Abstract
There is a lack of information of exposure to organochlorine pesticides (OCP) and some metals, such as lead (Pb) and arsenic (As), both of which were used as arsenicals pesticides, in children living in the major agricultural areas of Mexico. The objective of this study was to assess the exposure of children to different OCP, As, and Pb in the Yaqui and Mayo valleys of Sonora to generate population baseline levels of these toxins. A cross-sectional study was undertaken in 165 children (age 6–12 years old) from 10 communities from both valleys during 2009. Blood samples were analyzed for OCP and Pb and first morning void urine for inorganic As (InAs). All of the blood samples had detectable levels of dichlorodiphenyltrichloroethylene (p,p′-DDE) ranging from 0.25 to 10.3 μg/L. However lindane, dichlorodiphenyltrichloroethane (p,p′-DDT), aldrin, and endosulfan were detected in far less of the population (36.4, 23.6, 9.1, and 3 %, respectively). Methoxychlor and endrin were not found in any sample. The average value of Pb in this population was 3.2 μg Pb/dL (range 0.17–9.0) with 8.5 % of the samples having levels <5.0 μg Pb/dL. Urinary As levels ranged from 5.4 to 199 μg As/L with an average value of 31.0 μg As/L. Levels > 50 μg/L were observed in 12.7 % of the samples. Our results show that is important to start a risk-reduction program to decrease exposure to these toxins in Mexican communities. In addition, the results can be used to establish the baseline levels of exposure to these toxins in this agricultural region and may be used as a reference point for regulatory agencies.
Persistent organic pollutants (POPs) are resistant to breakdown and are associated with different health problems, including teratogenic and carcinogenic effects (Avalos and Ramírez 2003; Van et al. 2001). POPs are persistent, toxic, and bioaccumulative in nature because they are biomagnified through the food chain (Gao et al. 2008).
In Mexico, exposure to pesticides, metals, and other toxic substances occurs in agricultural, industrial, and mining communities (Trejo-Acevedo et al. 2009). Most of the information reported on population exposure concerns the south and central regions of Mexico, and there are very limited exposure data for the major agricultural areas of Northwest Mexico (SEMARNAT-INE-CONACYT 2008).
The Stockholm Convention on Persistent Organic Pollutants, which came into force in 2004, outlawed the use of 12 industrial chemicals, including DDT, aldrin, endrin, dieldrin, heptacloro, hexachlorobenzene (HCB), mirex, toxaphene, and polychlorinated biphenyls (Pérez-Maldonado et al. 2006). As part of Mexico’s commitment to this agreement, it must prepare and start the National Plan of Implementation that pursues the definition of projects and specific activities to decrease and eliminate OCP. As a consequence of these requirements, in 2008, the major environmental agencies in Mexico—Ministry of Environmental and Natural Resources (SEMARNAT) and the National Institute of Ecology (INE), together with the National Council of Science and Technology (CONACYT)—issued calls to submit proposals to generate information regarding the levels of OCP in human serum of people living in high-risk communities in different States of Mexico and of vulnerable populations, which include the major agricultural valleys in Sonora. Thus, SEMARNAT will have a baseline of the type and extent of exposure of human populations before beginning the start-up of the National Implementation Plan of the Stockholm Convention (SEMARNAT-INE-CONACYT 2008).
The Yaqui and Mayo valleys, located at the southern end of the State of Sonora, could be considered “high-risks areas” because they are two of the most important agricultural areas in Mexico. Due to the availability of water from the Yaqui and Mayo rivers, the Yaqui valley is considered the “cradle of the green revolution” for agriculture. The Yaqui and Mayo valleys have modern irrigation systems, with approximately 266,673 ha under irrigation for the Yaqui valley and 129,514 ha of agricultural lands in the Mayo valley. The Yaqui and Mayo valleys produce ~2.7 million tons/y of crops, mainly wheat, corn, safflower, soy bean, cotton, tomato, potato, chili, watermelon, and zucchini (SAGARPA 2011).
Historically, large amounts of pesticides have been used in the Yaqui and Mayo valleys (Cantu-Soto et al. 2011). Organochlorine, organophosphate, pyrethroids, and carbamates were the most important pesticides used in both valleys, but now endosulfan is the only organochlorine pesticide still in use in this area, with an estimated application of 0.91 L/ha (Rodríguez 2009). In addition, since the 1950s, there have been reports of heavy applications of lead (Pb) arsenate and sodium arsenates as components in herbicides and insecticide mixtures (Cejudo 2009; Woolson 1975). Therefore, it is important to know the exposure levels of Pb and arsenic (As) to these vulnerable populations.
Children are often considered the most vulnerable to a variety of environmental contaminants, and they reflect present trends of environmental exposure more accurately than adults (Trejo-Acevedo et al. 2009). Reports of OCP in children from the Yaqui and Mayo valleys are limited. Only data for the Yaqui valley exists, but this information was obtained in the early 1990s when heavy application of these pesticides occurred in this region. For example, García and Meza (1991) reported the presence of DDT, hexachlorohexane, aldrin, dieldrin, endrin, HCB, and heptachlorine in blood samples of newborns from Pueblo Yaqui. In addition, breast milk from their mothers had high levels of these compounds. Environmental exposures during critical periods of development are likely to contribute to adverse outcomes in childhood and later life stages (Makri et al. 2004). For example, Guillette et al. (1998) used an anthropological approach to evaluate preschool children exposed to pesticides in which they compared two groups of children in the South of Sonora: one group from an agrarian region exposed to pesticide and the other group living in the foothills where pesticide use was avoided. The results showed decreased stamina, gross and fine eye-hand coordination, and short-term memory.
The objective of this study was to assess the exposure to OCP, As, and Pb in children from two of the major agricultural areas in Sonora, Mexico. The goal of this study was to establish baseline exposure levels to OCP in this vulnerable and at-risk population as a point of reference for the assessment of trends over time.
Materials and Methods
Ethical Issues
Our experimental protocol was approved by the Public Health Department of the Sonora State and the Technological Institute of Sonora. Signed consent for each participant and signed parental consent for each child were obtained.
Study Sites
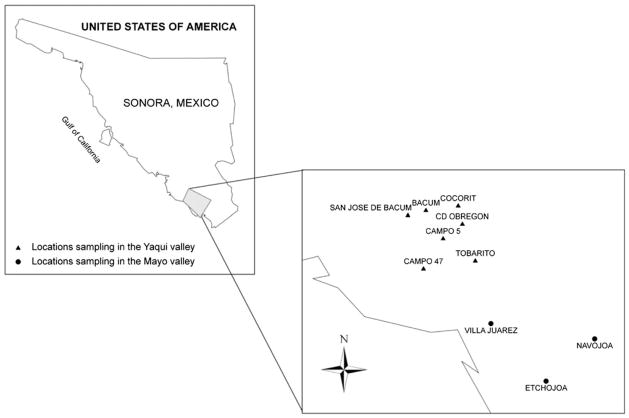
Biomonitoring was focused on communities in the Yaqui and Mayo valleys. Ten communities with agriculture activity were chosen from the valleys to conduct a cross-sectional study, 7 in the Yaqui (Cocorit, Bácum, Campo 5, SJ de Bácum, Cd. Obregón, Tobarito, and Campo 47) and 3 in the Mayo (Navojoa, Etchojoa, and Villa Juárez) (Fig. 1). From February to May 2009, medical personnel from the health department in each of the towns recruited 165 children between 6 and 12 years old for the study. All of the children had similar socioeconomic status, nutritional habits, and health services. Ultimately, the participation rate was >98 %.
Fig. 1.
Localities including in the biological sampling from the Yaqui and Mayo valleys, Sonora, Mexico
Urine Collection
First morning void urine samples were obtained in 100 mL polypropylene bottles, kept on ice, and transported to the Technological Institute of Sonora where they were frozen at −20 °C. Samples were shipped at −60 °C to the University of Arizona and stored at −80 °C until the analysis was performed.
Blood Collection
Blood was drawn from study subjects for Pb and pesticide analysis. The samples were collected by trained personnel from the local Ministry of Health of Cd. Obregón. For Pb determination, blood samples were drawn from a cubital vein into vacutainer tubes containing ethylenediaminetetraacetic acid for plasma collection. The plasma samples were kept on ice and shipped at 4–8 °C to the University of San Luis, Potosí, Mexico, where they were stored 4–8 °C until the analysis was performed. For pesticide analysis, blood was collected in vacutainer SST (serum separation tubes) without anticoagulant for serum separation. Blood samples were allowed to clot at room temperature. The clot was carefully separated from the tube with a wooden probe to ensure phase separation on centrifugation. After centrifugation for 14 min at 2,500 rpm, the serum was transferred with hexane-rinsed Pasteur pipettes to hexanerinsed vials. The vials were stored in the freezer at a temperature of approximately −25 °C before extraction (it is important to mention that all blood samples from Cocorit [n = 12] were lost during the process of clot separation; thus, we could not analyze them for pesticides).
Analysis of OCP, As, and Pb in Biological Samples
Analysis of OCP
All reagents for pesticide extraction (gas chromatography [CG]-quality aluminum oxide power and glass wool) were purchased from Mallinckrodt Baker Inc. (New Jersey, USA). CG-quality solvents (acetone, hexane) were purchased from Honeywell Burdick & Jackson (Michigan, USA). Reference standards of organochlorines were purchased from ULTRA Scientific Inc. (Rhode Island, USA). Pesticide analysis was performed using liquid–liquid microextraction according to Dale et al. (1970) with some modifications: 300 μL serum was extracted with 15 mL hexane at room temperature and the sample was vortexed for 10 s at maximum speed; this procedure was repeated four times. In each extraction, hexane was recovered in conical glass tubes of 50 mL volume. The eluate was evaporated to dryness with compressed air using N-EVAP equipment (model no 11155-RT). The sample was reconstituted with 100 μl/L hexane and then placed into a 2 mL vial with 0.3 mL insert (C4010-630; National Scientific). Eight OCP were analyzed (HCB, lindane, aldrin, methoxychlor, p,p′-DDT, p,p′-DDE, p,p′-DDD, and endosulfan). Quantification was performed using an HP 7890A gas chromatograph equipped with an electron microcapture detector and 7683B series injector autosampler (Agilent Technologies). A DB-5 (J&W Scientific) GC column (30 m × 0.25 mm ID, 0.25-μm film thickness [5 % phenyl and 95 % dimethylpolysiloxane]) was used. Column temperature was as follows: initial 110 °C (1 min), final 280 °C (2 min) (rate 15 °C/min up to 280 °C). Injector temperature was 270 °C operated in pulsed splitless mode. The carrier gas used was helium at a liner velocity of 2.3 mL/min. To determinate the quality of the method, a recovery study was performed on 10 spiked replicates of the blank serum samples (the pesticide levels in these blank were lower than limits of detection). The spiked serum samples (2.0–20 ppb [or μg/L] depending on the pesticide) showed mean recovery values from 84 to 101 %. The variation coefficient was <18 %, indicating excellent repeatability of the method. The calibration curve for each pesticide was performed from 0 to 30 ppb and obtained regression coefficients (R2) of >0.99. The detection limits for all compounds were between 0.2 and 0.5 μg/L.
Urinary As analysis
Reference urine samples containing As (III), MM(V), and DMA (V) were obtained from Institut National de Santé Publique (Québec, Canada). Sodium arsenite was from Thermo Fisher Scientific (Massachusetts, USA). Sodium arsenate and DMA were from Sigma-Aldrich (Missouri, USA), and MMA was purchased from Chem Service, Inc. (Pennsylvania, USA). Frozen urine was thawed, diluted twofold using Milli-Q water, and filtered with a Millex-HV filter (0.45-μm syringe driven filter unit; Millipore) before injection (Francesconi et al. 2002; Mandal et al. 2001). The high performance liquid chromatography system consisted of an Agilent 1100 high performance liquid chromatograph (Agilent) with a reverse phase C18 column (Prodigy 3 μm ODS (3) 150 × 4.60 mm; Phenomenex, Torrance, CA), with an Agilent 7500a inductively coupled plasma–mass spectrometer used as detector for the analysis of five As species: As(III), As(V), MMA(V), DMA(V), and arsenobetaine (Meza et al. 2004). Due to difficulties in the timely transfer of samples across the United States–Mexican border, analysis of unstable MMA(III) and DMA(III) metabolites was not attempted.
The detection limit, quality control, precision, and accuracy of this analytical method were performed as previously reported (Meza et al. 2004). In addition, reference urine samples containing As(III), MMA(V), and DMA(V) were used. Analyses of these standards yielded recoveries between 80 and 109 % with coefficient of variation values <4 %.
Pb Analysis
Plasma samples were treated with a matrix modifier (diammonium hydrogenphosphate-Triton-X-100 in the presence of 0.2 % nitric acid) according to Jasso-Pineda et al. (2007), and the samples were analyzed using a Perkin Elmer 3110 atomic absorption spectrophotometer using a graphite furnace. For quality control, a CDC WS2H proficiency testing blood material (method code 3851; Wisconsin State Laboratory of Hygiene, Toxicology Section, Madison, WI) was used, and the accuracy was determined to be 98 %.
Statistical Analysis
Arithmetic means (including range) were calculated for the variables studied. Concentrations of total As and Pb were transformed to a log scale to calculate the significance of the differences between means for each town using analysis of variance (ANOVA) and Bonferroni correction. All statistical analyses were computed using Software Stata 9.0 software (College Station, Texas, USA, 2002).
Results
Characteristics of the Children and Study Sites
Children from the 10 communities, except Cocorit and Bácum where 12.12 % of the children participants were Native Indian Yaquis, had similar socioeconomic status, nutritional habits, and ethnicity. All participant groups were generally similar with comparable distribution by age, sex, and length of residence (Table 1).
Table 1.
Characteristics of the sampled sites and children from the Yaqui and Mayo valleys, Sonora, Mexico
| Town | Children sampled (n) | Sex (M/F) | Age (y) | Residence time (y) | Characteristics of the site |
|---|---|---|---|---|---|
| Cocorit | 12 | 6/6 | 9.0 (8–11) | 8.1 (2–11) | Community adjacent to the agricultural fields (27° 34′.314″N; 109° 57′.328″W) |
| Bácum | 8 | 4/4 | 7.8 (6–12) | 9 (6–12) | Community adjacent to the agricultural fields (27° 33′.190″N; 110° 05′.196″W) |
| Campo 5 | 18 | 10/8 | 8.5 (7–11) | 8.5 (7–11) | Community surrounded by agricultural fields (27° 26′.207″N; 110 01′.388″W) |
| SJ de Bácum | 27 | 15/12 | 9.1 (6–11) | 8.5 (2–12) | Community surrounded by agricultural fields (27° 30′.797″N; 110° 09′.129″W) |
| Campo 47a | 22 | 12/10 | 9.7 (7–11) | 8.7 (2–11) | Rural community surrounded by agricultural fields (27° 20′.540″N; 110° 05′.275″W) |
| Tobarito | 13 | 7/6 | 9.6 (6–12) | 8.4 (4–11) | Community surrounded by agricultural fields (27° 21′.562″N; 109° 53′.862″W) |
| Cd. Obregón | 12 | 7/5 | 9.4 (7–12) | 9.5 (7–12) | Community adjacent to agricultural fields (27° 26′.206″ N; 109° 55′.672″W) |
| Etchojoa | 19 | 11/8 | 7.3 (6–10) | 7.5 (4–9) | Community surrounded by agricultural fields (26° 54′.836″N; 109° 37′.779″W) |
| Villa Juárez | 17 | 10/7 | 9.8 (6–12) | 8.7 (2–12) | Community surrounded by agricultural fields (27° 07′.387″N; 109° 51′.060″W) |
| Navojoa | 17 | 11/6 | 9.6 (6–12) | 8.8 (6–12) | Community adjacent to agricultural fields (27° 05′.074″N; 109° 27′.249″W) |
A community with a population <2,500 residents is considering “rural” according to National Institute of Statistics Geography and Informatics criteria
Levels of OCP
Although p,p′-DDT was only found in 25.5 % of the children (mean 0.38 μg/L), 100 % of the children (n = 153) had detectable concentrations of p,p′-DDE (mean 1.24 μg/L) (Table 2). This shows chronic and past exposure to p,p′-DDT. Lindane was found in the 39.2 % of the samples (mean 0.36 μg/L) followed by aldrin (mean 0.43 μg/L) in 9.80 % of the samples and endosulfan (mean 0.25 μg/L) in 3.9 % of the samples.
Table 2.
Serum levels and presence of OCP in children from the Yaqui and Mayo valleys in Sonora, Mexico
| Pesticide | Occurrence (n = 153) | Occurrence (%)a | Mean (μg/L)b | Range (μg/L) |
|---|---|---|---|---|
| Lindane | 60 | 39.2 | 0.36 | 0.25–1.0 |
| Aldrin | 15 | 9.80 | 0.43 | 0.25–0.75 |
| Endrin | 0 | 0 | ND | ND |
| Endosulfan | 6 | 3.9 | 0.25 | 0.25 |
| p,p′-DDE | 153 | 100 | 1.24 | 0.25–10.3 |
| p,p′-DDD | 1 | 0.65 | 0.75 | 0.75 |
| p,p′-DDT | 39 | 25.5 | 0.38 | 0.25–1.0 |
| Methoxychlor | 0 | 0 | ND | ND |
ND nondetected
Percentage of samples giving a quantitative instrument response
Values are arithmetic means
The towns with the highest levels of p,p′-DDE, p,p′-DDT, lindane, and aldrin were from the Mayo valley: Villa Juárez, Etchojoa, and Navojoa (Table 3). Endosulfan was detected at the same concentration in both valleys, with a mean value of 0.25 μg/L for SJ de Bácum, Campo 47, and Etchojoa.
Table 3.
Serum levels of OCP in children from the Yaqui and Mayo valleys in Sonora, Mexico
| Community | p,p′-DDEa | p,p′-DDTa | Lindanea | Aldrina | Endosulfana |
|---|---|---|---|---|---|
| Yaqui valley (n = 100) | |||||
| Bácum (n = 8) | 0.54 (0.25–1.0) | 0.25 (n = 1)b | ND | ND | ND |
| Campo 5 (n = 18) | 0.90 (0.50–3.0) | 0.25 (n = 2)b | 0.50 (n = 2)b | 0.33 (0.25–0.5) (n = 3)b | ND |
| SJ de Bácum (n = 27) | 0.64 (0.25–2.5) | 0.50 (n = 1)b | 0.28 (0.25–0.50) (n = 20)b | 0.25 (n = 4)b | 0.25 (n = 2)b |
| Campo 47 (n = 22) | 1.17 (0.25–8.0) | 0.34 (0.25–0.50) (n = 8)b | 0.30 (0.25–0.50) (n = 17)b | ND | 0.25 (n = 2)b |
| Tobarito (n = 13) | 0.58 (0.25–1.0) | ND | ND | ND | ND |
| Cd. Obregón (n = 12) | 0.65 (0.25–2.25) | 0.25 (n = 1)b | ND | ND | ND |
| Mayo valley (n = 53) | |||||
| Etchojoa (n = 19) | 2.2 (0.50–5.8) | 0.52 (0.25–1.0) (n = 11)b | 0.30 (0.25–0.50) (n = 9)b | 0.38 (0.25–0.5) (n = 2)b | 0.25 (n = 2)b |
| Villa Juárez (n = 17) | 2.2 (0.25–10.3) | 0.25 (n = 3)b | 0.25 (n = 6)b | ND | ND |
| Navojoa (n = 17) | 2.1 (0.50–7.8) | 0.34 (0.25–0.75) (n = 12)b | 0.92 (0.25–1.0) (n = 6)b | 0.63 (0.25–0.75) (n = 6)b | ND |
ND nondetected
ppb (or μg/L)
Mean value of positive samples (n)
Blood Pb Levels
All of the children had detectable levels of Pb in their blood (mean 3.2) (Table 4). A portion (8.5 %) of the samples had blood Pb values >5.0 μg Pb/dL, but none of the samples exceeded 10 μg Pb/dL. The towns with the highest levels were Tobarito, Cocorit, and Bácum at 3.8, 3.7 and 3.7 μg Pb/dL, respectively.
Table 4.
Blood Pb levels in children from the Yaqui and Mayo valleys, Sonora, Mexico
| Community | Pb (μg/dL)a | Range (μg/dL) | > 5 μg/dL (%) | > 10 μg/dL (%) |
|---|---|---|---|---|
| All communities (n = 165) | 3.2 | 0.17–9.0 | 8.50 | 0 |
| Cocorit (n = 12) | 3.7 | 2.1–8.6 | 16.7 | 0 |
| Bácum (n = 8) | 3.7 | 0.98–9.0 | 25.0 | 0 |
| Campo 5 (n = 18) | 3.1* | 1.2–5.0 | 0.0 | 0 |
| SJ deBácum (n = 27) | 3.1 | 1.5–5.9 | 3.7 | 0 |
| Campo 47 (n = 22) | 2.9* | 1.2–5.2 | 4.5 | 0 |
| Tobarito (n = 13) | 3.8 | 1.9–4.9 | 0.0 | 0 |
| Cd. Obregón (n = 12) | 3.4* | 1.1–8.2 | 16.7 | 0 |
| Etchojoa (n = 19) | 2.3* | 0.17–5.3 | 10.5 | 0 |
| Villa Juárez (n = 17) | 3.4 | 0.98–7.9 | 5.9 | 0 |
| Navojoa (n = 17) | 3.5* | 1.9–5.2 | 17.6 | 0 |
ANOVA p<0.05 compared with other communities (n = 165)
Values are arithmetic means
Urinary As Levels
All of the children had detectable levels of urinary As (mean 30.9 μg As/L) (Table 5) with 12.7 % of the samples having values >50 μg As/L. The towns with the highest levels were Cocorit, Bácum, Tobarito, and Villa Juárez at 31.0, 32.3, 37.6, and 34.7 μg As/L, respectively. Urine from the children from Cd. Obregón and Navojoa had the highest level of urinary As (62.4 and 40.5 μg As/L, respectively).
Table 5.
Urinary InAs levels in children from the Yaqui and Mayo valleys, Sonora, Mexico
| Communities (n = 65) | InAsa (μg As/L) | Range (μg/L) | > 50 μg As/L (%) |
|---|---|---|---|
| All communities | 30.9 | 5.4–198.8 | 12.7 |
| Cocorit | 31.0 | 10.9–57.2 | 16.7 |
| Bácum | 32.3 | 10.3–66.6 | 25.0 |
| Campo 5 | 20.4 | 9.2–51.4 | 5.5 |
| SJ de Bácum | 26.9* | 5.4–198.8 | 3.7 |
| Campo 47 | 24.4* | 5.9–44.6 | 0.0 |
| Tobarito | 37.6 | 8.5–134.1 | 15.4 |
| Cd. Obregón | 62.4* | 32.1–94.1 | 75.0 |
| Etchojoa | 16.4* | 6.4–35.6 | 0.0 |
| Villa Juárez | 34.7 | 10.0–78.9 | 17.6 |
| Navojoa | 40.5* | 10.1–190.3 | 11.8 |
InAs inorganic arsenic = sum of [s(III), As(V), MMA(V), and DMA(V)
ANOVA p<0.05 compared with other communities (n = 165)
Values are arithmetic means
Discussion
Pesticide Levels
Public health programs in Mexico used DDT from 1957 until 2000 as the insecticide of choice to control disease-transmitting organisms (Pérez-Maldonado et al. 2006). p,p′-DDE, the major metabolite of DDT, was detectable in 100 % of the children’s serum samples and at concentrations greater than any of the other pesticides. These findings are consistent with worldwide reports that ~90–100 % of the population have detectable concentrations of p,p′-DDE (Waliszewski et al. 2001). Concentrations of p,p′-DDE and p,p′-DDT (means = 1.24 and 0.38 μg/L, respectively) (Table 2), were substantially lower than those reported in children by Trejo-Acevedo et al. (2009) in some endemic regions of malaria in the south of Mexico (547 ± 356 to 22,284 ± 7439 μg/L for DDE and nondetectable [ND] to 613 ± 476 μg/L for DDT). Our results were greater than those reported in children from the United States with mean values of 0.65 ± 0.96 ng/g serum for p,p′-DDE and 0.03 ± 0.02 ng/g serum for p,p′-DDT (Sexton et al. 2006). Although p,p′-DDT was detected in only 25.5 % of the children, in the human body, p,p′-DDT is slowly dechlorinated to p,p′-DDD and p,p′-DDE, with the latter being even more persistent than the parent compound. Because 100 % of children had detectable concentrations of p,p′-DDE in their serum, the contamination in these children appears to have resulted from previous exposure to technical-grade DDT and/or from intake of p,p′-DDE residues present in fatty foods (e.g., meat, milk, eggs). The ratio of (p,p′-DDE + p, p′-DDD/p,p′-DDT) is often used as an index for assessing the residence time of p,p′-DDT in the environmental. A value >1.0 indicates that the application was performed in the past, and a value <1.0 indicates a relatively recent application (Cheng et al. 2008). In this study, this ratio p,p′-DDE/p,p-DDT was 3.3 > 1.0, which is an indication of past exposure to DDT. This information, along with the low frequency of p,p′-DDT in serum samples (25.5 %), suggests past exposure to this compound in this region. Recent studies reported by Orduño (2010) described values of p,p′-DDE and p,p′-DDT in serum samples (range 0.30–4.30 and ND to 1.3 μg/L, respectively) in children from Pótam (Yaqui Indian community), located in southern Sonora, that are similar to the levels found in our samples.
Because aldrin was historically applied as pesticide in both the Yaqui and Mayo valleys, children could have been exposed by inhalation or ingestion to this agent. Although aldrin was detected in only 9.80 % of children with a mean value of 0.43 μg/L (range 0.25–0.75) (Table 2), this does not preclude that more children could have been exposed because aldrin is rapidly metabolized to dieldrin in the body (Smith 1991). Surprisingly, no dieldrin was detected in these children. There are some reports that the storage of dieldrin is decreased when both aldrin and DDT are fed simultaneously to dogs; however, when these compounds are combined in rats, the storage of dieldrin is markedly decreased, whereas the storage of DDT is uninfluenced. In addition, aldrin has been detected only in trace amounts in the blood of people who make and formulate it (Smith 1991). In Mexico, studies about the presence of aldrin in human population are very scarce, and there are only two reports in children. Trejo-Acevedo et al. (2009) evaluated 229 children from 8 states in Mexico (Chiapas, Veracruz, San Luis Potosí, Coahuila, Guanajuato, Zacatecas, Durango, and Querétaro), and no detectable levels were noted. In contrast, in nursing babies from Pueblo Yaqui, Sonora (n = 11), aldrin was found in serum samples from the infants (16.9 ± 45.3 μg/L) (García and Meza 1991); these concentrations are greater than those found in our study population. Lindane has been used in this agricultural area since 1972 and is still permitted in the formulation of pharmaceuticals and indoor applications (Avalos and Ramírez 2003). Lindane was detected in the 39.2 % of children with an average of 0.36 μg/L (range 0.25–1.0) (Table 2). Although the blood concentration of lindane was the lowest reported compared with children’s blood levels in other parts of Mexico, the results are consistent with the past use of lindane-based pesticides in the region (Trejo-Acevedo et al. 2009). It should be mentioned that when this study was performed, lindane was in a commercial shampoo used for the outbreak of lice in these localities.
Some types of organochlorine pesticides are still being used in Mexico despite their prohibition in some industrialized countries. This is the case with endosulfans, which were also included in this study because of their widespread use in this region. Endosulfan was detected in the 3.9 % of children with a mean value of 0.25 μg/L (Table 2). There are no published reports of the levels of endosulfan in children in Mexico, and more attention should be paid to endosulfans because they have been reported as endocrine disruptors (Bejarano 2009). Our study clearly found the presence of OCP residues in children living in agricultural areas of northwest Mexico and showed that OCP are detectable in the body despite their ban a few decades ago and may reflect historical exposure to pesticides that are no longer in use.
Blood Pb Levels
It was important for this study to include the analysis of Pb and As because these chemicals were sprayed as arsenical pesticides in both valleys. Both the Centers for Disease Control and Prevention and the World Health Organization recommend that blood Pb in children be <10 μg Pb/dL. In our study, children from both valleys had blood Pb levels (mean ≤ 3.2 μg Pb/dL) lower than this guideline. This was surprising because Pb arsenate was use as a pesticide in past decades in both valleys (Cejudo 2009). Even the current Pb levels in soil from these valleys are lower than the permissible levels (Meza-Montenegro et al. 2012). Caution is still needed because recent studies have shown that cognitive deficits, as well as developmental and behavioral problems, have occurred in children with blood Pb levels just below 10 μg Pb/dL (Hui-Li et al. 2008; Lanphear et al. 2009). There were some children from Bácum and Cocorit in whom blood Pb levels are closer to 10 μg/dL (9.0 and 8.6 μg/dL, respectively) (Table 4). Both communities are close to the Yaqui river where levels of Pb have been detected at greater than Mexican guideline in a battery of wells constructed as a drinking water source for these towns (Hernández 2006). In addition, the major contribution of Pb exposure in these communities could be due to leaded gasoline. Cd. Obregón, Navojoa, Tobarito, and Villa Juárez are large communities with agriculture activities and have high traffic density. Although Mexico has increased its use of Pb-free gasoline, there are reports that some gasolines still contains traces of this metal (Del Rio-Salas et al. 2012).
Urinary As Levels
Urinary As levels in children were measured because both farmers in both valleys previously used As compounds as pesticides. Normal total urinary As values are <50 μg As/L (in the absence of seafood consumption in the previous 48 h); values >200 μg As/L are considered abnormal (Agency for Toxic Substances and Disease Registry 2000). Children from the largest communities (Tobarito, Cd. Obregón, Villa Juárez, and Navojoa) had greater As levels in urine compared with children living in the smaller areas, and 12.7 % of the urine samples had values >50 μg As/L (Table 5). Although Meza (2011) reported As concentrations in residential and agricultural soils in those towns with a range of 12.7–19.4 and 12.7–27.0 mg As/Kg, respectively, and As concentrations in drinking water with a range of 3.9–30.2 μg As/L, no statistical association was found between As levels in environmental samples and urinary As excretion. Recent studies in some towns from these valleys have shown that drinking water may not be the largest contributor to urinary As concentrations (Roberge et al. 2012), and that food consumption or dust inhalation may be other important routes of As exposure for the children living in these larger areas.
Conclusion
The present study reports for first time the presence of OCP residues, Pb, and As in children living in two of the major agricultural areas of southern end Sonora, Mexico, and show that OCP are detectable in their body despite their ban a few decades ago and may reflect historical exposure to pesticides that are no longer in use. The heavy application of these compounds, mainly on cotton fields and during malaria campaigns, is responsible for their presence in soil, water, and biological samples of the population. Although few blood Pb levels were close to the guideline value, some precaution should be taken because adverse health effects in children at concentrations <10 μg Pb/dL have been reported. Similar concerns occur relative to children with urinary As levels >50 μg As/L. Accordingly, it is imperative to initiate the National Implementation Plan of the Stockholm Convention to decrease exposure to these toxicants and improve risk reduction in children from Mexican communities. In addition, our results can be used to establish baseline levels of exposure to these toxins in this agricultural region and may be used as a reference point for regulatory agencies.
Acknowledgments
This work was supported by FOMIX-CONACYT-SONORA (Grant No. SON-2005-C01-22879) and Superfund (NIEHS Grant 04940). Thank to Leticia Carrizales and Mike Kopplin for supporting the analysis of Pb and As, respectively.
Contributor Information
Maria M. Meza-Montenegro, Email: mmeza@itson.mx, Department of Natural Resources, ITSON, 5 de Febrero 818 Sur, Zona Centro, 85000 Cd. Obregon, Sonora, Mexico
Ana I. Valenzuela-Quintanar, Department of Food Sciences, CIAD, A.C., Carretera a la Victoria, Km 0.6, 83000 Hermosillo, Sonora, Mexico
José J. Balderas-Cortés, Department of Natural Resources, ITSON, 5 de Febrero 818 Sur, Zona Centro, 85000 Cd. Obregon, Sonora, Mexico
Leticia Yañez-Estrada, Unidad Pediátrica Ambiental, UASP, Av. Venustiano Carranza 2405, 78210 San Luis Potosí, Mexico.
Maria L. Gutiérrez-Coronado, Department of Food Sciences, CIAD, A.C., Carretera a la Victoria, Km 0.6, 83000 Hermosillo, Sonora, Mexico
Alberto Cuevas-Robles, Department of Natural Resources, ITSON, 5 de Febrero 818 Sur, Zona Centro, 85000 Cd. Obregon, Sonora, Mexico.
A. Jay Gandolfi, College of Pharmacy, University of Arizona, 1703 E. Mabel Street, Tucson, AZ 85724-5024, USA.
References
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Arsenic. United States Department of Health and Human Services, Public Health Services; Atlanta, GA: 2000. [Google Scholar]
- Avalos M, Ramírez J. La situación del Lindano en México. Rev Gaceta Ecológica. 2003;69:93–100. [Google Scholar]
- Bejarano GF. La regulación internacional de los plaguicidas altamente peligrosos y sus implicaciones para México. In: Sánchez SE, Ortíz HL, Olvera VA, Folch MJ, editors. La situación de los plaguicidas en. México: Impactos y perspectivas. Cuernavaca, Morelos; 2009. pp. 56–59. [Google Scholar]
- Cantu-Soto EU, Meza-Montenegro MM, Valenzuela-Quintanar AI, Félix-Fuentes A, Grajeda-Cota P, Balderas-Cortes JJ, et al. Residues of organochlorine pesticides in soils from Southern Sonora, Mexico. Bull Environ Contam Toxicol. 2011;87:556–560. doi: 10.1007/s00128-011-0353-5. [DOI] [PubMed] [Google Scholar]
- Cejudo EA. Doctoral dissertation. Technological Institute of Sonora; Sonora, Mexico: 2009. Pesticides most marketed of domestic and urban application in Ciudad Obregón, Sonora. [Google Scholar]
- Cheng H, Fu S, Liu Y, Li D, Zhou J, Xia X. Organochlorine pesticides in the soil in Linfen, China. Bull Environ Contam Toxicol. 2008;81:599–603. doi: 10.1007/s00128-008-9544-0. [DOI] [PubMed] [Google Scholar]
- Dale WE, Miles JW, Gaines TB. Quantitative method for determination of DDT metabolites in blood serum. J Assoc Off Anal Chem. 1970;53:1287–1292. [Google Scholar]
- Del Rio-Salas R, Ruiz J, De la O-Villanueva M, Valencia-Moreno M, Moreno-Rodríguez V, Gómez-Alvarez A, et al. Tracing geogenic and anthropogenic sources in urban dusts: insights from lead isotopes. Atmos Environ. 2012;60:202–210. [Google Scholar]
- Francesconi KA, Tanggaard R, McKenzie CJ, Goesser W. Arsenic metabolites in human urine after ingestion of an arsenosugar. Clin Chem. 2002;48:92–101. [PubMed] [Google Scholar]
- Gao F, Jia J, Wang X. Ocurrence and ordination of dichlorodiphenyltrichloroethane and hexachlorocyclohexane in agricultural soils from Guangzhou, China. Arch Environ Contam Toxicol. 2008;54:155–166. doi: 10.1007/s00244-007-9023-3. [DOI] [PubMed] [Google Scholar]
- García BM, Meza MM. Principal ways of insecticide contamination in new born lactant babies residents of Pueblo Yaqui, Sonora, Mexico. Rev ITSON-DIEP. 1991;1:33–42. [Google Scholar]
- Guillette EA, Meza MM, Aguilar MG, Soto AD, Garcia IE. An anthropological approach to the evaluation of preschool children exposed to pesticides in Mexico. Environ Health Perspect. 1998;106:347–353. doi: 10.1289/ehp.98106347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández MS. Doctoral dissertation. Technological Institute of Sonora; Sonora, Mexico: 2006. Determination of trace heavy metals in well water of Guaymas-Empalme, Sonora. [Google Scholar]
- Hui-Li W, Xiang-Tao C, Bin Y, Fan-Li M, Shu W, Ming-Liang T, et al. Case-control study of blood lead levels and attention deficit hyperactivity disorder in Chinese children. Environ Health Perspect. 2008;116:1401–1406. doi: 10.1289/ehp.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasso-Pineda Y, Espinoza-Reyes G, González-Mille D, Razo SI, Carrizales L, Torres-Dosal A, et al. An integrated health risk assessment approach to the study of mining sites contaminated with arsenic and lead. Integr Environ Assess Manag. 2007;3:344–350. [PubMed] [Google Scholar]
- Lanphear B, Taylor M, Jones A, Gulson B, Lopez L, Cebrian M, et al. [Accessed 17 June 2012];The Perth Declaration for the Global Reduction of Childhood Lead Exposure. 2009 Available at: http://www.pacificbasin.org/PBC_2009_Conference/THE%20PERTH%20DECLARATION%20FOR%20THE%20GLOBAL%20REDUCTION%20OF%20CHILDHOOD%20LEAD%20EXPOSURE.pdf.
- Makri A, Goveia M, Balbus J, Parkin R. Children’s susceptibility to chemicals: a review by developmental stage. J Toxicol Environ Health B. 2004;7:417–435. doi: 10.1080/10937400490512465. [DOI] [PubMed] [Google Scholar]
- Mandal BK, Ogra Y, Susuki KT. Identification of dimethylarsinous and monomethylarsonous acids in human urine of the arsenic affected areas in West Bengal India. Chem Res Toxicol. 2001;14:371–378. doi: 10.1021/tx000246h. [DOI] [PubMed] [Google Scholar]
- Meza MM. Assessment of health risk from exposure to pesticides, lead and arsenic in children from the Yaqui and Mayo Valleys, Sonora. Final Technical Report. 2011 Project No. SON-2005-CO1-22879. Fondo Mixto Conacyt-Gobierno del Estado de Sonora. [Google Scholar]
- Meza MM, Kopplin MJ, Burgess JL, Gandolfi AJ. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora Mexico. Environ Res. 2004;96:119–126. doi: 10.1016/j.envres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Meza-Montenegro MM, Gandolfi AJ, Santana-Alcántar ME, Klimecki TW, Aguilar-Apodaca MG, Del Rio-Salas R, et al. Metals in residential soils and cumulative risk assessment in Yaqui and Mayo agricultural valleys, Northern Mexico. Sci Total Environ. 2012;433:472–481. doi: 10.1016/j.scitotenv.2012.06.083. [DOI] [PubMed] [Google Scholar]
- Orduño VR. Doctoral dissertation. Technological Institute of Sonora; Sonora, Mexico: 2010. Levels of organochlorine pesticides in children from Potam community, and evaluation of possible routes of exposure. [Google Scholar]
- Pérez-Maldonado I, Athanasiadou M, Yáñez L, Gonzáles-Amaro R, Bergman A, Díaz-Barriga F. DDE-induced apoptosis in children exposed to the DDT metabolite. Sci Total Environ. 2006;370:343–351. doi: 10.1016/j.scitotenv.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Roberge J, O’Rourke MK, Meza-Montenegro MM, Gutiérrez-Millán LE, Burgess JL, Harris RB. Binational arsenic exposure survey: methodology and estimated arsenic intake from drinking water and urinary arsenic concentrations. Int J Environ Res Pubic Health. 2012;9:1051–1067. doi: 10.3390/ijerph9041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez VS. Doctoral dissertation. Technological Institute of Sonora; Sonora, Mexico: 2009. Major agricultural pesticides sold in the Yaqui and Mayo valleys in the period 2007–2008. [Google Scholar]
- SAGARPA Monitor AGROECONÓ MICO. [Accessed June 2012];2011 Available at: http://www.sagarpa.gob.mx/agronegocios/Documents/pablo/Documentos/monitor%20estados/Sonora.pdf.
- SEMARNAT-INE-CONACYT. Fondo Sectorial de Investigación Ambiental: Convocatoria SEMARNAT-INE-CONACYT 2008/01 2008 [Google Scholar]
- Sexton K, Adgate JL, Fredrickson AL, Ryan AD, Needham LL, Ashley DL. Using biologic markers in blood to assess exposure to multiple environmental chemicals for inner-city children 3–6 years of age. Environ Health Perspect. 2006;114:453–459. doi: 10.1289/ehp.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG. Chlorinated hydrocarbon insecticides. In: Hayes WJ, Laws ER, editors. Handbook of pesticide toxicology. Academic; San Diego: 1991. pp. 731–868. [Google Scholar]
- Trejo-Acevedo A, Díaz-Barriga F, Carrizales L, Domínguez G, Costilla R, Ize-Lema I, et al. Exposure assessment of persistent organic pollutants and metals in Mexican children. Chemosphere. 2009;74:974–980. doi: 10.1016/j.chemosphere.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Van WE, Henk BV, der Bank H, Verdoorn GH, Hofmann D. Persistent organochlorine pesticides detected in blood and tissue samples of vultures from different localities in South Africa. Comp Biochem Physiol C Toxicol Pharmacol. 2001;129:243–264. doi: 10.1016/s1532-0456(01)90201-7. [DOI] [PubMed] [Google Scholar]
- Waliszewski SM, Aguirre AA, Infazon RM, Silva CS, Siliceo J. Organochlorine pesticide levels in maternal adipose tissue, maternal blood serum, umbilical blood serum, and milk from inhabitants of Veracruz, Mexico. Arch Environ Contam Toxicol. 2001;40:432–438. doi: 10.1007/s002440010194. [DOI] [PubMed] [Google Scholar]
- Woolson EA. Arsenical pesticides. A symposium sponsored by the Division of Pesticide Chemistry at the 168th meeting of the American Chemical Society; Atlantic City. 1975. [Accessed 12 Oct 2012]. Available at: http://pubs.acs.org. [Google Scholar]