Abstract
Background
Mast cells play a central role in allergic and inflammatory diseases. Several reports indicated role of peroxisome proliferator-activated receptor gamma (PPARγ) on mast cell function. However, there is no report about the role of PPARγ on differentiation of mast cells from the progenitors. In this study, we investigated the role of PPARγ in regulating bone marrow-derived mast cell maturation and the therapeutic implications for mast cell-related diseases such as atopic or contact dermatitis.
Methods
We used in vitro cell culture system for mast cell differentiation from bone marrow-progenitors using specific ligands and lentiviral-mediated short hairpin RNA of PPARγ, and in vivo murine dermatitis models.
Results
Activation of PPARγ inhibited the maturation of bone marrow progenitors into connective tissue-type mast cells (CTMCs) through up-regulation of GATA-4 and GATA-6 resulting in a decrease in expression of histidine decarboxylase and mast cell histamine content. In comparison, the differentiation of bone marrow progenitors into CTMCs was significantly accelerated by the knockdown of PPARγ expression by lentiviral-mediated short hairpin RNA. Peroxisome proliferator-activated receptor gamma ligand administration to mice inhibited the maturation of mast cells resulting in attenuation of atopic and contact dermatitis via diminishment of the number of mature mast cells.
Conclusion
Our results indicate that PPARγ is one of master regulators on mast cell maturation and potentially useful for the therapy in various disorders involving mast cell activation.
Keywords: allergic disease, differentiation, dermatitis, mast cell, peroxisome proliferator-activated receptor gamma
Mast cells are considered to play an important role in many allergic diseases and inflammatory responses (1–5). In mammals, there are two distinct mast cell subpopulations, connective tissue-type (CTMCs) and mucosal-type mast cells (MMCs) (1–5). These cell types differ in location, staining characteristics, protease expression pattern, and histamine content. For example, CTMCs, which are considered to be mature mast cells, express the mouse mast cell protease (MMCP)-4, 5, 6 and 7, and contain a histamine content that is at least ten-times higher than that of MMCs. However, it remains unclear what factors or mechanisms are involved in directing the differentiation of immature mast cells into either CTMCs or MMCs.
Peroxisome proliferator-activated receptor gamma (PPARγ) is a nuclear receptor that was shown to play a critical role in adipocyte differentiation and insulin sensitivity (6, 7). Peroxisome proliferator-activated receptor gamma expression in adipose tissue is extremely higher than other organs such as kidney, stomach, heart, liver and brain. Recently, various inflammatory cells, such as lymphocytes, neutrophils and macrophages, have been shown to be potential targets for the PPARγ-mediated inhibitory functions, because of the expression of PPARγ in such cell types (7). However, other potential target cell types that may be important to achieving inhibition of inflammatory and allergic diseases are mast cells.
Recently, PPARγ expression has been described in mouse bone marrow-derived mast cells (BMMCs), although the function in this cell type is still unclear (8). In this regard, we have recently reported that PPARγ expressed in neural stem cell dramatically suppresses their differentiation into maturate neurons (9). This suggests the possibility that bone-marrow progenitors might be targets of PPARγ-mediated regulation of differentiation into mature mast cells.
In this study, we investigated the role of PPARγ in regulating BMMC maturation and the therapeutic implications of this for mast cell-related diseases such as atopic or contact dermatitis.
Methods
Reagents
The PPARγ-specific ligands, rosiglitazone and pioglitazone were kind gifts from Glaxo SmithKline (Tokyo, Japan) and Takeda Pharmaceutics (Osaka, Japan), respectively.
Preparation and culture of bone marrow-derived mast cells
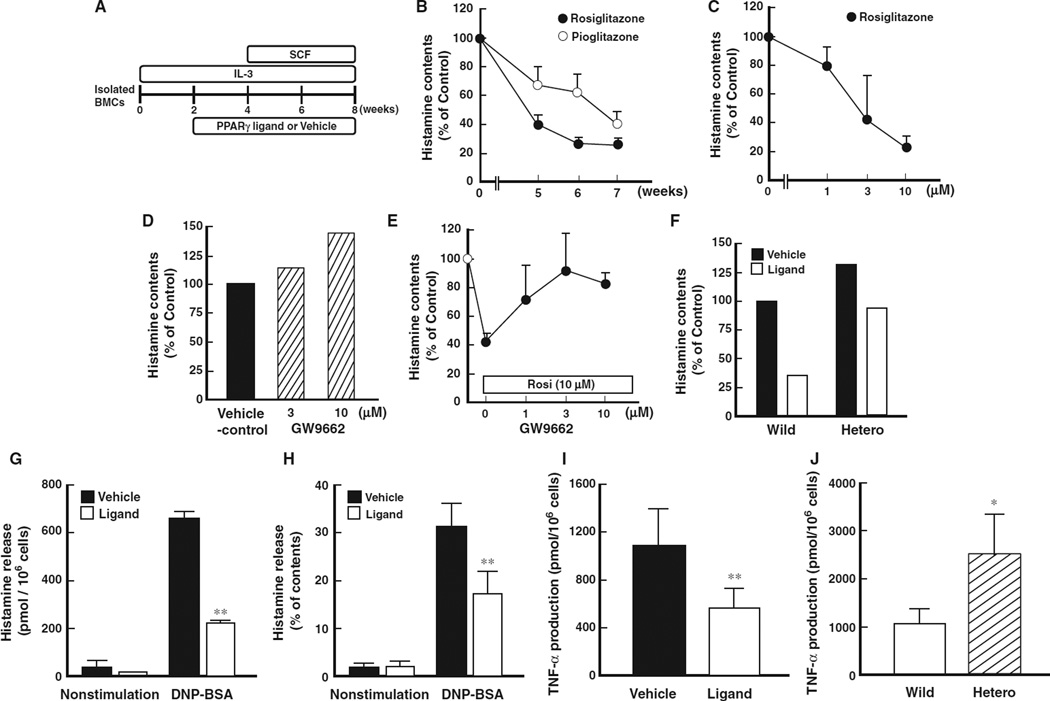
All mice were treated humanely according to the National Institutes of Health and AERI-BBRI Animal Care and Use Committee guidelines. All animal experiments were approved by the institutional animal care and use committee of Osaka University and Yokohama City University School of Medicine. Bone marrow-derived mast cells were collected from the femur and tibia and differentiated according to the methods previously (1–5). After 4 weeks, stem cell factor (SCF, 50 ng/ml) was added in culture medium. Peroxisome proliferator-activated receptor gamma-specific ligands, rosiglitazone and pioglitazone (0.3–10 µM), were administered to the culture medium at 2 weeks after the start of culture (Fig. 3A).
Figure 3.
Effect of PPARγ ligands on histamine contents and release of bone marrow-derived mast cells. (A) Schematic protocol of bone marrow-derived progenitor cell culture. (B) Time-course alterations of histamine content of bone marrow-derived mast cells (BMMCs) treated with PPARγ ligand, rosiglitazone or pioglitazone. Data represent mean ± SEM from five to seven independent experiments. (C) Dose-dependent alteration of histamine content of BMMCs treated with PPARγ ligand, rosiglitazone. Data represent mean ± SEM from three to five independent experiments. (D) Effect of PPARγ-specific antagonist, GW 9662, on histamine contents. Data represent mean from three independent experiments. (E) Recovery of decreased histamine content in BMMCs by the treatment with PPARγ-specific antagonist in the presence of PPARγ agonist (rosiglitazone, 10 µM, Rosi). Data represent mean ± SEM from three to five independent experiments. (F) Effect of PPARγ on the decrease in histamine contents using bone marrow-progenitors prepared from heterozygous PPARγ-deficient mice. Open column represents the histamine content in the presence of PPARγ agonist (rosiglitazone, 10 µM). Data represent mean from 3 independent experiments. (G) Effect of PPARγ ligand (rosiglitazone, 10 µM) on DNP-BSA antigen-stimulated histamine release from BMMCs. Data represent mean ± SEM from five to seven independent experiments. **P < 0.01 vs vehicle control. (H) Effect of PPARγ ligand (rosiglitazone, 10 µM) on DNP-BSA antigen-stimulated histamine release from BMMCs. Percentage of histamine release was calculated as ‘net histamine release/total contents of histamine × 100’. Data represent mean ± SEM from five to seven independent experiments. **P < 0.01 vs vehicle control. (I) Effect of PPARγ ligand (rosiglitazone, 10 µM) on DNP-BSA antigen-stimulated TNF-α production from BMMCs. Data represent mean ± SEM from four to seven independent experiments. **P < 0.01 vs vehicle control. (J) Effect of PPARγ on TNF-α production using bone marrow-progenitors prepared from heterozygous PPARγ-deficient mice. Data represent mean from three independent experiments. *P < 0.05 vs wild type.
Histamine release assay
BMMCs were sensitized with 0.2 µg/ml anti-DNP IgE for 24 h and washed. The IgE-sensitized cells were stimulated with 20 ng/ml of DNP-BSA in PIPES buffer at 37°C for 30 min, and supernatant and pellet were collected. Histamine contents both in the supernatant and pellets were measured by HPLC and content in supernatant expressed as ‘histamine release’ (10).
RNA isolation and RT-PCR analysis
Total RNA was extracted from the BMMCs, and first-strand cDNA was generated from 1 µg of RNA by using oligo (dT)12–18 primer and SuperScriptIII RNase H Reverse Transcriptase (Invitrogen) according to the manufacturer’s protocol. The reaction mixture was amplified with the oligonucleotides specific for HDC, MMCP-1, MMCP-2, MMCP-4 and β-actin (11–13).
Staining of BMMCs
BMMCs were fixed in Carnoy’s solution. To investigate the maturation of mast cells, granules in BMMCs were stained with toluidine blue. To distinguish MMC-like immature mast cells and CTMCs, alcian blue-safranin staining was performed. CTMCs were stained with a red and MMC-like immature mast cells were stained with a light blue color (1–5).
Lentiviral vector mediated knockdown of PPARγ
Lentiviral vector (LV) based PPARγ-knockdown system was previously described (9, 14). BMMCs were infected with LV-shRNA-P15, which expresses an effective short hairpin RNA (shRNA) effective against murine PPARγ, or LV-shRNA-Scramble, which expresses control shRNA, at different multiplicities of infection (MOI; 12.5 and 25) at 4 weeks and subcultured until analysis. Transduction efficiencies, which can be detected by enhanced green fluorescent protein (EGFP) expression, were about 40% at 12.5 and about 60% at 25 MOI.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays were performed using ChIP Assay Kit (Upstate, New York, USA) according to the manufacturer’s protocol.
The transcriptional regulatory regions of target genes were assumed based on DBTSS (Database of Transcriptional Start Sites, http://dbtss.hgc.jp/index.html). The primers were used for PCR to correspond to regions flanking the PPRE within the HDC, GATA-4 and GATA-6 gene promoters are as follows: Sense: 5′TCCTCTGTCTGTCTGCCAGG3′; antisense: 5′ACCACTGAAGCCAAACCGGC3′ (for HDC), sense: 5′CGGAGTCCAGAGATTTCCAG3′; antisense: 5′AAGTGTGCGGTCACACGGTG3′ (for GATA-4), sense: 5′AGGGGCGAGGTAGGGAATAC3′; antisense: 5′TCCCAGCCCCTCCTTCCAAA3′ (for GATA-6). Densitometric quantification for PCR products of anti-PPARγ immunoprecipitants relative to the input was calculated.
Murine dermatitis models
NC/Nga mice (3 weeks male, CLEA Japan, Tokyo, Japan) were purchased and housed under conventional conditions. The murine-atopic dermatitis model was established according to a method previously (15). Spontaneous dermatitis similar to human atopic dermatitis was observed for 4–5 weeks. The murine-contact dermatitis model was established according to a method previously (16). NC/Nga mice were sensitized with picryl chloride on ventral skin. On day 4, the mice were challenged with 0.03 ml of 1% picryl chloride in acetone : olive oil (1 : 4). Ear thickness was measured by a dial thickness gauge.
Statistical analysis
All results are expressed as mean ± SEM. Statistical comparisons were made using Student t-test or Scheffe’s method after analysis of variances (anova). The results were considered significantly different at P < 0.05.
Results
PPARγ ligands attenuate atopic and contact dermatitis in mice
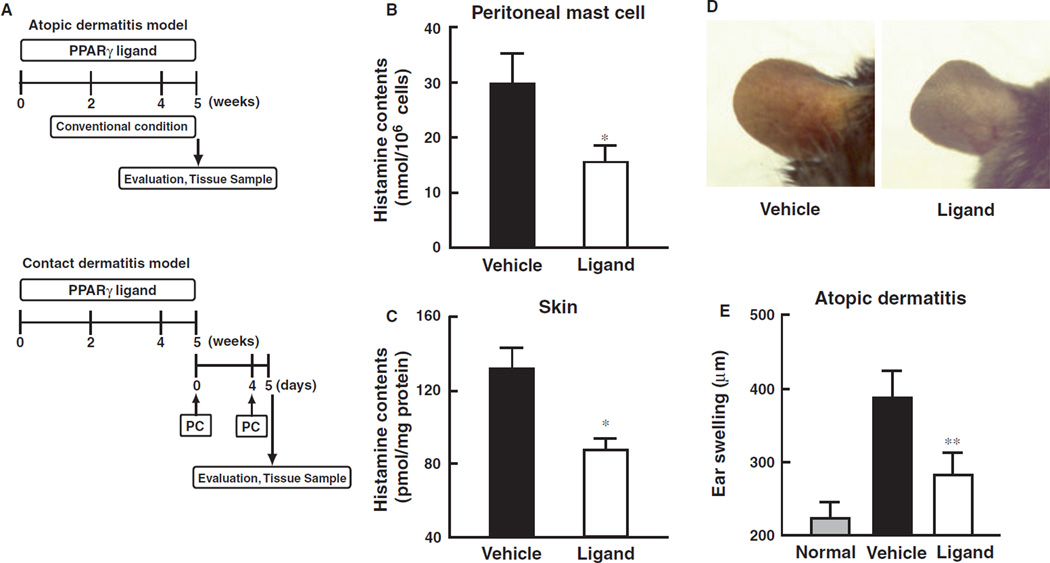
In pathological skin conditions, histamine is involved in the induction of itching, flaring and edema, and therefore, the contribution of activated mast cells are important (15, 16). However, regulatory systems of mast cell maturation in allergic dermatitis are still unclear. We, therefore, investigated the effects of PPARγ ligands on the development of atopic and contact dermatitis (Fig. 1A). Administration of PPARγ ligand in the diet significantly decreased the histamine content of both peritoneal mast cells and skin tissues (Fig. 1B,C). In NC/Nga mice, edema (especially ear swelling) associated with atopic dermatitis was observed. The atopic dermatitis spontaneously induced in NC/Nga mice was significantly inhibited by treatment with a PPARγ ligand (Fig. 1D,E). These results clearly indicate that activation of PPARγ pathways by ligand administration decreases histamine levels in association with attenuation of atopic dermatitis.
Figure 1.
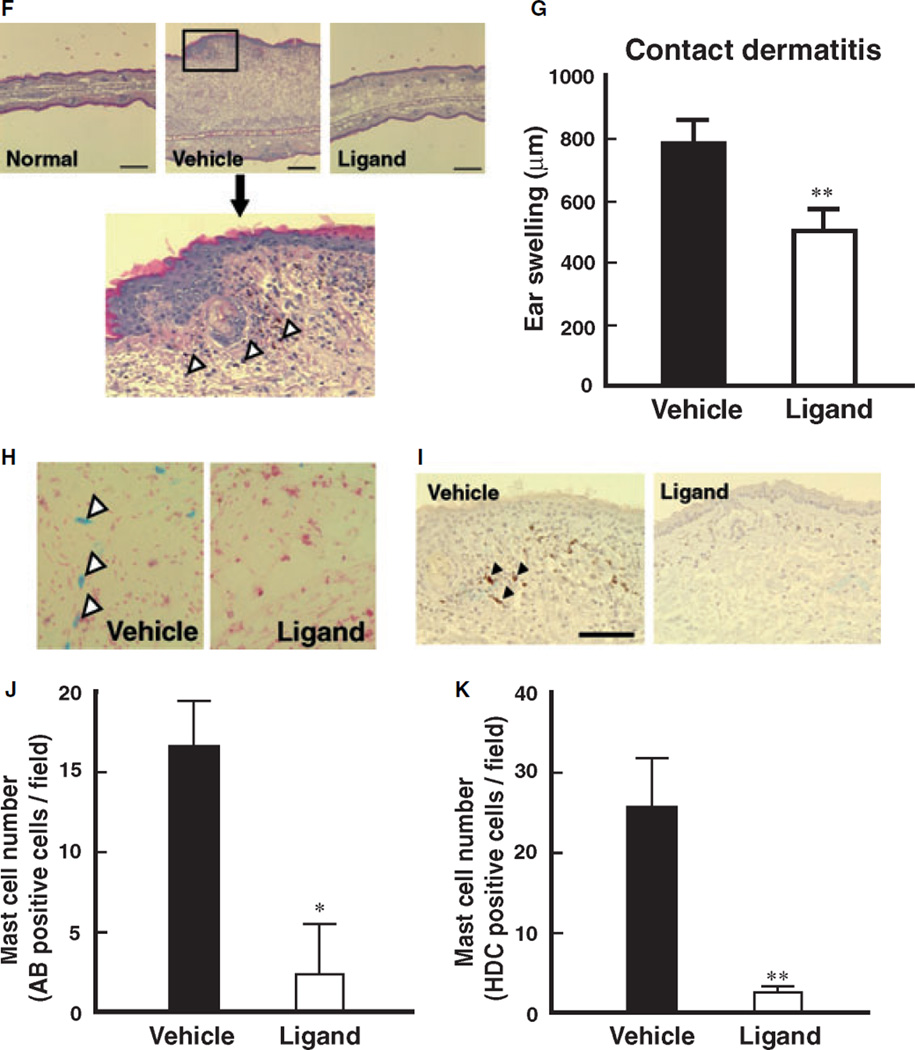
Attenuation of PPARγ ligand on atopic and contact dermatitis models in mice. (A) Schematic protocol of murine-atopic (upper panel) and contact dermatitis (lower panel) models. Dietary administration of PPARγ ligand, pioglitazone (approximately 25.60–28.08 mg/kg), was performed according to our previous study. In the murine-atopic dermatitis model, NC/Nga mice were housed under conventional conditions for 4 weeks. Spontaneous dermatitis similar to human atopic dermatitis was observed. In the murine-contact dermatitis model, NC/Nga mice were sensitized with picryl chloride (PC) on the ventral skin at day 0. On the day 4, the mice were challenged with picryl chloride (PC) on ear. Ear thickness was measured by a dial thickness gauge. (B and C) Histamine content of skin (B) and peritoneal mast cells (C) prepared from mice treated with PPARγ ligand (pioglitazone) or vehicle. Data represent mean ± SEM from five to seven independent animals. *P < 0.05 vs vehicle control. (D) Macroscopic observation atopic dermatitis of ear from vehicle-control or ligand. (E) Effect of PPARγ ligand, pioglitazone, on atopic dermatitis model. Ear thickness, as an index of edema, was expressed as ear swelling (µm). Normal group was before initiation of dermatitis. Data represent mean ± SEM from five to seven independent animals. **P < 0.01 vs vehicle control. (F) Typical microscopic pictures of ears from mice of normal, vehicle, and PPARγ ligand-treated group on antigen-induced contact dermatitis model. Hematoxylin-eosin stain was performed. Scale bar represents 200 µm. Lower panel represents large magnification of ear from vehicle group. Arrowheads represent infiltrated inflammatory cells. (G) Effect of PPARγ ligand, pioglitazone, on dermatitis model. Ear thickness, as an index of edema, was expressed as ear swelling (µm). Data represent mean ± SEM from five to seven independent animals. **P < 0.01 vs vehicle control. (H) Microscopic observations of the increase in mature mast cells in tissues from vehicle-control or PPARγ ligand treated mice on contact dermatitis model. Representative photomicrographs of mouse ear sections were stained with alcian blue-nuclear fast red from vehicle-control or PPARγ ligand treated mice. In this condition, mature mast cells are stained as a blue color indicated by arrowheads. (I) Immunohistochemical staining of HDC in ear tissues of mice from vehicle, and PPARγ ligand-treated groups on contact dermatitis model. HDC expressing cells are shown by a brown color (arrow heads). Scale bar represents 50 µm. (J) Alteration of alcian blue-positive cell number in ear tissues of mice from vehicle, and PPARγ ligand-treated groups on contact dermatitis model. Alcian blue-nuclear fast red positive cell number was counted and expressed as matured mast cell number per field. Each column represents mean ± SEM from five to seven independent animals. *P < 0.05 vs vehicle control. (K) Alteration of HDC positive-cell number in ear tissues of mice from vehicle, and PPARγ ligand-treated groups on contact dermatitis model. HDC positive cell number was counted and expressed as matured mast cell number per field. Each column represents mean ± SEM from seven to seven independent animals. **P < 0.01 vs vehicle control.
To further clarify the contribution of PPARγ-mediated inhibition of allergic dermatitis, we investigated the effect of PPARγ ligand on contact dermatitis in mice. Application of picryl chloride to NC/Nga mice induced significant edema in association with contact dermatitis in vehicle-treated control mice in comparison to that observed in non-treated normal mice (Fig. 1F,G). Accumulation of inflammatory cells was also observed in the skin (Fig. 1F, large magnification). Antigen-induced contact dermatitis was dramatically attenuated by treatment with a PPARγ ligand. Mature mast cells that are represented by alcian blue-positive and HDC-positive cells were markedly decreased by treatment with a PPARγ ligand in the skin of mice induced to exhibit contact dermatitis in comparison to the vehicle treated control (Fig. 1H,I). The elevated numbers of mature mast cells in the dermatitic tissues were significantly decreased by treatment with a PPARγ ligand (Fig. 1J,K). Treatment with the PPARγ ligand to mice without the onset of dermatitis also diminished the HDC-positive cell number in comparison to the vehicle-treated control (1.2 ± 0.6 vs 8.3 ± 2.6 cells/field). These results indicate that activation of PPARγ by ligand treatment diminishes the number of mature mast cells in the skin in association with attenuation of allergic dermatitis.
Administration of PPARγ ligand to attenuates histamine release and inflammatory mediator production by peritoneal mast cells
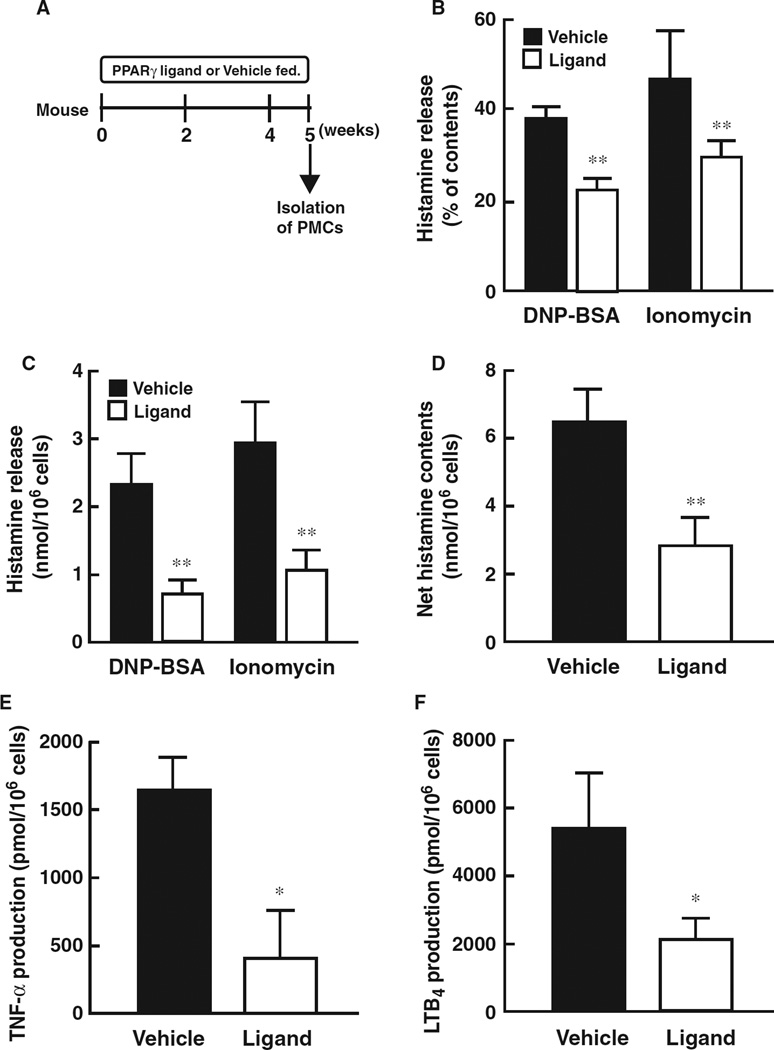
Peritoneal mast cells (PMCs) were isolated from PPARγ-ligand administered mice, and histamine release and inflammatory mediator production was measured (Fig. 2A). Antigen-stimulated and ionomycin-induced histamine release was significantly decreased in PMCs isolated from PPARγ-ligand treated mice compared to that observed from vehicle treated mice (Fig. 2B,C). Net histamine content in PMCs isolated from PPARγ-ligand treated mice was significantly lower than that detected from vehicle treated mice (Fig. 2D). In addition, antigen-stimulated inflammatory mediator production, such as TNF-α and LTB4, in PMCs isolated from PPARγ-ligand treated mice was diminished compared to that detected in vehicle treated mice (Fig. 2E,F). These results show that PPARγ ligands effectively suppress the histamine release and inflammatory mediator production by PMCs in vivo.
Figure 2.
Administration of PPARγ ligand to whole body attenuates histamine release and inflammatory mediator productions from peritoneal mast cells. (A) Schematic protocol of dietary administration of PPARγ ligand, pioglitazone (approximately 25.60– 28.08 mg/kg). Five weeks after the administration of PPARγ ligand, peritoneal mast cells (PMCs) were isolated and used for experiments. (B and C) Effect of PPARγ ligand administration on antigen-stimulated (DNP-BSA) or ionomycin-induced histamine release from isolated PMCs. Data represent mean ± SEM from four to seven independent experiments. **P < 0.01 vs vehicle control. (D) Net histamine content of PMCs isolated from PPARγ-ligand or vehicle treated mice. Data represent mean ± SEM from four to seven independent experiments. **P < 0.01 vs vehicle control. (E and F) Antigen-stimulated inflammatory mediator production, such as TNF-α (E) and LTB4 (F), on PMCs isolated from PPARγ-ligand treated mice. Data represent mean ± SEM from four to seven independent experiments. *P < 0.01 vs vehicle control.
PPARγ ligands decrease histamine content and release of BMMCs
To clarify the details of the role of PPARγ pathway on mast cell maturation, we used in vitro culture system of BMMCs. Bone marrow-derived progenitor cells were isolated and cultured with PPARγ ligands in the presence of IL-3 and SCF to differentiate into mast cells (Fig. 3A). Treatment with PPARγ ligands significantly diminished histamine content of BMMCs in a time- and concentration-dependent fashion (Fig. 3B,C). No significant difference in total cell numbers between the vehicle- and PPARγ ligand-treated groups was observed at the range of the concentrations (data not shown).
Treatment with a PPARγ-specific antagonist, GW9662, resulted in an increase in histamine content compared to that of vehicle control (Fig. 3D). The decrease in mast cell histamine content induced by PPARγ ligand treatment was also reversed by addition of GW9662 (Fig. 3E) and T90067 (data not shown). The histamine content of BMMCs from heterozygous PPARγ-deficient mice (14, 17) was higher than that observed in wild-type mice. More importantly, the histamine content decreased after treatment of BMMCs with a PPARγ ligand (Fig. 3F). This decrease was significantly less in heterozygous PPARγ-deficient mice in comparison to that observed from wild-type mice (Fig. 3F). These results provide definitive evidence that PPARγ regulates the histamine content of mast cell progenitors. The decrease in histamine content by PPARγ ligands was also associated with a significant decrease in the quantity of antigen-stimulated histamine release (Fig. 3G). Similar results were also observed when cells were treated with other histamine-releasing agents such as compound 48/80 and ionomycin (data not shown). In addition, the ability of histamine release (percentage of histamine release relative to total histamine content) was significantly decreased by treatment with a PPARγ ligand (Fig. 3H). To investigate whether the decrease in histamine content induced by treatment with PPARγ ligands is also associated with suppression of inflammatory mediator production, we determined antigen-stimulated TNF-α production from BMMCs cultured with PPARγ ligand. Antigen-stimulated TNF-α production from BMMCs cultured with PPARγ ligand was significantly decreased compared to that of vehicle treated controls (Fig. 3I). In comparison, TNF-α production from heterozygous PPARγ-deficient mouse BMMCs was greater than that observed from wild type mice (Fig. 3J). These results indicate that activation of PPARγaffects not only histamine content, but also the inflammatory mediator production of BMMCs.
PPARγ ligands inhibit maturation of BMMCs
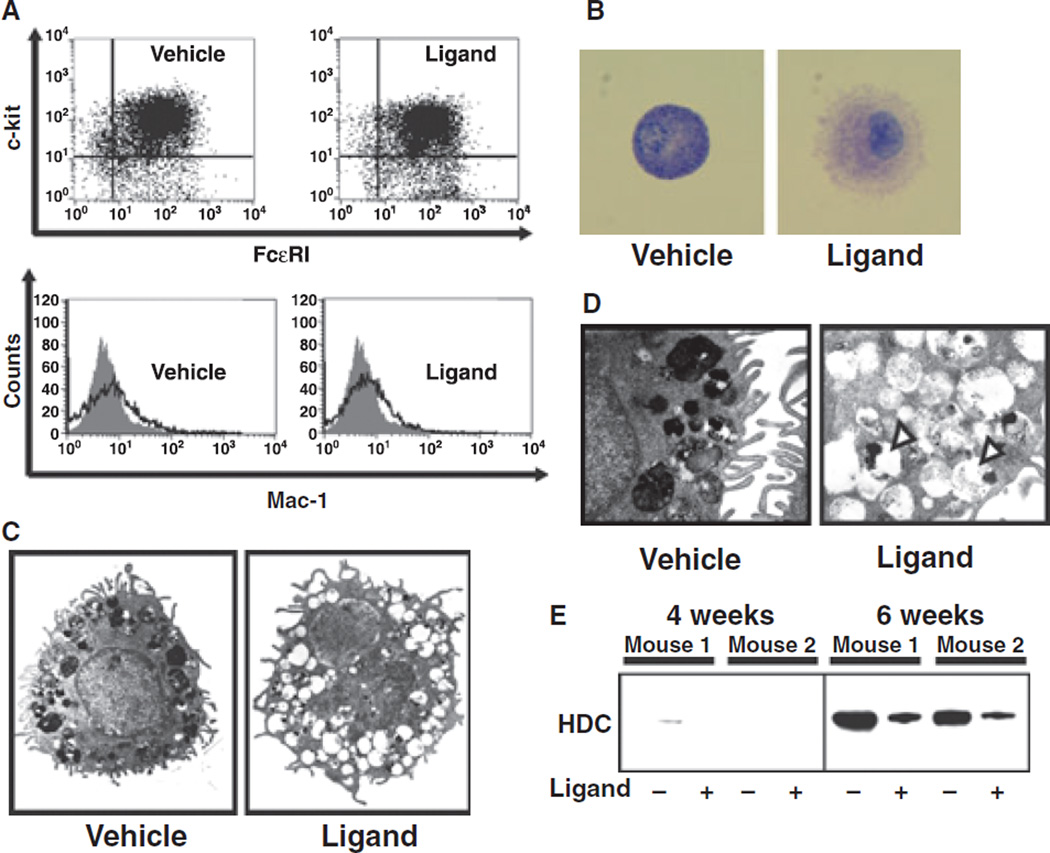
To show that PPARγ ligand treated cells do not differentiate into other cell types such as macrophages, T cells or B cells as an explanation for the decrease in histamine content observed, we analyzed cell surface markers of PPARγ ligand-treated cells by flow cytometry. Both the vehicle- and ligand-treated groups similarly expressed c-kit and FcεRI, cell surface markers of typical mast cells (Fig. 4A, upper panel). No expression of Mac-1, a marker of macrophages, was observed either in vehicle- or ligand-treated groups (Fig. 4A, lower panel). We also confirmed that other cell surface markers such as CD3 (T-cell marker), B220 (B-cell marker), F4/80 (activated macrophage marker) and NK pan marker were the same in both vehicle- and ligand-treated groups (data not shown). These results indicate that treatment of BMMCs with the reagents resulted in mast cell differentiation (purity of 91.4 + 5.2%) and that ligation with PPARγ did not induce differentiation into other cell types. Taken together, the decrease in mast cell histamine content observed after PPARγ ligand treatment was independent of the pattern of cell surface molecule expression associated with mast cell differentiation and not likely due to direction into a non-mast cell fate.
Figure 4.
Effect of PPARγ ligands on maturation of bone marrow-derived progenitor cells. (A) Upper panel: Flow cytometric confirmation of mast cell. Expression of two specific mast cell markers, c-kit and FcεRI, was investigated. Both markers expressed cells (90%) are defined mast cells. Lower panel: Flow cytometric analysis of Mac-1, a macrophage marker, expression. Black line represents with Mac-1 antibody and grey picture represents without antibody, respectively. (B) Typical microscopic picture of toluidine blue staining of BMMCs treated with PPARγ ligand (rosiglitazone, 10 µM) or vehicle. Mature granules in BMMCs were stained blue with the toluidine blue staining. (C) Electron microscopic pictures of BMMCs treated with PPARγ ligand (rosiglitazone, 10 µM) or vehicle. (D) High magnification of electron microscopic pictures of BMMCs treated with PPARγ ligand (rosiglitazone, 10 µM) or vehicle. White arrowhead represents empty granule. (E) Alteration of protein levels of HDC in BMMCs treated with ligand (+, rosiglitazone, 10 µM) or vehicle (−) for 4 or 6 weeks: Typical photo of the data of western blot analysis. Same mouse number indicates same individual mouse, respectively. (F) Arbitrary units of the alteration of protein levels of HDC in BMMCs treated with ligand (rosiglitazone 10 µM, open column) or vehicle (closed column) for 6 weeks. Arbitrary units were calculated using the expression of GAPDH as an internal control. Each column represents mean ± SEM from five independent experiments. **P < 0.01 vs vehicle control. (G) Alteration in mRNA levels of HDC, MMCP-1, MMCP-2 and MMCP-4 of BMMCs treated with ligand (rosiglitazone, 10 µM) or vehicle. β-actin was used as internal control. (H) Arbitrary units of alteration in mRNA levels of HDC, MMCP-1, MMCP- 2 and MMCP-4 of BMMCs treated with ligand (rosiglitazone 10 µM, open column) or vehicle (closed column). Arbitrary units were calculated using the expression of β-actin as an internal control. Each column represents mean ± SEM from three independent experiments. **P < 0.01 vs vehicle control for each molecule. (I) Typical microscopic pictures of alcian blue-safranin staining of BMMCs treated with PPARγ ligand (rosiglitazone, 10 µM) or vehicle. Connective tissue type mast cells were stained a red color and mucosal type mast cells were stained a light or dark blue color. Lower panels represent large magnified pictures. (J) Percentage of safranin-positive (connective tissue type) mast cells treated with PPARγ ligand (rosiglitazone 10 µM, open circles) or vehicle (closed circles). Number of safranin positive cells was calculated per total number of cells and expressed as percentage. Each point represents mean ± SEM from three to five independent experiments. (K) Alteration of protein levels of GATA-3, 4, 5, and 6 on BMMCs treated with ligand (+; rosiglitazone, 10 µM) or vehicle (−) for 4 or 6 weeks. (L) Arbitrary units of alteration in protein levels of GATA-3, GATA-5, GATA-4 and GATA-6 of BMMCs treated with ligand (rosiglitazone 10 µM, open column) or vehicle (closed column) for 6 weeks. Arbitrary units were calculated using the expression of GAPDH as an internal control. Each column represents mean ± SEM from four independent experiments. **P < 0.01 vs vehicle control for each molecule. (M) Typical photos of Chromatin immunoprecipitation (ChIP) assay. Cells were treated with PPARγ ligand (rosiglitazone, 10 µM) or vehicle for 24 h, then harvested. The chromatin fractions were immunoprecipitated with 10 µg of the anti-PPARγ antibody. After incubation at 4°C overnight, the DNA-protein complexes were immunoprecipitated with protein G agarose. After washing the DNA-protein complexes, DNA was extracted with phenol/chloroform, precipitated, re-dissolved, and used as templates for polymerase chain reaction (PCR). Input and antibody controls were performed at the same number of PCR cycles as the immunoprecipitated complexes. (N) ChIP assay. Densitometric quantification for PCR products of anti-PPARγ immunoprecipitants relative to the input was calculated. The values were calculated as follows: ([signal intensities of anti-PPARγ precipitates] − [control Ig precipitates])/(input DNA).
Toluidine-blue staining for granules in BMMCs revealed a disorder of granule formation within mast cells exposed to PPARγ ligand treatment in comparison with vehicle treatment (Fig. 4B). To confirm these observations more definitely, we investigated the ultra-structure of PPARγ ligand-treated cells using electron microscopy. Mature granule formation, that is characterized by full granule content, was observed in vehicle-treated BMMCs (Fig. 4C,D, left panels). In contrast, immature granule formation, that is characterized by empty granules, was observed in PPARγ ligand-treated BMMCs (Fig. 4C,D, right panels). However, fusion of histamine-containing granules to cell membrane was not observed indicating that the empty granules were not due to de-granulation by PPARγ ligand. These results indicate that PPARγ likely inhibits the maturation of granules containing histamine in BMMCs resulting in a decrease in histamine content.
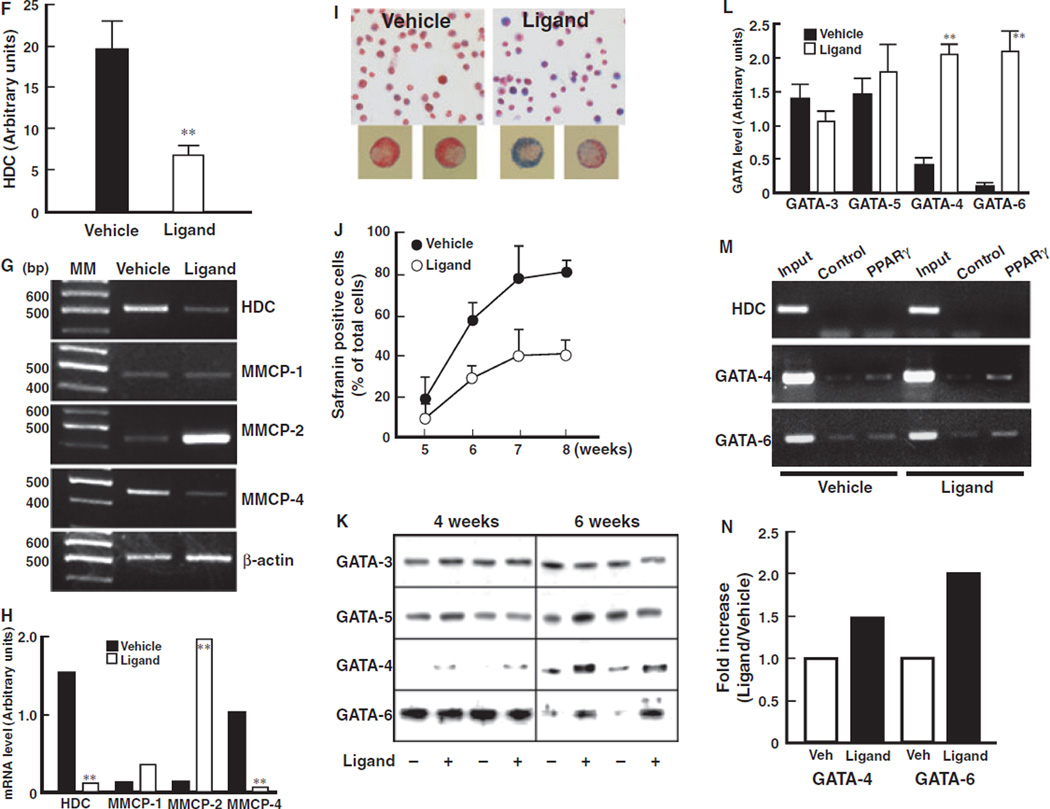
Histidine decarboxylase (HDC) is a critical enzyme that is involved in the synthesis of endogenous histamine in mammals (18, 19). HDC is also considered to be one of the indices of mast cell maturation (19). Treatment with PPARγ ligand significantly inhibited the expression of HDC protein in BMMCs at 6 weeks (Fig. 4E,F). The expression of HDC mRNA levels was also inhibited by treatment with PPARγ ligand (Fig. 4G,H). These results indicate that the decrease in histamine content observed in BMMCs treated with PPARγ ligands is due, to a significant extent, to inhibition of HDC expression. We hypothesized that PPARγ may affect the maturation of mast cells from bone marrow-progenitors into CTMCs. Expression of MMCP-4, an important marker of CTMCs, was decreased by treatment with a PPARγ ligand (Fig. 4G,H). In contrast, expression of MMCP-2, a critical marker of MMCs, was significantly increased by treatment with PPARγ ligand. These assessments of mRNA levels were confirmed by DNA microarray analysis (Table 1). The protein levels of MMPs were also altered by the treatment with PPARγ ligands (data not shown). These results indicate that PPARγ activation may inhibit the bone marrow-progenitor differentiation into mature CTMCs.
Table 1.
DNA microarray
| Gene name | Fold increase |
|---|---|
| GATA-1 | 1.260687 |
| GATA-2 | 1.007550 |
| GATA-3 | 0.957856 |
| GATA-4 | 2.012635 |
| GATA-5 | 0.882848 |
| GATA-6 | N/A |
| MMCP-1 | N/A |
| MMCP-2 | 4.537260 |
| MMCP-4 | N/A |
| MMCP-5 | 0.783791 |
| MMCP-6 | 0.695547 |
| MMCP-7 | N/A |
| MMCP-8 | 0.267900 |
| MMCP-9 | 1.124920 |
| Mast cell carboxypeptidase A3 | 0.754739 |
| Histidine decarboxylase | 0.204730 |
N/A, not applicable.
Cells were collected at 5 weeks after the start of experiment when cells were applied the treatment with SCF for a week. Total RNA was prepared and applied to a mouse microarray system (Sigma Genosis Japan, Sapporo, Japan). Expression of genes was detected by the intensity of Cy3 and Cy5. Alteration of the major mast cell-related genes on cells treated with PPARγ-ligand (rosiglitazone, 10 µM) was expressed as fold increase in comparison with those treated with vehicle.
To confirm this inhibition of differentiation, we performed alcian blue-safranin staining, which shows a specific red color in mature CTMCs and a blue color in MMC-like immature mast cells (1–5). Vehicle-treated bone marrow-progenitors almost totally differentiated into CTMCs by 7–8 weeks by this measure (Figs 4I and J, approximately 80%). In contrast, PPARγ ligand-treatment significantly inhibited the maturation of bone marrow-progenitors into CTMCs. Taken together, these results strongly suggest that PPARγ inhibits the maturation of bone marrow-progenitors into CTMCs.
PPARγ ligands inhibit the maturation of mast cells through up-regulation of GATA-4 and GATA-6
It has been reported that GATA family members of transcription factors regulate differentiation of several cell types (20, 21). GATA-4 expression was not observed in vehicle or ligand-treated groups at 4 weeks of differentiation. Treatment with PPARγ ligands for 6 weeks, however, significantly increased the expression of GATA-4 in BMMCs (Figs 4K and L). In contrast, high level GATA-6 expression was observed in both the vehicle and ligand-treated groups at 4 weeks with a significant diminution at 6 weeks in the vehicle-treated, but not ligand-treated group. No significant differences were observed in the expression of GATA-3 and GATA-5 in the context of PPARγ ligand treatment.
It has been reported that GATA-6 is essential for early embryonic development (21). In contrast, it has also been reported that over-expression of GATA-6 blocks the late differentiation of precursor cells of heart in Xenopus embryos (22). These reports suggest that maintenance of high level GATA-6 expression inhibits the later phase of cell differentiation, although GATA-6 is necessary for the early phase of development. In addition, GATA-4 and GATA-6 have been observed to negatively regulate HDC gene expression in gastric epithelium (23). These observations are consistent with our present data that PPARγ up-regulates the protein levels of GATA-4 and maintains high levels of GATA-6 consistent with a role for these proteins in inhibiting bone marrow cell maturation into CTMCs. This is supported by DNA microarray analysis of PPARγ ligand-treated cells which revealed an up-regulation of MMCP-2 and GATA-4 consistent with the inhibition of differentiation into CTMCs (Table 1). Taken together, these studies suggest that PPARγ ligand-mediated inhibition of bone marrow-progenitor maturation to CTMCs occurs through up-regulation of GATA-4 and maintenance of high levels of GATA-6 which results in decreased HDC protein levels and histamine content.
We performed ChIP assays. An increased binding of PPARγ to the GATA-6 gene promoter region in BMMCs treated with PPARγ ligand was detected (Fig. 4M,N). In contrast, binding of PPARγ to the transcriptional regulatory region of the HDC gene was not detected. In addition, low level of PPARγ binding to the transcriptional regulatory region of GATA-4 gene was detected. These results indicate that activation of PPARγ directly regulates the transcription of GATA-6 and, to a lesser extent, GATA-4, but not HDC. Because GATA-6 protein expression in BMMCs is down-regulated by SCF, but is maintained by treatment with PPARγ ligand, we suggested that maintenance of GATA-6 expression levels by PPARγ might inhibit the differentiation of bone marrow-progenitors into CTMCs.
Analysis of differentiation on PPARγ-knockdown BMMCs
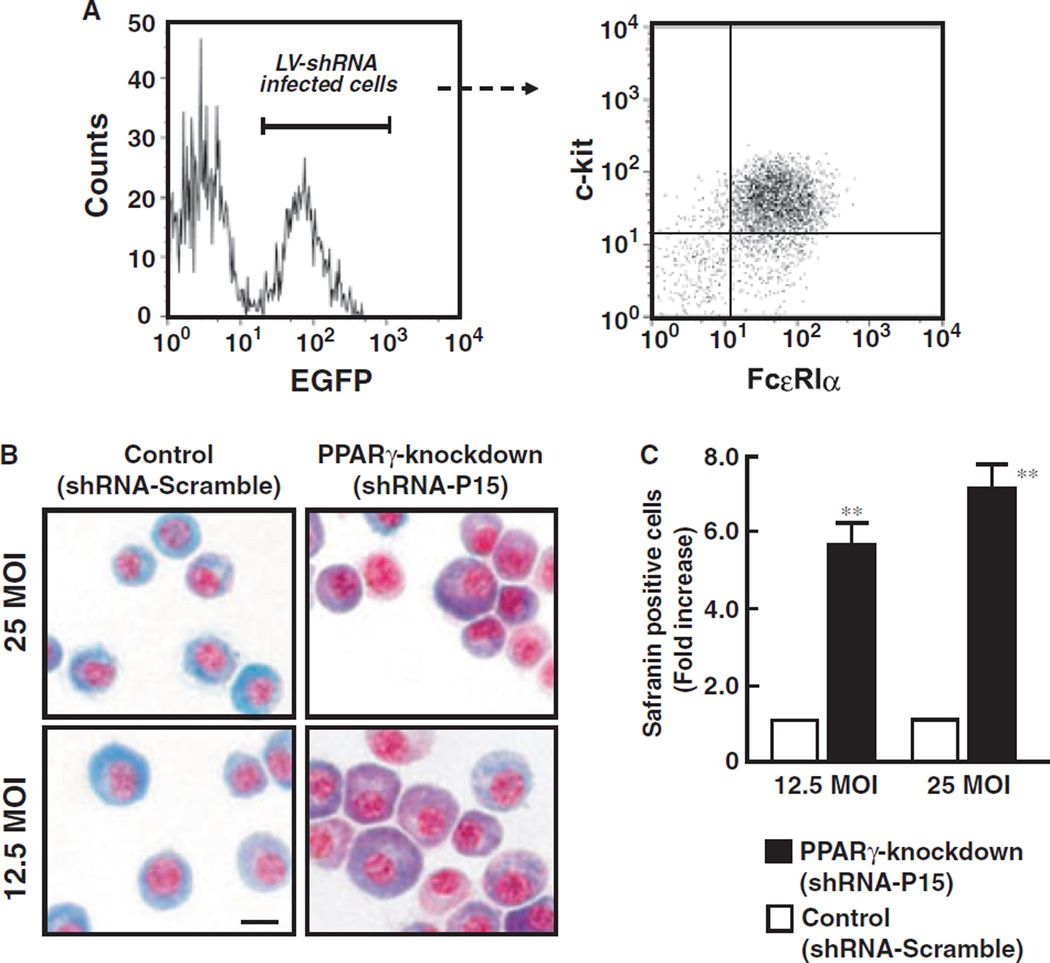
To prove that PPARγ negatively regulates BMMC differentiation, we investigated whether knockdown of PPARγ by RNA interference could affect the differentiation of BMMCs. We used a lentiviral vector-mediated short hairpin RNA (LV-shRNA) system to effectively knockdown of PPARγ1 and PPARγ2 as previously described (9, 14). Treatment with shRNA-P15, which effectively inhibits PPARγ expression, significantly increased number of the safranin-positive stained cells consistent with formation of mature CTMCs (Fig. 5B,C). These results clearly indicate that activation of PPARγ inhibits the bone marrow-progenitor differentiation into CTMCs, but deficiency of PPARγ stimulates the differentiation into CTMCs.
Figure 5.
Knockdown of PPARγ by LV-shRNA enhances differentiation of bone marrow-progenitors into CTMCs. (A) LV-shRNA infected cells can be identified by expression of EGFP. A flow-cytometory gate was set on EGFP-positive cells, and the expression of c-kit and FcεRIα was analyzed. (B) Typical photographs of LV-shRNA-P15 (PPARγ-knockdown) or LV-shRNA-Scramble (Control) infected BMMCs. After culture, cells were sorted and stained with alcian blue-safranin O. Safranin-positive cells (red color) represent CTMCs. Bar indicates 10 µM. (C) Percentage of safranin-positive cells on LV-shRNA infected BMMCs. Each column represents mean ± SD.
Discussion
In the present study, we show the inhibitory effects of PPARγ on mast cell maturation and the therapeutic utility of PPARγ ligands for mast cell-related diseases such as atopic or contact dermatitis.
In this paper, we clearly showed that long-term administration of PPARγ ligand to the whole animal significantly suppresses mast cell functions such as histamine release, TNF-α production and LTB4 production. As direct treatment with PPARγ ligand on isolated PMCs demonstrated weak inhibition of mast cell functions (data not shown), the suppressive effect of PPARγ on mast cell functions related to inflammatory responses are likely due to the attenuation of mast cell maturation by long-term treatment rather than direct action. In fact, mRNA levels of TNF-α and 5-lipoxygenase, a limiting enzyme to synthesize the LTB4, were decreased by the treatment with PPARγ ligand by DNA microarray analysis (data not shown).
Administration of PPARγ ligand significantly decreased the histamine content of both peritoneal mast cells and skin tissues resulting in an attenuation of atopic dermatitis. These results clearly indicate that activation of PPARγ by ligand administration decreases histamine levels resulting in amelioration of atopic dermatitis.
To clarify the contribution of PPARγ-mediated pathway on allergic dermatitis, we investigated the effects of PPARγ ligand on contact dermatitis in mice. Antigen-induced contact dermatitis was dramatically attenuated by treatment with PPARγ ligands which was associated with a decrease in mature mast cell accumulation in the skin. These results clearly indicate that continuous activation of PPARγ pathways by ligand treatment diminishes the number of mature mast cells in the skin resulting in the attenuation of allergic dermatitis. These results provide support for approaches in the treatment of severe allergic dermatitis via inhibition of excessive mast cell maturation in skin.
To clarify the exact role of PPARγ on mast cell maturation, we used in vitro culture system of BMMCs. Treatment with PPARγ ligands significantly diminished the histamine content of BMMCs prepared from mice in a time- and concentration-dependent fashions. The decrease in mast cell histamine content after PPARγ ligand treatment was reversed by the addition of PPARγ antagonists indicating that the reduction in histamine was related to a PPARγ-mediated pathway. Under our experimental conditions, most of the bone marrow progenitors differentiated into mast cells even in the presence of PPARγ ligand indicating that the decrease in mast cell histamine content induced by PPARγ-ligand treatment occurred independently of differentiation into either cell type. The ability of histamine release was significantly decreased by treatment with a PPARγ ligand. The electron microscopy and toluidine blue staining of BMMCs revealed a dramatic inhibition of mature granule formation which are known to contain a significant quantity of histamine within mast cells treated with PPARγ-ligands. These results may indicate that PPARγ inhibits the maturation of granules containing histamine in BMMCs.
It is well known that HDC is the most critical enzyme in the synthesis of endogenous histamine and is involved in the maturation of mast cells. In fact, it has been reported that deficiency of HDC led to deficits in granule formation resulting not only in diminished histamine contents but also in alteration of granule integrity (12). Treatment with PPARγ ligands significantly inhibited the expression of HDC protein and mRNA in BMMCs. These results indicate that the decrease in histamine content observed in BMMCs treated with PPARγ ligands is due predominantly to the inhibition of HDC expression. We focused on the involvement of PPARγ in the maturation of mast cells from bone marrow-progenitors into CTMCs. The results of protease expression and histamine content strongly indicate that activation of the PPARγ pathway inhibits the bone marrow-progenitor differentiation into ‘mature’ CTMCs. The results of alcian blue-safranin staining also support this conclusion. To prove the negative regulation of BMMC differentiation induced by PPARγ, we investigated whether knockdown of PPARγ expression by RNA interference could affect the differentiation of BMMCs. Knockdown of PPARγ by shRNA in bone marrow-progenitors significantly increased the quantity of mature CTMCs. Taken together, PPARγ inhibits the maturation of bone marrow-progenitors into CTMCs, but deficiency of PPARγ stimulates the differentiation into CTMCs.
Our results presented here indicate that high-level expression of GATA-4 and GATA-6 may inhibit the differentiation of progenitor cells into mature mast cells. In fact, the results of our present study clearly show that activation of the PPARγ pathway maintains the high-level expression of GATA-4 and GATA-6 in BMMCs after the addition of SCF. This activation of PPARγ up-regulates the protein levels of GATA-4 and GATA-6 resulting in inhibition of bone marrow-progenitor cell maturation into CTMCs. We also demonstrated the increased binding of PPARγ to the GATA-6 and, to a lesser extent, GATA-4 gene promoter regions in BMMCs treated with PPARγ ligand. In contrast, the binding of PPARγ to the transcriptional regulatory region of HDC gene was not detected in the present study. These results indicate that activation of PPARγ directly regulates the transcription of GATA-6 and possibly GATA-4, but not HDC. Because GATA-6 protein expression in BMMCs is down-regulated by SCF but is maintained by treatment with a PPARγ ligand, we suggest that maintenance of GATA-6 expression level may inhibit the differentiation of bone marrow-progenitors into CTMCs. PPARγ ligands likely inhibit the maturation of bone marrow-progenitor to mature CTMCs through up-regulation of GATA-4 and GATA-6 resulting in a decrease in HDC protein levels and histamine content. This pathway of GATA-4 and GATA-6 regulation of mast cells that is mediated by PPARγ as described here is novel mechanism to negatively regulate mast cell maturation.
In conclusion, we reveal a role for PPARγ in mediating the negative regulation of mast cell maturation and its involvement in allergic diseases. Our findings may open new methods of study for developing novel therapeutic applications of PPARγ ligands in mast cell-mediated diseases
Acknowledgments
This work was supported in part by a grant (Tokuteiryouiki C13204072, to A.N.) from the Ministry of Education, Culture, Sports, Science and Technology, grants (15590227 and 18592028, to K.W.) from the Japan Society for the Promotion of Science. R.S.B. was supported by NIH DK44319, DK51362 and DK53056 and the Harvard Digestive Disease Center.
Abbreviations
- BMMCs
bone marrow-derived mast cells
- CTMCs
connective tissue-type mast cells
- MMCs
mucosal-type mast cells
- MMCP
mouse mast cell protease
- PPARc
peroxisome proliferator-activated receptor gamma.
References
- 1.Katz HR, Austen KF, Caterson B, Stevens RL. Secretory granules of heparin-containing rat serosal mast cells also possess highly sulfated chondroitin sulfate proteoglycans. J Biol Chem. 1986;261:13393–13396. [PubMed] [Google Scholar]
- 2.Huang RY, Blom T, Hellman L. Cloning and structural analysis of MMCP-1, MMCP-4 and MMCP-5, three mouse mast cell-specific proteases. Eur J Immunol. 1991;21:1161–1621. doi: 10.1002/eji.1830210706. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Krilis SA. Mast-cell growth and differentiation. Allergy. 1998;54:306–312. doi: 10.1034/j.1398-9995.1999.00086.x. [DOI] [PubMed] [Google Scholar]
- 4.Gurish MF, Boyce JA. Mast cell growth, differentiation, and death. Clin Rev Allergy Immunol. 2002;22:107–118. doi: 10.1385/CRIAI:22:2:107. [DOI] [PubMed] [Google Scholar]
- 5.Kambe N, Hiramatsu H, Shimonaka M, Fujino H, Nishikomori R, Heike T, et al. Development of both human connective tissue-type and mucosal-type mast cells in mice from hematopoietic stem cells with identical distribution pattern to human body. Blood. 2004;103:860–867. doi: 10.1182/blood-2003-04-1160. [DOI] [PubMed] [Google Scholar]
- 6.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARgamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 7.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama H, Nonaka T, Kishimoto T, Komoriya K, Tsuji K, Nakahata T. Peroxisome proliferator-activated receptors are expressed in mouse bone marrow-derived mast cells. FEBS Lett. 2000;467:259–262. doi: 10.1016/s0014-5793(00)01169-8. [DOI] [PubMed] [Google Scholar]
- 9.Wada K, Nakajima A, Katayama K, Kudo C, Shibuya A, Kubota N, et al. Peroxisome proliferator-activated receptor γ-mediated regulation of neural stem cell proliferation and differentiation. J Biol Chem. 2006;281:12673–12681. doi: 10.1074/jbc.M513786200. [DOI] [PubMed] [Google Scholar]
- 10.Yamatodani A, Fukuda H, Wada H, Iwaeda T, Watanabe T. High-performance liquid chromatographic determination of plasma and brain histamine without previous purification of biological samples: cation-exchange chromatography coupled with post-column derivatization fluorometry. J Chromatogr. 1985;344:115–123. doi: 10.1016/s0378-4347(00)82012-5. [DOI] [PubMed] [Google Scholar]
- 11.Serafin WE, Reynolds DS, Rogelj S, Lane WS, Conder GA, Johnson SS, et al. Identification and molecular cloning of a novel mouse mucosal mast cell serine protease. J Biol Chem. 1990;265:423–429. [PubMed] [Google Scholar]
- 12.Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 13.Safina F, Tanaka S, Inagaki M, Tsuboi K, Sugimoto Y, Ichikawa A. Expression of L-histidine decarboxylase in mouse male germ cells. J Biol Chem. 2002;277:14211–14215. doi: 10.1074/jbc.M200702200. [DOI] [PubMed] [Google Scholar]
- 14.Katayama K, Wada K, Miyoshi H, Ohashi K, Tachibana M, Furuki R, et al. RNA interfering approach for clarifying the PPARgamma pathway using lentiviral vector expressing short hairpin RNA. FEBS Lett. 2004;560:178–182. doi: 10.1016/S0014-5793(04)00100-0. [DOI] [PubMed] [Google Scholar]
- 15.Habu Y, Seki S, Takayama E, Ohkawa T, Koike Y, Ami K, et al. The mechanism of a defective IFN-gamma response to bacterial toxins in an atopic dermatitis model, NC/Nga mice, and the therapeutic effect of IFN-gamma, IL-12, or IL-18 on dermatitis. J Immunol. 2001;166:5439–5447. doi: 10.4049/jimmunol.166.9.5439. [DOI] [PubMed] [Google Scholar]
- 16.Fuchibe K, Nabe T, Fujii M, Mizutani M, Danno K, Koda A, et al. Delayed type allergic itch-associated response induced by toluene-2,4-diisocyanate in hairless mice. J Pharmacol Sci. 2003;93:47–54. doi: 10.1254/jphs.93.47. [DOI] [PubMed] [Google Scholar]
- 17.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 18.Ohtsu H, Kuramasu A, Tanaka S, Terui T, Hirasawa N, Hara M, et al. Plasma extravasation induced by dietary supplemented histamine in histamine-free mice. Eur J Immunol. 2002;32:1698–1708. doi: 10.1002/1521-4141(200206)32:6<1698::AID-IMMU1698>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Wiener Z, Andrasfalvy M, Pallinger E, Kovacs P, Szalai C, Erdai A, et al. Bone marrow-derived mast cell differentiation is strongly reduced in histidine decarboxylase knockout, histamine-free mice. International Immunol. 2002;14:381–387. doi: 10.1093/intimm/14.4.381. [DOI] [PubMed] [Google Scholar]
- 20.Dimaline R, Campbell BJ, Watson F, Sandvik AK, Struthers J, Noble PJ. Regulation of GATA-6 transcription factor in gastric endocrine cells. Gastroenterol. 1997;112:1559–1567. doi: 10.1016/s0016-5085(97)70037-4. [DOI] [PubMed] [Google Scholar]
- 21.Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–732. [PubMed] [Google Scholar]
- 22.Gove C, Walmsley M, Nijjar S, Bertwistle D, Guille M, Partington G, et al. Over-expression of GATA-6 in Xenopus embryos blocks differentiation of heart precursors. EMBO J. 1997;16:355–368. doi: 10.1093/emboj/16.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson F, Kiernan RS, Dimaline R. GATA proteins are potential negative regulators of HDC gene expression in the gastric epithelium. Biochim Biophys Acta. 2002;1576:198–202. doi: 10.1016/s0167-4781(02)00301-9. [DOI] [PubMed] [Google Scholar]