Abstract
Background
Bariatric surgery results in dramatic weight loss and improves metabolic syndrome and Type 2 diabetes (T2DM). However, previous studies have noted that morbidly obese patients with T2DM experience less weight loss benefits than non-diabetic patients following bariatric surgery. We sought to determine longitudinal effects of laparoscopic Roux-en-Y gastric bypass (LRYGB) on percent excess BMI loss (%EBMIL) and clinical metabolic syndrome parameters in patients with T2DM compared to appropriately matched cohort without T2DM.
Methods
Retrospective cohort analysis of T2DM patients (n=126) to non-T2DM patients (n=126) matched on age (M=48.1±9.5), sex (81% Female), race (81% Caucasian) and pre-surgical body mass index (BMI; M=49.3±9.5). Lipids, glucose, hemoglobin A1c, blood pressure (BP), co-morbidities of obesity, medications for co-morbidities and T2DM medications were collected at baseline, 6 months and 12 months post-surgery. %EBMIL was collected at 1, 3, 6, 9 and 12 months post-surgery. One-way ANOVAs with effect sizes estimates were conducted to compare the two groups.
Results
As expected, T2DM subjects had significantly greater pre-surgical HbA1c, blood glucose, blood pressure and lipid parameters at baseline vs. non-T2DM (all p's<0.05). At 1, 3, 6, 9, and 12 months after LRYRB, both groups had similar reduction in %EBMIL (p>0.10). At 6 months, there was a significant reduction in HbA1c, blood glucose and lipid in the T2DM cohort compared to pre-surgical levels (p<0.0001). At 12 months, these values were not different to that of the non-T2DM subjects (p>0.10).
Conclusion
When matched on appropriate factors associated with weight loss outcomes, severely obese patients with T2DM have similar post-LRYGB weight loss outcomes in the first twelve months following surgery compared to non-T2DM patients. Further, T2DM surgical patients achieved significant improvement in metabolic syndrome components.
Keywords: Type 2 Diabetes Mellitus, Morbid obesity, Roux-en-Y anastomosis, Weight loss, Metabolic Syndrome, Gastric bypass, Bariatric surgery, Insulin
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease and treatment initially consists primarily of a combination of lifestyle adjustments and oral hypoglycemic agents. However, single, or even multiple oral hypoglycemic agents may not be a permanent solution due to continuing decline in pancreatic β-cell function [1, 2, 3].For those who initiate insulin therapy, less than half achieve an A1c of ≤7% [4].
Approximately 50-90% of T2DM patients are also obese, and use of hypoglycemic agents, especially insulin, may exacerbate weight gain. Studies have shown improvement in insulin sensitivity following small amounts of weight loss [5]. Thus, achieving resolution of one may depend on the resolution of the other. Gastric bypass surgery induces massive weight loss and can result in biochemical remission of diabetes in the majority of patients, especially in those with early duration of diabetes and higher degree of β-cell function at pre-surgery. In these patients, insulin sensitivity improves proportionally to weight loss, while β-cell glucose sensitivity increases disproportionally to weight loss [6].
Furthermore, excess body fat and especially abdominal adiposity is a predisposition to metabolic syndrome, which is characterized by hypertension, inflammation and hyperlipidemia [7]. The presence of metabolic syndrome is associated with an increased risk of a cardiovascular event. Eighty percent of T2DM patients also have metabolic syndrome [8], a series of risk factors that includes hypertension and hyperlipidemia, which increase the chances of a cardiovascular event.
Studies have shown that cases of diabetes can be reversed within days of the surgery, even prior to significant weight loss [9], although the mechanism behind restoration of euglycemia and insulin sensitivity is not well understood. In particular, LRYGB is one of the most common forms of bariatric surgery and has been shown to be effective at inducing remission of diabetes in 83.7% of patients and reducing excess body weight by 61.6% [10]. Pories et al. showed similar results: glycemic control was significantly improved within days of the surgery and trends for total body weight loss parallel those of excess weight loss, with patients losing a mean of 102 lbs after one year and 70% of excess body weight after two years [9].
Despite the excellent resolution rates of diabetes post-LRYGB, not all T2DM patients who undergo LRYGB achieve remission. Some studies have suggested that T2DM patients do not lose as much weight as non-T2DM patients [11, 12, 13]. However, some of these studies did not control for gender [14], despite studies that have shown that gender affects weight loss outcome [15]. A recent retrospective cohort study following 42 patients three years after LRYGB suggests that recurrence or worsening of T2DM is associated with a lower preoperative BMI. However, the authors suggested this was because the patients with lower BMIs had a more severe stage of T2DM, as indicated by insulin use [11].Moreover, race plays a role in weight loss outcomes; African Americans lost 12% less weight compared to whites one year after gastric bypass[16]. Finally, patients with higher BMIs often evidence less percent excess body weight loss when compared to patients with lower BMIs[17].
Thus, our study, which was designed to match for factors associated with weight loss outcomes including: age, gender, race and pre-surgical percent excess BMI, provides a comprehensive view as to differences in weight loss and related outcomes in T2DM and non-T2DM patients based on metabolic and clinical factors. We hypothesized that non-T2DM patients would have better weight loss outcomes compared to T2DM patients. However, we predicted that T2DM patients would have a markedly improved metabolic profile compared to baseline.
Materials and Methods
All adult patients who underwent LRYGB at the Cleveland Clinic between 2005-2010 were included in the study. Patients who had laparoscopic gastric adjustable banding, laparoscopic sleeve gastrectomy, revisional procedures or did not have a 12-month post-LRYGB visit at the time of study initiation were excluded. A total of 265 patients who underwent LRYGB between 2005-2010 at the Cleveland Clinic were identified, of which 252 were included. Thirteen patients were excluded as repeats, later found to not have a 12-month visit or were unmatched.
Patients were identified as having T2DM if they met any of the following criteria: fasting blood glucose ≥126 mg/dl, HbA1c > 6.0, on at least one hypoglycemic agent and/or an ICD-9 code consistent with T2DM in their electronic health record. Of the 252 patients, 126 were classified as having T2DM. Patient groups were frequency-matched on age, sex, race and pre-surgical BMI (Table 1).
Table 1. Baseline characteristics of subjects.
| All patients n=252 | T2DM n=126 | Non-T2DM n=126 | |
|---|---|---|---|
| % Female | 81 | 81 | 81 |
| Age | 48.1 ± 9.5 | 48.4 ± 9.7 | 47.8 ± 9.4 |
| Ethnicity | |||
| White (%) | 81 | 80 | 84.6 |
| Black (%) | 15.9 | 18.4 | 13.8 |
| Other (%) | 2.8 | 1.6 | 1.6 |
| Pre-Surgical BMI | 49.3 ± 9.5 | 47.5 ± 8.4 | 48 ± 8.1 |
Continuous variables are listed as mean±SD.
The following patient variables were collected at baseline, six months and twelve months after surgery: number of medications for obesity-related conditions (including T2DM medications), number of T2DM medications, insulin use (if applicable, yes or no), lipid panel values (triglycerides, cholesterol, LDL, HDL and VLDL), fasting blood glucose, HbA1c and systolic and diastolic blood pressures. Baseline was defined as the date of visit with a psychologist within the Bariatric and Metabolic Institute. If the psychologist report was incomplete, a physician report within two months of the visit with the psychologist was used. Physician notes from the Bariatric and Metabolic Institute were preferred, followed by those of staff endocrinologists and internists. Percent excess percent BMI (%EBMI) lost was also collected at 1, 3, 6, 9 and 12 months post-LRYGB. At each time interval, patients were evaluated on whether they met American Diabetes Association recommended targets: an HbA1c of ≤7%, blood pressure of ≤130/80 and LDL ≤ 100; only patients with complete longitudinal information were included in this analysis.
We also classified T2DM patients into two groups based on treatment of diabetes as an indicator of severity of disease to determine differences in weight loss outcomes; one group included patients who were non-insulin dependent (diet and exercise controlled or on oral hypoglycemic agents) while the second group included patients who were insulin-users. Of the 126 T2DM patients, 86 were classified as non-insulin dependent while 39 were insulin-users, and the status of one patient was not known.
Continuous variables were analyzed through one-way ANOVA with effect size estimates between the T2DM patients and non-T2DM patients. For categorical variables such as insulin use, chi-square tests were performed to look at changes in insulin use in the T2DM group. Multivariate analyses of variance were used to examine the effects of time on total T2DM medications in the T2DM group and total medications in the full cohort. Level of significance was set at p<0.05. All statistical analysis was performed on SPSS 17.
Results
As expected, T2DM patients exhibited higher FBG, HbA1c levels and were on more medications at baseline than the non-T2DM group (p<0.001, Table 2).T2DM patients also had elevated diastolic blood pressures (p<0.01), with systolic blood pressures approaching significance (p=0.095). Also as expected, T2DM patients displayed significantly higher VLDL and LDL levels compared to non-T2DM patients at baseline (p<0.05).
Table 2. Univariate analysis of clinical and metabolic changes in subjects.
| Non-diabetic | Diabetic | |||||||
|---|---|---|---|---|---|---|---|---|
| Timepoint | Characteristic | n | mean (±SD) | n | mean (±SD) | 95% CI | p-value | Significant |
| Baseline | HDL | 93 | 50.4 (±12.1) | 87 | 47.8 (±14.4) | (47.2, 51.1) | 0.196 | |
| VLDL | 90 | 30.4 (±14.7) | 83 | 37.7 (±21.0) | (31.3, 36.7) | 0.009 | * | |
| LDL | 93 | 116.9 (±37.1) | 87 | 96.1 (±35.1) | (101.2, 111.8) | <0.001 | * | |
| A1c | 23 | 5.6 (±0.4) | 73 | 7.5 (±1.4) | (6.3, 6.9) | <0.001 | * | |
| SBP | 115 | 140.9 (±17.5) | 120 | 137.1 (±17.1) | (136.8, 141.2) | 0.095 | ||
| DBP | 115 | 82.3 (±9.4) | 120 | 78.8 (±9.9) | (79.3, 81.8) | 0.006 | * | |
| # Medications | 97 | 3.0 (±2.4) | 108 | 5.1 (±4.0) | (3.6, 4.5) | <0.001 | * | |
| 6 MO | HDL | 48 | 51.7 (±12.3) | 66 | 50.4 (±11.9) | (48.1, 53.3) | 0.575 | |
| VLDL | 44 | 23.7 (±17.8) | 61 | 24.1 (±11.1) | (21.7, 26.7) | 0.877 | ||
| LDL | 48 | 93.7 (±31.6) | 66 | 94.5 (±31.5) | (88.2, 100.0) | 0.893 | ||
| A1c | 16 | 5.4 (±0.2) | 66 | 6.2 (±1.0) | (5.5, 6.0) | 0.005 | * | |
| SBP | 92 | 128.1 (±15.8) | 107 | 131.4 (±21.5) | (127.1, 132.4) | 0.231 | ||
| DBP | 92 | 78.2 (±10.4) | 107 | 77.3 (±11.1) | (76.2, 79.2) | 0.553 | ||
| # Medications | 93 | 3.4 (±2.6) | 109 | 4.3 (±3.3) | (3.4, 4.2) | 0.035 | * | |
| 12 MO | HDL | 47 | 59.1 (±14.2) | 47 | 58.5 (±15.7) | (55.7, 61.8) | 0.837 | |
| VLDL | 43 | 18.6 (±8.1) | 41 | 21.8 (±10.4) | (18.2, 22.2) | 0.121 | ||
| LDL | 47 | 88.2 (±29.1) | 47 | 92.5 (±28.3) | (84.5, 96.2) | 0.465 | ||
| A1c | 20 | 5.3 (±0.4) | 50 | 6.0 (±0.9) | (5.5, 5.9) | 0.002 | * | |
| SBP | 88 | 126.9 (±16.3) | 85 | 129.2 (±19.7) | (125.3, 130.8) | 0.399 | ||
| DBP | 88 | 77.0 (±8.4) | 85 | 75.2 (±10.2) | (74.7, 77.5) | 0.189 | ||
| # Medications | 84 | 3.3 (±2.9) | 88 | 4.2 (±3.5) | (3.3, 4.3) | 0.064 | ||
indicates significance at p<0.05
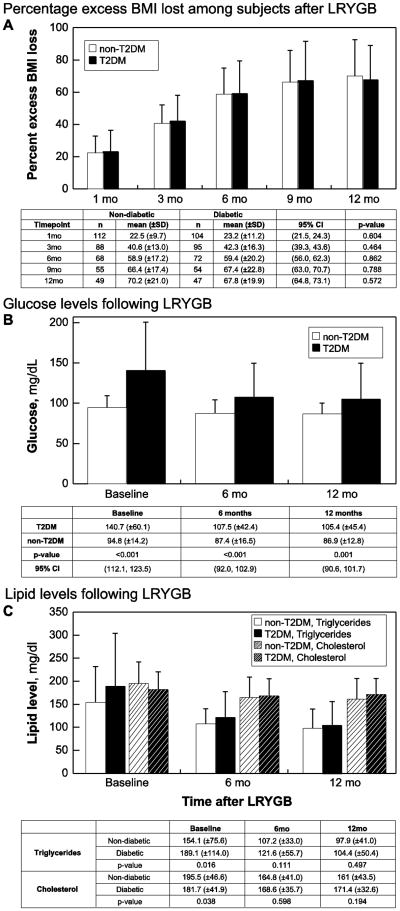
In T2DM patients, mean FBG and HbA1c levels approached normal values over 12 months, but were increased as compared to the non-T2DM group. HbA1c levels reduced from 7.5±1.4 % at baseline to 6.15±1.0% at six months to 5.98±0.8 % at 12 months, suggesting that a majority of the patients experienced biochemical remission for T2DM and that variability between patients decreased. No significant changes in glycemic control were noted in the non-T2DM group over 12 months.
Medication usage showed mixed results in the two groups. The overall number of medications for the non-T2DM group remained fairly constant while those in the T2DM group reduced over time. At baseline, T2DM patients were on an average of 5.1 medications, but by 12 months post-surgery, they were on an average of 4.2 medications. At baseline, T2DM patients were on a significantly higher number of medications compared to non-T2DM patients (p<0.001), but by 12 months post-surgery, there was no difference in the number of medications for T2DM patients (p=0.064). Within the T2DM group, oral hypoglycemic and insulin use decreased over time; at baseline, patients were on an average of 1.07±1.083 oral hypoglycemic agents and 20.5% were on insulin. Twelve months after surgery, patients were on an average of 0.37±0.809 oral hypoglycemic agents and 5.9% were on insulin and of these, three began insulin therapy after bariatric surgery. These results suggest major overall improvements in glycemic control of T2DM patients.
Patients in both groups displayed a marked decrease in % EBMI (Fig. 1a), although weight loss became less pronounced at 6 months post-surgery. Differences in weight loss outcomes at each time point were not significant between T2DM and non-T2DM patients, but by 12 months post-surgery, patients collectively achieved a %EBMI loss of 69.0 ± 20.1.There were no differences in % EMBI lost between insulin-users and non-insulin-users within the T2DM group, depending on severity of diabetes as defined by insulin use. Twelve months after surgery, insulin users averaged a %EBMI loss of 66.3±18.9, while non-insulin-users averaged a %EBMI loss of 67.2 ± 19.6 (p=0.60). This observation suggests that diabetic severity is not a factor in achieving weight loss outcomes similar to non-T2DM patients. Furthermore, no correlation between the HbA1c at 12 months and excess %BMI lost (r = -0.156, P = 0.39) was noted, suggesting other factors beyond weight loss may result in improved glycemic control.
Figure 1.
Comparisons of the T2DM and non-T2DM groups after LYRGB through (A) Percent excess BMI lost as a measure of weight loss, (B) glucose, in mg/dL, and (C) triglyceride (solid) and cholesterol (hashed) levels depicted on the y-axes. Values are listed as mean (±SD).
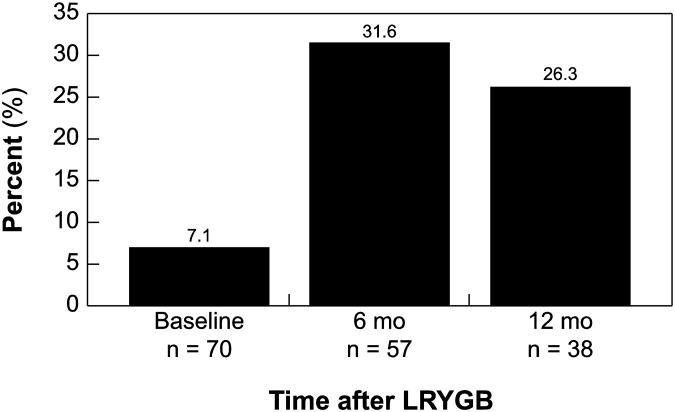
Overall, patients in both groups achieved lower lipid levels after surgery. Post-surgery, cholesterol, triglyceride and FBG levels rapidly declined (Fig. 1b and 1c); at six and twelve months post-LRYGB, lipid values and glucose parameters of T2DM patients approached those of non-T2DM patients (Table 2 and Fig. 1). Patients who did not have diabetes also had lower lipid levels, but the improvement was not as pronounced compared to the T2DM patients. Furthermore, the percent of patients meeting ADA recommended guidelines increased post-LYRGB (Fig. 1d). While only 26.3% of T2DM patients achieved the recommended guidelines compared to 45% of non-T2DM patients 12 months after surgery, T2DM patients had more severe hyperlipidemia, hypertension and hyperglycemia. Overall, these results suggest the benefits of increased insulin sensitivity and improvements in co-morbidities regardless of diabetes status, although a greater benefit was conferred upon the T2DM group.
Discussion
Bariatric surgery has become an increasingly popular treatment option for severely obese patients, often improving or extinguishing many related co-morbidities such as diabetes. In Buchwald's meta-analysis, 83.7% of T2DM patients who underwent gastric bypass went into remission[10]. Previous literature suggests that after patients undergo LRYGB, non-T2DM appear to achieve significantly better weight loss outcomes than T2DM [18]. However, research has shown that any amount of weight loss improves glycemic and metabolic status [5], so the potential for less weight loss should not discourage bariatric surgery as an effective intervention for T2DM. Weight regain is also thought to increase the risk for a relapse in glycemic control and diabetes status[19]. In one study [20], non and late-remitters of diabetes following LRYGB experienced less weight loss than non-diabetic obese individuals and had reduced baseline pancreatic ß cell function suggesting that diabetes severity is linked to less weight loss. Several of these studies did not control for factors that have been shown to be associated with weight loss, such as age [21], gender [12, 14], ethnicity [16] and baseline BMI. Thus, this retrospective cohort study sought to improve upon these studies by matching for those factors as well as race and pre-surgical BMI between the two groups.
We did not detect changes in weight loss outcomes in our study based on diabetes status. These results parallel those of a prospective observational study with matched controls, which found that diabetes remission is tied to the control and duration of diabetes [12]. However, our results are in contrast to another study, which found that a smaller percentage of T2DM patients achieved ≥50% excess weight loss compared to non-T2DM patients three years post-surgery [13]. However, our data only spans one year after LRYGB and our results parallel those of Junior et al. at the one year mark [13]. Further studies are warranted to understand weight loss and weight regain effects over a longer period of time after gastric bypass surgery.
Severity of diabetes also did not yield differences in weight loss outcomes. In our study, we grouped patients based on medication status: diet and exercise only, oral hypoglycemics, and insulin. Some studies have shown that insulin use is a negative predictor of diabetes remission [11, 18], suggesting that insulin use is a marker of diabetes severity. In another study, Carbonell et al. found an inverse relationship between severity of diabetes and percent excess weight loss [14]. However, patients in the study were considered to have T2DM if their FBG ≥ 150 mg/dl, which is a less stringent requirement for diagnosis compared to our study. Thus, this observation provides a unique opportunity to further study the effects of pre-surgical diabetes medication type against weight loss outcomes.
Additionally, T2DM patients achieved a marked metabolic benefit through alteration of lipid levels to a more favorable profile and by decreases in blood pressures equalizing values of non-T2DM patients, indicating that LRYGB is effective at decreasing the severity of hyperlipidemia and hypertension for T2DM patients. However, the reductions in lipid levels for non-T2DM patients over time were smaller compared to T2DM patients; we attribute this to having an initial lower level of lipids pre-surgery, making it more difficult to achieve comparable reductions against T2DM patients. While LDL levels were significantly lower in the T2DM group, we hypothesize that patients in that group were more aggressively treated with medications than non-T2DM patients prior to surgery, which may have undermined our results. Overall, our data confirm those of other studies [10, 22], which have shown vast improvements in lipid and blood pressure statuses.
Unlike a similar previous study which included only T2DM patients who used insulin and/or hypoglycemic agents [12], we included T2DM patients who were diet and exercise controlled, allowing for inclusion of an important subgroup within T2DM patients. We also stratified patients on their diabetic status based on use of insulin though we did not find differences in weight loss outcomes at any point between these groups.
Our study was limited by the loss of patients to follow-up, creating a selection bias, likely for patients who are most concerned for their health. Individuals who are adherent with follow-up appointments may also be more adherent with the post-operative recommendations resulting in better weight loss and metabolic outcomes. We also used ICD-9 codes as one method of verifying diabetic status among the patients, which raises questions of reliability. However, patients without an ICD-9 code for T2DM but were actually diabetic likely also displayed more objective signs, such as use of hypoglycemic agents or an elevated FBG or HbA1c.
With the incidence of diabetes and obesity increasing worldwide, bariatric surgery and especially LRYGB has surged in popularity as a treatment option. The results of our study show that %EBMI loss is equivalent regardless of diabetic status, while metabolic syndrome parameters improve to equalize T2DM patients to non-T2DM patients. This suggests that metabolic surgery is a viable treatment option and should be encouraged for severely obese diabetic patients. Additional studies in the future are needed to address long-term weight loss management after bariatric surgery to allow patients to remain in euglycemia and remission of diabetes.
Figure 2.
Percentage of T2DM patients meeting ADA guidelines, defined to be LDL cholesterol < 100, blood pressure 130/80, and HbA1c < 7.0%.
Acknowledgments
Funding: This work was supported by a grant from the Ray A. and Robert L. Kroc Summer Research Fellowship (KY). SRK receives grant support by American Diabetes Association and both SRK and PRS are supported by National Institutes of Health RO1 DK089547-01 NIDDK/NIH.
Abbreviations
- ANOVA
Analysis of Variance
- BP
Blood pressure
- BMI
body mass index
- %EBMIL
percent excess BMI loss
- FBG
fasting blood glucose
- HbA1c
hemoglobin A1c
- HDL
high density lipoprotein
- ICD-9
International Classification of Diseases ninth revision
- LRYGB
laparoscopic Roux-en-Y gastric bypass
- LDL
low density lipoprotein
- T2DM
Type 2 Diabetes Mellitus
- VLDL
very low density lipoprotein
Footnotes
Competing Interests: SRK and PRS disclose grant support from Ethicon Endo-surgery, Inc. PRS is also on the scientific advisory board of SurgiQuest, Barosense, and Surgical Excellence; acts as consultant for C.R. Bard, Ethicon Endo-surgery, Inc., Baxter Healthcare, Stryker, Cardinal Health, and W.L. Gore & Associates, Inc.; and acts in a fiduciary capacity to the MISS Surgery Symposium, Physician Review of Surgery and RemedyMD. LJH discloses grant support from Nutrisystems, Inc. The other authors (KY, VG) have nothing to disclose.
References
- 1.U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249–58. [PubMed] [Google Scholar]
- 2.Cook MN, Girman CJ, Stein PP, et al. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care. 2005;28:995–1000. doi: 10.2337/diacare.28.5.995. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 4.Koro CE, Bowlin SJ, Bourgeois N, et al. Glycemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27:17–20. doi: 10.2337/diacare.27.1.17. [DOI] [PubMed] [Google Scholar]
- 5.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nannipieri M, Mari A, Anselmino M, et al. The Role of {beta}-Cell Function and Insulin Sensitivity in the Remission of Type 2 Diabetes after Gastric Bypass Surgery. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2011-0446. In press. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 8.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–9. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–50. doi: 10.1097/00000658-199509000-00011. discussion 50-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 11.DiGiorgi M, Rosen DJ, Choi JJ, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis. 2010;6:249–53. doi: 10.1016/j.soard.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Hall TC, Pellen MG, Sedman PC, et al. Preoperative factors predicting remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery for obesity. Obes Surg. 2010;20:1245–50. doi: 10.1007/s11695-010-0198-8. [DOI] [PubMed] [Google Scholar]
- 13.Junior WS, do Amaral JL, Nonino-Borges CB. Factors Related to Weight Loss up to 4 Years after Bariatric Surgery. Obes Surg. 2011 doi: 10.1007/s11695-011-0420-3. In press. [DOI] [PubMed] [Google Scholar]
- 14.Carbonell AM, Wolfe LG, Meador JG, et al. Does diabetes affect weight loss after gastric bypass? Surg Obes Relat Dis. 2008;4:441–4. doi: 10.1016/j.soard.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Melton GB, Steele KE, Schweitzer MA, et al. Suboptimal weight loss after gastric bypass surgery: correlation of demographics, comorbidities, and insurance status with outcomes. J Gastrointest Surg. 2008;12:250–5. doi: 10.1007/s11605-007-0427-1. [DOI] [PubMed] [Google Scholar]
- 16.Anderson WA, Greene GW, Forse RA, et al. Weight loss and health outcomes in African Americans and whites after gastric bypass surgery. Obesity (Silver Spring) 2007;15:1455–63. doi: 10.1038/oby.2007.174. [DOI] [PubMed] [Google Scholar]
- 17.Ortega E, Morinigo R, Flores L, et al. Predictive factors of excess body weight loss 1 year after laparoscopic bariatric surgery. Surg Endosc. doi: 10.1007/s00464-011-2104-4. [DOI] [PubMed] [Google Scholar]
- 18.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chikunguwo SM, Wolfe LG, Dodson P, et al. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 6:254–9. doi: 10.1016/j.soard.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Nannipieri M, Mari A, Anselmino M, et al. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab. 96:E1372–9. doi: 10.1210/jc.2011-0446. [DOI] [PubMed] [Google Scholar]
- 21.Camastra S, Gastaldelli A, Mari A, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54:2093–102. doi: 10.1007/s00125-011-2193-6. [DOI] [PubMed] [Google Scholar]
- 22.White S, Brooks E, Jurikova L, et al. Long-term outcomes after gastric bypass. Obes Surg. 2005;15:155–63. doi: 10.1381/0960892053268282. [DOI] [PubMed] [Google Scholar]