Abstract
Many frail older adults are thin, weak, and undernourished; this component of frailty remains a critical concern in the geriatric field. However, there is also strong evidence that excessive adiposity contributes to frailty by reducing the ability of older adults to perform physical activities and increasing metabolic instability. Our scoping review explores the impact of being obese on physical frailty in older adults by summarizing the state of the science for both clinical markers of physical function and biomarkers for potential underlying causes of obesity-related decline. We used the five-stage methodological framework of Arksey and O’Malley to conduct a scoping review of randomized trials of weight loss and/or exercise interventions for obesity (BMI ≥ 30 kg/m2) in older adults (aged > 60 yrs), examining the outcomes of inflammation, oxidative stress, and lipid accumulation in muscle, as well as direct measures of physical function. Our initial search yielded 212 articles; exclusion of cross-sectional and observational studies, cell culture and animal studies, disease-specific interventions, and articles published before 2001 led to a final result of 21 articles. Findings of these trials included the following major points: The literature consistently confirmed benefits of lifestyle interventions to physical function assessed at the clinical level. Generally speaking, weight loss alone produced a greater effect than exercise alone and the best outcomes were achieved with a combination of weight loss and exercise, especially exercise programs that combined aerobic, resistance, and flexibility training. Weight loss interventions tended to reduce markers of inflammation and/or oxidative damage when more robust weight reduction was achieved and maintained over time, whereas exercise did not change markers of inflammation. However, participation in a chronic exercise program did reduce the oxidative stress induced by an acute bout of exercise. Weight loss interventions consistently reduced lipid accumulation in the muscle; however, in response to exercise, three studies showed an increase and two a decrease in muscle lipid infiltration. In summary, this scoping review identified strong clinical evidence that weight reduction and/or exercise interventions can improve physical function and biomarkers of physical dysfunction among overweight/obese older adults, supporting the suggestion that excessive adiposity contributes to physical frailty. However, the evidence also suggests a complexity of metabolic influences, both systemically and within muscle, which has not been elucidated to date. Considerable further study is needed to examine the mechanisms by which lifestyle interventions influence physical frailty before the net impact of such interventions can be fully understood.
Keywords: Obesity, frailty, older adults, physical function, inflammation, oxidative stress, muscle lipid infiltration
Introduction and Background
Age-related frailty, characterized as a state of high vulnerability to adverse health events (1), is a particular challenge to both health care providers and affected elders because of its wide-ranging impact on health, functional independence, and quality of life. Its etiology is multi-faceted, but common pathways to frailty include acute or chronic illness, inflammation, malnutrition and inactivity.(2) Physical disability and frailty are extensively entwined (3, 4); models of frailty consistently include functional deficits as an important component of the profile.(1, 2, 5) The purpose of this review is to highlight important associations between late life obesity and physical frailty by exploring the influences of lifestyle interventions for obesity on functional outcomes and related metabolic variables in this population.
Frailty was once thought of primarily as a wasting disorder (6–8) and there remains no doubt that rapid weight loss and decreased muscle mass and strength are important hallmarks. However, a strong and growing body of evidence indicates that excessive adiposity can also lead to physical frailty, especially when it occurs in concert with diminished muscle mass and/or strength. At the outset, let us underscore that, for older adults, being obese (BMI of 30 kg/m2 or more; obese (OB)) is a very distinct condition from that of being mildly to moderately overweight (BMI >25<30 kg/m2; overweight (OW)) in terms of health outcomes; in fact, being OW is not linked with detrimental effects on morbidity and mortality in older adults.(9, 10) In contrast, markedly excessive adiposity contributes to an array of negative health outcomes in the older population, leading to significant morbidity, disability, decreased quality of life and, in some cases, increased rates of mortality. OB is associated with new cases of coronary heart disease (11), fatal and nonfatal myocardial infarction (12), and cardiovascular disease mortality.(13) Type 2 diabetes is also closely linked with the growing prevalence of OB and compounds the cardiovascular risk in older individuals.(14) The number of older adults who are obese (BMI ≥ 30 kg/m2; OB) is increasing exponentially, driven by the overall obesity epidemic and large cohorts of adults reaching old age. More than one third of U.S. (15) adults over age 60 have body weights (BW) in the OB range and the prevalence of geriatric OB continues to rise. A recent flurry of published reviews confirms the recent recognition of this problem in geriatric populations.(16–21)
The deterioration in functional status caused by late life obesity supersedes that typically associated with aging alone.(22–25) Being OB leads to decreased muscle strength and reduced aerobic capacity (25), reduced ability to perform physical tasks (26), and increased likelihood of injury.(27) Moreover, as subsequently explained, excessive adiposity and muscle deterioration are cyclically reinforcing, so that physical frailty can be markedly progressive in older adults with BMIs in the OB range. Ultimately, being OB impacts self-care and Activities of Daily Living (ADLs) and can lead to early nursing home placement and increased costs of nursing care.(28–32)
In the following sections, the underlying mechanisms by which being OB contributes to physical frailty are more fully explored. In addition to obesity-related health conditions, oxidative stress, inflammation, and reduced muscle quality due to lipid infiltration are important potential harbingers of accelerated functional decline in obese older adults. (25)
Oxidative Stress and Inflammation
Age-associated increases in markers of oxidative stress, accumulation of oxidative damage, and systemic inflammation are well recognized. These observed changes led to the oxidative stress theory of aging, which hypothesizes that oxidative stress and the related inflammation cause cellular and molecular damage when reactive oxygen species (ROS) overwhelm antioxidant defenses, leading to progressive deleterious changes over time.(33) The ROS imbalance not only leads to structural damage to macromolecules, including lipids, proteins and nucleic acids, but it also activates transcriptional factors that could up-regulate pro-inflammatory cytokines leading to a chronic state of low-grade inflammation.(33) ROS and inflammation may or may not always be related, but the relationship nevertheless appears to be complex and multifactorial with mechanisms for triggering a pathological cascade being influenced by many things including environmental, genetic, or aging factors.
Adipose tissue is highly metabolically active; the abundant macrophages present there produce an array of signaling molecules. When there is an excess of adipose stores, the cellular milieu remodels to activate a number of stress pathways, including those that increase oxidative stress. During over-nutrition, adipocytes release more ROS and pro-inflammatory cytokines, leading to a continuous state of “simmering” inflammation; when the inflammatory cascade of obesity is coupled with age-related inflammation, the net effect of increased catabolism and blunted anabolism is highly detrimental to muscle.(34) This shift toward catabolism, coupled with direct muscle damage resulting from elevated ROS, leads to reduced lean mass and contributes to muscle weakness, slowed movement, exhaustion, and difficulty with mobility that severely limits the ability of OB older adults to perform physical functions essential for maintaining their independence.
Lipid Deposition in Skeletal Muscle
Due to the central role of skeletal muscle in human mobility and metabolic function, any deterioration of the contractile or material properties of muscle is strongly detrimental to physical function.(35) Both aging and obesity are associated with morphological changes resulting from an increased deposition of lipid within muscle fibers. Age-related lipid infiltration contributes to frailty by reducing muscle strength (36) and increasing mobility disability.(37) Obesity is also associated with a marked increase in lipid accumulation within muscle fibers.(38, 39) This lipid infiltration decreases muscle density and precipitates a loss of muscle strength independent of changes in muscle mass.(40, 41) Thus for older adults who are also obese, there can be a loss of muscle strength that is considerably disproportionate to loss of muscle mass, indicative of a reduction in muscle quality due to lipid infiltration and reflected in marked functional impairment.(25, 42)
Approach and Methods
The purpose of this scoping review was to assess the breadth of scientific evidence linking excess adiposity in older adults with detrimental effects on function; to accomplish this; we reviewed the impact of lifestyle interventions, namely weight loss diets and exercise regimens, on these parameters. We used the five-stage methodological framework of Arksey and O’Malley (43) to conduct this scoping review. The steps followed were: (1) identify the research questions; (2) identify relevant studies; (3) select studies for more detailed analysis; (4) chart the data; and (5) collate, summarize, and report the results. We included interventions with outcomes related to function at the molecular/cellular, tissue, and whole body level. The lifestyle interventions we considered were limited to exercise (EX) and weight loss (WL) interventions using dietary calorie restriction. We did not include studies of diet composition (except in the context of a weight loss or exercise trials) or other interventions for obesity such as pharmacologic treatments or bariatric surgery. While not every trial we reviewed had a minimum enrollment BMI of 30, all of the trials we reviewed reported a group mean BMI that was obese (range 29.8 to 39.0). We excluded from consideration studies published before 2001, cross-sectional and observational studies, studies using cell culture, animal studies, and interventions that were disease specific, as well as studies of subjects under age 60 years and those for which no subject age was specified. One exception was made to this age criteria. Due to a scarcity of lifestyle intervention studies with oxidative stress and/or inflammation as an outcome, studies with middle aged individuals were included in our review. These studies are noted in the text and table when they are mentioned. With regards to lipid infiltration of muscle, we included studies with a variety of approaches for measuring muscle lipid content, including muscle biopsies and computed tomography (CT) scans, as well as the more precise approach of proton magnetic resonance spectroscopy (3H-MRS).(44, 45)
The literature search began by consulting with a reference librarian to determine best approach methods for mapping the existing research. Electronic databases PubMed, EBSCO, and OVID were used and search strategies were developed using key words (e.g., frail, obese, lipid infiltration, inflammation, reactive oxygen species, physical function, aged, older, geriatric, and age factors) and medical subject heading terms to identify primary indexed and un-indexed articles. Review articles and the reference lists of primary articles were used to identify additional pertinent studies. Searchers were saved in My NCBI, and abstracts were carefully read, and relevant studies selected and reviewed in full. Selection of relevant studies was independently confirmed by at least two of the authors and all three authors participated in the analysis and interpretation of findings for each topic. To identify commonality and gaps, data was collected and organized by author, year of publication, origin, study population, intervention design, outcomes of measures, and key findings.
Results of Systematic Review
Overview
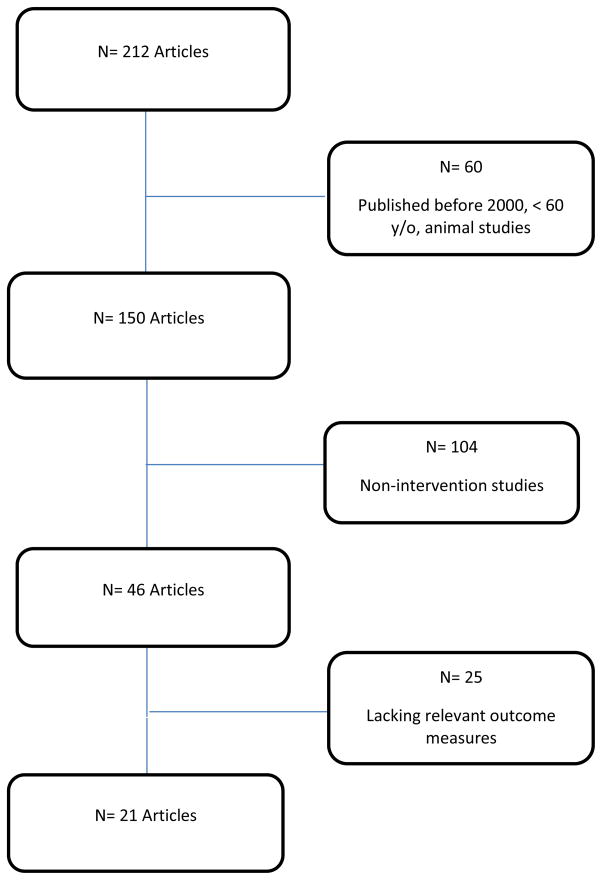
The flow chart shown in Figure 1 illustrates the process by which 21 studies were selected from the original 212 papers identified by the literature search. The most common reason for excluding studies was that they did not meet the criteria of being a prospective lifestyle intervention trial. The 21 papers represented 20 distinct major trials; in one case, two articles were written about different aspects of the same subjects within the same trial.
Figure 1.
Article Search Flow Chart
The characteristics, outcomes, and key findings for each of the 21 trials included in this review are listed in Tables 1–3. Eighteen of the studies originated from the United States with one study from Australia, Canada, and Italy. Interventions were published between the years 2001 and 2013 and 2011 was the most prolific year, comprising 28.6% of the studies published. This was followed by 2008, comprising 23.8% of the studies. The studies’ populations included both men and women, with the exception of 3 studies that include only women.(46–48) In the following sections, we discuss findings regarding the impact of lifestyle interventions on measures of physical function and then “drill down” to examine what has been found regarding the influence of these interventions on the underlying conditions of oxidative stress, inflammation, and lipid deposition in muscle.
Table 1.
Intervention Studies with Physical Function as an Outcome
| Article | Origin | Study population | Intervention | Outcomes | Important Findings |
|---|---|---|---|---|---|
| Anton et al, 2011(46) | Gainesville, FL; U.S. |
N= 34 Age: 63.7±4.5 yrs Gender: Women only BMI: Control: 35.8±6.8 kg/m2, WL + EX: 37.8±5.5 kg/m2 Health: Mild to moderate functional impairment |
Design: Randomized control trial Arms: Control (n=17) WL+EX (n=17) Duration: 6 mo |
Walking speed; SPPB; knee extension isokinetic; anthropometrics |
Weight loss: Control: −0.23±4.08 kg; WL+EX: −5.95±4.08 kg Function: Walking speed increased more in WL+EX compared to control (0.16±0.03 m/s vs. 0.02±0.03 m/s); WL+EX and control increased in SPPB, with greater increases in WL+EX (1.82±1.24 vs 0.80±1.20) Other: WL+EX decreased BW greater than control (5.95±0.992 vs.0.23±0.99 kg) |
| Villareal et al, 2011(49) | St. Louis, MO; U.S. |
N= 93 Age: Control: 69±4; WL, EX, and WL+EX:70±4 yrs Gender: Control: 67% women; WL: 65% women; EX: 62% women; WL+EX: 57% women BMI: control: 37.3±4.7; WL: 37.2±4.5; EX: 36.9±5.4; WL+EX: 37.2±5.4 kg/m2 Health: Mild to moderate frailty Sedentary lifestyle |
Design: Randomized control trial Arms: Control (n=27) WL (n=26) EX (n=26) WL+EX (n=28) Duration: 12 mo |
PPT; VO2 max; FSQ score; 1-RM; dynamic balance (obstacle course); static balance (single limb leg stance time); gait speed; SF-36; body comp by DXA; BMD; anthropometrics |
Weight loss: Control: −0.1±3.5 kg,<1%; WL: −9.7±5.4 kg, 10%; EX: −0.5±3.6 kg, 1%; WL+EX: −8.6±3.8 kg, 9% Function: WL+EX and EX arms significantly improved in all functional measures compared to control. WL significantly improved compared to control in all functional measures except strength and gait speed. PPT and VO2 improved more in WL+EX than WL or EX. Similar improvements were seen between WL+EX and EX arms in FSQ, 1-RM and gait speed, and between WL+EX and WL in single limb leg stance time. Other: Significant and similar decreases in BW and FM in WL and WL+EX arm. LMM increased in EX and decreased less in the WL+EX compared to WL. BMD increased in EX and decreased in control, WL and WL+EX. |
| Villareal et al, 2011(50) | St. Louis, MO; U.S. |
N= 9 Age: 70±2 yrs Gender: 44% women BMI: 39±5 kg/m2 Health: Moderately frail; sedentary |
Design: Single arm trial Arm: EX (N=9) Duration: 3 mo |
1-RM; VO2 max; ROM; chair rise; gait speed; single limb leg stance time; body comp by DXA |
Weight loss: −0.3kg Function: Chair rise speed decreased; upper body 1-RM increased ~20%; lower body 1-RM increased 10–20%; VO2 increased ~15%. ROM, gait speed, and one-leg stand also increased. Other: FM decreased; LMM and appendicular LM increased. |
| Davidson et al, 2009(54) | Kingston, Ontario; Canada |
N= 117 Age: Women: 67.4±5.1 yrs; Men: 67.7±5.1 yrs Gender: 58.0% women BMI: Women: 30.5±2.0 kg/m2; Men: 30.4±2.7 kg/m2 Health: Abdominal obesity, Sedentary |
Design: Randomized control trial Arms: Control (n=28) Resistance EX (n= 36) Aerobic EX (n=37) Combined EX (n=35) Duration: 6 mo |
Chair stands; 2-minute step; 8-ft-up-and-go; seated arm curl; VO2 max; anthropometrics; body composition by MRI |
Weight loss: Control; 0.28±0.37 kg; Resistance EX: −0.64±0.37 kg; Aerobic EX: −2.77±0.33 kg; Combined EX: −2.31±0.33 kg Function: Chair stands; 2-minute step; 8-ft-up- and-go; seated arm curl improved in all EX arms, with combined EX having greater improvements than Aerobic EX. VO2 increased in Aerobic EX and Combined EX. Other: BW and total fat decreased in Aerobic and Combined EX compared to control and Resistance EX arms. Abdominal fat decreased in Aerobic EX and Combined EX compared to control only; LMM increased in Resistance EX ad Combined EX compared to control and Aerobic EX |
| Frimel al. 2008(53) | St. Louis, MO; U.S. |
N= 30 Age: WL: 70.3±4.8 yrs; WL+EX: 68.7±4.3 yrs Gender: 60% women BMI: WL: 36.9±4.9kg/m2; WL+EX:36.7±5.1kg/m2 Health: Mild to moderate frailty; sedentary |
Design: Randomized, parallel groups Arms: WL (n=15) WL+EX (n=15) Duration: 6 mo |
1-RM; body comp by DXA; anthropometrics |
Weight loss: WL: −10.7±4.5 kg, 10.6±4.6%; WL+EX: −9.7±4.0 kg, 100±3.9% Function: WL+EX increased in upper and lower extremity strength (1-RM). Other: WL and WL+EX decreased in BW and FM; WL+EX lost less FFM (1.8±1.5 vs 5.4±3.7 kg), and upper (0.1±0.2 vs 0.2±0.2 kg) and lower (0.9±0.8 vs 2.0±0.9 kg) extremity LMM compared to WL arm. |
| Miller et al, 2006(51) | Winston-Salem, NC; U.S. |
N= 87 Age: Control: 69.3±0.9 yrs; WL+EX : 69.7±0.9 yrs Gender: WS: 60.5% women; WL+EX : 63.6% BMI: Control : 34.3±3.9 kg/m2; WL: 34.9±4.9 kg/m2 Health: Symptomatic knee OA; difficulty with 1 or more: lifting and carrying groceries, walking one-quarter mile, getting in and out of a chair, or going up and down stairs |
Design: Randomized control trial Arms: Control (n=43) WL+EX (n=44) Duration: 6 mo |
WOMAC; 6-minute walk distance test; stair climb test; body comp by DXA; anthropometrics |
Weight loss: Control: −0.1±0.7 kg; WL+EX: −8.3 ±0.7 kg Function: Compared to control WL+EX had improvements in WOMAC score in WL+EX; walking distance; faster stair climb in WL+EX. Other: BW, WC, FM, and FFM decreased in WL+EX compared to control. |
| Villareal et al, 2006(52) | St. Louis, MO; U.S. |
N= 27 Age: Control: 71.1±5.1 yrs; WL+EX : 69.4±4.6 yrs Gender: Both BMI: Control: 39.0±5.0 kg/m2; EX+WL: 38.5±5.3 kg/m2 Health: Mild to moderate frailty |
Design: Randomized control trial Arms: Control (n=10) WL+EX (n=17) Duration: 6 mo |
PPT; VO2 max; FSQ score; 1-RM; knee extensor and flexor strength; dynamic balance (obstacle course); static balance (single limb leg stance time); gait speed; SF-36; body comp by DXA; anthropometric |
Weight loss: Control: 0.7±2.7 kg; WL+EX: −8.2 ±5.7 kg Function: WL+EX increased in VO2; FSQ score; knee extension and flexor strength; gait speed; physical function (SF-36); role limitations (SF-36); vitality (SF-36); and change in health (SF-36). WL+EX improved one leg limb stand and obstacle course time. Other: BW and FM decreased in WL+EX. No difference between LMM loss in control and WL+EX. |
| Jensen et al, 2005(24) | Nashville, TN; U.S. | N= 18 Age: 64.1±4.8 yrs Gender: Women only BMI: 39.1±6.6 kg/m2 |
Design: Single arm trial Arm: WL (N=18) Duration: 3 mo |
Modified PPT; SF-36; Lifespan Assessment questionnaire; pedometer; body comp by BEI; anthropometrics |
Weight loss: −4.3±5.5 kg Function; Increase in PPT; physical functioning (SF-36); vitality (SF-36); and step count Other: Dec in BW; BMI; waist and hip circumference; FM; LMM. |
| Messier et al, 2004(55) | Winston-Salem, NC; U.S. | N= 252 Age: healthy lifestyle: 69±0.1 yrs; WL: 68±0.7 yrs; EX: 69±0.8 yrs; WL+EX: 69±0.8 yrs Gender: Control: 68% women; WL: 72% women; EX: 74% women; WL+EX: 74% women BMI: healthy lifestyle: 34.2±0.6 kg/m2; WL: 34.5±0.6 kg/m2; EX: 34.2±0.6 kg/m2; WL+EX: 34.0±0.7 kg/m2 Health: Knee pain, radiographic evidence of knee OA, sedentary, self-reported physical disability |
Design: Randomized control trial Arms: Control (n=78) WL (n=82) EX (n=80) WL+EX (n=76) Duration: 18 mo |
WOMAC; 6-minute walk; timed stair-climb; anthropometrics |
Weight loss: Control: 1.2% WL: 4.5%; EX: 3.7%; WL+EX: 5.7% Function: WL+EX decreased in WOMAC score compared to control; WL+EX and WL decreased in WOMAC score compared to baseline scores. 6-minute walk distance increased in WL+EX and EX compared to control and stair-climb time decreased in WL+EX. Other: WL+EX and WL decreased in BW compared to control. |
BEI = bioelectric impedance; BMD= bone mineral density; BW = body weight; EX = exercise intervention; FM = fat mass; FSQ = functional status questionnaire; LMM = lean muscle mass; PPT =physical performance test; 1-RM = 1 repetition maximum; ROM = range of motion; SF-36 = short form health survey; SPPB = short physical performance battery; VO2 max= cardiorespiratory fitness; WC = waist circumference; WL = weight loss intervention; WL+EX = weight loss and exercise intervention; WOMAC= Western Ontario and McMaster Universities Osteoarthritis Index.
Table 3.
Intervention Studies with Muscle Lipid (ML) as an Outcome
| Article | Origin | Study population | Intervention | Outcomes | Important Findings |
|---|---|---|---|---|---|
| Solomon et al, 2013(69) | Cleveland, Ohio; U.S. |
N= 15 Mean age: 65±1yrs Gender: 55% women BMI: 35.0± 1.0 kg/m2 Health: Sedentary |
Design: Randomized, parallel groups Arms: Ex+low (n=4) or high (n= 11) GI diet Duration: 12 wks |
ML by 3H-MRS of soleus muscle. |
Weight change: −8.6 ± 1.1% for both groups ML: Unchanged. |
| Haus et al, 2011(67) | Cleveland, OH; U.S. |
N= 36 Mean age: 66±1 yrs Gender: 83.3 % women BMI: 32.9 ± 3.2 kg/m2 Health: Sedentary |
Design: Randomized, parallel groups Arms: Ex+low (n=7) or high GI diet (n=8) (eucaloric) Duration: 7 days |
ML by 3H-MRS of soleus muscle. |
Weight change: −1.7±0.6 kg in low GI and −1.9±0.6 in high GI. ML: Increased, 2.3±1.3 mmol/kg in low GI and 1.4±0.9 in high GI. No group difference. |
| Dube et al, 2011(64) | Pittsburgh, PA; U.S. |
N= 16 Mean age: 67.2±4.0 yrs Gender: 56.2% women BMI: 30.6 ± 0.8 kg/m2 Health: Impaired glucose tolerance or elevated FBS |
Design: Randomized, parallel groups Arms: Aerobic EX (n = 8) WL diet (n = 8) Duration: 16 wks |
ML by histochemistry of muscle biopsy. Body comp by DXA. |
Weight change: −8.5±1.5% in WL; −1.8±0.9% in EX ML: Decreased in WL (−16.0±3.2%); increased in EX (40.8±18.2%) Other: Fat mass decreased greater in WL than in EX. FFM decreased in WL, increased slightly in EX. |
| Solomon et al, 2008(66) | Cleveland, OH; U.S. |
N= 23 Mean age: 66 ± 1 yrs Gender: Both BMI: 33.2± 1.4 kg/m2 Health: Impaired glucose tolerance |
Design: Randomized, parallel groups Arms: EX (n=12); EX+ WL (n=11) Duration: 12 wks |
ML by histochemistry of muscle biopsy. Body comp by hydrostatic weighing. Anthropometrics. |
Weight change: −3.3 kg in EX; −7.9 kg in EX+WL ML: Decreased −25.9±12.4% in EX and −34.3±17.6% in EX+WL. No group difference. Other: No change in FFM. EX+WL had greater decreases in body and fat mass. |
| Dube et al, 2008(68) | Pittsburgh, PA; U.S. |
N= 25 Mean age: 66.4±0.8 yrs Gender: 64% women BMI: 30.3±0.7 kg/m2 Health: Sedentary |
Design: Single arm trial Arm: EX Duration: 16 wks |
ML by histochemistry of muscle biopsy. Body comp by DXA. |
Weight change: −1.3 kg ML: Increased 21%. Other: Decreased body fat, FFM unchanged. |
| Santanast o et al, 2011(65) | McKeespo rt, PA; U.S. |
N= 36 Mean age: 70.3±5.9 yrs Gender: 83.3 % women BMI: 32.9±3.2 kg/m2 Health: Sedentary |
Design: Randomized, parallel groups Arms: EX+ WL, n= 21 ; EX + Education (EX+Ed), n= 15 Duration: 24 weeks |
ML by CT of thigh. Also CT of abdomen; body comp by DXA; anthropometrics; SPPB; knee extensor strength, CHAMPS questionnaire. |
Weight change: −4.9±4.8 kg in EX+WL; −1.0±3.5 kg in EX+Ed ML: Decreased in thigh of both groups, −18.1±17.5 cm in EX+WL and −5.4±9.9 cm in EX+Ed but group effect P<0.07. Other: EX+WL decreased body fat, FFM, subcutaneous total visceral fat, SAT, VAT, and thigh fat, waist circumference and knee extensor strength and increased SPPB score. |
| Mazzali et al, 2006(47) | Verona, Italy |
N= 15 Mean age:69.2±7.9 yrsa Gender: Women only BMI: 35.5±4.0 kg/m2 Health: Post-menopausal, no hormone replacement |
Design: Single arm trial Arm: WL Duration: 12 weeks |
ML by CT of thigh. Body comp by DXA. Anthropometrics. |
Weight loss: −4.66±2.72 kg (5.4%) ML: Reduced by 2.15±1.59 cm. Other: Reduced subcutaneous muscle fat, other stuff |
Anthropometrics = body weight, height, BMI, waist circumference; CHAMPS = Community Health Activities Model Program for Seniors physical activity questionnaire; CT = computed tomography; EX = exercise intervention; FFM = Fat free mass; GI = Glycemic index. 3H-MRS = proton magnetic resonance spectroscopy; ML = Muscle lipid content; SPPB: Short Physical Performance Battery; WL = weight loss intervention.
Age data only available for larger group of 35 subjects.
Interventions Affecting Physical Function
The search of the physical function topic yielded 9 distinct intervention trials, of which 5 were randomized control trials, 2 were randomized intervention trials with parallel treatment groups, and 2 were single armed trials (Table 1). At baseline, mean BMIs ranged from 30.5±2.0 to 39.1±6.6 kg/m2 and mean ages were 63.7±4.5 to 71.1±5.1 years. Two studies included women only, while the others ranged from 44 to 74% women. The interventions applied in these trials included 4 weight loss interventions, 4 exercise interventions and 6 weight loss plus exercise interventions. Durations ranged from 3 to 18 months.
Physical function outcomes were measured a number of ways, including gait speed (46, 49–52), short physical performance battery (SPPB) (46), Physical Performance Test (PPT) (48, 49, 52), daily step count(46), cardiorespiratory fitness (VO2) (49, 50, 52), strength (1-RM) (49, 50, 53), Functional Status questionnaire (FSQ) (49, 52), isokinetic strength (46, 52), SF-36 (48, 49, 52), range of motion (50), obstacle course time (49, 52), 30 second chair stands(54), 2-minute step(54), 8-ft-up-and-go(54), seated arm curl (54), Western Ontario and McMaster University Osteoarthritis Index scores (WOMAC)(51, 55), 6-minute walk (51, 55), stair-climb (51, 55), Life Span Assessment (LSA) (48), Medical Outcomes Survey 36-Item Short-Form Health Survey (SF-36) (52). Other outcome variables related to obesity were assessed, including body mass (46, 48–55); body composition by DXA (49–53), MRI (50), or bioelectrical impedance analysis (48); and waist circumference.(54)
Weight loss interventions (WL)
Weight loss through calorie reduction was assessed in four studies with mean weight reductions ranging from 4.3±5.5 to 10.7±4.5 kg. Of those, three illustrated significant improvements in physical function.(48, 49) Jensen et al,(48) found improvements in the number of daily steps (4027±2515 to 5883±3214 steps), PPT score (21.6±4.5 to 22.7±4.2) and SF-36 physical function score (53.8±24.9 to 62.5±26.7) in a 3-month WL intervention. In a 12 month intervention, Villareal et al,(49) identified physical function improvement including, improved PPT score (28.6±1.9 to 31.7±1.4), VO2 (17.6±2.2 to 19.3±2.3 ml/kg/min), FSQ score (31.6±2.0 to 32.9±1.5), obstacle course completion time (11.0±2.2 to 9.9±1.1 sec), and one-legged stand (11.7±8.7 to 16.4±5.0) in their WL arm compared to the control. Messier et al, (55) reported significant within-group changes in the WOMAC physical function score (23±1 to 19±1); however, no significant between-group changes were found. Of the four, only one WL arm failed to show improvements in physical function. Despite a 10.7±4.5 kg weight loss in the WL-arm, Frimel et al,(53) was unable to find a significant improvement in physical function, defined as 1-RM.
Exercise interventions (EX)
Four studies assessed the impact of EX on physical function in OB, frail older adults. Villareal and coworkers conducted two of the four interventions. The first of the Villareal studies,(50) a 3 month intervention including multi-component exercises (flexibility, strength, and balance), found improvements in VO2 (17.4±2.8 to 19.5±3.1 ml/kg/min); 1-RM bench press (93±8 to 115±9 lb), leg press (106±10 to 127±10 lb), knee flexion (107±8 to 128±10 lb), seated row (122±10 to 134±9 lb); range of motion measured by knee flexion (126±3.0 to 130±2 deg), shoulder flexion (157±5 to 162±4 deg), and hamstring flexion (69±5 to 78±4 deg); chair rise (17.3±2.4 to 14.2±1.8 sec); gait speed (81±4 to 88±2 m/min); and one-legged stand (10.7±3.1 to 16.5±3.9 sec). In a 12-month intervention by the same group (49), similar improvements in physical function following an EX intervention including, PPT score (27.1±3.5 to 31.1±2.5); VO2 (17.4±3.5 to 18.8±1.0 ml/kg/min); FSQ score (29.8±3.3 to 31.6±2.7); 1-RM mean for all exercises (519±187 to 693±166 lb); obstacle course completion time (10.9±3.3 to 9.4±1.4 sec); gait speed (76.0±18.3 to 84.2±15.5 m/min); and one-legged stand (13.4±10.4 to 16.8±5.9 sec) were observed. In a 6 month study, Davidson et al.(54) studies 3 EX arms, namely resistance EX only, aerobic EX only, and resistance and aerobic EX combined, and found all measures of physical function (chair stands, arms curls, 2-min step test, and 8-ft up-and-go) significantly improved in all EX arms when compared to the control. The resistance and aerobic EX combined arm also had significantly greater improvements compared to the aerobic EX arm in number of chair stands (5.9±0.5 vs 4.00±0.5), arm curls (7.9±0.7 vs 5.1±0.7), and 2-min step test (26.9±2.0 vs 17.0±2.0 steps), but no difference was found when compared to resistance EX arm. Finally, in an 18-month intervention conducted by Messier et al,(53) 6-minute walk distances (424.2±11.4 to 472.7±13.2 m) significantly improved in the EX arm, although another measure of physical function, WOMAC physical function score, showed no exercise effect.
Combination interventions (EX+WL)
Six interventions combined EX+WL and examined the effects on physical function in obese, frail older adults. Anton et al, (46) found that a 6-month EX+WL intervention significantly improved SPPB score (9.0±0.8 to 10.8 ±1.2) and walking speed (0.91±0.27 to 1.07±0.12 m/s) compared to the control; however, leg strength was not increased. In a 12 month intervention, Villareal et al,(49), discovered that participants in the WL+EX arm significantly improved in all measures of physical function, PPT score (28.0±2.9 to 33.4±2.4); VO2 (17.3±3.5 to 20.4±2.4 ml/kg/min); FSQ score (30.5±3.5 to 32.7±2.6); 1-RM mean for all exercises (539±218 to 703±124 lb); obstacle course completion time (10.7±3.3 to 9.0±2.2 sec); gait speed (72.9±14.9 to 89.8±42.3 m/min); and one-legged stand (10.5±9.5 to 18.4±7.8 sec). Additionally, in response to a 6-month intervention in a similar population, Villareal et al,(52) reported significant improvements in physical function in the EX+WL arm. Similar to the previous results, PPT score (29.4±2.2 to 31.9±2.2); VO2 (16.4±2.3 to 18.1±3.2 ml/kg/min); FSQ score (31.0±4.0 to 33.9±3.2); knee extension (80.2±17.1 to 89.3±15.3 ft/lb); knee flexion (50.3±16.1 to 61.0±15.1 ft/lb); obstacle course completion time (11.9±1.9 to 10.5±1.7 sec); gait speed (71.5±12.9 to 76.4±11.8 m/min); one-legged stand (6.8±8.0 to 11.9±9.3 sec); and FS-36 physical function score (60.0±21.0 to 83.2±13.9) all significantly improved from baseline and compared to the control. Another 6-month multi-component intervention for frail, older adults assessed the impact of an EX+WL arm on physical function by measuring improved strength using 1-RM.(53) The EX+WL arm increased 1-RM for bicep curls (167±108 to 286±133 kg); seated row (467±218 to 698±403 kg); bench press (432±208 to 635±256 kg), leg press (525±275 to 915±647 kg), knee flexion (550±270 to 792±380 kg); and knee extension (549±254 to 958±510 kg) whereas no significant improvements were seen with the WL only group. Miller et al,(51) compared a WL+EX arm to a weight stable control. Compared to the control, participants in the WL+EX significantly improved their WOMAC function scores (24.0±1.5 to 15.6±1.8), 6-minute walk (436.5±13.0 to 590.3±10.3 m), and stair climb time (9.2±0.5 to 7.7±0.5 sec). Finally, Messier et al,(55) reported significant improvements in the EX+WL arm following an 18-month intervention. Participants in the EX+WL arm significantly improved their WOMAC function score by 23%; additionally stair-climb time (11.0±0.7 to 8.5±0.8 sec) and 6-minute walk distance (416.2±11.3 to 477.8±13.1 m) also improved.
Interventions Affecting Markers of Oxidative Stress and Inflammation
Searching oxidative stress and inflammation as a function of age and obesity yielded 5 distinct randomized controlled intervention trials that used weight reduction, exercise or a combination of both; 3 of these trials had a control group (Table 2). The mean age of the participants was over 65 years of age (ranging 66.5 ± 1 to 71.2 ± 2.1 years) in 3 of the 5 studies (56)(57)(58) and, as previously noted, 2 of the studies (59)(60) had younger participants (ranging 43.0 ± 10.2 to 56.6 ± 1.1 years). Mean BMIs in the experimental groups at baseline ranged from 29.4 ± 0.7 to 38.5 ± 6.8 kg/m2. All 5 studies had both male and female subjects; the percentage of female subjects in the 4 studies that provided this information ranged from 50 to 74%. The intervention arms in these trials were of three types: exercise (resistance alone or resistance plus aerobic) (EX), diet-induced weight-loss (WL), or a combination of exercise and weight loss (EX+WL). Intervention durations ranged from 12 weeks to 18 months, although regular supervision was not provided after 6 months.
Table 2.
Intervention Studies with Inflammation or Oxidative Stress as an Outcome
| Article | Origin | Study population | Intervention | Outcomes | Important Findings |
|---|---|---|---|---|---|
| Davis et al, 2010(59)a | Maryland; U.S. |
N= 90 Age: MD: 43.0 ±10.2 yrs; FB: 45.1 ±11.6 yrs Gender: MD: 66.7% women; FB: 75.6% BMI: MD: 38.5 ±6.8 kg/m2; FB: 44.1±6.4 kg/m2 |
Design: Randomized trial Arms: Medifast Food-based Duration: 40 weeks |
CRP, ULP; Satiety; anthropometric; Body comp via BIA |
Weight loss: MD: 12.3%; FB: 6.7% Inflammation/ROS: CRP decreased for both arms; significant interaction between baseline CRP and intervention groups and time. Other: Weight loss was greater in MD while maintenance was better in FB. MD decreased in body and visceral fat and increased in LMM. |
| Tsai et al, 2008(60)a | Perth, Australia |
N= 37 Age: WL: 56.6 ±1.1 yrs; Control: 56.6 ±1.0 yrs Gender: WL: 61.5% women; Control: 50% women BMI: 33.8 ±0.8 kg/m2 Health: Met |
Design: RCT Arms: WL Weight maintenance (control) Duration: 16 weeks |
Plasma and urinary 20-HETE and F2-isoprostanes; Fasting glucose; insulin; gamma-GT; urinary sodium and Cr; total plasma arachidonic acid |
Weight loss: WL: 4.4%; Control: 0% Inflammation/ROS: No changes in urinary or plasma 20–HETE or F2-isoprostantes compared to control. |
| Lambert et al, 2008(58) | St. Louis, MO; U.S. |
N= 16 Age: 69±1 yrs Gender: EX: 50% women; WL: 50% women BMI: 38±2 kg/m2 Health: Frail; difficulty or need for assistance in two IADLS of one ADL |
Design: Randomized trial Arms: EX WL Duration: 12 weeks |
Skeletal muscle mRNAs for toll-like receptor-4; mechano-growth factor, TNF-α; IL-6; HsCRP; body comp via DXA |
Weight loss: WL: 7.1%; EX: 0% Inflammation/ROS: EX decreased TLR-4 mRNA; IL-6 mRNA; TNF-α mRNA; EX increased MGF mRNA |
| Vincent et al, 2006(57) | Charlottesville, VA; U.S. |
N= 49 Age: Normal WT Non-trained 70.9±1.4 yrs; Normal WT RX-Trained 68.1±1.5 yrs; OW/OB Non-trained 71.2±2.1 yrs; OW/OB RX-trained 66.5±1.2yrs Gender: Both (% female unknown) BMI: Normal WT Non-trained 23.9±0.5 kg/m2; Normal WT RX-Trained 22.8±0.5 kg/m2; OW/OB Non-trained 29.4±0.7 kg/m2; OW/OB RX-trained 29.8±0.9 kg/m2 Health: Sedentary |
Design: RCT Arms: EX Control Duration: 6 mo |
PEROXs; TBARS; homocysteine; lipoprotein a; cholesterol; HDL; body comp via DXA; VO2 |
Weight loss: Not available Inflammation/ROS: EX decreased EX induced PEROXs, TBARS, and homocysteine in both OW/OB and normal WT. Inverse correlation between the change in FM and PEROXs and the changes in strength and homocysteine. |
| Nicklas et al, 2004(56) | Winston-Salem, NC; U.S |
N= 252 Age: Control: 69±0.1 yrs; WL: 68±0.7 yrs; EX: 69±0.8 yrs; WL+EX: 69±0.8 yrs Gender: Control: 68% women; WL: 72% women; EX: 74% women; WL+EX: 74% women BMI: Control: 34.2±0.6 kg/m2; Diet only: 34.5±0.6 kg/m2; EX only: 34.2±0.6 kg/m2; WL+EX: 34.0±0.7 kg/m2 Health: Knee pain, radiographic evidence of knee OA, sedentary, self-reported physical disability |
Design: RCT Arms: Control WL EX WL+EX Duration: 18 mo |
IL-6; TNF-α; IL-6sR; sTNFR1; sTNFR2; CRP |
Weight loss: Control: 2.3% WL: 12.8%; EX: 4.1%; WL+EX: 8.2% Inflammation/ROS: WL decreased C-reactive protein, IL-6, and sTNFR1. Dec in sTNFR1 correlated with decreased in BW |
ADL = Activities of daily living; BIA = bioelectrical impedance; EX = exercise intervention; CRP = C-reactive protein; FB=Food based; HsCRP = high sensitivity C-reactive protein; IADL = Instrumental activities of daily living; IL-6= Interleukin 6; IL-6sR = soluble IL-6 receptor; MD=Medifast; MGF=mechano-growth factor; N20-HETE = 20-hydroxyyeicosatetraenoic acid; OA = osteoarthritis; PEROXs = serum lipid peroxidation; RX=resistance training; sTNFR1=soluble TNF-α receptor 1; sTNFR2 = soluble TNF-α receptor 2; TBARS= thiobarituric-reactive acid substances; TLR-4= toll-like receptor-4ULP = urine lipid peroxides; WL = weight loss intervention.
Mean age of participants is less than 60 yrs.
The impact of interventions on systemic oxidative stress, oxidative damage to selected macromolecules (namely, lipids) and systemic inflammation was assessed by a variety of methods. Two of the 5 studies examined oxidative damage. Two measured inflammatory markers, and one had both a marker of oxidative damage and inflammation. The markers of oxidative damage included end products of lipid peroxidation, namely serum lipid peroxidation (PEROXs), thiobarituric-reactive acid substances (TBARS), urine lipid peroxides (ULP), and plasma and urinary F2-isoprostanes. Two markers of oxidative stress, 20-hydroxyeicosatetraenoic acid (20-HETE) and γ-glutamyl transferase (γ-GT), as well as one measurement of oxidative substrate (total plasma arachidonic acid) were analyzed as well. The markers of inflammation included: interleukin 6 (IL-6), soluble IL-6 receptor (IL-6sR), tumor necrosis factor (TNF)-α, soluble TNF-α receptor 1 (sTNFR1), soluble TNF-α receptor 2 (sTNFR2), C-reactive protein (CRP), and high-sensitivity CRP.
Weight loss interventions (WL)
Three studies examined the impact of a WL intervention on oxidative and/or inflammation. Two of the studies showed that weight loss from calorie reduction alone ameliorates either oxidative stress or inflammation. In Davis et al,(59) WL of either 12.3% or 6.7% body weight resulted in a significant reduction of CRP at 40 weeks when compared to baseline values, but only the group achieving the greatest weight loss of 12.3% resulted in a significant reduction of ULP at 40 weeks. CRP was also reduced in both Medifast and food-based weight loss groups after a 16 week active weight loss period, but with only a significant within group decline in the FB group. For baseline CRP values, a significant interaction was found for intervention groups and time (p=0.04). For ULP, a significant interaction was also found between intervention groups and time (p=0.05). Another weight-loss intervention leading to a 12.8% reduction in body weight after 18 months using diet alone resulted in significantly greater reductions in concentrations of CRP (P = 0.01), IL-6 (P = 0.009), and sTNFR1 (P = 0.007) than did no weight-loss treatment.(56) Changes in sTNFR1 but not in CRP or IL-6 correlated with changes in bodyweight.(56) A third study with calorie restriction yielding a 4.4% weight loss after 16 weeks did not change 20-HETE or F2-isoprostanes levels in the urine or plasma, and the plasma levels of γ-GT did not change either.(60)
Exercise intervention
EX was assessed in 3 studies. Nicklas et al,(56) reported that EX in the form of weight training plus walking did not have a significant effect on any inflammatory biomarkers. However, in a second study, EX defined as resistance training was shown lowered exercise-induced oxidative damage in both older obese and normal weight elders when compared to controls at 6 months.(57) There was also a noted inverse correlation between the change in fat mass and PEROXs values at 6 months (r = −0.329, P < 0.05). Finally, A 12 week EX intervention consisting of aerobic activity and resistance training reduced the levels of mRNAs for IL-6 and TNF-α, but did not change serum concentrations of any inflammatory markers before or after exercise.(58)
Combination interventions (EX+WL)
Only one study investigated the effects of EX in combination with WL on changes inflammation. In Nicklas et al,(56) participants lost 8.2% of their body weight after 18 months of a calorie-reduced diet together with weight training and walking, but EX+WL had no significant impact on any inflammatory biomarkers.
Interventions Affecting Lipid Infiltration into Muscle
A search of this topic yielded 7 distinct intervention trials. Five were randomized intervention trials with parallel treatment groups; 2 were single armed trials (Table 3). The mean ages of subjects studied were similar, ranging from 65 ± 1 to 70 ± 6 years. Baseline BMIs ranged from 30.3 ± 0.7 to 35.5 ± 4.0 kg/m2. One study included women only; the other studies also had a majority of women, with percent female subjects ranging from 55 to 83%. In all studies, subjects were sedentary at baseline; in 2 studies, subjects had impaired glucose tolerance at baseline. The intervention arms in these trials were of 3 types: exercise with little or no weight loss (EX), weight loss alone (WL), and a combination of exercise and significant weight loss (EX+WL). The durations of the interventions ranged from 7 days to 24 weeks, with all but one intervention lasting at least 12 weeks.
The lipid compartment within the musculature that is most central to this review is the intramyocellular lipid (IMCL); IMCL exists as fat droplets within the muscle cells and provides a fuel for the myocytes.(44) IMCL levels are well known to be related to insulin resistance (61) and have also been shown to be affected by exercise and diet.(45) IMCL is best measured using the aforementioned 3H-MRS technique. However, because only two relevant studies revealed by our search used the 3H-MRS technique, we also included three studies that used histochemical analysis of a muscle biopsy and two studies that determined muscle lipid content via a CT scan of a cross-sectional area of the mid-thigh. These methods are less precise than 3H-MRS because they do not discriminate between intra- and inter-musclular lipid deposition.(61)
In addition to body mass, a variety of other outcomes related to obesity and function were assessed in these trials; these included body composition by dual energy X-ray absorptiometry (DXA) or hydrostatic weighing, abdominal fat by CT, waist circumference, function by Short Physical Performance Battery (62), physical activity by Community Health Activities Model Program for Seniors questionnaire (63) and knee extensor strength.
WL and combination interventions (EX+WL)
Two studies of a WL only intervention had physiologically important changes in body weight. (47, 64) In both of these trials, a significant reduction in muscle lipid was observed. Dube et al.(64) reported a 16% reduction in muscle lipid assessed pre- and post-intervention in muscle biopsies from 8 subjects who lost 8.5% of their baseline weight via a low-fat, reduced calorie diet. Mazzali et al.(47) measured a 2.15 ± 1.59 cm reduction in muscle lipid content in a single armed trial of WL in 15 postmenopausal women who reduced body weight by 4.7% over 12 weeks. A reduction in muscle lipid was also reported for the 2 trials that included intervention arms of EX+WL.(65, 66)
Exercise interventions (EX)
The results of studies of EX alone and its effects on muscle lipid content are not nearly as consistent as those of WL. Of the six trials that included an exercise arm, muscle lipid was decreased in two trials, increased in three trials, and unchanged in one trial. Santanasto et al,(65) reported an exercise-induced reduction in muscle lipid content in 15 OW/OB subjects participating in a 6-month program that included aerobic, resistance and balance training and Solomon et al.(66) reported a 25.9% decrease in muscle lipid in a 12-week EX trial of exercise. In contrast, Dube et al,(64) assessed pre- and post-intervention muscle biopsies in 8 subjects who completed a 16 week aerobic exercise program and found an increase of more than 40% in muscle lipid content. Two additional EX interventions also reported increases in muscle lipids.(67, 68) One laboratory conducted 2 separate studies of EX combined with a low or high glycemic index diet. Both of these studies used 3H-MRS of soleus muscle to quantify muscle lipids, however muscle lipid was found to be unchanged in the 3 month trial (69) but increased in the 7-day trial (66). The finding of no change in the three-month trial might be explained by the fact that subjects in both treatment groups lost 8.6% loss of their baseline weight; the lowering of muscle lipid by weight loss has already been mentioned and this could have “cancelled out” the expected EX effect in these subjects.
Discussion
Interventions Affecting Physical Function
This scoping review has confirmed robust beneficial effects of lifestyle interventions on physical function and body composition in obese older adults. Improvements in an array of functional measures were identified in WL arms, EX arms, and WL+ EX arms. Notwithstanding the functional improvements observed with WL, however, it should be noted that LBM was not preserved in WL interventions, a potentially negative consequence for frail older adults.(70) More research is needed to determine the health consequences of WL only interventions that are unable to preserve LBM. In regards to EX only, physical function improved in response to a variety of exercise modalities (aerobic, resistance and combined exercise), but the greatest improvements were seen with the combination of exercise components.(50, 54) Weight reduction was only apparent in aerobic and combined exercise; however, LBM was increased in resistance and combined exercise. Thus, combined exercise was more advantageous than aerobic or resistance exercise alone. The most beneficial physical function and body composition improvements were evident in the WL+EX arms, particularly those that incorporated multi-component exercise interventions (balance, flexibility, strength, and aerobic). These finding illustrate the benefit and increasing need for combining multicomponent exercise with weight loss to achieve the most effective lifestyle intervention for physical frailty in obese older adults.
Interventions Affecting Markers of Oxidative Stress and Inflammation
Scoping the literature for randomized controlled trials highlighted the lack of studies demonstrating a link between total fat mass, systemic inflammation, oxidative stress and damage to muscle tissue, and changes in strength and physiologic function. A number of observational studies have associated markers of oxidative stress or inflammation with measures of frailty such as slow gait speed.(71, 72) These studies have suggested that adiposity induces oxidative stress and thus grip strength(73) and that inflammation is associated with reduced grip strength and reduced walking speed in older subjects with the highest body fat percentage.(74) But it is also known that inflammation only partially explains observed mobility limitations.(75)
The evidence identified in our scoping review advances the notion that WL is beneficial but that for both systemic inflammation and/or markers of oxidative stress there may be a threshold of weight reduction that needs to be obtained in order to achieve the benefits. However, the impact of exercise on inflammation and/or oxidative stress in older obese adults is more difficult to interpret because there was no weight loss in some studies, the length of exercise was variable, and outcome markers were different in each of the 3 studies. Exercise alone alters the production of inflammatory cytokines, and it appears that it also changes inflammatory gene expression as well as repair mechanisms for skeletal muscle.(56) Of all 5 studies related to inflammation and oxidative stress only the study by Vincent et al, (57)showed any correlation of muscle function to a biochemical marker. In this case, older overweight or obese adults who underwent resistance training for 6 months had a 6% reduction in homocysteine levels, illustrating a significant correlation with the increase in muscle strength to the decline in homocysteine levels. Considerable further study will be required to fully elucidate the influence of lifestyle interventions for obesity on inflammation and oxidative damage and the implications of these changes for muscle structure and physical function, but caution must be taken when interpreting the resulting biomarkers from either ROS or inflammation because these processes may represent separate pathways with different triggers for each.
Interventions Affecting Lipid Infiltration into Muscle
In the case of studies examining changes in muscle lipid content as an outcome, the consensus of the available literature was that, in addition to the mechanical benefit of reducing the proportion of fat mass to muscle mass in the body, a reduction in body weight is directly beneficial to muscle function via its ability to lower the amount of lipid accumulation there. With regards to the impact of EX, however, we found considerable disagreement amongst the findings, an observation that has a strong precedent in the literature. In fact, increased intramuscular lipid following an EX intervention is well supported by reports of an “athlete paradox”. (44, 76). For over a decade, it has been recognized that chronically exercised individuals, who are clearly insulin-sensitive, also have high muscle lipid content (77, 78). The fact that both obesity and endurance exercise increase lipid in muscle underscores the complexity of the relationship between muscle lipid content and muscle function and emphasizes our limited understanding how lipid infiltration ultimately influences muscle function.
We recognize the limitation that the studies of muscle lipid infiltration used different methodologies. However, the findings on the impact of exercise on muscle lipid do not seem to be explained by differences in method of assessment. As previously noted, the two studies using 3H-MRS of soleus muscle to quantify muscle lipids (67, 69) did not agree in their findings on exercise effects. The same is true for the studies measuring lipids by muscle biopsy—two found an exercise-induced increase (64, 68) while one found a decrease (66). As there was only one study using CT to examine exercise effects (65), we cannot determine if that method would yield more consistent results. Similarly to the observations with measurement techniques, study duration did not appear to influence the findings on exercise and lipid change.
In summary, this scoping review strongly supports the conclusion that weight loss and exercise interventions improve function and biomarkers of physical frailty among obese seniors. Interventions with a weight loss component appear to have a more robust effect on these outcomes than interventions using exercise alone. When obese individuals lose weight, and thus fat stores, function directly improves by an increase in the proportion of muscle to adipose tissue. Put quite simply, there is less body mass to transport and more muscle to do it with. Yet, the influence of having excess body fat on physical frailty goes beyond the “mechanical,” as do the potential benefits of lifestyle intervention. Based on the limited literature available, it seems that oxidative stress and inflammation may be reduced using lifestyle interventions, but much further study is needed to understand the extent to which these changes might produce functional benefits. Lipid infiltration due to obesity likely impairs muscle function and our review provides fairly strong support for improvements in response to weight loss intervention. However, the impact of exercise on lipid muscle content and the extent to which this influences muscle function cannot be conclusively determined from the available evidence. Thus, while the scientific literature to date argues strongly for the clinical benefits of lifestyle interventions for obesity in older adults with compromised physical mobility, the mechanisms by which these interventions influence functional status are not well delineated and need further exploration.(69)
Acknowledgments
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number T32AG000029. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research reported in this publication was also supported by Donald W. Reynolds Foundation FD~AGE Grant and the Health Resources and Services Administration Fellowship Grant, Grant Number D01HP08791.
References
- 1.Gielen E, Verschueren S, O’Neill TW, Pye SR, O’Connell MD, Lee DM, et al. Musculoskeletal frailty: a geriatric syndrome at the core of fracture occurrence in older age. Calcif Tissue Int. 2012;91(3):161–77. doi: 10.1007/s00223-012-9622-5. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Gallucci M, Ongaro F, Meggiolaro S, Antuono P, Gustafson DR, Forloni GL, et al. Factors related to disability: evidence from the “Treviso Longeva (TRELONG) study”. Arch Gerontol Geriatr. 2011;52(3):309–16. doi: 10.1016/j.archger.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 5.Cooper C, Dere W, Evans W, Kanis JA, Rizzoli R, Sayer AA, et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int. 2012;23(7):1839–48. doi: 10.1007/s00198-012-1913-1. [DOI] [PubMed] [Google Scholar]
- 6.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24(3):455–69. vi. doi: 10.1016/j.cger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2010;65(4):377–81. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 8.Bowen ME. The relationship between body weight, frailty, and the disablement process. J Gerontol B Psychol Sci Soc Sci. 2012;67(5):618–26. doi: 10.1093/geronb/gbs067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman IM. Obesity paradox during aging. Interdiscip Top Gerontol. 2010;37:20–36. doi: 10.1159/000319992. [DOI] [PubMed] [Google Scholar]
- 10.van Uffelen JG, Berecki-Gisolf J, Brown WJ, Dobson AJ. What is a healthy body mass index for women in their seventies? Results from the Australian longitudinal study on women’s health. J Gerontol A Biol Sci Med Sci. 2010;65(8):847–53. doi: 10.1093/gerona/glq058. [DOI] [PubMed] [Google Scholar]
- 11.Bales CW, Buhr G. Is Obesity Bad for Older Persons? A Systematic Review of the Pros and Cons of Weight Reduction in Later Life. J Am Med Dir Assoc. 2008;9(5):302–12. doi: 10.1016/j.jamda.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Harris TB, Launer LJ, Madans J, Feldman JJ. Cohort Study of Effect of Being Overweight and Change in Weight on Risk of Coronary Heart Disease in Old Age. BMJ. 1997;314(7097):1791–4. doi: 10.1136/bmj.314.7097.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dey DK, Lissner L. Obesity in 70-year-old Subjects as a Risk Factor for 15-year Coronary Heart Disease Incidence. Obes Res. 2003;11(7):817–27. doi: 10.1038/oby.2003.113. [DOI] [PubMed] [Google Scholar]
- 14.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The Effect of Age on the Association between Body-mass Index and Mortality. N Engl J Med. 1998;338(1):1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 15.Mathus-Vliegen EM. Obesity and the elderly. J Clin Gastroenterol. 2012;46(7):533–44. doi: 10.1097/MCG.0b013e31825692ce. [DOI] [PubMed] [Google Scholar]
- 16.Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65years and older: A review of the controversy. Exp Gerontol. 2013 doi: 10.1016/j.exger.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decaria JE, Sharp C, Petrella RJ. Scoping review report: obesity in older adults. Int J Obes (Lond) 2012;36(9):1141–50. doi: 10.1038/ijo.2012.29. [DOI] [PubMed] [Google Scholar]
- 18.Darmon P. Intentional weight loss in older adults: useful or wasting disease generating strategy? Curr Opin Clin Nutr Metab Care. 2013 doi: 10.1097/MCO.0b013e32835f503f. [DOI] [PubMed] [Google Scholar]
- 19.Howel D. Waist circumference and abdominal obesity among older adults: patterns, prevalence and trends. PLoS One. 2012;7(10):e48528. doi: 10.1371/journal.pone.0048528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathus-Vliegen EM. Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: a guideline. Obes Facts. 2012;5(3):460–83. doi: 10.1159/000341193. [DOI] [PubMed] [Google Scholar]
- 21.Samper-Ternent R, Al Snih S. Obesity in Older Adults: Epidemiology and Implications for Disability and Disease. Rev Clin Gerontol. 2012;22(1):10–34. doi: 10.1017/s0959259811000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topinkova E. Aging, disability and frailty. Ann Nutr Metab. 2008;52 (Suppl 1):6–11. doi: 10.1159/000115340. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Guo X. Obesity and functional disability in elderly Americans. J Am Geriatr Soc. 2008;56(4):689–94. doi: 10.1111/j.1532-5415.2007.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen GL. Obesity and functional decline: epidemiology and geriatric consequences. Clin Geriatr Med. 2005;21(4):677–87. v. doi: 10.1016/j.cger.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010;13(1):46–51. doi: 10.1097/MCO.0b013e32833309cf. [DOI] [PubMed] [Google Scholar]
- 26.Gates DM, Succop P, Brehm BJ, Gillespie GL, Sommers BD. Obesity and presenteeism: the impact of body mass index on workplace productivity. J Occup Environ Med. 2008;50(1):39–45. doi: 10.1097/JOM.0b013e31815d8db2. [DOI] [PubMed] [Google Scholar]
- 27.Schmier JK, Jones ML, Halpern MT. Cost of obesity in the workplace. Scand J Work Environ Health. 2006;32(1):5–11. doi: 10.5271/sjweh.970. [DOI] [PubMed] [Google Scholar]
- 28.Bradway C, DiResta J, Fleshner I, Polomano RC. Obesity in nursing homes: a critical review. J Am Geriatr Soc. 2008;56(8):1528–35. doi: 10.1111/j.1532-5415.2008.01821.x. [DOI] [PubMed] [Google Scholar]
- 29.Galanos AN, Pieper CF, Cornoni-Huntley JC, Bales CW, Fillenbaum GG. Nutrition and function: is there a relationship between body mass index and the functional capabilities of community-dwelling elderly? J Am Geriatr Soc. 1994;42(4):368–73. doi: 10.1111/j.1532-5415.1994.tb07483.x. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins KR. Obesity’s effects on the onset of functional impairment among older adults. Gerontologist. 2004;44(2):206–16. doi: 10.1093/geront/44.2.206. [DOI] [PubMed] [Google Scholar]
- 31.Jensen GL, Friedmann JM. Obesity Is Associated with Functional Decline in Community-Dwelling Rural Older Persons. Journal of the American Geriatrics Society. 2002;50(5):918–23. doi: 10.1046/j.1532-5415.2002.50220.x. [DOI] [PubMed] [Google Scholar]
- 32.Rolland Y, Lauwers-Cances V, Cristini C, Abellan van Kan G, Janssen I, Morley JE, et al. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am J Clin Nutr. 2009;89(6):1895–900. doi: 10.3945/ajcn.2008.26950. [DOI] [PubMed] [Google Scholar]
- 33.Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2007;292(1):R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 34.Ershler WB. A gripping reality: oxidative stress, inflammation, and the pathway to frailty. Journal of Applied Physiology. 2007;103(1):3–5. doi: 10.1152/japplphysiol.00375.2007. [DOI] [PubMed] [Google Scholar]
- 35.Sakuma K, Yamaguchi A. Molecular Mechanisms in Aging and Current Strategies to Counteract Sarcopenia. Curr Aging Sci. 2010;3(2):90–101. doi: 10.2174/1874609811003020090. [DOI] [PubMed] [Google Scholar]
- 36.Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 37.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 38.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49(4):467–72. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 39.Moro C, Galgani JE, Luu L, Pasarica M, Mairal A, Bajpeyi S, et al. Influence of gender, obesity, and muscle lipase activity on intramyocellular lipids in sedentary individuals. J Clin Endocrinol Metab. 2009;94(9):3440–7. doi: 10.1210/jc.2009-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 41.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90(6):2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 42.Johannsen DL, Ravussin E. Obesity in the elderly: is faulty metabolism to blame? Aging health. 2010;6(2):159–67. doi: 10.2217/ahe.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arksey H, O’Malley L. Scoping Studies: Towards a Methodological Framework. International Journal of Social Research Methodology. 2005;8(1):19–32. [Google Scholar]
- 44.Machann J, Stefan N, Schick F. (1)H MR spectroscopy of skeletal muscle, liver and bone marrow. Eur J Radiol. 2008;67(2):275–84. doi: 10.1016/j.ejrad.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 45.Boesch C, Machann J, Vermathen P, Schick F. Role of proton MR for the study of muscle lipid metabolism. NMR Biomed. 2006;19(7):968–88. doi: 10.1002/nbm.1096. [DOI] [PubMed] [Google Scholar]
- 46.Anton SD, Manini TM, Milsom VA, Dubyak P, Cesari M, Cheng J, et al. Effects of a weight loss plus exercise program on physical function in overweight, older women: a randomized controlled trial. Clin Interv Aging. 2011;6:141–9. doi: 10.2147/CIA.S17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazzali G, Di Francesco V, Zoico E, Fantin F, Zamboni G, Benati C, et al. Interrelations between fat distribution, muscle lipid content, adipocytokines, and insulin resistance: effect of moderate weight loss in older women. Am J Clin Nutr. 2006;84(5):1193–9. doi: 10.1093/ajcn/84.5.1193. [DOI] [PubMed] [Google Scholar]
- 48.Jensen GL, Roy MA, Buchanan AE, Berg MB. Weight loss intervention for obese older women: improvements in performance and function. Obes Res. 2004;12(11):1814–20. doi: 10.1038/oby.2004.225. [DOI] [PubMed] [Google Scholar]
- 49.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring) 2011;19(2):312–8. doi: 10.1038/oby.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller GD, Nicklas BJ, Davis C, Loeser RF, Lenchik L, Messier SP. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity (Silver Spring) 2006;14(7):1219–30. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- 52.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166(8):860–6. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 53.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40(7):1213–9. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med. 2009;169(2):122–31. doi: 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- 55.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 56.Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BW, Loeser RF, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. The American Journal of Clinical Nutrition. 2004;79(4):544–51. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 57.Vincent HK, Bourguignon C, Vincent KR. Resistance Training Lowers Exercise-Induced Oxidative Stress and Homocysteine Levels in Overweight and Obese Older Adults. Obesity. 2006;14(11):1921–30. doi: 10.1038/oby.2006.224. [DOI] [PubMed] [Google Scholar]
- 58.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. Journal of Applied Physiology. 2008;105(2):473–8. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis LM, Coleman C, Kiel J, Rampolla J, Hutchisen T, Ford L, et al. Efficacy of a meal replacement diet plan compared to a food-based diet plan after a period of weight loss and weight maintenance: a randomized controlled trial. Nutr J. 2010;9:11. doi: 10.1186/1475-2891-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsai IJ, Croft KD, Mori TA, Falck JR, Beilin LJ, Puddey IB, et al. 20-HETE and F2-isoprostanes in the metabolic syndrome: the effect of weight reduction. Free Radical Biology and Medicine. 2009;46(2):263–70. doi: 10.1016/j.freeradbiomed.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 61.Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr. 2007;85(3):662–77. doi: 10.1093/ajcn/85.3.662. [DOI] [PubMed] [Google Scholar]
- 62.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 63.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Dube JJ, Amati F, Toledo FG, Stefanovic-Racic M, Rossi A, Coen P, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia. 2011;54(5):1147–56. doi: 10.1007/s00125-011-2065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santanasto AJ, Glynn NW, Newman MA, Taylor CA, Brooks MM, Goodpaster BH, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. 2011;2011 doi: 10.1155/2011/516576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Solomon TP, Sistrun SN, Krishnan RK, Del Aguila LF, Marchetti CM, O’Carroll SM, et al. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol. 2008;104(5):1313–9. doi: 10.1152/japplphysiol.00890.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haus JM, Solomon TP, Lu L, Jesberger JA, Barkoukis H, Flask CA, et al. Intramyocellular lipid content and insulin sensitivity are increased following a short-term low-glycemic index diet and exercise intervention. Am J Physiol Endocrinol Metab. 2011;301(3):E511–6. doi: 10.1152/ajpendo.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294(5):E882–8. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solomon TP, Haus JM, Cook MA, Flask CA, Kirwan JP. A Low-Glycemic Diet Lifestyle Intervention Improves Fat Utilization during Exercise in Older Obese Humans. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82(4):872–8. doi: 10.1093/ajcn/82.4.872. quiz 915–6. [DOI] [PubMed] [Google Scholar]
- 71.Baptista G, Dupuy AM, Jaussent A, Durant R, Ventura E, Sauguet P, et al. Low-grade chronic inflammation and superoxide anion production by NADPH oxidase are the main determinants of physical frailty in older adults. Free Radic Res. 2012;46(9):1108–14. doi: 10.3109/10715762.2012.692784. [DOI] [PubMed] [Google Scholar]
- 72.Wu IC, Shiesh SC, Kuo PH, Lin XZ. High oxidative stress is correlated with frailty in elderly chinese. J Am Geriatr Soc. 2009;57(9):1666–71. doi: 10.1111/j.1532-5415.2009.02392.x. [DOI] [PubMed] [Google Scholar]
- 73.Komatsu F, Kagawa Y, Kawabata T, Kaneko Y, Ishiguro K. Relationship of dietary habits and obesity to oxidative stress in Palauan people: compared with Japanese and Mongolian people. Curr Aging Sci. 2009;2(3):214–22. doi: 10.2174/1874609810902030214. [DOI] [PubMed] [Google Scholar]
- 74.Stenholm S, Rantanen T, Heliovaara M, Koskinen S. The mediating role of C-reactive protein and handgrip strength between obesity and walking limitation. J Am Geriatr Soc. 2008;56(3):462–9. doi: 10.1111/j.1532-5415.2007.01567.x. [DOI] [PubMed] [Google Scholar]
- 75.Stenholm S, Koster A, Alley DE, Houston DK, Kanaya A, Lee JS, et al. Joint association of obesity and metabolic syndrome with incident mobility limitation in older men and women--results from the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010;65(1):84–92. doi: 10.1093/gerona/glp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amati F, Dube JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 2011;60(10):2588–97. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86(12):5755–61. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 78.Russell AP, Gastaldi G, Bobbioni-Harsch E, Arboit P, Gobelet C, Deriaz O, et al. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: a case of good vs. bad lipids? FEBS Lett. 2003;551(1–3):104–6. doi: 10.1016/s0014-5793(03)00875-5. [DOI] [PubMed] [Google Scholar]