Abstract
The sympathoexcitatory effects of insulin are well-established, although the exact mechanisms by which insulin stimulates the sympathetic nervous system are not completely understood. The majority of research supports a primary role for the central nervous system in the gradual and sustained rise in muscle sympathetic nerve activity (MSNA) in response to hyperinsulinemia; in addition, recent studies in animals suggests carotid body chemoreceptors respond to increases in systemic insulin levels. Intermittent activation of the carotid chemoreceptors, similar to that seen in patients with sleep apnea, can result in sensory long term facilitation and may contribute to the observed rise in baseline MSNA in this population. Consistent with this idea, insulin exposure results in sustained increases in MSNA that persist even when plasma insulin levels return to baseline. We propose the carotid chemoreceptors contribute to insulin-mediated sympathoexcitation and the persistent rise in MSNA in patients with sustained hyperinsulinemia. If the carotid chemoreceptors sense and respond to changes in systemic insulin levels, these organs may provide a viable target for the treatment of disorders known to exhibit sustained hyperinsulinemia and sympathoexcitation including, but not limited to, obesity, hypertension, sleep apnea, metabolic syndrome, cardiovascular disease, and diabetes.
OBESITY, SYMPATHOEXCITATION, AND THE CAROTID CHEMORECEPTORS
The increased prevalence of obesity worldwide has resulted in a large increase in obesity-related disorders including hypertension, insulin resistance, sleep apnea, and type II diabetes. Each of these disorders is associated with an increase in the activity of the sympathetic nervous system, which has been shown to predict the development of cardiovascular disease and subsequent complications [1-4]. The rise in activity of the sympathetic nervous system in obesity-related disorders may be due to a number of factors, including increases in circulating leptin, insulin, free fatty acids, and inflammatory mediators [5]. Additionally, heightened sympathetic nervous system activity in obese adults may be secondary to obstructive sleep apnea, which has been linked to increased carotid chemoreceptor activation due to repeated intermittent bouts of nocturnal hypoxia (i.e. repeated desaturations during sleep) [6]. The purpose of this paper is to explore a novel additional sympathoexcitatory mechanism: insulin-mediated sensitization and activation of the carotid body chemoreceptors.
The carotid chemoreceptors are sensory organs located within the carotid body at the bifurcation of the common carotid artery. The carotid bodies sense and respond to changes in circulating oxygen and carbon dioxide pressures, temperature, and pH [7]. Activation of the carotid chemoreceptors increases afferent nerve activity and results in increased ventilation and reflex activation of the sympathetic nervous system. Activity of the sympathetic nervous system in humans can be examined using microneurography – a technique first described by Vallbo and colleagues [8]. Microneurography requires insertion of a tungsten microelectrode percutaneously into a peripheral nerve containing post-ganglionic sympathetic efferent nerve fibers directed toward skeletal muscle and the resultant measure of muscle sympathetic nerve activity (MSNA) is highly related to whole body sympathetic activity [9, 10].
SENSORY LONG-TERM FACILITATION
Whereas a single hypoxic exposure can increase activity of the sympathetic nervous system, intermittent hypoxic stimuli lead to prolonged activation of the chemoreceptors via a mechanism termed “sensory long term facilitation” [11]. In animal models, it has been demonstrated that repeat, acute (15-30 second) exposures to hypoxia can lead to long-lasting (~1 hour) activation of the carotid body and resultant increase in afferent activity [11-13]. In support of this idea in humans, both acute sustained and/or intermittent asphyxia (~20 min) results in significant increases in sympathetic activity (MSNA) that persist for at least 20 minutes after the stimuli are removed [14, 15] (Figure 1). This long-lasting effect of chemoreceptor activation likely plays an important role in increased baseline levels of sympathetic nerve activity in adults with sleep apnea, in addition to the effects of sympathoexcitation on other conditions such as hypertension and cardiovascular disease risk. Treatment of sleep apnea with continuous positive airway pressure (CPAP) to reduce the number of hypoxic events occurring during sleep has been shown to reduce chemoreceptor activity, baseline sympathetic activation, and cardiovascular morbidity/mortality [16-19].
FIGURE 1. Long-term facilitation.
After acute exposure to intermittent asphyxia, the rise in MSNA persists even after subjects return to room air breathing. Data adapted from (Xie et al 2000).
INSULIN-MEDIATED SYMPATHOEXCITATION
Insulin is released from pancreatic beta cells in response to increased blood glucose levels (such as that observed after a meal). Increases in plasma insulin concentrations are known to increase activity of the sympathetic nervous system directed toward skeletal muscle [20-24]. Taking into consideration the dose-dependent vasodilatory effects of insulin in the periphery [25] and blood volume shifts to the mesenteric circulation, the rise in muscle sympathetic nerve activity (MSNA) with hyperinsulinemia contributes to the preservation of blood pressure after a meal. Consistent with this concept, patients with autonomic failure exhibit a reduction in blood pressure after intravenous infusion of insulin [26].
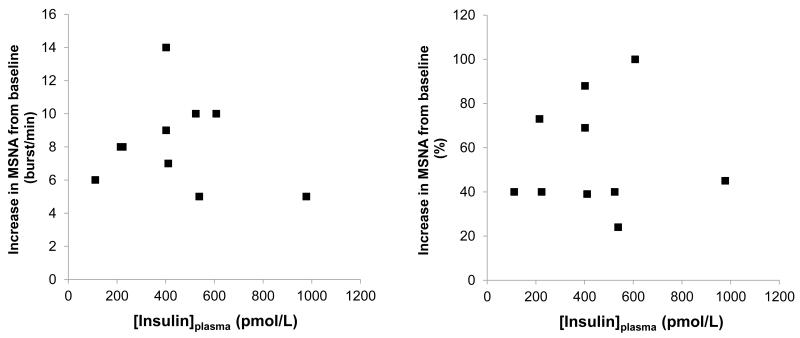
Despite this logic, the relationship between the rise in MSNA and plasma insulin levels may not be linearly-related. In the 1990s, a number of studies examined the sympathoexcitatory effects of insulin in humans. In the majority of studies, hyperinsulinemic euglycemic clamps were employed and MSNA was measured with microneurography. In studies in which a low dose of insulin was followed by a higher dose, the rise in MSNA in response to increases in plasma insulin appeared to be dose-dependent [23, 24, 27, 28]. However, Vollenweider and colleagues challenged this view, showing when low- and high-dose hyperinsulinemic euglycemic clamps were conducted on separate days, a dose-dependent effect was not observed [29]. To further examine this possibility, we completed a search of the literature and compiled results from 8 studies that measured MSNA in humans during a hyperinsulinemic euglycemic clamp [20, 21, 24, 29-33]. In support of Vollenweider and colleagues, a dose-dependent rise in MSNA with hyperinsulinemia is not apparent (See Figure 2).
FIGURE 2. Lack of a dose-response relationship between plasma insulin and MSNA.
After combining results from 8 studies measuring MSNA in humans during 60-minutes of a hyperinsulinemic euglycemic clamp, a dose-dependent rise in MSNA is not observed, such that higher plasma insulin levels do not appear to result in greater sympathoexcitation (Data adapted from: Vollenweider et al 1994; Vollenweider et al 1993; Vollenweider et al 1995; Young et al 2010; Scherrer et al 1993; Anderson et al 1992; Anderson et al 1991; Hoffman et al 1997).
INSULIN, SYMPATHOEXCITATION, AND THE CENTRAL NERVOUS SYSTEM
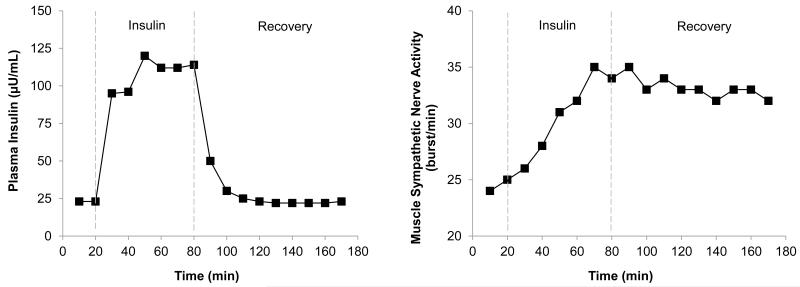
In addition to the apparent lack of a dose-response relationship between insulin and MSNA, the rise in MSNA in response to a hyperinsulinemic euglycemic clamp is both gradual [20, 21, 24, 29], and sustained [30], such that MSNA remains increased even after plasma insulin levels return to baseline (See Figure 3). The majority of research has focused on a role for the central nervous system in the rise in sympathetic activity with hyperinsulinemia. Thus, the gradual rise and fall in insulin-mediated sympathoexcitation, and potential lack of a dose-response relationship, has primarily been attributed to the time needed for insulin to cross the blood brain barrier [34-36] and the saturation of receptors needed to facilitate this transfer [37, 38]. In support of this idea, an approximate 30-minute delay occurs between an increase in systemic insulin levels and a rise in cerebrospinal levels of insulin in dogs [39, 40] and an increase in the concentration of insulin in peripheral lymphatic vessels mirrors the rise in MSNA [41]. Once in the brain, insulin acts within the arcuate nucleus to increase sympathetic activity via pathways in the paraventricular nucleus of the hypothalamus and the rostral ventrolateral medulla [42-45]. The persistent rise in MSNA after plasma insulin concentrations have returned to baseline [24] is also consistent with the half-life of insulin within cerebrospinal fluid (~140 min; [39]) and changes in signaling pathways and genomic events within the brain that unlikely to be rapidly reversed [46]. For more, see Reviews [25, 47]. Interestingly, without activation of insulin receptors within the arcuate nucleus, the sympathoexcitatory response to acute insulin exposure is lost [48]. However, responses to repeated or more prolonged elevations of insulin – commonly observed in hyperinsulinemic conditions – may not rely on the same control mechanisms; this temporal distinction has not been adequately examined.
FIGURE 3.
The rise in MSNA in response to a hyperinsulinemic euglycemic clamp is both gradual and sustained, such that MSNA remains increased after plasma insulin levels return to baseline. Data adapted from (Anderson et al 1992).
POSSIBLE ROLE FOR THE CAROTID CHEMORECEPTORS?
Despite the large body of knowledge supporting an important role for the central nervous system in the rise in MSNA with hyperinsulinemia, sensors within the periphery (i.e. the carotid bodies) may also contribute to insulin-mediated sympathoexcitation. Although clear scientific evidence is limited, these ideas are supported by early research showing insulin injected into the carotid artery of anesthetized dogs results in an increase in arterial blood pressure that: a) is not observed when similar doses are given systemically and b) can be abolished by ganglionic blockade [49]. More recent studies reported stimulation of breathing with insulin [50, 51]; a response which was absent following carotid sinus nerve resection. Furthermore, carotid bodies express insulin receptors and insulin activates the carotid body, as evidenced by increased neurotransmitter release with insulin exposure [50].
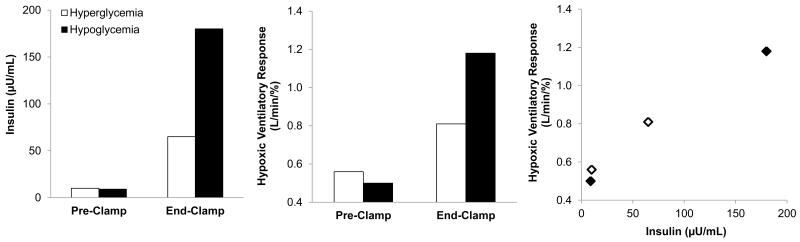
Unfortunately, parallel data are not available in humans. The closest data available come from Ward and colleagues (2007), who examined the effect of changes in glucose on chemoreceptor sensitivity as measured by the ventilatory response to hypoxia [52]. Contrary to their hypothesis, both hypo- and hyperglycemia augmented the hypoxic ventilatory response (HVR), suggesting hypo- and hyperglycemia activate the carotid body. Interestingly, both conditions were achieved using a hyperinsulinemic clamp and closer examination of the results uncovered a potential dose-response relationship between insulin and carotid body chemoreflex activity (HVR) (Figure 4; [52]) – however these results are confounded by changes in plasma glucose levels and are thus difficult to interpret. Although the relationship between hyperinsulinemia and carotid chemoreceptor activity were not directly examined, such relationships suggest a potential link between hyperinsulinemia, the carotid chemoreceptors, and sympathoexcitation in humans.
FIGURE 4.
The rise in chemoreceptor sensitivity, as measured by the ventilatory response to hypoxia, mirrors the increase in plasma insulin during a hyperinsulinemic clamp. However, these results are confounded by changes in plasma glucose levels and are thus difficult to interpret. Data adapted from (Ward et al 2007).
INSULIN-MEDIATED SENSORY LONG-TERM FACILITATION?
Despite the aforementioned evidence, the sustained rise in MSNA after systemic insulin levels return to baseline remains perplexing. On one hand, this effect may be the result of a prolonged half-life of insulin present within the central nervous system and/or insulin-mediated changes in signaling pathways within the brain. On the other hand, the “recovery” phase of MSNA after a hyperinsulinemic clamp is reminiscent of a prolonged effect of carotid body activation previously attributed to “sensory long-term facilitation” (See above). Interestingly, the magnitude of this “sensory long-term facilitation,” at least in animals, appears to be independent of the severity of the stimulus (i.e. no dose-response); for example, intermittent exposure to either 5% or 10% oxygen resulted in the same increase in carotid body afferent activity [11]. Although primarily correlative at this time, these findings are consistent with the lack of a dose-dependent effect of plasma insulin levels on reflex increases in MSNA in humans (Figure 2). For more, see Review [53]. Taken together, insulin – especially during repeated and/or more prolonged exposures - may work at very similar locals under very similar conditions as that observed with intermittent hypoxia, thus resulting in reflex increases in MSNA and contributing to increases in basal activity levels. Furthermore, it may be this sustained effect of insulin at the level of the carotid chemoreceptors which contributed to apparent dose-dependent increases in MSNA during two-step hyperinsulinemic euglycemic clamps (See section “Insulin-mediated Sympathoexcitation”).
CLINICAL IMPLICATIONS
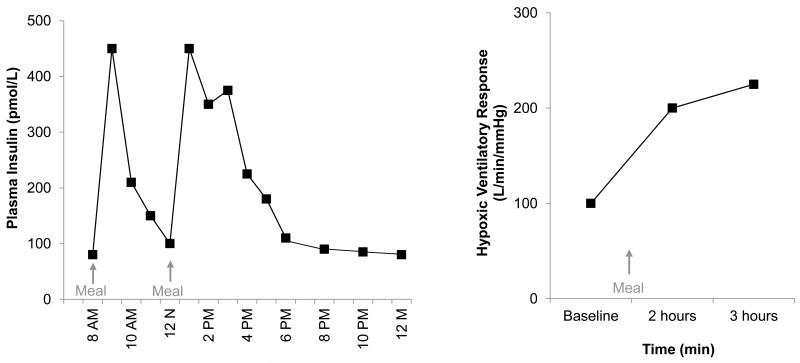
Plasma insulin levels fluctuate drastically throughout the day (See Figure 5); for example, plasma insulin concentrations can increase 7-fold after a meal and remain elevated for approximately 4 hours in healthy adults [54-56]. Consequently, periodic insulin exposures mirror the intermittent exposures to hypoxia commonly observed in recurrent apneas (i.e. sleep apnea) – including sustained increases in ventilation and carotid chemoreceptor sensitivity [57]. Such fluctuations are likely exacerbated in insulin resistant adults where post-feeding insulin spikes are dramatically increased [54, 58]. Furthermore, “snacking” and/or irregular meals have been shown to result in higher peak insulin levels when compared with regular feedings [59, 60]. As described above, a growing body of literature suggests activation of the carotid chemoreceptors via intermittent hypoxia induces functional plasticity (sensory long term facilitation) that persists after termination of the stimulus.
FIGURE 5.
Plasma insulin levels fluctuate drastically throughout the day in healthy, nondiabetic individuals. Data adapted from (Jeppesen et al 1995). Chemoreceptor sensitivity, as measured by the ventilatory response to hypoxia, increases significantly after a meal. Data adapted from (Zwillich et al 1977).
Given the similarities in the responses to hypoxia and insulin, we speculate repeated and/or prolonged exposures to high levels of insulin contribute to functional plasticity at the level of the carotid chemoreceptor, which then act independently or in combination with known central effects. Such relationships may contribute to increased baseline MSNA, and resultant increases in cardiovascular disease risk. In support of this idea, hyperinsulinemia is associated with increased cardiovascular, coronary, and all-cause mortality, independent of other risk factors [61]. Interestingly, “snacking” has also been linked to increased risk of type 2 diabetes [62] and cancer [63].
Altogether, it is reasonable to speculate fluctuations in systemic insulin may lead to very similar sensory long term facilitation of the chemoreceptors and may provide an important link between disorders of sympathoexcitation and cardiovascular disease risk such as hyperinsulinemia, sleep apnea, hypertension, and the metabolic syndrome. Thus, it may not be surprising:
Insulin resistance is reversed with acute supplemental oxygen (which should “turn off” the carotid body chemoreceptors) in patients with chronic obstructive lung disease [64].
Increased chemoreflex sensitivity and insulin resistance observed in adults with metabolic syndrome both improve with CPAP (continuous positive airway pressure) treatment [65].
The number of apneic events during sleep (a possible measure of chemoreceptor activity) is reduced very early after bariatric surgery (12 weeks; [66]), when dramatic effects on insulin resistance are often observed prior to significant reductions in weight.
Carotid sinus nerve resection in rodents was recently shown to blunt diet-induced insulin resistance and carotid body over-activation [50] and carotid body denervation is being considered as a potential therapeutic target for sympathetically-mediated diseases in humans [67].
Taken together, these provocative data from both animals and humans support a potential link between insulin, the carotid chemoreceptors, and sensitivity to peripheral stimuli.
SUMMARY
Provocative evidence from a variety of animal and human studies suggests the carotid bodies are sensitive to insulin, although definitive data are lacking. We propose the gradual rise and persistent increase in sympathetic activity in response to repeated and/or prolonged increases in systemic insulin levels are mediated, in part, through activation of the carotid chemoreceptors. To support and/or refute the proposed hypothesis, studies using direct recordings of afferents exiting the carotid body chemoreceptors in response to changes in insulin levels, in addition to measures of MSNA in humans are needed. It will be important to examine both the time-course and the magnitude of insulin-mediated sympathoexcitation. Most importantly, these findings must be translated to and studied in disease populations, with the goal of identifying the carotid chemoreceptors as a viable target for the treatment for disorders of sustained hyperinsulinemia (i.e. obesity, hypertension, metabolic syndrome, type II diabetes) and their physiological consequences.
Acknowledgments
We acknowledge our sources of funding: NIH R01 DK090541 (MJJ), NIH P01 HL090554 (NRP), T32 DK07352 (JKL). The funding sources had no involvement in the manuscript.
Support: NIH R01 DK090541 (MJJ), NIH P01 HL090554 (NRP), T32 DK07352 (JKL)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST: There are no conflicts to disclose.
REFERENCES
- [1].Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. doi: 10.1161/01.hyp.34.4.724. [DOI] [PubMed] [Google Scholar]
- [2].Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- [3].Lambert E, Dawood T, Schlaich M, Straznicky N, Esler M, Lambert G. Single-unit sympathetic discharge pattern in pathological conditions associated with elevated cardiovascular risk. Clin Exp Pharmacol Physiol. 2008;35:503–507. doi: 10.1111/j.1440-1681.2008.04905.x. [DOI] [PubMed] [Google Scholar]
- [4].Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- [5].Smith MM, Minson CT. Obesity and adipokines: effects on sympathetic overactivity. J Physiol. 2012;590:1787–1801. doi: 10.1113/jphysiol.2011.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385–390. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- [7].Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- [9].Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, et al. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491(Pt 3):881–887. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, et al. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, et al. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci. 2009;29:4903–4910. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xie A, Skatrud JB, Crabtree DC, Puleo DS, Goodman BM, Morgan BJ. Neurocirculatory consequences of intermittent asphyxia in humans. J Appl Physiol. 2000;89:1333–1339. doi: 10.1152/jappl.2000.89.4.1333. [DOI] [PubMed] [Google Scholar]
- [15].Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol. 1995;79:205–213. doi: 10.1152/jappl.1995.79.1.205. [DOI] [PubMed] [Google Scholar]
- [16].Vanderveken OM, Boudewyns A, Ni Q, Kashyap B, Verbraecken J, De Backer W, et al. Cardiovascular implications in the treatment of obstructive sleep apnea. J Cardiovasc Transl Res. 2011;4:53–60. doi: 10.1007/s12265-010-9238-y. [DOI] [PubMed] [Google Scholar]
- [17].Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–2335. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- [19].Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA. Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest. 2007;131:1406–1413. doi: 10.1378/chest.06-2580. [DOI] [PubMed] [Google Scholar]
- [20].Vollenweider P, Tappy L, Randin D, Schneiter P, Jequier E, Nicod P, et al. Differential effects of hyperinsulinemia and carbohydrate metabolism on sympathetic nerve activity and muscle blood flow in humans. J Clin Invest. 1993;92:147–154. doi: 10.1172/JCI116542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Scherrer U, Vollenweider P, Randin D, Jequier E, Nicod P, Tappy L. Suppression of insulin-induced sympathetic activation and vasodilation by dexamethasone in humans. Circulation. 1993;88:388–394. doi: 10.1161/01.cir.88.2.388. [DOI] [PubMed] [Google Scholar]
- [22].Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–2640. doi: 10.1161/01.cir.89.6.2634. [DOI] [PubMed] [Google Scholar]
- [23].Berne C, Fagius J, Pollare T, Hjemdahl P. The sympathetic response to euglycaemic hyperinsulinaemia. Evidence from microelectrode nerve recordings in healthy subjects. Diabetologia. 1992;35:873–879. doi: 10.1007/BF00399935. [DOI] [PubMed] [Google Scholar]
- [24].Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Scherrer U, Sartori C. Insulin as a vascular and sympathoexcitatory hormone: implications for blood pressure regulation, insulin sensitivity, and cardiovascular morbidity. Circulation. 1997;96:4104–4113. doi: 10.1161/01.cir.96.11.4104. [DOI] [PubMed] [Google Scholar]
- [26].Brown RT, Polinsky RJ, Baucom CE. Euglycemic insulin-induced hypotension in autonomic failure. Clin Neuropharmacol. 1989;12:227–231. doi: 10.1097/00002826-198906000-00009. [DOI] [PubMed] [Google Scholar]
- [27].Spraul M, Ravussin E, Baron AD. Lack of relationship between muscle sympathetic nerve activity and skeletal muscle vasodilation in response to insulin infusion. Diabetologia. 1996;39:91–96. doi: 10.1007/BF00400418. [DOI] [PubMed] [Google Scholar]
- [28].Hoffman A, Fischer Y, Gilhar D, Raz I. The effect of hyperglycaemia on the absorption of glibenclamide in patients with non-insulin-dependent diabetes mellitus. Eur J Clin Pharmacol. 1994;47:53–55. doi: 10.1007/BF00193478. [DOI] [PubMed] [Google Scholar]
- [29].Vollenweider P, Randin D, Tappy L, Jequier E, Nicod P, Scherrer U. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest. 1994;93:2365–2371. doi: 10.1172/JCI117242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Anderson EA, Balon TW, Hoffman RP, Sinkey CA, Mark AL. Insulin increases sympathetic activity but not blood pressure in borderline hypertensive humans. Hypertension. 1992;19:621–627. doi: 10.1161/01.hyp.19.6.621. [DOI] [PubMed] [Google Scholar]
- [31].Hoffman RP, Sinkey CA, Anderson EA. Hypoglycemic symptom variation is related to epinephrine and not peripheral muscle sympathetic nerve response. J Diabetes Complications. 1997;11:15–20. doi: 10.1016/1056-8727(95)00082-8. [DOI] [PubMed] [Google Scholar]
- [32].Vollenweider L, Tappy L, Owlya R, Jequier E, Nicod P, Scherrer U. Insulin-induced sympathetic activation and vasodilation in skeletal muscle. Effects of insulin resistance in lean subjects. Diabetes. 1995;44:641–645. doi: 10.2337/diab.44.6.641. [DOI] [PubMed] [Google Scholar]
- [33].Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol. 2010;588:3593–3603. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- [35].Margolis RU, Altszuler N. Insulin in the cerebrospinal fluid. Nature. 1967;215:1375–1376. doi: 10.1038/2151375a0. [DOI] [PubMed] [Google Scholar]
- [36].Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- [37].Banks WA, Jaspan JB, Kastin AJ. Selective, physiological transport of insulin across the blood-brain barrier: novel demonstration by species-specific radioimmunoassays. Peptides. 1997;18:1257–1262. doi: 10.1016/s0196-9781(97)00198-8. [DOI] [PubMed] [Google Scholar]
- [38].Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997;18:1423–1429. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- [39].Schwartz MW, Sipols A, Kahn SE, Lattemann DF, Taborsky GJ, Jr., Bergman RN, et al. Kinetics and specificity of insulin uptake from plasma into cerebrospinal fluid. Am J Physiol. 1990;259:E378–383. doi: 10.1152/ajpendo.1990.259.3.E378. [DOI] [PubMed] [Google Scholar]
- [40].Schwartz MW, Bergman RN, Kahn SE, Taborsky GJ, Jr., Fisher LD, Sipols AJ, et al. Evidence for entry of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. Quantitative aspects and implications for transport. J Clin Invest. 1991;88:1272–1281. doi: 10.1172/JCI115431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Castillo C, Bogardus C, Bergman R, Thuillez P, Lillioja S. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J Clin Invest. 1994;93:10–16. doi: 10.1172/JCI116932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011;57:435–441. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate Nucleus Injection of an Anti-Insulin Affibody Prevents The Sympathetic Response to Insulin. Am J Physiol Heart Circ Physiol. 2013 doi: 10.1152/ajpheart.00081.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol. 2011;589:1643–1662. doi: 10.1113/jphysiol.2011.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension. 2010;55:284–290. doi: 10.1161/HYPERTENSIONAHA.109.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, et al. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Muntzel MS, Anderson EA, Johnson AK, Mark AL. Mechanisms of insulin action on sympathetic nerve activity. Clin Exp Hypertens. 1995;17:39–50. doi: 10.3109/10641969509087053. [DOI] [PubMed] [Google Scholar]
- [48].Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am J Physiol Heart Circ Physiol. 2013;304:H1538–1546. doi: 10.1152/ajpheart.00081.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pereda SA, Eckstein JW, Abboud FM. Cardiovascular responses to insulin in the absence of hypoglycemia. Am J Physiol. 1962;202:249–252. [Google Scholar]
- [50].Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes. 2013 doi: 10.2337/db12-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bin-Jaliah I, Maskell PD, Kumar P. Indirect sensing of insulin-induced hypoglycaemia by the carotid body in the rat. J Physiol. 2004;556:255–266. doi: 10.1113/jphysiol.2003.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ward DS, Voter WA, Karan S. The effects of hypo- and hyperglycaemia on the hypoxic ventilatory response in humans. J Physiol. 2007;582:859–869. doi: 10.1113/jphysiol.2007.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Prabhakar NR. Sensory plasticity of the carotid body: role of reactive oxygen species and physiological significance. Respir Physiol Neurobiol. 2011;178:375–380. doi: 10.1016/j.resp.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tchobroutsky G, Kopf A, Eschwege E, Assan R. Serial postprandial plasma insulin levels in 117 subjects with and without diabetes. Diabetes. 1973;22:825–833. doi: 10.2337/diab.22.11.825. [DOI] [PubMed] [Google Scholar]
- [55].Reaven GM, Chen YD, Hollenbeck CB, Sheu WH, Ostrega D, Polonsky KS. Plasma insulin, C-peptide, and proinsulin concentrations in obese and nonobese individuals with varying degrees of glucose tolerance. J Clin Endocrinol Metab. 1993;76:44–48. doi: 10.1210/jcem.76.1.8421101. [DOI] [PubMed] [Google Scholar]
- [56].Jeppesen J, Hollenbeck CB, Zhou MY, Coulston AM, Jones C, Chen YD, et al. Relation between insulin resistance, hyperinsulinemia, postheparin plasma lipoprotein lipase activity, and postprandial lipemia. Arterioscler Thromb Vasc Biol. 1995;15:320–324. doi: 10.1161/01.atv.15.3.320. [DOI] [PubMed] [Google Scholar]
- [57].Zwillich CW, Sahn SA, Weil JV. Effects of hypermetabolism on ventilation and chemosensitivity. J Clin Invest. 1977;60:900–906. doi: 10.1172/JCI108844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hollenbeck C, Reaven GM. Variations in insulin-stimulated glucose uptake in healthy individuals with normal glucose tolerance. J Clin Endocrinol Metab. 1987;64:1169–1173. doi: 10.1210/jcem-64-6-1169. [DOI] [PubMed] [Google Scholar]
- [59].Marmonier C, Chapelot D, Louis-Sylvestre J. Metabolic and behavioral consequences of a snack consumed in a satiety state. Am J Clin Nutr. 1999;70:854–866. doi: 10.1093/ajcn/70.5.854. [DOI] [PubMed] [Google Scholar]
- [60].Farshchi HR, Taylor MA, Macdonald IA. Regular meal frequency creates more appropriate insulin sensitivity and lipid profiles compared with irregular meal frequency in healthy lean women. Eur J Clin Nutr. 2004;58:1071–1077. doi: 10.1038/sj.ejcn.1601935. [DOI] [PubMed] [Google Scholar]
- [61].Pyorala M, Miettinen H, Laakso M, Pyorala K. Plasma insulin and all-cause, cardiovascular, and noncardiovascular mortality: the 22-year follow-up results of the Helsinki Policemen Study. Diabetes Care. 2000;23:1097–1102. doi: 10.2337/diacare.23.8.1097. [DOI] [PubMed] [Google Scholar]
- [62].Mekary RA, Giovannucci E, Willett WC, van Dam RM, Hu FB. Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr. 2012;95:1182–1189. doi: 10.3945/ajcn.111.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wei JT, Connelly AE, Satia JA, Martin CF, Sandler RS. Eating frequency and colon cancer risk. Nutr Cancer. 2004;50:16–22. doi: 10.1207/s15327914nc5001_3. [DOI] [PubMed] [Google Scholar]
- [64].Jakobsson P, Jorfeldt L. Oxygen supplementation increases glucose tolerance during euglycaemic hyperinsulinaemic glucose clamp procedure in patients with severe COPD and chronic hypoxaemia. Clin Physiol Funct Imaging. 2006;26:271–274. doi: 10.1111/j.1475-097X.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- [65].Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 2008;134:686–692. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- [66].Varela JE, Hinojosa MW, Nguyen NT. Resolution of obstructive sleep apnea after laparoscopic gastric bypass. Obes Surg. 2007;17:1279–1282. doi: 10.1007/s11695-007-9228-6. [DOI] [PubMed] [Google Scholar]
- [67].Paton JF, Sobotka PA, Fudim M, Engleman ZJ, Hart EC, McBryde FD, et al. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013;61:5–13. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]