Abstract
Aims/Introduction
Previously, it was observed that long‐term ingestion of a probiotic strain Lactobacillus casei Shirota (LcS) ameliorates insulin resistance and glucose intolerance in rats fed a high‐fat diet. In the present study, we examined its possible role in the autonomic nervous system during LcS‐induced modulations in glucose and lipid metabolism or cardiovascular functions.
Materials and Methods
The present study examined the effects of intragastric (IG) LcS injection on autonomic nerve tones in anesthetized rats by electrophysiological method.
Results
We found that an IG injection of LcS suppressed neural activity of sympathetic nerves supplying the white adipose tissue of urethane‐anesthetized rats in a dose‐dependent manner, whereas sympathetic nerve outflow to brown adipose tissue was not affected by the IG LcS injection. Furthermore, the IG LcS injection reduced efferent sympathetic nerve outflow to the adrenal gland and liver, but did not alter gastric vagal nerve activity, renal sympathetic nerve activity, as well as mean arterial pressure. To test the involvement of afferent vagal nerves and the abdominal organs, we examined the adrenal sympathetic response to an LcS injection in rats with ablated afferent vagal nerves, and found that the adrenal sympathetic nerve response to LcS was inhibited in vagotomized rats. In addition, we found that oral ingestion of LcS attenuated the hyperglycemic response to glucose loading and blood glycerol levels in conscious rats.
Conclusions
Our data suggest that LcS might affect tissue‐specific autonomic nerves through the afferent vagal nerve pathway to modulate glucose and lipid metabolism.
Keywords: Adipose tissue, Afferent vagal nerve, Hyperglycemia
Introduction
Probiotics are beneficial for various illnesses, such as enteropathy, inflammation, immunologic diseases or metabolic syn‐drome1, and have recently been used for preventing obesity, diabetes and hypertension2. We previously observed that acute injection of Lactobacillus johnsonii La1 into the intestine lowers blood pressure (BP) in urethane‐anesthetized rats2, and that chronic ingestion suppresses hyperglycemia after a 2‐deoxy‐D‐glucose injection4. Furthermore, acute oral ingestion of Lactobacillus paracasei ST11 stimulates thermogenesis in the brown adipose tissue (BAT) of rats3, and chronic ingestion attenuates the high‐fat diet‐induced increase in abdominal fat weight3. These findings suggest that ingesting a Lactobacillus beverage might have antihypertensive, antidiabetic and antiobesity effects.
The autonomic nervous system supplying peripheral tissues contributes towards the regulation of homeostasis, including BP, blood glucose, and lipid and energy metabolism. Sympathetic nerve outflow to the kidney is implicated in modulating cardiovascular function by regulating the renin–angiotensin system5. In addition, sympathetic nerve outflow to the liver and adrenal grand is involved in blood glucose regulation by stimulating gluconeogenesis6, and the sympathetic nerves that innervate the adipose tissue‐containing BAT or white adipose tissue (WAT) and the stomach contribute toward regulation of lipid metabolism and feeding behavior, respectively7. We previously showed that an intraduodenal injection of L. johnsonii La1 decreases renal and adrenal sympathetic nerve activities (ASNA), but increases vagal nerve outflow to the stomach in anesthetized rats2. In contrast, injecting L. paracasei ST11 into the intestine activates sympathetic neural activities innervating the BAT and WAT3. Thus, ingestion of some Lactobacillus strains might affect the autonomic nervous system, and regulate cardiovascular and metabolic functions.
One study found that long‐term ingestion of another probiotic strain, L. casei strain Shirota (LcS), improves insulin resistance and glucose intolerance in dietary obese mice9, suggesting that LcS might prevent obesity‐associated metabolic abnormalities through the autonomic nervous system. However, it has not been determined whether LcS affects autonomic nerve activities to regulate not only glucose metabolism, but also thermogenesis, lipid metabolism and cardiovascular functions. Thus, in the present study, we examined the acute effects of an intragastric (IG) LcS injection on the autonomic nervous system in anesthetized rats, and the effects of oral ingestion of LcS on hyperglycemia after oral glucose loading in conscious rats.
Materials and Methods
Animals
We used male Wistar rats (weighing 250–275 g) in the present experiment. Animals were housed in a temperature‐controlled room with a 12‐h light–dark cycle (07.00–19.00 h). Food and water were freely available. Animals were adapted to the experimental environment for at least 1 week before the experiment. All animal care and handling procedures were approved by the Institutional Animal Care and Use Committee of Ritsumeikan University.
Electrophysiological Recording
On the day of the experiment, food was removed 3–4 h before surgery. Under anesthesia induced by intraperitoneal injection of 1.2 g/kg urethane (when it was insufficient, 0.2–0.3 g/kg of urethane was added), a polyethylene catheter was inserted into the carotid vein for intravenous injection, and another catheter was inserted into the carotid artery for blood pressure determination. The rat was then cannulated through the trachea, and fixed in a stereotaxic apparatus. Body temperature was maintained at 36.5–37.0°C using a heating pad, and monitored with a thermometer inserted into the rectum. Using a dissecting microscope, the left sympathetic nerve innervating the adrenal grand or the kidney was exposed through an incision to the left flank, and a recording of the ASNA or renal sympathetic nerve activity (RSNA), respectively, was made. For recording gastric vagal nerve activity (GVNA) the gastric branch of the ventral subdiaphragmatic vagal nerve was identified and exposed after incision of the abdomen midline. For recording brown adipose tissue‐sympathetic nerve activity (BAT‐SNA), the left sympathetic nerve innervating interscapular BAT was exposed through a left dorsal incision. For recording white adipose tissue‐sympathetic nerve activity (WAT‐SNA), an abdominal blood vessel supplying the testis and WAT of the epididymis was located, and the nerve bundle was exposed as described previously10. For recording liver‐sympathetic nerve activity (Liv‐SNA), the celiac and liver branch of the ventral splanchnic nerve was identified and exposed on the liver artery after incision of the abdomen midline. The nerve was attached to a pair of stainless steel electrodes, and then hooked up to electrodes for recording. The electrodes were fixed with a silicon gel (liquid A and liquid B; Kagawa kikai Co., Kagawa, Japan) to prevent dehydration and for electrical insulation. The rat was allowed to stabilize for 30–40 min after being placed on the recording electrodes.
Electrical changes in autonomic nerve activities were amplified 2000–5000 times with a band path of 100–1000 kHz, and monitored by an oscilloscope. Raw data of nerve activity was converted to standard pulses by a window discriminator, which separated discharge from electrical background noise that remained post‐mortem. Both the discharge rates and the neurogram were sampled with a Power‐Lab analog‐to‐digital converter for recording and data analysis on a computer. Data were obtained as described previously2. The catheter in the left femoral artery was connected to a BP transducer (DX‐100; Nihon Kohden, Tokyo, Japan), and the output signal of the transducer was amplified in a BP amplifier (AP641G; Nihon Kohden). Two needle electrodes were placed under the skin at the right arm and left leg to record an electrocardiogram. The signal was amplified with a bioelectric amplifier (AB‐620G; Nihon Kohden). These data were sampled with the Power‐Lab, and stored on a hard disk for off‐line analysis to calculate mean arterial pressure (MAP) and heart rate (HR).
Baseline measurements of ASNA, RSNA, GVNA, BAT‐SNA, WAT‐SNA and Liv‐SNA were made 5 min before the IG injection of LcS (0.5 × 108 or 1 × 108/mL, BLP (BioLactis Powder) Yakult Co., Ltd., Tokyo, Japan) dissolved with sterile water, heat‐killed LcS (1 × 108/mL) or placebo (1 mL, probiotics‐free; Yakult Co., Ltd.) dissolved with sterile water. In the case of IG, injections of these agents (1 mL) were carried out by hand gilding a syringe for 2 min. After the injection, nerve activities were recorded for 60 min. To confirm successful recordings of efferent nerve activity, at the end of the experiment, an intravenous injection of hexamethonium chloride (10 mg/kg), a postganglionic blocker, was used to show that the postganglionic efferent autonomic tone was suppressed.
Vagotomy
In some rats (n = 5), to treat vagal nerve denervation (VND), afferent vagotomy was carried out 2 weeks before the LcS injection in the electrophysiological experiment. The stomach was retracted through a midline abdominal incision to cut the subdiaphragmatic vagus nerve, and the nerve bundles of the anterior and posterior vagi were dissected from the esophagus and sectioned using an ophthalmic clip. The abdominal organs were then returned to their normal positions. Control rats (n = 4) underwent sham operations that did not include the aforementioned incisions. LcS (1 × 108) or heat‐killed LcS (1 × 108/mL) was injected into the stomach.
Oral Glucose Tolerance Test and Blood Parameters Measurement
Surgical catheterizations were carried out under anesthesia with intraperitoneal pentobarbital (35 mg/kg) before the experiment. A total of 3–4 days after surgery, the animals were fasted overnight, and glucose at 2 g/kg was administered orally by gavage. Blood samples were collected from a chronically indwelling silicone catheter implanted in the left carotid artery, with its end at a point just outside the atrium, and were carried out before, and 15, 30, 60, 90 and 120 min after the oral glucose tolerance test (OGTT). LcS (1 × 108, 1 × 109), heat‐killed LcS (1 × 109/mL) or placebo (1 mL) orally was injected 30 min before the OGTT. Measurement of plasma glucose levels was carried out by FUJI DRY‐CHEM (Fujifilm Corporation, Tokyo, Japan), and measurement of plasma insulin levels was carried out by an enzyme‐linked immunosorbent assay kit (RTU; Shibayagi Co., Ltd., Gunma, Japan). In addition, to investigate the effects of oral injection of LcS or placebo on blood levels of glycerol and glucose in awake rats, blood was sampled before, and 30, 60, 90 and 120 min after injection; and as described previously3, measurement of plasma glycerol levels was carried out by an enzyme assay kit (Glycerol UV‐method; R‐Biopharm AG, Darmstadt, Germany), and measurement of plasma FFA levels was carried out by (NEFA C; WAKO, Osaka, Japan).
Data Analyses
The autonomic nervous data, BP and HR measured during each 5‐min period after injection of all agents were analyzed by digital signal processing and appropriate statistical analyses. All data were expressed as means ± standard error of the mean. Percent changes from baseline values were calculated for the nerve activities, and absolute value changes from the baseline were calculated for BP and HR. Two‐way analysis of variance (anova), or anova with post‐hoc test was applied to compare group responses or blood parameter responses between the groups, respectively. P < 0.05 was considered as statistically significant.
Results
Effects of IG Injection of LcS on Autonomic Nerve Activities and Cardiovascular Function
As shown in Figure 1, we showed sympathetic and parasympathetic responses to IG injection of LcS or placebo, and typical recordings or time course data of WAT‐SNA and BAT‐SNA before and after injection are described in Figure 1. In the LcS group, a decrease in WAT‐SNA was detected in a dose‐dependent manner, and the response was caused gradually (Figure 1b), whereas BAT‐SNA was not changed by IG injection of LcS (Figure 1d). In addition, IG injection of heat‐killed LcS reduced WAT‐SNA (Figure 1c). IG injection of placebo did not alter the BAT‐SNA and WAT‐SNA.
Figure 1.
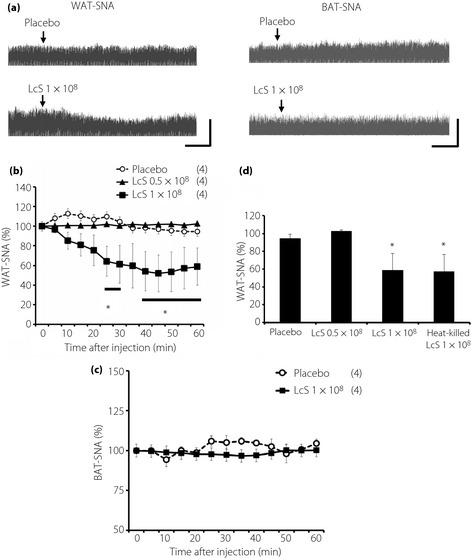
Effects of intragastric (IG) injection of Lactobacillus casei Shirota (LcS) on neural activities of sympathetic nerves supplying to the adipose tissue in urethane‐anesthetized rats. (a) Raw tracing data of white adipose tissue sympathetic nerve activity (WAT‐SNA) and brown adipose tissue sympathetic nerve activity (BAT‐SNA) before and after IG injection of placebo or 1 × 108 LcS. Time‐course data of responses of (b) WAT‐SNA and (c) BAT‐SNA to IG injection of placebo or LcS (0.5 × 108, 1 × 108). (d) Responses of WAT‐SNA to heat‐killed LcS is shown as a bar graph. Values are expressed means ± standard error of the mean. The numbers of animals used are shown in parentheses. The significance of difference between values of groups after injection was analyzed by anova. P < 0.05 vs the placebo group.
Figure 2.
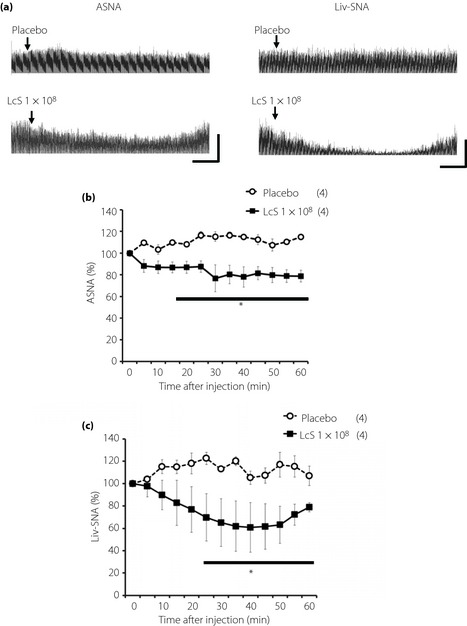
Effects of intragastric (IG) injection of Lactobacillus casei Shirota (LcS) on neural activities of sympathetic nerves supplying to the adrenal gland or liver in urethane‐anesthetized rats. (a) Raw tracing data of adrenal sympathetic nerve activity (ASNA) and liver sympathetic nerve activity (Liv‐SNA) before and after IG injection of placebo or 1 × 108 LcS. Time‐course data of responses of (b) ASNA and (c) Liv‐SNA to IG injection of placebo or LcS (1 × 108). Values were expressed mean ± standard error of the mean. The numbers of animals used are shown in parentheses. The significance of difference between values of groups after injection was analyzed by anova. P < 0.05 vs the placebo group.
Figure 3.
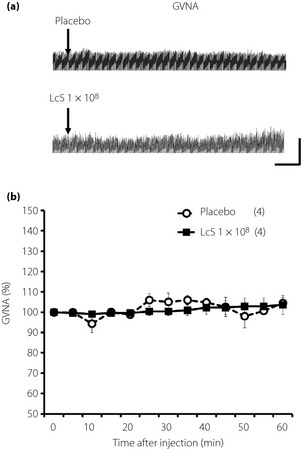
Effects of intragastric (IG) injection of Lactobacillus casei Shirota (LcS) on neural activity of parasympathetic nerves supplying to the stomach in urethane‐anesthetized rats. (a) Raw tracing data of gastric vagal nerve activity (GVNA) before and after IG injection of placebo or 1 × 108 LcS. (b) Time‐course data of responses of GVNA to IG injection of the placebo or LcS (1 × 108). Values were expressed mean ± standard error of the mean. The numbers of animals used are shown in parentheses. The significance of difference between values of groups after injection was analyzed by anova.
Typical recordings or time‐course data of ASNA and Liv‐SNA before and after injection of LcS or placebo are described in Figure 2. In the LcS group, decreases in ASNA and Liv‐SNA were detected, and these responses were caused gradually (Figure 1b,c). IG injection of a placebo did not alter the ASNA and Liv‐SNA.
Typical recordings or time‐course data of GVNA before and after injection of LcS or placebo are described in Figure 3. Neither LcS injection nor placebo injection caused significant changes of GVNA. In addition, LcS injection did not alter responses of RSNA, MAP and HR in anesthetized rats (Figure 4).
Figure 4.
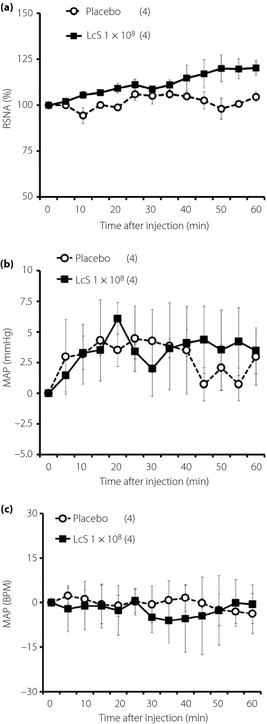
Effects of intragastric (IG) injection of Lactobacillus casei Shirota (LcS) injection of LcS on neural activity of sympathetic nerve supplying to the kidney and cardiovascular function in urethane‐anesthetized rats. Time‐course data of responses of (a) renal sympathetic nerve activity (RSNA), (b) mean arterial pressure (MAP) and (c) heart rate (HR) to IG injection of placebo or LcS (1 × 108) are shown. Values are expressed as mean ± standard error of the mean. The numbers of animals used are shown in parentheses. The significance of difference between values of groups after injection was analyzed by anova.
Possible Role of Afferent Vagal Nerve Pathway in the Efferent Sympathetic Responses to IG Injection of LcS
We examined the possible role of the abdominal afferent autonomic nerves in the adrenal sympathetic response to IG injection of LcS, and found that the reducing effect of LcS on the ASNA was eliminated in the VND rats (Figure 5). In addition, IG injection of heat‐killed LcS suppressed ASNA in sham rats, whereas it did not alter ASNA in VND rats (Figure 5).
Figure 5.
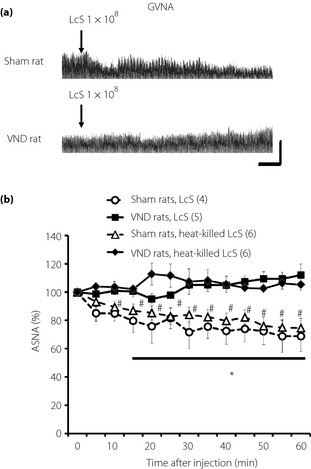
Possible role of abdominal afferent vagal nerve in decrease in adrenal sympathetic nerve activity (ASNA) as a result of intragastric (IG) injection of Lactobacillus casei Shirota (LcS). (a) Typical raw data of ASNA before and after IG injection of LcS in a rat‐treated abdominal vagotomy (VND rat) or in a sham‐operated rat (sham rat). (b) Time‐course data of responses of ASNA to IG injection of LcS or heat‐killed LcS in rats treated vagotomy (VND rats) or in sham‐operated rats (sham rats). Values were expressed as mean ± standard error of the mean. The numbers of animals used are shown in parentheses. The significance of difference between values of groups after injection was analyzed by anova. *P < 0.05 vs sham rats, LcS. #P < 0.05 vs sham rats, heat‐killed LcS.
Effects of Oral Injection of LcS on Hyperglycemia During OGTT and Plasma Glycerol Levels in Awake Rats
We showed that injection of 1 × 109 LcS or heat‐killed LcS significantly lowered blood glucose levels compared with the placebo group in OGTT, whereas a small dose of LcS (1 × 108) injection did not alter the hyperglycemic response in OGTT (Figure 6a). However, in responses of plasma insulin levels in OGTT, there was not a significant difference between all groups (placebo, 1 × 109 LcS, heat‐killed LcS; Figure 6b). Homeostasis model assessment insulin resistance index (mean ± standard error of the mean) in rats injected with placebo, 1 × 109 LcS or heat‐killed LcS was 3.0 ± 1.6, 2.4 ± 0.5 or 3.3 ± 1.0, respectively.
Figure 6.
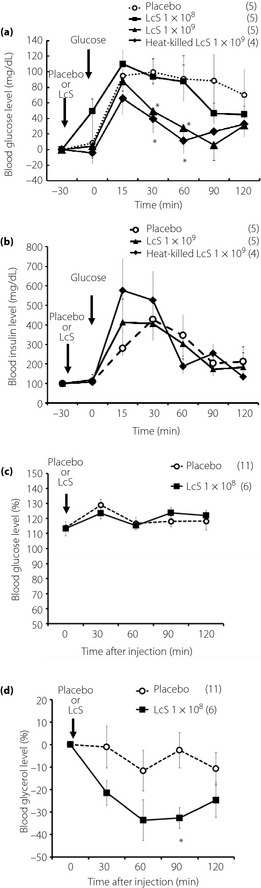
Effects of oral injection of Lactobacillus casei Shirota (LcS) on hyperglycemic response and plasma glycerol level in awake rats. (a) Time‐course data of hyperglycemic response by oral glucose solution after injection of placebo, LcS (1 × 108, 1 × 109) or heat killed LcS. (b) Time‐course data of plasma insulin levels in oral glucose tolerance test. The (c) blood glucose levels and (d) plasma glycerol levels changes after oral injection of placebo or LcS (1 × 108) in conscious rats. Values were expressed as mean ± standard error of the mean. The numbers of animals used are shown in parentheses. Significance of difference between values of groups after injection was analyzed by anova. P < 0.05 vs placebo group.
In the LcS group, blood glucose level was not changed for 120 min after injection (Figure 6c), but blood glycerol level was significantly lowered compared with the placebo group (Figure 6d). In addition, in plasma FFA levels after injection of LcS, there was not a significant difference between LcS group with placebo group ([0 min], placebo group 0.46 ± 0.03 mEq/L; LcS group 0.52 ± 0.04 mEq/L [90 min after injection]; placebo group 0.81 ± 0.05 mEq/L; LcS group 0.9 ± 0.01 mEq/L).
Discussion
Our previous studies showed that an intraduodenal injection of certain kinds of Lactobacillus beverages alters autonomic nerve activities2; and after a 2‐deoxy‐D‐glucose injection, one probiotic suppressed hyperglycemia4 or lowered BP2 in rats, whereas other probiotics accelerated thermogenesis and stimulated lipid metabolism3. The present data suggest that Lactobacillus might affect the autonomic nervous system, and regulates cardiovascular functions, as well as blood glucose and energy metabolism. In the present study, we found that IG injection of LcS decreased sympathetic nerve activity (SNA) in WAT, ASNA and Liv‐SNA in anesthetized rats, whereas oral ingestion of LcS suppressed hyperglycemic responses to oral glucose loading and plasma glycerol levels in conscious rats. In addition, the suppressing action of the LcS injection on ASNA was eliminated in abdominal afferent vagal nerve‐ablated rats. These results suggest that ingesting LcS might affect autonomic nerve outflow in a tissue‐specific manner through the abdominal afferent vagal nerve signal, and regulate glucose and lipid metabolism. To the best of our knowledge, this is the first report to show autonomic nervous regulation by LcS. However, high doses of bacteria were required to suppress hyperglycemia evoked by oral glucose loading in conscious rats compared with the autonomic responses to LcS in anesthetized rats. The reason for the excess amounts of LcS used to inhibit the blood glucose response to oral glucose loading in conscious rats could not be determined in the present study. One possibility is that conscious rats might have reduced sensitivity to LcS during the oral glucose tolerance test experiment than anesthetized rats during the autonomic experiment.
We confirmed that the LcS injection affected efferent WAT‐SNA, ASNA and Liv‐SNA in a tissue‐specific manner; whereas BAT‐SNA, renal sympathetic nerve activity and gastric vagal nerve activity did not change after LcS administration. Because WAT‐SNA and Liv‐SNA contribute toward regulation of lipid metabolism in the WAT and glucose metabolism in the liver, respectively6, the present data agrees with previous findings, which suggest that decreases in WAT‐SNA and Liv‐SNA after LcS administration lead to lowering of blood glycerol levels and suppression of hyperglycemia after oral glucose intake. In contrast, renal sympathetic nerves, which are implicated in cardiovascular regulation, remained unchanged after LcS injection. It seemed that autonomic responses to LcS might be closely related to some homeostatic functions, such as the cardiovascular system or the metabolic regulatory system. However, it is unclear why the IG LcS injection causes tissue‐specific autonomic nervous system responses. We speculate that the specificity of the neuroanatomical pathway in the efferent autonomic nerves, such as the efferent autonomic nerves innervating the abdominal tissues, have an independent neural pathway in the brain. The neural connection between the paraventricular nucleus and the suprachiasmatic nucleus in the hypothalamus is the center for regulating the efferent sympathetic nerve branch that innervates the adrenal gland11, whereas the neural route between the dorsomedial hypothalamus and the median preoptic area is the center for regulating the sympathetic nerve branch that innervates the BAT12. Thus, it is possible that differences in central transmission or site specificity might have caused tissue‐specific autonomic nerve responses to LcS injection.
The afferent autonomic nerve reflex in the abdominal organs plays an important role in regulating the gastrointestinal response to efferent autonomic modulation through the brain for various nutritional components or hormones10. We previously found that IG injections of Lactobacillus15, oolong tea14 or monosodium glutamate10 affect efferent autonomic nerve discharges through the afferent vagal nerve in the digestive organs. In agreement with previous studies, we suggest that the afferent vagal nerve in the abdominal organs is implicated in the ASNA response to LcS injection, as evidenced by the inhibition of the reducing effects of the LcS injection to the stomach on ASNA in VND rats. This is a novel finding, and shows that oral LcS ingestion might act on the digestive organ sensors and cause a reflex response in the afferent vagal nerve, resulting in efferent signal changes in the ASNA through the central nervous system. As shown here, local vagal denervation was treated by limiting innervations to the abdominal organs, such as the stomach, intestine or liver by ablating the vagal branches of gastric nerves, celiac nerves and hepatic nerves, respectively. Previous studies have confirmed that monosodium glutamate selectively acts on the stomach and stimulates the afferent vagal signal16. We hypothesized a possible role for the afferent vagal signal in the stomach during the LcS‐induced efferent autonomic response, and examined the effect of a LcS injection into the stomach on afferent gastric vagal outflow. Our preliminary study determined that an IG LcS injection did not alter afferent vagal nerve outflow; however, it activated the afferent celiac nerve outflow (data not shown). Thus, it might be concluded that LcS stimulates afferent neural activity of the intestinal vagal nerve by affecting intestinal organ sensors, but the possible role of tissue‐specific afferent pathways must be determined during the autonomic responses to an LcS injection.
Live Lactobacillus generally reaches the gastrointestinal organs, but some Lactobacillus are killed during digestion17. When live LcS are consumed, LcS survives transit through the gastrointestinal tract. Thus, to verify the importance of live LcS for ASNA regulation after an IG injection, we examined the effect of heat‐killed LcS on ASNA, and found that in anesthetized rats it had an effect on ASNA. Thus, we suggest that an unknown effective component, except for live LcS, reaches the intestine and stimulates the afferent vagal nerve, and modulates efferent autonomic nerves through the central nervous system. We previously confirmed that an intraduodenal injection of some other Lactobacillus strains cause autonomic changes through histaminergic neurotransmission containing presynaptic H3‐receptor and postsynaptic H1‐receptor2. The histaminergic H3‐ and H1‐receptors contribute to sympathetic inhibition and sympathetic acceleration, respectively18, and sympathetic suppression by L. johnsonii La1 is mediated by the central histamine H3‐receptor2, suggesting that central histamine nerves with the H3‐receptor are implicated in LcS‐induced ASNA suppression. However, this hypothesis is unclear, so further experiments will be required to determine the relationship between autonomic modulation by LcS and the histaminergic nervous system in the brain.
In conclusion, we found that IG LcS injection suppressed WAT‐SNA, ASNA and Liv‐SNA in anesthetized rats, and inhibited blood glycerol levels and the hyperglycemic response after oral glucose ingestion in conscious rats. In addition, we confirmed that the adrenal sympathetic response was abolished in VND rats. These results suggest that orally ingested LcS might reach the gastrointestinal organs and stimulate the afferent vagal nerve, thus modulating efferent sympathetic nerve outflow, and glucose and lipid metabolism. Thus, LcS supplementation might have an antidiabetic action through the autonomic nervous system by reflex regulation from afferent vagal nerves to efferent sympathetic nerves.
Acknowledgements
We acknowledge that Dr Jun Satomi (Ritsumeikan University) kindly gave us experimental support. This research was supported by grants from the Yakult Bio‐Science Foundation. No conflicts of interest, financial or otherwise, are declared by the authors.
J Diabetes Invest 2014; 5: 153–161
References
- 1.Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients 2011; 3: 637–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanida M, Yamano T, Maeda K, et al Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane‐anesthetized rats. Neurosci Lett 2005; 389: 109–114 [DOI] [PubMed] [Google Scholar]
- 3.Tanida M, Shen J, Maeda K, et al High‐fat diet‐induced obesity is attenuated by probiotic strain Lactobacillus paracasei ST11 (NCC2461) in rats. Obes Res Clin Prac 2008; 2: 159–169 [DOI] [PubMed] [Google Scholar]
- 4.Yamano T, Tanida M, Niijima A, et al Effects of the probiotic strain Lactobacillus johnsonii strain La1 on autonomic nerves and blood glucose in rats. Life Sci 2006; 79: 1963–1967 [DOI] [PubMed] [Google Scholar]
- 5.Nakamura A, Johns EJ. Renal nerves, renin, and angiotensinogen gene expression in spontaneously hypertensive rats. Hypertension 1995; 25: 581–586 [DOI] [PubMed] [Google Scholar]
- 6.Niijima A. Neural mechanisms in the control of blood glucose concentration. J Nutr 1989; 119: 833–840 [DOI] [PubMed] [Google Scholar]
- 7.Vander Tuig JG, Knehans AW, Romsos DR. Reduced sympathetic nervous system activity in rats with ventromedial hypothalamic lesions. Life Sci 1982; 30: 913–920 [DOI] [PubMed] [Google Scholar]
- 8.Shen J, Niijima A, Tanida M, et al Olfactory stimulation with scent of grapefruit oil affects autonomic nerves, lipolysis and appetite in rats. Neurosci Lett 2005; 380: 289–294 [DOI] [PubMed] [Google Scholar]
- 9.Naito E, Yoshida Y, Makino K, et al Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet‐induced obesity mice. J Appl Microbiol 2011; 110: 650–657 [DOI] [PubMed] [Google Scholar]
- 10.Tanida M, Satomi J. Effects of intragastric injection of glutamate on efferent sympathetic nerve activity in rats. Neurosci Lett 2011; 491: 211–215 [DOI] [PubMed] [Google Scholar]
- 11.Buijs RM, la Fleur SE, Wortel J, et al The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol 2003; 464: 36–48 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Kerman IA, Laque A, et al Leptin‐receptor‐expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci 2011; 31: 1873–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Date Y, Murakami N, Toshinai K, et al The role of the gastric afferent vagal nerve in ghrelin‐induced feeding and growth hormone secretion in rats. Gastroenterology 2002; 123: 1120–1128 [DOI] [PubMed] [Google Scholar]
- 14.Tanida M, Tsuruoka N, Shen J, et al Effects of oolong tea on renal sympathetic nerve activity and spontaneous hypertension in rats. Metabolism 2008; 57: 526–534 [DOI] [PubMed] [Google Scholar]
- 15.Tanida M, Nagai K. Electrophysiological analysis of the mechanism of autonomic action by Lactobacilli. Biosci Microflora 2011; 30: 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitamura A, Sato W, Uneyama H, et al Effects of intragastric infusion of inosine monophosphate and L: ‐glutamate on vagal gastric afferent activity and subsequent autonomic reflexes. J Physiol Sci 2011; 61: 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gänzle MG, Hertel C, van der Vossen JM, et al Effect of bacteriocin‐producing lactobacilli on the survival of Escherichia coli and Listeria in a dynamic model of the stomach and the small intestine. Int J Food Microbiol 1999; 48: 21–35 [DOI] [PubMed] [Google Scholar]
- 18.Tanida M, Kaneko H, Shen J, et al Involvement of the histaminergic system in renal sympathetic and cardiovascular responses to leptin and ghrelin. Neurosci Lett 2007; 413: 88–92 [DOI] [PubMed] [Google Scholar]


