factor- κB and nitrogen-activated protein kinase signal transduction cascades. Increased inflammation in cag+ H. pylori strains is also noted and importantly, human studies indicate that patients infected with CagA+ H. pylori are at a higher risk of developing gastric cancer than those infected with Cag− strains (Jones et al., 2010, Muller, 2012). Mice appear to be incompatible with cag+ H. pylori strains carrying functionally active T4SS. H. pylori strains rapidly switch off the cag PAI and down-regulate proinflammatory responses. The widely used H. pylori SS1 strain has a complete cag PAI, but is unable to induce IL-8 or to deliver CagA into gastric epithelial cells in vitro. Using PMSS1 (H. pylori SS1 prior to serial mouse passage) with an intact functional CagA elicits more robust inflammation during its initial colonization in C57BL mice, but this feature is lost upon prolonged colonization, presumably due to functional loss of Cag, which recapitulates the in vivo behavior of SS1 (Arnold et al., 2011). Other mouse-adapted H. pylori strains used in different laboratories carry mutations or gene deletions in the cag-PAI and are unable to translocate and tyrosine-phosphorylate CagA.
A number of other very useful murine model of gastric cancer, including genetically engineered mice (e.g. p27 (Kip1−/−); IL1β), have been developed (Judd et al., 2004, Rogers and Fox, 2004, Zavros et al., 2005, Tu et al., 2008). Indeed, in many of the models of gastric cancer, a long period of achlorhydria, associated with presumed bacterial overgrowth, appears to be the common denominator in mouse models of GAC (Zavros et al., 2005, Lertpiriyapong et al., 2013, Lofgren et al., 2011). It is likely that various mouse models will continue to be developed and used to dissect the role of gastric Helicobacter infection and the associated immune response to precisely determine the cancer pathways most relevant to humans.
Gerbils
The Mongolian gerbil has been shown to have particular relevant features that have proven useful in addressing the potential of H. pylori to induce gastric cancer and the role cofactors play in the carcinogenic process. Mongolian gerbils were first reported as a model for experimental H. pylori infection in 1991 (Yokota et al., 1991). Japanese investigators noted premalignant lesions consisting of intestinal metaplasia, atrophy, and gastric ulcers in gerbils after experimental infection with H. pylori (Hirayama et al., 1996, Honda et al., 1998a). In one study, acute gastritis with erosions of the gastric mucosa occurred shortly after infection, whereas gastric ulcers, cystica profunda, and atrophy with intestinal metaplasia were observed at 3–6 months after H. pylori infection (Honda et al., 1998a). Following these findings, others in Japan have noted that gerbils infected with H. pylori from periods ranging from 15 to 18 months developed gastric adenocarcinoma (Honda et al., 1998a, Watanabe et al., 1998).
When infected with H. pylori for 62 weeks, 37% of the gerbils developed adenocarcinoma in the pyloric region, although vascular invasion and metastases were not observed (Watanabe et al., 1998). In another report, H. pylori induced gastric adenocarcinoma at 15 months post inoculation, but again the authors also did not record metastases or vascular invasion (Honda et al., 1998b). Importantly, the histological progression in the gerbil closely resembled that observed in humans, in terms of the early appearance of intestinal metaplasia, well-differentiated histological patterns of the gastric malignancy, and location of the cancer in the antrum. Similar to findings in humans, the gerbils also had gastric ulcer disease in conjunction with gastric cancer (Hansson et al., 1996). The development of metaplasia with production of predominantly acid sialomucins was associated with tumor development. Whether the cancers arose directly form these metaplastic cells is unknown, but the tumors clearly originated deep in the gastric glands, in close proximity to these metaplastic cells (Watanabe et al., 1998).
Although most of the gastric tumors in this H. pylori gerbil model originated in the pyloric region of the stomach, significant changes in the oxyntic mucosa consistent with chronic atrophic gastritis were seen (Watanabe et al., 1998). Glandular tissue in the gastric body and fundus were atrophied and replaced by hyperplastic epithelium of the pseudopyloric type. The differences between this lesion and gastric atrophy in humans is that the gerbil corpus is not "thinner" consequent to the pseudopyloric hyperplasia (Wang et al., 1998, Watanabe et al., 1998). The diagnosis of atrophy in H. pylori-infected gerbils (as well as in mice) is not involved with the thickness of the mucosa but rather with the loss of oxyntic (parietal and chief) cell populations within the gastric glands. Accumulating data supports that the parietal cell may regulate key differentiation decisions within the gastric glands, and ablation of parietal cells using transgenic technology (Li et al., 1996) or their loss to infection with H. felis and H. pylori infection leads to altered glandular differentiation and neck cell proliferation; this corresponds to changes in gastric acid and gastrin physiology. The expansion of an aberrant neck cell (“regenerative hyperplasia” or "pseudopyloric hyperplasia") in the gerbils is similar to that observed in the H. felis mouse model. This lineage has been shown to be spasmolytic polypeptide (SP) -positive (Wang et al., 1998), and this SP-positive lineage also develops in H. pylori-associated gastric cancers in humans (Schmidt et al., 1998). H. pylori induced loss of oxyntic glandular tissue suggests that the gerbil becomes achlorhydric before the development of gastric cancer. Although the precise effects of chronic H. pylori infection on gastric acid secretion are not fully elucidated, serum gastrin levels, considered a risk factor in gastric cancer in the H. pylori infected gerbil, are increased (Rieder et al., 2005, Franco et al., 2005). Importantly, gerbils infected with H. pylori SS1 develop gastritis and dysplasia; however, these experiments published to date do not result in gastric cancer in the absence of carcinogens such as MNU (Martin et al., 2010, Suzuki et al., 2002, Court et al., 2002).
Unlike mice, gerbils colonize efficiently with cag+ H. pylori strains and the importance in pathogenic potential of cagA in H. pylori strains has been shown in gerbils. Mongolian gerbils were infected for 32 weeks either with H. pylori cag+ strain B128 or with the isogenic mutant strain B128Δ cytotoxin-associated gene (cagY) or B128ΔcagA, defective in T4SS or in the production of its effector protein CagA, respectively (Rieder et al., 2005). H. pylori B128-infected gerbils harbored high numbers of bacteria in the gastric antrum and corpus, whereas B128Δ cagY and B128Δ cagA colonized the antrum more densely than the corpus. All infected animals had robust antral inflammation and epithelial cell proliferation. B128-infected, but not mutant-infected, gerbils had severe transmural inflammation with large lymph aggregates, increased proliferation, gastric atrophy, and mucous gland metaplasia in the corpus. Plasma gastrin levels and gastric pH values were significantly increased only in H. pylori WT B128-infected gerbils. In all infected gerbils, proinflammatory cytokines interleukin 1β, interferon γ, and growth-regulated protein were increased in the antrum, but only in wild type-infected animals was an increase seen in the corpus mucosa (Rieder et al., 2005).
A recent study from Japan provided compelling evidence that the pattern of H. pylori colonization depends on the acid output and that this influences the long-term progression to neoplasia (Hagiwara et al., 2011). They examined the potential effect of proton pump inhibitor (PPI) therapy on progression of H. pylori-associated disease. The male gerbils were divided into four groups: H. pylori (ATCC43504) positives and negatives, with and without administration of a PPI (omeprazole 100 mg/kg body weight/day). At the end point of the 6 month experiment, the results provided support for previously published literature in humans. H. pylori-negative male gerbils did not develop gastritis, metaplasia, or cancer irrespective of PPI treatment; whereas all H. pylori-positive gerbils developed gastritis and most also developed metaplasia during the later stages of the disease. Treatment with omeprazole in H. pylori-positive animals accelerated progression to atrophy and enhanced hypergastrinemia, resulting in a significantly increased incidence of gastric cancer. The authors emphasized that hypergastrinemia might be promoting the development of H. pylori gastric cancer (Hagiwara et al., 2011). The findings support previous findings showing the increased risk factor of gastric atrophy in H. pylori-infected patients on PPI therapy (Kuipers, 2006, Kuipers et al., 1996, Kuipers et al., 1995, Lundell et al., 2006). In humans, it remains to be shown whether chronic PPI therapy in H. pylori-infected individuals also increases the further progression to metaplasia, dysplasia and neoplasia in the proximal stomach, and whether there are protective effects of hypogastrinemia on the gastric antrum (Tomita et al., 2011) are also observed with PPI therapy. These studies will require longer-term follow-up of this subset of patients (Fox and Kuipers, 2011).
The unusual susceptibility of this animal species to gastric adenocarcinoma using standard cag+ H. pylori strains underscores the overriding importance of host factors in determining the outcome from gastric Helicobacter spp. infection. It is important to note that many laboratories- particularly those outside of Japan- were unable to document the carcinogenic potential of H. pylori in the gerbil model. However, investigators in the U.S. using H. pylori strain B128, a Cag+ strain (7.13) serially passaged in gerbils have now documented rapid onset gastric adenocarcinoma in gerbils. This study serves to highlight the importance of bacterial factors as well as host susceptibility in cancer induction (Franco et al., 2005).
Dietary Factors and Gastric Cancer
Fat
Epidemiological evidence supports the premise that both H. pylori and obesity are independent risk factors for gastric cancer (Ahmed and Sechi, 2005, Chung and Leibel, 2006). Obesity induced inflammation underlies increasingly common diseases, such as type II diabetes and atherosclerosis (Kanneganti and Dixit, 2012, James et al., 2012, Ferrante, 2007, Zuniga et al., 2010). Importantly, several studies employing mouse models and focusing on the direct effect of adipose-derived factors on established tumors, have shown that obesity promotes cancers (e.g. colorectal and pancreatic cancer) and implicate systemic inflammation as an important component of the tumorigenic process (Moon et al., 2013, Zyromski et al., 2009). Helicobacter-dependent gastric cancer in mice and humans results from a sustained gastric immune response involving both lymphoid and myeloid derived cells. The population of CD11b/Gr1, immature myeloid cells (IMC), increase in the gastric tissue and circulation during the early stages of gastric helicobacter infection and have been implicated in cancer initiation (Asfaha et al., 2013). IMCs are considered a heterogeneous cell population; myeloid-derived suppressor cells (MDSCs), a component of IMCs, suppress cytotoxic CD8 T cells and effectively prevent their antitumor activity and promote the survival of transformed epithelial cells (Pylayeva-Gupta et al., 2012, Ostrand-Rosenberg and Sinha, 2009, Wang et al., 2013). Helicobacter-associated gastric cancer is also correlated with an increased T helper (Th1) and TH-17 immune response (Shi et al., 2010, Fox et al., 2000). Despite its exact role, an elevated Th17 has been positively associated with increased severity of gastritis, whereas its diminution is linked to decreased gastric dysplasia (Wang et al., 2009a). Cytokines and growth factors such as IL6 and granulocyte-macrophage colony-stimulating factors (GM-CSF) also play a role in recruitment and activation of MDSCs (Pylayeva-Gupta et al., 2012, Ostrand-Rosenberg and Sinha, 2009).
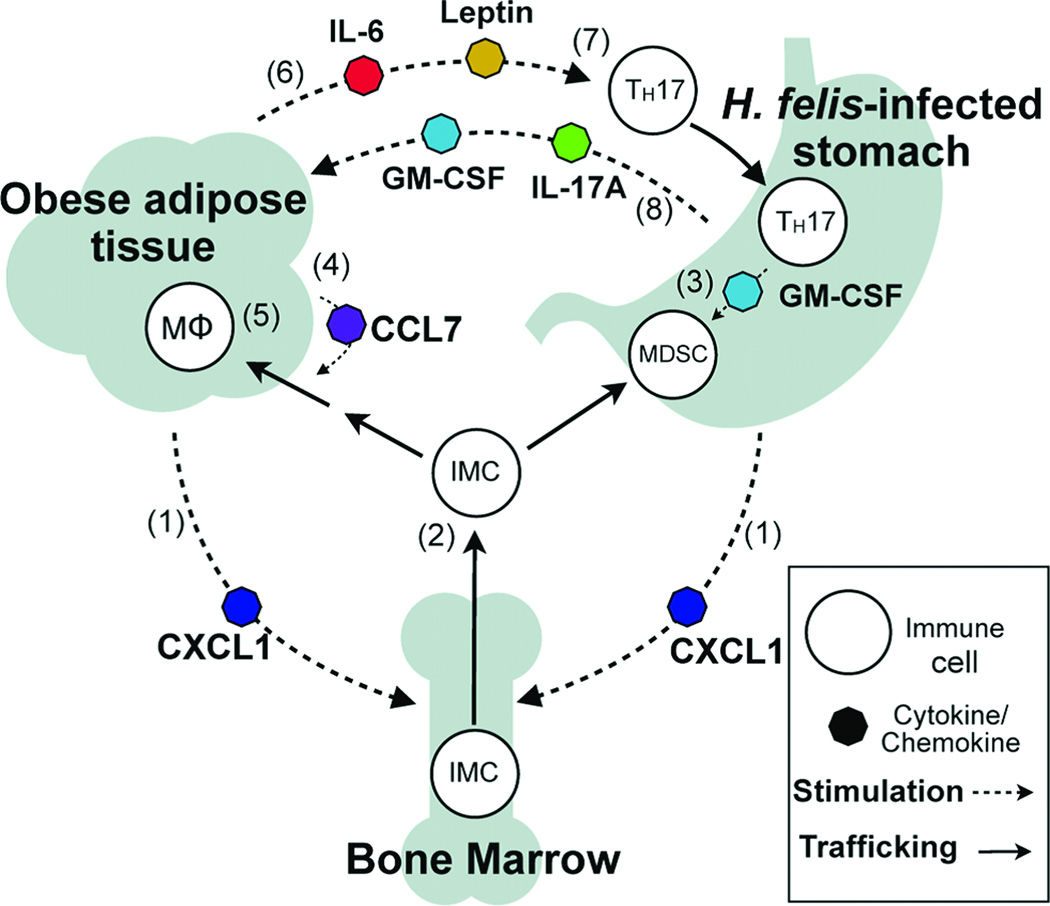
A recently completed study utilized H. felis infected C57/BL male mice maintained on a high fat diet (HFD), 45% of dietary calories from fat, to study the effect of obesity on immune modulation and the establishment of a protumorigenic gastric microenvironment (Ericksen et al., 2013). Mice on the HFD develop adipose inflammation, hyperglycemia, and insulin resistance (Zuniga et al., 2010, Collins et al., 2004). The authors reported that H. felis infected mice in the HFD developed accelerated gastric cancer compared to infected mice fed a lean diet (13% fat). Obese mice had increased bone marrow-derived IMCs in both gastric tissue and blood, as well as increased numbers of CD4 T cells, IL17A, GM-CSF, and pro-survival gene expression in gastric tissue. Phosphorylated STAT3 was increased and likely played a role in promoting cellular transformation as well as regulating inflammatory gene expression. Analysis of adipose tissue in infected obese mice had increased accumulations of macrophages, increased IL6, C-C motif ligand 7 and leptin. Obesity and helicobacter-induced gastric inflammation in concert also increased serum proinflammatory cytokines, including IL6, a cytokine known to promote tumor progression. The authors concluded that obesity accelerated Helicobacter-associated gastric cancer via cytokine mediated cross-talk between inflamed gastric and adipose tissue by augmenting immune responses at both tissue sites and contributing to a protumorigenic gastric microenvironment (Ericksen et al., 2013) (Figure 3).
Figure 3.
Schematic hypothesis of inflammatory cross-talk during Helicobacter felis infection and diet-induced obesity. CXCL1 produced by adipose and gastric tissues (1) elevates circulating bone marrow-derived immature myeloid cells (IMCs) (2). IMCs recruited to gastric tissue by CXCL1 encounter TH17-associated cytokines, including GM-CSF, and develop into myeloid-derived suppressor cells (3). Adipose CCL7 secretion increases IMC recruitment to adipose (4), resulting in accumulation of adipose tissue macrophages (MΦ) (5). Inflamed adipose produces IL-6 and leptin (6), promoting TH17 differentiation (7). TH17-associated cytokines IL-17A and GM-CSF produced in the stomach signal to adipose (8) to increase CCL7 production and amplify adipose inflammation. CCL, C-C motif ligand; CXCL, Chemokine C-X-C motif ligand; GM-CSF, granulocyte macrophage-colony stimulating factor. (Reprinted with permission (Ericksen et al., 2013)
Folic Acid
Studies have supported the protective role of dietary folic acid against specified cancers. Results of these studies have varied and mechanisms responsible for its protective effective are not defined. Because a global reduction in DNA methylation is an early and consistent observation during carcinogenesis, it has been argued that reversal or protection against DNA hypomethylation provided by folic acid supplementation is a possible mechanism explaining folic acid chemopreventive effect (Kim, 2004, Song et al., 2000b, Song et al., 2000a). H. pylori infection and development of precancerous lesions has been associated with a loss of global DNA methylation (Cravo et al., 1996). Lower blood leukocyte (DNA methylation) levels were also observed in gastric cancer patients compared to matched controls (Hou et al., 2010). A recent study also found that high dietary folate increased survival rates in gastric cancer patients compared with low folic acid intake in advanced gastric cancer (Shitara et al., 2010). Interestingly, folic acid supplementation also reduced the incidence of N-ethyl-N-nitroguanidine-induced gastric adenocarcinoma in beagles (Xiao et al., 2002). Though not described in this report, commercially available beagles have a high incidence of gastric helicobacter infection (Lee et al., 1992). Whether Helicobacter promoted ENNG carcinogenesis in the dog is unresolved but should be considered.
In a study conducted in H. felis or control INS/GAS male mice animals were fed a rodent diet containing 2 (control) or 8 (supplemented) mg FA/kg of diet (Research Diets, Newark, NJ). The diets were administered either at weaning or later at 8 and 16 weeks post infection (Gonda et al., 2012). All mice were sacrificed at 28 weeks post infection. The authors measured global DNA methylation in gastric tissue by H-dCTP incorporation assay and expressed data as a percentage of methyl-C content compared with uninfected non-transgenic FVB WT mice. For the first time, the authors found reduced global DNA methylation in the stomach of male H. felis infected INS/GAS mice compared to WT uninfected mice; a significant effect was noted at 22 weeks post infection, and because more severe at 28 weeks post infection. There was a significant 18% reduction in folate content in infected INS/GAS mice, reflecting a deficiency of methyl donors in inflamed, dysplastic tissues. Serum homocysteine levels, an inverse measurement of methyl donor status, were also significantly elevated in H. felis infected mice at 22 and 28 weeks post infection. Importantly, FA supplementation prevented the loss of global methylation in both dysplastic epithelial and gastric stromal myofibroblasts, measured by 3 independent assays, and markedly reduced gastric dysplasia and mucosal inflammation. FA supplementation also had an anti-inflammatory effect as indicated by gene expression profiling and IHC for lymphocyte markers in gastric tissue (Gonda et al., 2012). However, other studies have demonstrated that treatment with demethylating agents also results in a reduction in preneoplastic lesions in H. pylori-infected gerbils (Niwa et al., 2013).
Iron
Nutritionally, iron deficiency remains a common global disorder affecting 500–600 million humans (Zimmermann and Hurrell, 2007, WHO/UNICEF/UNU, 2001), with children being particularly susceptible to iron deficiency anemia (IDA). A variety of clinical syndromes are recognized with IDA, including reduced growth, mental retardation, increased susceptibility to infectious microorganisms, and in extreme cases, mortality (Stoltzfus, 2001). H. pylori has been linked to IDA and interestingly, H. pylori-infected individuals are refractory to iron supplementation, but IDA can be reversed in these patients with H. pylori eradication (Barabino et al., 1999, Kurekci et al., 2005, Choe et al., 2000, Choe et al., 1999, Marignani et al., 1997). Hypochlorhydria, a marker for atrophic gastritis noted in chronic H. pylori infections, and sometimes observed with acute H. pylori infections, is considered a risk factor for IDA because under non-acidic conditions in the stomach, there is an inability to reduce ferric iron to absorbable ferrous (Fe2+) iron (Windle et al., 2007, Charlton and Bothwell, 1983, Annibale et al., 2003). Also under iron restricted conditions, the virulence factors CagA and VacA in H. pylori are increased; iron release due to mucosal injury affords better access of H. pylori to the host’s iron stores, and allows the organism to bind and utilize iron from hemoglobin, lactoferrin, and transferrin (Senkovich et al., 2010). In a recent report, investigators determined operable mechanisms of iron deficiency in H. felis infected INS/GAS male mice over a 9 month period (Thomson et al., 2012). They noted that the infected mice, apparently on a standard mouse diet (although not stated) had parietal cell loss and hypochlorhydria accompanied by decreased serum iron and transferrin saturation, as well as hyperferritinemia, and increased total iron binding capacity (Thomson et al., 2012). The H. felis infected INS/GAS mice at 9 months post infection also had decreased gastric expression of iron metabolism regulators hepcidin (expressed in parietal cells and capable of regulatory acid secretion- (Schwarz et al., 2012)), Bmp4, and Bmp6, but increased expression of the iron efflux protein, ferroportin, iron absorption genes and Lcn2, a siderophore-binding protein. The mice had low serum iron concentrations at 6 and 9 months post infection, and at 9 months infected mice also had lowered transferrin, reflecting systemic iron deficiency. Iron storage capacity, measured by serum ferritin, was initially increased at 3 months in infected mice. The authors hypothesized that the H. felis infected mice were attempting to sequester iron, making it unavailable for H. felis colonizing the stomach. However, by 6 and 9 months, H. felis infected mice had significantly reduced serum ferritin, presumably due to overall paucity of the systemic iron (and thereby negating the need for iron storage) (Thomson et al., 2012). Others have observed similar patterns of initial elevation in serum ferritin at 10 weeks post H. felis infection followed by a reduction at 30 weeks post infection on WT mice maintained on an iron restricted diet (Keenan et al., 2004). The authors further speculated that the marked changes in gastric iron transporters may also play a role in the rapid development of gastric cancer noted in gastric Helicobacter spp. infection in male, INS/GAS hypergastrinemic mice, known to be more susceptible to gastric cancer compared to their female counterparts (Fox et al., 2003a, Fox et al., 2003b). The data generated with the H. felis model are Cag-independent given H. felis lacks a cag PAI. It will be interesting to ascertain if H. pylori SS1 or PMSS1 infected male INS/GAS mice will also have iron deficiency.
Importantly, iron deficiency has also been associated with stomach cancer along with other gastrointestinal tumors (Pra et al., 2009, Akiba et al., 1991, Harrison et al., 1997). H. pylori-associated gastric cancer has been recently used to explore the role of dietary iron as a cofactor in cag+ H. pylori (strain 7.13) associated gastric cancer in gerbils (Noto et al., 2013). The authors clearly demonstrated that iron depletion accelerated H. pylori-induced premalignant and malignant gastric lesions in a cagA dependent manner (Noto et al., 2013) (Figure 4) (Table 2). Importantly, irrespective of dietary iron status, infection with a cagA isogenic H. pylori mutant significantly attenuated inflammation, dysplasia, and gastric adenocarcinoma, highlighting the importance of cagA in gastric cancer development. Furthermore, in gerbils maintained on an iron deficient diet, isogenic cagA mutants of H. pylori have impaired ability to colonize, suggesting that cagA plays a role in H. pylori iron acquisition (Tan et al., 2011). To determine whether the severity of gastric lesions under conditions of iron depletion were specific for H. pylori, gerbils either on iron replete or iron depleted diets were exposed to ethanol-induced inflammation. Proteomic analysis of in vivo adapted H. pylori harvested from gerbils on replete or deplete iron diets indicated that there was increased abundance of proteins that mediated bacterial-host interactions (Helicobacter sp. Q enolase) and cag T4SS function (CagA) in strains from the iron depleted group (Noto et al., 2013).
Figure 4.
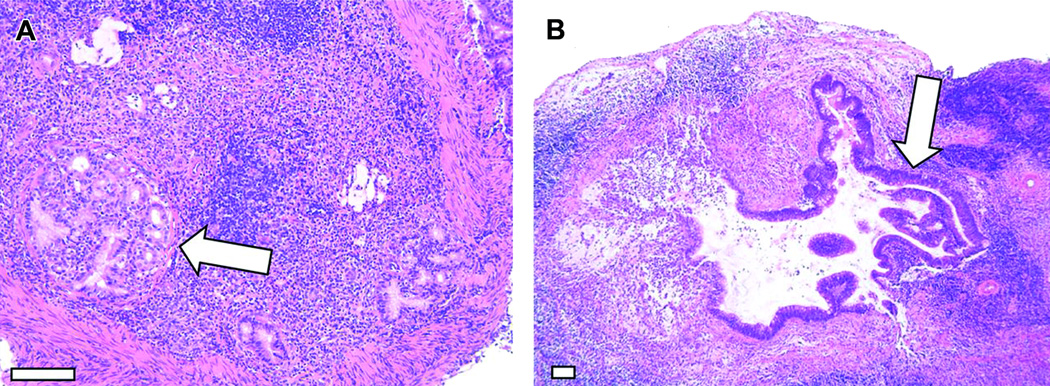
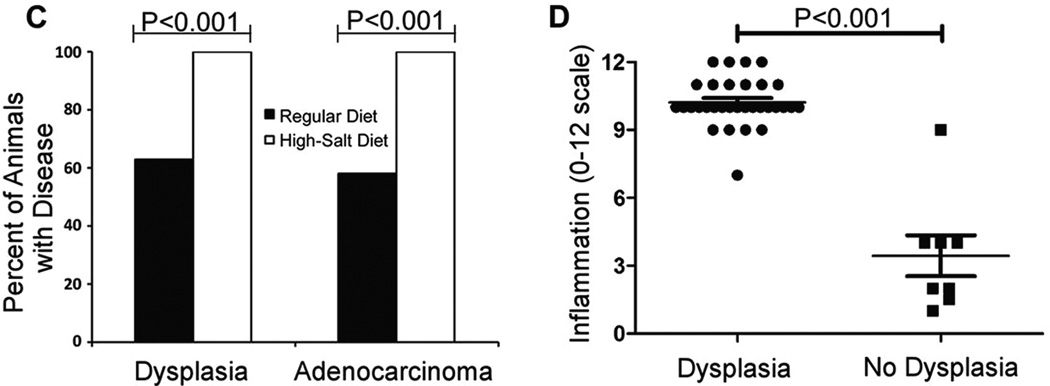
Analysis of gastric dysplasia and adenocarcinoma. Gerbils infected with WT H. pylori maintained on either a regular diet or a high salt diet. A–B) Representative micrographs of gastric tissue derived from WT-infected animals maintained on a high salt diet (arrows indicate tumors). Magnification bars indicate 100 µm. C) Gastric tissues were evaluated for dysplastic lesions and invasive adenocarcinoma. Only animals infected with the WT strain exhibited these abnormalities. Gastric cancer and dysplasia were detected significantly more frequently in the WT-infected high salt diet group than in WT-infected regular diet counterparts (p<0.0001). D) WT-infected animals maintained on a regular diet and WT infected animals maintained on a high salt diet were analyzed. The total gastric inflammation score (scale of 0–12) was higher in WT-infected animals with gastric dysplasia than in WT-infected animals without dysplastic lesions. Horizontal bars indicate mean inflammation score + SEM for each group. (Modified with permission (Gaddy et al., 2013)
Table 2.
Iron depletion in gerbils increases the frequency and severity of gastric inflammation, dysplasia, and adenocarcinoma
| 2 weeks (n = 48) | 6 weeks (n = 58) | 12 weeks (n = 39) | ||||
|---|---|---|---|---|---|---|
| Iron-replete (n = 24) |
Iron-depleted (n = 24) |
Iron-replete (n = 29) |
Iron-depleted (n = 29) |
Iron-replete (n = 20) |
Iron-depleted (n = 19) |
|
| Gastritis | 3/24 (13%) | 8/24 (33%) | 16/29 (55%) | 23/29 (79%) | 18/20 (90%) | 18/19 (95%) |
| Dysplasia | 2/24 (8%) | 5/24 (21%) | 9/29 (31%) | 18/29 (62%)^ | 9/20 (45%) | 14/19 (74%) |
| Adenocarcinoma | 1/24 (4%) | 2/24 (8%) | 3/29 (10%) | 13/29 (45%)^ | 7/20 (35%) | 11/19 (58%) |
Statistical significance (P ≤ 0.05) was determined by χ2 tests comparing iron-depleted gerbils to iron-replete gerbils
(Reprinted with permissions (Noto et al., 2013))
Further, using scanning electron microscopy, the authors indicated that the H. pylori isolated from the iron-depleted gerbils and grown on human gastric epithelial cells had significantly more T4SS pili on their surface, compared to H. pylori isolated from gerbils on iron-replete diets. This data suggested that the increased assembly of the T4SS pili was attributable to H. pylori adaptation under iron depletion (Noto et al., 2013). This observation was tested using in vitro iron growth conditions with similar findings; the H. pylori grown in iron deficient media had significantly more T4SS pili then the H. pylori grown in iron-replete media (Noto et al., 2013).
To compare data collected in the gerbil model, the authors compared H. pylori isolated from Colombian patients with the lowest serum ferritin levels to strains isolated with the highest ferritin levels. These H. pylori isolates from subjects with low serum ferritin levels induced significantly (p < 0.05) higher levels of IL8 compared with H. pylori strains isolated from patients with the highest ferritin levels (individuals with gastritis only, or with intestinal metaplasia). The authors argued that the ability of H. pylori strains from a low ferritin serum level patient to induce more severe pathology was more likely to have accelerated the diseases early in the carcinogenic process. This hypothesis needs further longitudinal evaluations of H. pylori-infected populations on low dietary iron intake (Noto et al., 2013).
Salt
Historically, the role of salt in gastric cancer has received considerable attention in the literature (Geboers et al., 1985, Tuyns, 1988). The deleterious effect of salt is based on epidemiological studies, biochemical analyses and in vivo experimentation (Joossens and Geboers, 1981, Takahashi and Hasegawa, 1986). In a case control Belgium study, consisting of 1087 cases (587 males, 500 females) of gastrointestinal cancer and 2914 controls, the individuals were asked to respond to their taste for salt (Tuyns, 1988). The question asked was “At the time you eat your food, do you add salt?”. The possible responses were ‘never’, ‘sometimes’, or ‘always’. The relative risks of developing gastric cancer were adjusted for age, sex, and province where the patient lived. The relative risk for development of gastric cancer for individuals with high salt intake was 1.78 (confidence limits 1.19–2.75), which was statistically significant; however the noted increase may be related to another causal factor, e.g. H. pylori or other unknown factors of greater importance. The relative risk of 1.78 for gastric cancer in Belgian patients with high salt intake corresponds almost exactly to that noted by Correa studying black Americans in Louisiana (Correa, 1985). A similar increased relative risk for gastric cancer with adding salt to their diet was noted in an Italian multicentre case control study (Buiatti et al., 1990). Other studies have incriminated increased risk of gastric cancer due to ingestion of preserved, often salty food (Tajima and Tominaga, 1985), preference for salty foods (Tuyns, 1988, You et al., 1988), or actual measurement of salt consumption (Correa et al., 1985, La Vecchia et al., 1987, Tsugane et al., 1991). In contrast, salt was not incriminated in a 1992 study (Friedman and Parsonnet, 1992). In this smaller case-control study on 200 individuals residing in the U.S., who subsequently developed gastric adenocarcinoma, and 200 age matched control participants were asked ‘Do you usually salt food before tasting it?’. Analysis of data indicated that there was not statistical evidence that routine salting of food lead to increased risk of gastric cancer (Friedman and Parsonnet, 1992). A joint WHO/FAO report, given the association of dietary salt and increased risk of gastric cancer rarely raised the odds ratio above two, concluded that high salt ingestion “probably increases the risk of stomach cancer” (WHO, 2003). Nevertheless, several more recent reports have analyzed the combinatory effects of high dietary salt and H. pylori infections in humans and have consistently shown increased rates of gastric cancer in these populations (Lee et al., 2003, Tsugane and Sasazuki, 2007, Wang et al., 2009b, D'Elia et al., 2012, Brenner et al., 2009, Krejs, 2010).
Given the late onset of gastric cancer in human populations, it is probable that both tumor initiators as well as tumor promoters play an important role in gastric carcinogenesis. In populations with a high risk for gastric cancer, dietary intake of fruits and vegetables is low, and the diet often consists of high starch and low protein. Both low protein and high starch may favor acid-catalyzed nitrosation in the stomach and possible mutagenesis of gastric epithelia (Berretta et al., 2012, Tsugane and Sasazuki, 2007). Because of the purported role of high nitrate diets and subsequent nitrosamine formation in the stomach, numerous studies have been conducted in rodents administering various nitrosamines known to be potent carcinogens. In experiments with rats dosed with MNNG, NaCl was shown to have a promotional effect of induction of gastric adenocarcinoma when salt is given intragastrically weekly or in the diet during 12–20 weeks exposure to MNNG in drinking water (Newberne et al., 1987, Takahashi et al., 1983, Tatematsu et al., 1975). It is also known that salt causes damage to rat gastric surface epithelium followed by proliferation of gastric epithelium (Charnley and Tannenbaum, 1985, Furihata et al., 1984, Ohgaki et al., 1989, Sorbye et al., 1988). In one study, 80% of rats given salt in the diet and MNNG in drinking water developed adenocarcinomas in the antrum, but not in the corpus (Takahashi et al., 1983). Also, ACI rats given a single initiation dose of MNNG [0.25 mL/10 g body weight MNNG in water (5 g/L)] by oral gavage and fed a 10% NaCl diet had significantly increased tumours in both forestomach and glandular stomach after being on the salt diet for 1 year (Watanabe et al., 1992).
Mice have also been used on a limited basis in studies addressing gastric damage due to salt intake. Mice (strain undesignated) fed a highly salted diet (salted codfish and yakuri sold for food consumption in Japan) developed acute gastric mucosal damage (Sato et al., 1959). Swiss/ICR mice fed salted (10% w/w NaCl) rice diets for 3–12 months were noted to have developed hypertrophy of the forestomach and atrophy (considered a marker of premalignancy in humans) of the glandular stomach (Kodama et al., 1984). Parietal cell loss and statistically increased epithelial proliferation were also noted in B6 129 mice fed a high salt diet (7.5%). A reduction in the parietal cells accounted for the atrophy observed in the fundus of the mice (Kodama et al., 1984). Although parietal cell loss has also been noted in C57BL and INS/GAS mice infected with H.felis or H. pylori, (Fox et al., 2002, Mohammadi et al., 1996, Wang et al., 2000, Fox et al., 2003a) these studies infer where uninfected mice receive salt in their diet that chronic mucosal damage per se may account for parietal cell loss in the fundus of mice.
More recent studies have incorporated NaCl in the diets of rodents infected with gastric helicobacters (Rogers et al., 2005, Fox et al., 1999). C57BL/6 mice infected with H. pylori SS1 for 4 months and maintained on a high-salt diet exhibited higher gastric urease activity, serum gastrin, bacterial colonization, and foveolar proliferation levels than did those on a basal diet; however, no meaningful effect of salt on gastric inflammation or oxyntic atrophy scores was observed (Fox et al., 1999). High salt likewise increased foveolar proliferation in uninfected but not H. pylori SS1 infected B6129 mice (Rogers et al., 2005). Thus, induction of proliferation in the absence of dysplasia does not seem to increase the risk of carcinogenesis in this model. In agreement with this concept, H. pylori SS1 infection was shown to induce DNA damage in Big Blue mice (containing a chromogen-inducible lambda phage transgene for in vitro mutation quantitation), but no increase in mutation frequency was evident in infected animals fed a high-salt diet (Touati et al., 2003). B6129 mice fed a high-salt diet showed a shift in anti-H. pylori humoral immunity from a Th1 to a Th2 phenotype. This humoral immunity shift is not specific to B6129 mice. Others observed a similar increase in Th2-associated IgG1 relative to Th1-associated IgG2a in H. pylori-infected INS-GAS mice on a FVB strain background (Fox et al., 2003a, Rogers et al., 2005). However, the salt-associated shift in humoral immunity to a Th2 pattern seemed to be H. pylori specific. The authors found no increase in total IgG1 or IgE consistent with hypersensitivity nor did they detect anti-parietal cell antibodies suggestive of autoimmune gastritis (Rogers et al., 2005).
Gerbils infected with H. pylori develop an antral gastritis/duodenitis that mimics the ulcerogenic form of the human disease (Ohkusa et al., 2003). Unlike mice, but similar to humans, the bacterial cag PAI seems to be a critical disease determinant in gerbils, particularly gastric cancer (Rieder et al., 2005). The gerbil model has proven highly useful in addressing questions specifically related to cagA and its association with high dietary salt and other H. pylori virulence determinants in vivo. In earlier studies, authors have shown a synergistic promoting effect of H. pylori and high salt on N-methyl-N-nitrosurea (MNU)–initiated gastric cancer in the gerbil model (Nozaki et al., 2002). However, chemical initiation was required to induce cancer, as no tumors developed in gerbils untreated with MNU regardless of H. pylori infection status or diet. Gerbils fed a high salt diet (2.5%) for 13 months were noted to have gastric atrophy and foveolar hyperplasia. However, the high salt diet did not promote gastric cancer in gerbils infected with H. pylori SS1, which does not express cagA (Bergin et al., 2003). However, in a recent study, investigators have demonstrated that a high dietary intake (8.75%) in a Mongolian gerbil model enhances H. pylori-induced carcinogenesis (Gaddy et al., 2013). The high-salt diet used approximates the concentration of sodium chloride in dried fish, preserved in 3 to 20% salt, pickled foods containing up to 25% salt, and soy sauce which has 19% salt. The administration of high-salt diet to uninfected gerbils did not result in gastric cancer. The authors concluded that the effects of a high-salt diet on gastric cancer were dependent on the presence of H. pylori infection. Importantly, the high salt diet increased incidence of gastric carcinoma in gerbils infected with WT cagA+ H. pylori strain 7.13 but not in animals infected with a cagA mutant strain (Gaddy et al., 2013). This observation is consistent with several parts of the world with high rates of gastric cancer, also, a high prevalence of cagA+ strains; a large percentage of these populations routinely have diets with a high salt concentration.
Others have reported that exposure to H. pylori to high-salt conditions in vitro leads to alterations in the expression of multiple H. pylori genes, included cagA and neutrophil activation protein (Liao et al., 2013, Loh et al., 2012). The authors also noted that cagA gene expression was elevated in the stomachs of WT-infected gerbils maintained on a high-salt diet compared to WT-infected animals maintained on a regular diet (Gaddy et al., 2013). Interestingly, both IL-1β transcription and iNOS transcription were significantly elevated in WT-infected gerbils maintained on a high-salt diet compared to the regular-diet fed to gerbils infected with WT-H. pylori. WT-infected animals maintained on a high-salt diet had markedly increased gastric pH compared to their regular diet WT-infected gerbil counterparts. This is consistent with the finding of atrophic gastritis and hypochlorhydria preceding the development of gastric cancer in humans. Parietal cells are reduced in the hypochlorhydric animals and likely accounts, at least in part, for the observed hypochlorhydria. They also hypothesized that hypochlorhydria could be due to perturbations in parietal cell function. To test this, the authors also measured hepcidin (produced in parietal cells), gastrin, and H,K-ATPase transcripts. The animals infected with WT H. pylori and maintained on a high-salt diet had decreased levels of all 3 transcripts compared to animals infected with WT H. pylori and maintained on a regular diet. They concluded that increased dietary salt consumption markedly alters the outcome of infected with cagA+ H. pylori strain 7.13 and proposed that high salt concentrations stimulate increased expression of cagA, and the actions of CagA lead to inflammation, hypochlorhydria, and enhanced carcinogenesis. They also raised the possibility that high salt concentrations lead to altered expression of multiple H. pylori genes (including cagA) as well as to the alterations in the host and that this constellation of alterations could enhance progression of carcinogenesis. Regardless of the mechanisms, their data argues that reduction in dietary salt could lower risk of gastric cancer in H. pylori-infected patients (Gaddy et al., 2013).
Vitamin C
Antioxidants, particularly vitamin C and b-carotene, have anticarcinogenic properties based on laboratory data as well as epidemiological evidence. Ascorbic acid scavenges nitrous oxide and makes the compound unavailable for nitrosation in the stomach. Furthermore, evidence indicates that many, but not all, patients with H. pylori gastritis have lowered ascorbic acid concentrations (Sobala et al., 1991, Sobala et al., 1993). Other investigators have shown that patients with precancerous lesions consisting of intestinal metaplasia and multifocal atrophic gastritis also have lowered ascorbate and elevated N-nitroso compound concentrations (Chen et al., 1990, Bartsch et al., 1988). Supplementation of vitamin C has been associated with reduced gastric cancer risk in some human studies (Su et al., 2000, Correa et al., 1998). Despite initial promising results in a prospective trial in a very high-risk gastric cancer population supplemented with vitamin C at 2 g per day for 6 years (Correa et al., 2000), a follow-up study on this population indicated that vitamin C supplementation over a 12-year period did not provide any lasting protection against gastric cancer (Mera et al., 2005).
To test whether H. pylori does indeed impair ascorbic acid secretion into the stomach, animals which need dietary supplementation of vitamin C should ideally be used to mimic vitamin C metabolism in humans. Of the Helicobacter sp. infected animal models presently available, non-human primates fulfill this criterion, but to date has not been used in answering this important question. The guinea pig is the only other laboratory animal that has dietary requirements for vitamin C. Published data indicated that H. pylori induced gastritis in this model (Shomer et al., 1998). Supplementation with vitamin C in the diet at 10-fold higher levels than a maintenance dose (3,102 mg/kg) for 6 weeks in guinea pigs, which lack L-gulono-γ-lactone oxidase similar to humans and gulo−/− mice, did not impact H. pylori colonization levels nor severity of gastritis (Taylor, 2007).
In a later study using vitamin C-deficient B6.129P2- Gulotm1Umc/mmcd (gulo−/−) mice lacking L-gulono-γ-lactone oxidase, authors compared gastric lesions and Th1 immune responses in H. pylori SS1-infected gulo−/− mice supplemented with low (33 mg/L) or high (3,300 mg/L) vitamin C in drinking water for 16 or 32 weeks (Lee et al., 2008b). Vitamin C levels in plasma and gastric tissue correlated with the dietary vitamin C supplementation levels in gulo−/− mice. H. pylori SS1 infection resulted in comparable gastritis and premalignant lesions in wildtype C57BL/6 and gulo−/− mice supplemented with high vitamin C, but lesions were less severe in gulo−/− mice supplemented with low vitamin C at 32 weeks post infection. The reduced gastric lesions in infected gulo−/− mice supplemented with low vitamin C correlated with reduced Th1-associated IgG2c, gastric IFN-γ and TNF-α mRNA and higher H. pylori colonization levels. These results in the H. pylori-infected gulo−/− mouse model suggest that although supplementation with a high level of vitamin C achieved physiologically normal vitamin C levels in plasma and gastric tissue, this dose of vitamin C did not protect gulo−/− mice from H. pylori-induced premalignant gastric lesions. In addition, less severe gastric lesions in H. pylori infected gulo−/− mice supplemented with low vitamin C correlated with an attenuated Th1 inflammatory response (Lee et al., 2008b).
It is interesting to note that low vitamin C-supplemented gulo−/− mice tended to have lower degrees of inflammation, oxyntic atrophy and dysplasia and significantly less severe epithelial defects, pseudopyloric metaplasia and foveolar hyperplasia relative to high vitamin C-supplemented gulo−/− and WT mice (Lee et al., 2008). Helicobacter-induced gastric disease in humans and mice is mediated by a Th1-predominant host immune response (Mohammadi et al., 1997, Fox and Wang, 2007, D'Elios et al., 1997). Gulo−/− mice supplemented with high vitamin C had a similar Th1-promoted inflammatory response to H. pylori as infected WT mice. Low vitamin C-supplemented, H. pylori-infected gulo−/− mice had less severe gastric lesions associated with suppressed Th1 responses as evidenced by reduced levels of plasma IgG2c, reduced expression of gastric IFN-γ and TNF-α mRNA, and increased H. pylori colonization levels. These data suggest that low dietary vitamin C impairs the host’s ability to sustain a proinflammatory response and conversely, that adequate vitamin C levels in the infected host may be important for maintaining a robust Th1 immune response to chronic H. pylori infection. Consistent with this report, others have reported attenuated Th1 immune responses in vitamin C-deprived gulo−/− mice. This was manifested by less severe pneumonia and suppressed pulmonary expression of proinflammatory IL-1β and TNF-α mRNA in vitamin C-deprived gulo−/− mice during the first few days of acute viral influenza (Li et al., 2006). In hosts with low or no vitamin C intake, attenuated Th1 responses to other types of infection, such as tuberculosis in humans or Klebsiella pneumoniae sepsis in gulo−/− mice, may also be important in predicting survival (Gaut et al., 2006, Hemila et al., 1999).
Using a mouse model of intestinal parasitic infection that causes a Th2 immune response, the effect of modulating the Th1-associated response to H. felis infection has been analyzed (Fox et al., 2000). In C57BL/6 mice coinfected with intestinal helminths and H. felis, reduced systemic Th1 immune responses and lower levels of Th1-mediated gastric cytokines were associated with increased H. felis colonization levels (a marker of less severe gastric pathology) and less severe premalignant lesions (Fox et al., 2000). In another series of mouse experiments, the authors demonstrated that lower bowel colonization with enteric helicobacters accentuated the severity of H. pylori gastritis. Colonization with these helicobacters led to a lower gastric Th1 immune response, apparently triggered by regulatory T cells sensitized by shared antigens between enteric Helicobacter spp. and H. pylori (Ge et al., 2011, Lemke et al., 2009). This may have direct relevance to the immune status of the African population where enteric helicobacters are commonly isolated from diarrheic children (Lastovica and le Roux, 2000). These results may in part explain the “African enigma” where helminth and enteric helicobacter infections are common and the incidence of gastric cancer is low, despite a high prevalence of H. pylori infection (Holcombe, 1992). The H. pylori-infected, low vitamin C-supplemented gulo−/− mouse model may offer another possible explanation to the African enigma. In Gambia, due to the impact of drought on the food supply, mean daily intake of vitamin C approaches zero for 7 months of the year, and is accompanied by low plasma vitamin C levels (Bates et al., 1994). It is tempting to speculate based on studies of the H. pylori infected gulo−/− mice on low vitamin C, that minimal vitamin C intake during H. pylori infection may be one of the factors contributing to a lower reported incidence of gastric cancer in some populations in Africa because of attenuated gastric immune responses resulting in less severe premalignant lesions. Although this hypothesis is consistent with a report of diminished mitogen responses of peripheral blood mononuclear cells from pigs affected by heritable vitamin C deficiency (Schwager and Schulze, 1998), the biological significance of these responses requires further studies. The authors concluded that high vitamin C supplementation in this model, similar to previous epidemiological studies in humans (Mera et al., 2005, Waring et al., 1996, Phull et al., 1999, Bjelakovic et al., 2004), did not prevent progression of H. pylori-induced gastritis and development of premalignant gastric lesions. In contrast, low dietary vitamin C resulted in less severe gastric disease by downregulating gastric and systemic Th1 immune responses to H. pylori infection.
References
- Ahmed N, Sechi LA. Helicobacter pylori and gastroduodenal pathology: new threats of the old friend. Annals of clinical microbiology and antimicrobials. 2005;4:1. doi: 10.1186/1476-0711-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba S, Neriishi K, Blot WJ, Kabuto M, Stevens RG, Kato H, Land CE. Serum ferritin and stomach cancer risk among a Japanese population. Cancer. 1991;67:1707–1712. doi: 10.1002/1097-0142(19910315)67:6<1707::aid-cncr2820670638>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Annibale B, Capurso G, Lahner E, Passi S, Ricci R, Maggio F, Delle Fave G. Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. GUT. 2003;52:496–501. doi: 10.1136/gut.52.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, Muller A. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011;140:199–209. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfaha S, Dubeykovskiy AN, Tomita H, Yang X, Stokes S, Shibata W, Friedman RA, Ariyama H, Dubeykovskaya ZA, Muthupalani S, Ericksen R, Frucht H, Fox JG, Wang TC. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology. 2013;144:155–166. doi: 10.1053/j.gastro.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabino A, Dufour C, Marino CE, Claudiani F, De Alessandri A. Unexplained refractory iron-deficiency anemia associated with Helicobacter pylori gastric infection in children: further clinical evidence. Journal of pediatric gastroenterology and nutrition. 1999;28:116–119. doi: 10.1097/00005176-199901000-00027. [DOI] [PubMed] [Google Scholar]
- Bartsch H, Ohshima H, Pignatelli B. Inhibitors of endogenous nitrosation. Mechanisms and implications in human cancer prevention. Mutation research. 1988;202:307–324. doi: 10.1016/0027-5107(88)90194-7. [DOI] [PubMed] [Google Scholar]
- Bates CJ, Prentice AM, Paul AA. Seasonal variations in vitamins A, C, riboflavin and folate intakes and status of pregnant and lactating women in a rural Gambian community: some possible implications. European journal of clinical nutrition. 1994;48:660–668. [PubMed] [Google Scholar]
- Bergin IL, Sheppard BJ, Fox JG. Helicobacter pylori infection and high dietary salt independently induce atrophic gastritis and intestinal metaplasia in commercially available outbred Mongolian gerbils. Digestive diseases and sciences. 2003;48:475–485. doi: 10.1023/a:1022524313355. [DOI] [PubMed] [Google Scholar]
- Berretta M, Cappellani A, Lleshi A, Di Vita M, Lo Menzo E, Bearz A, Galvano F, Spina M, Malaguarnera M, Tirelli U, Berretta S. The role of diet in gastric cancer: still an open question. Frontiers in bioscience : a journal and virtual library. 2012;17:1640–1647. doi: 10.2741/4009. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- Brandt S, Kwok T, Hartig R, Konig W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9300–9305. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S, Shafikhani S, Balachandran P, Jin S, Hartig R, Konig W, Engel J, Backert S. Use of a novel coinfection system reveals a role for Rac1, H-Ras, and CrkII phosphorylation in Helicobacter pylori-induced host cell actin cytoskeletal rearrangements. FEMS immunology and medical microbiology. 2007;50:190–205. doi: 10.1111/j.1574-695X.2007.00234.x. [DOI] [PubMed] [Google Scholar]
- Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, Bonaguri C, Cipriani F, Cocco P, Giacosa A, et al. A case-control study of gastric cancer and diet in Italy: I. Frequencies of food consumption. International journal of cancer. Journal international du cancer. 1990;44:611–616. doi: 10.1002/ijc.2910440409. [DOI] [PubMed] [Google Scholar]
- Cai X, Carlson J, Stoicov C, Li H, Wang TC, Houghton J. Helicobacter felis eradication restores normal architecture and inhibits gastric cancer progression in C57BL/6 mice. Gastroenterology. 2005;128:1937–1952. doi: 10.1053/j.gastro.2005.02.066. [DOI] [PubMed] [Google Scholar]
- Camargo MC, Mera R, Correa P, Peek RM, Jr, Fontham ET, Goodman KJ, Piazuelo MB, Sicinschi L, Zabaleta J, Schneider BG. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:1674–1687. doi: 10.1158/1055-9965.EPI-06-0189. [DOI] [PubMed] [Google Scholar]
- Charlton RW, Bothwell TH. Iron absorption. Annual review of medicine. 1983;34:55–68. doi: 10.1146/annurev.me.34.020183.000415. [DOI] [PubMed] [Google Scholar]
- Charnley G, Tannenbaum SR. Flow cytometric analysis of the effect of sodium chloride on gastric cancer risk in the rat. Cancer research. 1985;45:5608–5616. [PubMed] [Google Scholar]
- Chen VW, Abu-Elyazeed RR, Zavala DE, Haenszel W, Ktsanes VK, Rice J, Cuello C, Montes G, Correa P. Risk factors of gastric precancerous lesions in a high-risk Colombian population. II. Nitrate and nitrite. Nutrition and cancer. 1990;13:67–72. doi: 10.1080/01635589009514046. [DOI] [PubMed] [Google Scholar]
- Choe YH, Kim SK, Son BK, Lee DH, Hong YC, Pai SH. Randomized placebo-controlled trial of Helicobacter pylori eradication for iron-deficiency anemia in preadolescent children and adolescents. Helicobacter. 1999;4:135–139. doi: 10.1046/j.1523-5378.1999.98066.x. [DOI] [PubMed] [Google Scholar]
- Choe YH, Lee JE, Kim SK. Effect of helicobacter pylori eradication on sideropenic refractory anaemia in adolescent girls with Helicobacter pylori infection. Acta Paediatr. 2000;89:154–157. doi: 10.1080/080352500750028753. [DOI] [PubMed] [Google Scholar]
- Chung WK, Leibel RL. The links between obesity, leptin, and prostate cancer. Cancer J. 2006;12:178–181. doi: 10.1097/00130404-200605000-00004. [DOI] [PubMed] [Google Scholar]
- Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiology & behavior. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Correa P. Mechanisms of gastric carcinogenesis. In: Joossens JV, editor. Diet and Human Carcinogenesis. Amsterdam: Elsevier; 1985. pp. 109–115. [Google Scholar]
- Correa P, Fontham E, Pickle LW, Chen V, Lin YP, Haenszel W. Dietary determinants of gastric cancer in south Louisiana inhabitants. Journal of the National Cancer Institute. 1985;75:645–654. [PubMed] [Google Scholar]
- Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD, Mera R. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. Journal of the National Cancer Institute. 2000;92:1881–1888. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- Correa P, Malcom G, Schmidt B, Fontham E, Ruiz B, Bravo JC, Bravo LE, Zarama G, Realpe JL. Review article: Antioxidant micronutrients and gastric cancer. Alimentary pharmacology & therapeutics. 1998;12(Suppl 1):73–82. doi: 10.1111/j.1365-2036.1998.00006.x. [DOI] [PubMed] [Google Scholar]
- Court M, Robinson PA, Dixon MF, Crabtree JE. Gastric Helicobacter species infection in murine and gerbil models: comparative analysis of effects of H. pylori and H. felis on gastric epithelial cell proliferation. The Journal of infectious diseases. 2002;186:1348–1352. doi: 10.1086/344321. [DOI] [PubMed] [Google Scholar]
- Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. The Journal of biological chemistry. 1992;267:10570–10575. [PubMed] [Google Scholar]
- Cravo M, Pinto R, Fidalgo P, Chaves P, Gloria L, Nobre-Leitao C, Costa Mira F. Global DNA hypomethylation occurs in the early stages of intestinal type gastric carcinoma. GUT. 1996;39:434–438. doi: 10.1136/gut.39.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Houghton J, Finkel N, Carlson J, Wang TC. IFN-gamma infusion induces gastric atrophy, metaplasia and dysplasia in the absence of Helicobacter infection-a role for immune response in Helicobacter disease. Gastroenterology. 2003;124:A19. [Google Scholar]
- Cui G, Koh TJ, Chen D, Zhao CM, Takaishi S, Dockray GJ, Varro A, Rogers AB, Fox JG, Wang TC. Overexpression of glycine-extended gastrin inhibits parietal cell loss and atrophy in the mouse stomach. Cancer research. 2004;64:8160–8166. doi: 10.1158/0008-5472.CAN-04-0876. [DOI] [PubMed] [Google Scholar]
- Cui G, Takaishi S, Ai W, Betz KS, Florholmen J, Koh TJ, Houghton J, Pritchard DM, Wang TC. Gastrin-induced apoptosis contributes to carcinogenesis in the stomach. Laboratory investigation; a journal of technical methods and pathology. 2006;86:1037–1051. doi: 10.1038/labinvest.3700462. [DOI] [PubMed] [Google Scholar]
- D'Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr. 2012;31:489–498. doi: 10.1016/j.clnu.2012.01.003. [DOI] [PubMed] [Google Scholar]
- D'Elios MM, Manghetti M, De Carli M, Costa F, Baldari CT, Burroni D, Telford JL, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- Doenges JL. Spirochaetes in the gastric glands of the Macaus Rhesus and Human without definite history of related disease. Exp Biol Med. 1938;38:536–538. [Google Scholar]
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF, Jr, Rabkin CS. Interleuki-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, Fraumeni JF, Jr, Chow WH. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- Ericksen RE, Rose S, Westphalen CB, Shibata W, Muthupalani S, Tailor Y, Friedman RA, Han W, Fox JG, Ferrante AW, Jr, Wang TC. Obesity accelerates Helicobacter felis-induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and TH17 response. GUT. 2013 doi: 10.1136/gutjnl-2013-305092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. Journal international du cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. Journal of internal medicine. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- Foo JH, Culvenor JG, Ferrero RL, Kwok T, Lithgow T, Gabriel K. Both the p33 and p55 subunits of the Helicobacter pylori VacA toxin are targeted to mammalian mitochondria. Journal of molecular biology. 2010;401:792–798. doi: 10.1016/j.jmb.2010.06.065. [DOI] [PubMed] [Google Scholar]
- Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nature medicine. 2000;6:536–542. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- Fox JG, Blanco M, Murphy JC, Taylor NS, Lee A, Kabok Z, Pappo J. Local and systemic immune responses in murine Helicobacter felis active chronic gastritis. Infection and immunity. 1993;61:2309–2315. doi: 10.1128/iai.61.6.2309-2315.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer research. 1999;59:4823–4828. [PubMed] [Google Scholar]
- Fox JG, Kuipers EJ. Long-term proton pump inhibitor administration, H pylori and gastric cancer: lessons from the gerbil. GUT. 2011;60:567–568. doi: 10.1136/gut.2010.229286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JG, Lee A, Otto G, Taylor NS, Murphy JC. Helicobacter felis gastritis in gnotobiotic rats: an animal model of Helicobacter pylori gastritis. Infection and immunity. 1991;59:785–791. doi: 10.1128/iai.59.3.785-791.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JG, Rogers AB, Ihrig M, Taylor NS, Whary MT, Dockray G, Varro A, Wang TC. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer research. 2003a;63:942–950. [PubMed] [Google Scholar]
- Fox JG, Sheppard BJ, Dangler CA, Whary MT, Ihrig M, Wang TC. Germ-line p53-targeted disruption inhibits helicobacter-induced premalignant lesions and invasive gastric carcinoma through down-regulation of Th1 proinflammatory responses. Cancer research. 2002;62:696–702. [PubMed] [Google Scholar]
- Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. The Journal of clinical investigation. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JG, Wang TC, Rogers AB, Poutahidis T, Ge Z, Taylor N, Dangler CA, Israel DA, Krishna U, Gaus K, Peek RM., Jr Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003b;124:1879–1890. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M, Whitehead R, Gaus K, O'Brien DP, Romero-Gallo J, Peek RM., Jr Activation of beta-catenin by carcinogenic Helicobacter pylori. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg AS, Barron L. The presence of spirochetes in human gastric mucosa. Jour. D. D. 1940;7:443–445. [Google Scholar]
- Friedman GD, Parsonnet J. Salt intake and stomach cancer: some contrary evidence. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1992;1:607–608. [PubMed] [Google Scholar]
- Furihata C, Sato Y, Hosaka M, Matsushima T, Furukawa F, Takahashi M. NaCl induced ornithine decarboxylase and DNA synthesis in rat stomach mucosa. Biochemical and biophysical research communications. 1984;121:1027–1032. doi: 10.1016/0006-291x(84)90780-0. [DOI] [PubMed] [Google Scholar]
- Gaddy JA, Radin JN, Loh JT, Zhang F, Washington MK, Peek RM, Jr, Algood HM, Cover TL. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infection and immunity. 2013;81:2258–2267. doi: 10.1128/IAI.01271-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaut JP, Belaaouaj A, Byun J, Roberts LJ, 2nd, Maeda N, Frei B, Heinecke JW. Vitamin C fails to protect amino acids and lipids from oxidation during acute inflammation. Free radical biology & medicine. 2006;40:1494–1501. [Google Scholar]
- Ge Z, Feng Y, Muthupalani S, Eurell LL, Taylor NS, Whary MT, Fox JG. Coinfection with Enterohepatic Helicobacter species can ameliorate or promote Helicobacter pylori-induced gastric pathology in C57BL/6 mice. Infection and immunity. 2011;79:3861–3871. doi: 10.1128/IAI.05357-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geboers J, Joossens JV, Kesteloot H. Epidemiology of stomach cancer. In: Joossens JV, editor. Diet and Human Carcinogenesis. Amsterdam: Elsevier; 1985. pp. 81–95. [Google Scholar]
- Gonda TA, Kim YI, Salas MC, Gamble MV, Shibata W, Muthupalani S, Sohn KJ, Abrams JA, Fox JG, Wang TC, Tycko B. Folic acid increases global DNA methylation and reduces inflammation to prevent Helicobacter-associated gastric cancer in mice. Gastroenterology. 2012;142:824–833. e827. doi: 10.1053/j.gastro.2011.12.058. [DOI] [PubMed] [Google Scholar]
- Gwack J, Shin A, Kim CS, Ko KP, Kim Y, Jun JK, Bae J, Park SK, Hong YC, Kang D, Chang SH, Shin HR, Yoo KY. CagA-producing Helicobacter pylori and increased risk of gastric cancer: a nested case-control study in Korea. British journal of cancer. 2006;95:639–641. doi: 10.1038/sj.bjc.6603309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara T, Mukaisho K, Nakayama T, Sugihara H, Hattori T. Long-term proton pump inhibitor administration worsens atrophic corpus gastritis and promotes adenocarcinoma development in Mongolian gerbils infected with Helicobacter pylori. GUT. 2011;60:624–630. doi: 10.1136/gut.2010.207662. [DOI] [PubMed] [Google Scholar]
- Hansson LE, Nyren O, Hsing AW, Bergstrom R, Josefsson S, Chow WH, Fraumeni JF, Jr, Adami HO. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. The New England journal of medicine. 1996;335:242–249. doi: 10.1056/NEJM199607253350404. [DOI] [PubMed] [Google Scholar]
- Harrison LE, Zhang ZF, Karpeh MS, Sun M, Kurtz RC. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a case-control study in the U.S. Cancer. 1997;80:1021–1028. [PubMed] [Google Scholar]
- Hemila H, Kaprio J, Pietinen P, Albanes D, Heinonen OP. Vitamin C and other compounds in vitamin C rich food in relation to risk of tuberculosis in male smokers. American journal of epidemiology. 1999;150:632–641. doi: 10.1093/oxfordjournals.aje.a010062. [DOI] [PubMed] [Google Scholar]
- Hirayama F, Takagi S, Kusuhara H, Iwao E, Yokoyama Y, Ikeda Y. Induction of gastric ulcer and intestinal metaplasia in mongolian gerbils infected with Helicobacter pylori. Journal of gastroenterology. 1996;31:755–757. doi: 10.1007/BF02347631. [DOI] [PubMed] [Google Scholar]
- Holcombe C. Helicobacter pylori: the African enigma. GUT. 1992;33:429–431. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Fujioka T, Tokieda M, Gotoh T, Nishizono A, Nasu M. Gastric ulcer, atrophic gastritis, and intestinal metaplasia caused by Helicobacter pylori infection in Mongolian gerbils. Scandinavian journal of gastroenterology. 1998a;33:454–460. doi: 10.1080/00365529850171990. [DOI] [PubMed] [Google Scholar]
- Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer research. 1998b;58:4255–4259. [PubMed] [Google Scholar]
- Hou L, Wang H, Sartori S, Gawron A, Lissowska J, Bollati V, Tarantini L, Zhang FF, Zatonski W, Chow WH, Baccarelli A. Blood leukocyte DNA hypomethylation and gastric cancer risk in a high-risk Polish population. International journal of cancer. Journal international du cancer. 2010;127:1866–1874. doi: 10.1002/ijc.25190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. ARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Schistosomes, Liver Flukes and Helicobacter Pylori. International Agency for Research on Cancer; 1994. [PMC free article] [PubMed] [Google Scholar]
- James BR, Tomanek-Chalkley A, Askeland EJ, Kucaba T, Griffith TS, Norian LA. Diet-induced obesity alters dendritic cell function in the presence and absence of tumor growth. J Immunol. 2012;189:1311–1321. doi: 10.4049/jimmunol.1100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jones KR, Whitmire JM, Merrell DS. A Tale of Two Toxins: Helicobacter Pylori CagA and VacA Modulate Host Pathways that Impact Disease. Frontiers in microbiology. 2010;1:115. doi: 10.3389/fmicb.2010.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joossens JV, Geboers J. Nutrition and gastric cancer. Nutrition and cancer. 1981;2:250–261. doi: 10.1080/01635588109513691. [DOI] [PubMed] [Google Scholar]
- Judd LM, Alderman BM, Howlett M, Shulkes A, Dow C, Moverley J, Grail D, Jenkins BJ, Ernst M, Giraud AS. Gastric cancer development in mice lacking the SHP2 binding site on the I-6 family co-receptor gp130. Gastroenterology. 2004;126:196–207. doi: 10.1053/j.gastro.2003.10.066. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Dixit VD. Immunological complications of obesity. Nature immunology. 2012;13:707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- Karita M, Kouchiyama T, Okita K, Nakazawa T. New small animal model for human gastric Helicobacter pylori infection: success in both nude and euthymic mice. Am J Gastroenterol. 1991;86:1596–1603. [PubMed] [Google Scholar]
- Keenan JI, Peterson RA, Fraser R, Frampton CM, Walmsley TA, Allardyce RA, Roake JA. The effect of Helicobacter pylori infection and dietary iron deficiency on host iron homeostasis: a study in mice. Helicobacter. 2004;9:643–650. doi: 10.1111/j.1083-4389.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- Kim YI. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2004;13:511–519. [PubMed] [Google Scholar]
- Kodama M, Kodama T, Suzuki H, Kondo K. Effect of rice and salty rice diets on the structure of mouse stomach. Nutrition and cancer. 1984;6:135–147. doi: 10.1080/01635588509513817. [DOI] [PubMed] [Google Scholar]
- Krejs GJ. Gastric cancer: epidemiology and risk factors. Dig Dis. 2010;28:600–603. doi: 10.1159/000320277. [DOI] [PubMed] [Google Scholar]
- Kuipers EJ. Proton pump inhibitors and gastric neoplasia. GUT. 2006;55:1217–1221. doi: 10.1136/gut.2005.090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, Havu N, Festen HP, Liedman B, Lamers CB, Jansen JB, Dalenback J, Snel P, Nelis GF, Meuwissen SG. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. The New England journal of medicine. 1996;334:1018–1022. doi: 10.1056/NEJM199604183341603. [DOI] [PubMed] [Google Scholar]
- Kuipers EJ, Uyterlinde AM, Pena AS, Hazenberg HJ, Bloemena E, Lindeman J, Klinkenberg-Knol EC, Meuwissen SG. Increase of Helicobacter pylori-associated corpus gastritis during acid suppressive therapy: implications for long-term safety. Am J Gastroenterol. 1995;90:1401–1406. [PubMed] [Google Scholar]
- Kurekci AE, Atay AA, Sarici SU, Yesilkaya E, Senses Z, Okutan V, Ozcan O. Is there a relationship between childhood Helicobacter pylori infection and iron deficiency anemia? Journal of tropical pediatrics. 2005;51:166–169. doi: 10.1093/tropej/fmi015. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Negri E, Decarli A, D'Avanzo B, Franceschi S. A case-control study of diet and gastric cancer in northern Italy. International journal of cancer. Journal international du cancer. 1987;40:484–489. doi: 10.1002/ijc.2910400409. [DOI] [PubMed] [Google Scholar]
- Lastovica AJ, le Roux E. Efficient isolation of campylobacteria from stools. Journal of clinical microbiology. 2000;38:2798–2799. doi: 10.1128/jcm.38.7.2798-2799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Fox JG, Otto G, Murphy J. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology. 1990;99:1315–1323. doi: 10.1016/0016-5085(90)91156-z. [DOI] [PubMed] [Google Scholar]
- Lee A, Krakowka S, Fox JG, Otto G, Eaton KA, Murphy JC. Role of Helicobacter felis in chronic canine gastritis. Veterinary pathology. 1992;29:487–494. doi: 10.1177/030098589202900601. [DOI] [PubMed] [Google Scholar]
- Lee A, O'Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- Lee CW, Rickman B, Rogers AB, Ge Z, Wang TC, Fox JG. Helicobacter pylori eradication prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer research. 2008a;68:3540–3548. doi: 10.1158/0008-5472.CAN-07-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Wang XD, Chien KL, Ge Z, Rickman BH, Rogers AB, Varro A, Whary MT, Wang TC, Fox JG. Vitamin C supplementation does not protect L-gulono-gamma-lactone oxidase-deficient mice from Helicobacter pylori-induced gastritis and gastric premalignancy. International journal of cancer. Journal international du cancer. 2008b;122:1068–1076. doi: 10.1002/ijc.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IO, Kim JH, Choi YJ, Pillinger MH, Kim SY, Blaser MJ, Lee YC. Helicobacter pylori CagA phosphorylation status determines the gp130-activated SHP2/ERK and JAK/STAT signal transduction pathways in gastric epithelial cells. The Journal of biological chemistry. 2010;285:16042–16050. doi: 10.1074/jbc.M110.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. Journal of epidemiology / Japan Epidemiological Association. 2003;13:162–168. doi: 10.2188/jea.13.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke LB, Ge Z, Whary MT, Feng Y, Rogers AB, Muthupalani S, Fox JG. Concurrent Helicobacter bilis infection in C57BL/6 mice attenuates proinflammatory H. pylori-induced gastric pathology. Infection and immunity. 2009;77:2147–2158. doi: 10.1128/IAI.01395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertpiriyapong K, Whary MT, Muthupalani S, Lofgren JL, Gamazon ER, Feng Y, Ge Z, Wang TC, Fox JG. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. GUT. 2013 doi: 10.1136/gutjnl-2013-305178. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk RD, Johnson PT, David BC, Kraft WG, Morgan DR. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. Journal of medical microbiology. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Levi S, Beardshall K, Haddad G, Playford R, Ghosh P, Calam J. Campylobacter pylori and duodenal ulcers: the gastrin link. Lancet. 1989;1:1167–1168. doi: 10.1016/s0140-6736(89)92752-9. [DOI] [PubMed] [Google Scholar]
- Li Q, Karam SM, Gordon JI. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. The Journal of biological chemistry. 1996;271:3671–3676. [PubMed] [Google Scholar]
- Li W, Maeda N, Beck MA. Vitamin C deficiency increases the lung pathology of influenza virus-infected gulo−/− mice. The Journal of nutrition. 2006;136:2611–2616. doi: 10.1093/jn/136.10.2611. [DOI] [PubMed] [Google Scholar]
- Liao JH, Sun YH, Hsu CH, Lin YC, Wu SH, Kuo CJ, Huang CH, Chiou SH. Up-regulation of neutrophil activating protein in Helicobacter pylori under high-salt stress: structural and phylogenetic comparison with bacterial iron-binding ferritins. Biochimie. 2013;95:1136–1145. doi: 10.1016/j.biochi.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Lofgren JL, Whary MT, Ge Z, Muthupalani S, Taylor NS, Mobley M, Potter A, Varro A, Eibach D, Suerbaum S, Wang TC, Fox JG. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140:210–220. doi: 10.1053/j.gastro.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh JT, Friedman DB, Piazuelo MB, Bravo LE, Wilson KT, Peek RM, Jr, Correa P, Cover TL. Analysis of Helicobacter pylori cagA promoter elements required for salt-induced upregulation of CagA expression. Infection and immunity. 2012;80:3094–3106. doi: 10.1128/IAI.00232-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell L, Havu N, Miettinen P, Myrvold HE, Wallin L, Julkunen R, Levander K, Hatlebakk JG, Liedman B, Lamm M, Malm A, Walan A. Changes of gastric mucosal architecture during long-term omeprazole therapy: results of a randomized clinical trial. Alimentary pharmacology & therapeutics. 2006;23:639–647. doi: 10.1111/j.1365-2036.2006.02792.x. [DOI] [PubMed] [Google Scholar]
- Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- Marignani M, Angeletti S, Bordi C, Malagnino F, Mancino C, Delle Fave G, Annibale B. Reversal of long-standing iron deficiency anaemia after eradication of Helicobacter pylori infection. Scandinavian journal of gastroenterology. 1997;32:617–622. doi: 10.3109/00365529709025109. [DOI] [PubMed] [Google Scholar]
- Martin HR, Shakya KP, Muthupalani S, Ge Z, Klei TR, Whary MT, Fox JG. Brugia filariasis differentially modulates persistent Helicobacter pylori gastritis in the gerbil model. Microbes and infection / Institut Pasteur. 2010;12:748–758. doi: 10.1016/j.micinf.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Long term follow up of patients treated for Helicobacter pylori infection. GUT. 2005;54:1536–1540. doi: 10.1136/gut.2005.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimuro H, Suzuki T, Tanaka J, Asahi M, Haas R, Sasakawa C. Grb2 is a key mediator of helicobacter pylori CagA protein activities. Molecular cell. 2002;10:745–755. doi: 10.1016/s1097-2765(02)00681-0. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn SJ. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infection and immunity. 1996;64:238–245. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HS, Liu X, Nagel JM, Chamberland JP, Diakopoulos KN, Brinkoetter MT, Hatziapostolou M, Wu Y, Robson SC, Iliopoulos D, Mantzoros CS. Salutary effects of adiponectin on colon cancer: in vivo and in vitro studies in mice. GUT. 2013;62:561–570. doi: 10.1136/gutjnl-2012-302092. [DOI] [PubMed] [Google Scholar]
- Mulholland G, Ardill JE, Fillmore D, Chittajallu RS, Fullarton GM, McColl KE. Helicobacter pylori related hypergastrinemia is the result of selective increase in gastrin 17. Gut. 1993;34:757–761. doi: 10.1136/gut.34.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A. MALT Lymphoma: Clinicopathologic Features and Molecular Pathogenesis. In: Wang TC, editor. The Biology of Gastric Cancers. New York, NY: Springer; 2009. p. 91. [Google Scholar]
- Muller A. Multistep activation of the Helicobacter pylori effector CagA. The Journal of clinical investigation. 2012;122:1192–1195. doi: 10.1172/JCI61578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberne PM, Charnley G, Adams K, Cantor M, Suphakarn V, Roth D, Schrager TF. Gastric carcinogenesis: a model for the identification of risk factors. Cancer letters. 1987;38:149–163. doi: 10.1016/0304-3835(87)90210-2. [DOI] [PubMed] [Google Scholar]
- Niwa T, Toyoda T, Tsukamoto T, Mori A, Tatematsu M, Ushijima T. Prevention of Helicobacter pylori-induced gastric cancers in gerbils by a DNA demethylating agent. Cancer Prev Res (Phila) 2013;6:263–270. doi: 10.1158/1940-6207.CAPR-12-0369. [DOI] [PubMed] [Google Scholar]
- Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, Romero-Gallo J, Suarez G, Loh J, Slaughter JC, Tan S, Morgan DR, Wilson KT, Bravo LE, Correa P, Cover TL, Amieva MR, Peek RM., Jr Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. The Journal of clinical investigation. 2013;123:479–492. doi: 10.1172/JCI64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki K, Shimizu N, Inada K, Tsukamoto T, Inoue M, Kumagai T, Sugiyama A, Mizoshita T, Kaminishi M, Tatematsu M. Synergistic promoting effects of Helicobacter pylori infection and high-salt diet on gastric carcinogenesis in Mongolian gerbils. Japanese journal of cancer research : Gann. 2002;93:1083–1089. doi: 10.1111/j.1349-7006.2002.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H, Szentirmay Z, Take M, Sugimura T. Effects of 4-week treatment with gastric carcinogens and enhancing agents on proliferation of gastric mucosa cells in rats. Cancer letters. 1989;46:117–122. doi: 10.1016/0304-3835(89)90018-9. [DOI] [PubMed] [Google Scholar]
- Ohkusa T, Okayasu I, Miwa H, Ohtaka K, Endo S, Sato N. Helicobacter pylori infection induces duodenitis and superficial duodenal ulcer in Mongolian gerbils. GUT. 2003;52:797–803. doi: 10.1136/gut.52.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani M, Garcia A, Rogers AB, Ge Z, Taylor NS, Xu S, Watanabe K, Marini RP, Whary MT, Wang TC, Fox JG. Protective role of 17 beta -estradiol against the development of Helicobacter pylori-induced gastric cancer in INS-GAS mice. Carcinogenesis. 2007;28:2597–2604. doi: 10.1093/carcin/bgm150. [DOI] [PubMed] [Google Scholar]
- Ohtani M, Ge Z, Garcia A, Rogers AB, Muthupalani S, Taylor NS, Xu S, Watanabe K, Feng Y, Marini RP, Whary MT, Wang TC, Fox JG. 17 beta-estradiol suppresses Helicobacter pylori-induced gastric pathology in male hypergastrinemic INS-GAS mice. Carcinogenesis. 2011;32:1244–1250. doi: 10.1093/carcin/bgr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MJ, Costa AM, Costa AC, Ferreira RM, Sampaio P, Machado JC, Seruca R, Mareel M, Figueiredo C. CagA associates with c-Met, E-cadherin, and p120-catenin in a multiproteic complex that suppresses Helicobacter pylori-induced cell-invasive phenotype. The Journal of infectious diseases. 2009;200:745–755. doi: 10.1086/604727. [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phull PS, Price AB, White KL, Schorah CJ, Jacyna MR. Gastroduodenal mucosal vitamin-C levels in Helicobacter pylori infection. Scandinavian journal of gastroenterology. 1999;34:361–366. doi: 10.1080/003655299750026362. [DOI] [PubMed] [Google Scholar]
- Poppe M, Feller SM, Romer G, Wessler S. Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene. 2007;26:3462–3472. doi: 10.1038/sj.onc.1210139. [DOI] [PubMed] [Google Scholar]
- Pra D, Rech Franke SI, Pegas Henriques JA, Fenech M. A possible link between iron deficiency and gastrointestinal carcinogenesis. Nutrition and cancer. 2009;61:415–426. doi: 10.1080/01635580902803701. [DOI] [PubMed] [Google Scholar]
- Przemeck SM, Varro A, Berry D, Steele I, Wang TC, Dockray GJ, Pritchard DM. Hypergastrinemia increases gastric epithelial susceptibility to apoptosis. Regulatory peptides. 2008;146:147–156. doi: 10.1016/j.regpep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder G, Merchant JL, Haas R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology. 2005;128:1229–1242. doi: 10.1053/j.gastro.2005.02.064. [DOI] [PubMed] [Google Scholar]
- Rogers AB, Fox JG. Inflammation and Cancer I Rodent models of infectious gastrointestinal and liver cancer. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2004;286:G361–G366. doi: 10.1152/ajpgi.00499.2003. [DOI] [PubMed] [Google Scholar]
- Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer research. 2005;65:10709–10715. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- Roth KA, Kapadia SB, Martin SM, Lorenz RG. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol. 1999;163:1490–1497. [PubMed] [Google Scholar]
- Sakagami T, Shimoyama T, O'Rouke J. Back to the host: severity of inflammation induced by Helicobacter felis in different strains of mice. Am J Gastroenterol. 1994;89:1345. [Google Scholar]
- Sato T, Fukuyama T, Urata F. Studies of the causation of gastric cancer. I. Bleeding in the glandular stomach of mice by feeding with highly salted foods, and a comment on salted foods in Japan. Jap Med J. 1959;1835:5. [Google Scholar]
- Schmidt PH, Lee JR, Goldenring, Wright NA, Poulsom R. Association of an aberrant spasmolytic peptideexpressing cell lineage with gastric adenocarcinoma in humans. Gastroenterology. 1998;114:A673. [Google Scholar]
- Schwager J, Schulze J. Modulation of interleukin production by ascorbic acid. Veterinary immunology and immunopathology. 1998;64:45–57. doi: 10.1016/s0165-2427(98)00120-2. [DOI] [PubMed] [Google Scholar]
- Schwarz P, Kubler JA, Strnad P, Muller K, Barth TF, Gerloff A, Feick P, Peyssonnaux C, Vaulont S, Adler G, Kulaksiz H. Hepcidin is localised in gastric parietal cells, regulates acid secretion and is induced by Helicobacter pylori infection. GUT. 2012;61:193–201. doi: 10.1136/gut.2011.241208. [DOI] [PubMed] [Google Scholar]
- Senkovich O, Ceaser S, McGee DJ, Testerman TL. Unique host iron utilization mechanisms of Helicobacter pylori revealed with iron-deficient chemically defined media. Infection and immunity. 2010;78:1841–1849. doi: 10.1128/IAI.01258-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheh A, Ge Z, Parry NM, Muthupalani S, Rager JE, Raczynski AR, Mobley MW, McCabe AF, Fry RC, Wang TC, Fox JG. 17beta-estradiol and tamoxifen prevent gastric cancer by modulating leukocyte recruitment and oncogenic pathways in Helicobacter pylori-infected INS-GAS male mice. Cancer Prev Res (Phila) 2011;4:1426–1435. doi: 10.1158/1940-6207.CAPR-11-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Liu XF, Zhuang Y, Zhang JY, Liu T, Yin Z, Wu C, Mao XH, Jia KR, Wang FJ, Guo H, Flavell RA, Zhao Z, Liu KY, Xiao B, Guo Y, Zhang WJ, Zhou WY, Guo G, Zou QM. Helicobacter pylori-induced Th17 responses modulate Th1 cell responses, benefit bacterial growth, and contribute to pathology in mice. J Immunol. 2010;184:5121–5129. doi: 10.4049/jimmunol.0901115. [DOI] [PubMed] [Google Scholar]
- Shitara K, Muro K, Ito S, Sawaki A, Tajika M, Kawai H, Yokota T, Takahari D, Shibata T, Ura T, Ito H, Hosono S, Kawase T, Watanabe M, Tajima K, Yatabe Y, Tanaka H, Matsuo K. Folate intake along with genetic polymorphisms in methylenetetrahydrofolate reductase and thymidylate synthase in patients with advanced gastric cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:1311–1319. doi: 10.1158/1055-9965.EPI-09-1257. [DOI] [PubMed] [Google Scholar]
- Shomer NH, Dangler CA, Whary MT, Fox JG. Experimental Helicobacter pylori infection induces antral gastritis and gastric mucosa-associated lymphoid tissue in guinea pigs. Infection and immunity. 1998;66:2614–2618. doi: 10.1128/iai.66.6.2614-2618.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in I-4, but not IFN-gamma, gene-deficient mice. J Immunol. 2000;165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- Sobala GM, Pignatelli B, Schorah CJ. Simultaneous determination of ascorbic acid nitrite, total nitrosocompounds and bile acids in fasting gastric juice, and gastric mucosal histology implications for gastric carcinogenesis. Carcinogenesis. 1991;14:291–292. [Google Scholar]
- Sobala GM, Schorah CJ, Pignatelli B, Crabtree JE, Martin IG, Scott N, Quirke P. High gastric juice ascorbic acid concentrations in members of a gastric cancer family. Carcinogenesis. 1993;14:291–292. doi: 10.1093/carcin/14.2.291. [DOI] [PubMed] [Google Scholar]
- Song J, Medline A, Mason JB, Gallinger S, Kim YI. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer research. 2000a;60:5434–5440. [PubMed] [Google Scholar]
- Song J, Sohn KJ, Medline A, Ash C, Gallinger S, Kim YI. Chemopreventive effects of dietary folate on intestinal polyps in Apc+/−Msh2−/− mice. Cancer research. 2000b;60:3191–3199. [PubMed] [Google Scholar]
- Sorbye H, Svanes C, Stangeland L, Kvinnsland S, Svanes K. Epithelial restitution and cellular proliferation after gastric mucosal damage caused by hypertonic NaCl in rats. Virchows Archiv. A, Pathological anatomy and histopathology. 1988;413:445–455. doi: 10.1007/BF00716993. [DOI] [PubMed] [Google Scholar]
- Steele IA, Dimaline R, Pritchard DM, Peek RM, Jr, Wang TC, Dockray GJ, Varro A. Helicobacter and gastrin stimulate Reg1 expression in gastric epithelial cells through distinct promoter elements. American journal of physiology. Gastrointestinal and liver physiology. 2007;293:G347–G354. doi: 10.1152/ajpgi.00076.2007. [DOI] [PubMed] [Google Scholar]
- Stenstrom B, Zhao CM, Rogers AB, Nilsson HO, Sturegard E, Lundgren S, Fox JG, Wang TC, Wadstrom TM, Chen D. Swedish moist snuff accelerates gastric cancer development in Helicobacter pylori-infected wild-type and gastrin transgenic mice. Carcinogenesis. 2007;28:2041–2046. doi: 10.1093/carcin/bgm071. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ. Iron-deficiency anemia: reexamining the nature and magnitude of the public health problem. Summary: implications for research and programs. The Journal of nutrition. 2001;131:697S–700S. doi: 10.1093/jn/131.2.697S. discussion 700S–701S. [DOI] [PubMed] [Google Scholar]
- Su L, Fontham E, Ruiz B, Schmidt S, Correa P, Bravo L. Association of dietary antioxidants on the severity of gastritis in a high risk population. Annals of epidemiology. 2000;10:468. doi: 10.1016/s1047-2797(00)00135-6. [DOI] [PubMed] [Google Scholar]
- Supajatura V, Ushio H, Wada A, Yahiro K, Okumura K, Ogawa H, Hirayama T, Ra C. Cutting edge: VacA, a vacuolating cytotoxin of Helicobacter pylori, directly activates mast cells for migration and production of proinflammatory cytokines. J Immunol. 2002;168:2603–2607. doi: 10.4049/jimmunol.168.6.2603. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Miyazawa M, Nagahashi S, Mori M, Seto K, Kai A, Suzuki M, Miura S, Ishii H. Attenuated apoptosis in H. pylori-colonized gastric mucosa of Mongolian gerbils in comparison with mice. Digestive diseases and sciences. 2002;47:90–99. doi: 10.1023/a:1013219621422. [DOI] [PubMed] [Google Scholar]
- Tajima K, Tominaga S. Dietary habits and gastro-intestinal cancers: a comparative case-control study of stomach and large intestinal cancers in Nagoya, Japan. Japanese journal of cancer research : Gann. 1985;76:705–716. [PubMed] [Google Scholar]
- Takahashi M, Hasegawa R. Enhancing effects of dietary salt on both initiation and promotion stages of rat gastric carcinogenesis. In: Hayashi S, editor. Diet, Nutrition and Cancer. Utrecht: Japan Sci Sco Press; 1986. [PubMed] [Google Scholar]
- Takahashi M, Kokubo T, Furukawa F, Kurokawa Y, Tatematsu M, Hayashi Y. Effect of high salt diet on rat gastric carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine. Gann = Gan. 1983;74:28–34. [PubMed] [Google Scholar]
- Takaishi S, Cui G, Frederick DM, Carlson JE, Houghton J, Varro A, Dockray GJ, Ge Z, Whary MT, Rogers AB, Fox JG, Wang TC. Synergistic inhibitory effects of gastrin and histamine receptor antagonists on Helicobacter-induced gastric cancer. Gastroenterology. 2005;128:1965–1983. doi: 10.1053/j.gastro.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Takaishi S, Tu S, Dubeykovskaya ZA, Whary MT, Muthupalani S, Rickman BH, Rogers AB, Lertkowit N, Varro A, Fox JG, Wang TC. Gastrin is an essential cofactor for helicobacter-associated gastric corpus carcinogenesis in C57BL/6 mice. The American journal of pathology. 2009;175:365–375. doi: 10.2353/ajpath.2009.081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammer I, Brandt S, Hartig R, Konig W, Backert S. Activation of Abl by Helicobacter pylori: a novel kinase for CagA and crucial mediator of host cell scattering. Gastroenterology. 2007;132:1309–1319. doi: 10.1053/j.gastro.2007.01.050. [DOI] [PubMed] [Google Scholar]
- Tan S, Noto JM, Romero-Gallo J, Peek RM, Jr, Amieva MR. Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS pathogens. 2011;7:e1002050. doi: 10.1371/journal.ppat.1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu M, Takahashi M, Fukushima S, Hananouchi M, Shirai T. Effects in rats of sodium chloride on experimental gastric cancers induced by N-methyl-N-nitro-N-nitrosoguanidine or 4-nitroquinolin-1-oxide. Journal of the National Cancer Institute. 1975;55:101–106. doi: 10.1093/jnci/55.1.101. [DOI] [PubMed] [Google Scholar]
- Taylor PR. Prevention of gastric cancer: a miss. Journal of the National Cancer Institute. 2007;99:101–103. doi: 10.1093/jnci/djk026. [DOI] [PubMed] [Google Scholar]
- Thompson LJ, Danon SJ, Wilson JE, O'Rourke JL, Salama NR, Falkow S, Mitchell H, Lee A. Chronic Helicobacter pylori infection with Sydney strain 1 and a newly identified mouse-adapted strain (Sydney strain 2000) in C57BL/6 and BALB/c mice. Infection and immunity. 2004;72:4668–4679. doi: 10.1128/IAI.72.8.4668-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson MJ, Pritchard DM, Boxall SA, Abuderman AA, Williams JM, Varro A, Crabtree JE. Gastric Helicobacter infection induces iron deficiency in the INS-GAS mouse. PloS one. 2012;7:e50194. doi: 10.1371/journal.pone.0050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Takaishi S, Menheniott TR, Yang X, Shibata W, Jin G, Betz KS, Kawakami K, Minamoto T, Tomasetto C, Rio MC, Lerkowit N, Varro A, Giraud AS, Wang TC. Inhibition of gastric carcinogenesis by the hormone gastrin is mediated by suppression of TFF1 epigenetic silencing. Gastroenterology. 2011;140:879–891. doi: 10.1053/j.gastro.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati E, Michel V, Thiberge JM, Wuscher N, Huerre M, Labigne A. Chronic Helicobacter pylori infections induce gastric mutations in mice. Gastroenterology. 2003;124:1408–1419. doi: 10.1016/s0016-5085(03)00266-x. [DOI] [PubMed] [Google Scholar]
- Tsugane S, Akabane M, Inami T, Matsushima S, Ishibashi T, Ichinowatari Y, Miyajima Y, Watanabe S. Urinary salt excretion and stomach cancer mortality among four Japanese populations. Cancer causes & control : CCC. 1991;2:165–168. doi: 10.1007/BF00056209. [DOI] [PubMed] [Google Scholar]
- Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleuki-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S, Chi AL, Lim S, Cui G, Dubeykovskaya Z, Ai W, Fleming JV, Takaishi S, Wang TC. Gastrin regulates the TFF2 promoter through gastrin-responsive cis-acting elements and multiple signaling pathways. American journal of physiology. Gastrointestinal and liver physiology. 2007;292:G1726–G1737. doi: 10.1152/ajpgi.00348.2006. [DOI] [PubMed] [Google Scholar]
- Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infection and immunity. 1993;61:1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuyns AJ. Salt and gastrointestinal cancer. Nutrition and cancer. 1988;11:229–232. doi: 10.1080/01635588809513992. [DOI] [PubMed] [Google Scholar]
- Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol. 2013;190:794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- Wang SS, Asfaha S, Okumura T, Betz KS, Muthupalani S, Rogers AB, Tu S, Takaishi S, Jin G, Yang X, Wu DC, Fox JG, Wang TC. Fibroblastic colony-forming unit bone marrow cells delay progression to gastric dysplasia in a helicobacter model of gastric tumorigenesis. Stem Cells. 2009a;27:2301–2311. doi: 10.1002/stem.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TC, Bonner-Weir S, Oates PS, Chulak M, Simon B, Merlino GT, Schmidt EV, Brand SJ. Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. The Journal of clinical investigation. 1993;92:1349–1356. doi: 10.1172/JCI116708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- Wang TC, Fox JG. Helicobacter pylori and gastric cancer: Koch's postulates fulfilled? Gastroenterology. 1998;115:780–783. doi: 10.1016/s0016-5085(98)70159-3. [DOI] [PubMed] [Google Scholar]
- Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, Koh TJ, Fox JG. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–689. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Dockray GJ. Processing and proliferative effects of human progastrin in transgenic mice. The Journal of clinical investigation. 1996;98:1918–1929. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Terry PD, Yan H. Review of salt consumption and stomach cancer risk: epidemiological and biological evidence. World journal of gastroenterology : WJG. 2009b;15:2204–2213. doi: 10.3748/wjg.15.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring AJ, Drake IM, Schorah CJ, White KL, Lynch DA, Axon AT, Dixon MF. Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: effects of gastritis and oral supplementation. GUT. 1996;38:171–176. doi: 10.1136/gut.38.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Okamoto T, Takahashi T, Ogundigie PO, Ito A. The effects of sodium chloride, miso or ethanol on development of intestinal metaplasia after X-irradiation of the rat glandular stomach. Japanese journal of cancer research : Gann. 1992;83:1267–1272. doi: 10.1111/j.1349-7006.1992.tb02757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- WHO. Diet, nutrition and the prevention of chronic diseases. World Health Organization technical report series. 2003;916:i–viii. 1–149. backcover. [PubMed] [Google Scholar]
- WHO/UNICEF/UNU. Iron Deficiency Anemia Assessment, Prevention, and Control. Geneva: World Health Organization; 2001. [Google Scholar]
- Windle HJ, Kelleher D, Crabtree JE. Childhood Helicobacter pylori infection and growth impairment in developing countries: a vicious cycle? Pediatrics. 2007;119:e754–e759. doi: 10.1542/peds.2006-2196. [DOI] [PubMed] [Google Scholar]
- Xiao SD, Meng XJ, Shi Y, Hu YB, Zhu SS, Wang CW. Interventional study of high dose folic acid in gastric carcinogenesis in beagles. GUT. 2002;50:61–64. doi: 10.1136/gut.50.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota K, Kurebayashi Y, Takayama Y, Hayashi S, Isogai H, Isogai E, Imai K, Yabana T, Yachi A, Oguma K. Colonization of Helicobacter pylori in the gastric mucosa of Mongolian gerbils. Microbiology and immunology. 1991;35:475–480. doi: 10.1111/j.1348-0421.1991.tb01577.x. [DOI] [PubMed] [Google Scholar]
- You WC, Blot WJ, Chang YS, Ershow AG, Yang ZT, An Q, Henderson B, Xu GW, Fraumeni JF, Jr, Wang TG. Diet and high risk of stomach cancer in Shandong, China. Cancer research. 1988;48:3518–3523. [PubMed] [Google Scholar]
- Zavros Y, Eaton KA, Kang W, Rathinavelu S, Katukuri V, Kao JY, Samuelson LC, Merchant JL. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–2366. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- Zeaiter Z, Cohen D, Musch A, Bagnoli F, Covacci A, Stein M. Analysis of detergent-resistant membranes of Helicobacter pylori infected gastric adenocarcinoma cells reveals a role for MARK2/Par1b in CagA-mediated disruption of cellular polarity. Cellular microbiology. 2008;10:781–794. doi: 10.1111/j.1462-5822.2007.01084.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–520. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- Zuniga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, Butcher EC. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947–6959. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyromski NJ, Mathur A, Pitt HA, Wade TE, Wang S, Nakshatri P, Swartz-Basile DA, Nakshatri H. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery. 2009;146:258–263. doi: 10.1016/j.surg.2009.02.024. [DOI] [PubMed] [Google Scholar]