Abstract
Aims/Introduction
We examined secular trends in the prevalence of type 2 diabetes and prediabetes in community‐dwelling Japanese subjects.
Materials and Methods
A total of 2,490 subjects in 1988 and 2,852 subjects in 2002 aged 40–79 years underwent a 75‐g oral glucose tolerance test, and their glucose tolerance status was defined by the 1998 World Health Organization criteria.
Results
The age‐adjusted prevalence of type 2 diabetes increased significantly from 1988 to 2002 in men (14.6% in 1988 to 20.8% in 2002, P < 0.001) and women (9.1% in 1988 to 11.2% in 2002, P = 0.002). A significant rise in the age‐adjusted prevalence of prediabetes was also observed in both sexes (26.2% in 1988 to 35.3% in 2002, P < 0.001 for men; 22.5% in 1988 to 25.1% in 2002, P = 0.04 for women). In age‐stratified analysis, the prevalence of type 2 diabetes increased markedly over time in men aged 60–69 and 70–79 years (both P < 0.001) and women aged 70–79 years (P = 0.02). The prevalence of overall and central obesity increased significantly in men aged 60–69 and 70–79 years, and women aged 70–79 years from 1988 to 2002, whereas the frequency of regular exercise decreased significantly in men aged 70–79 years between the surveys.
Conclusions
Our findings suggest that the prevalence of type 2 diabetes and prediabetes increased significantly in both sexes from the 1980s to the 2000s in a general Japanese population, and that the increasing prevalence of obesity and the decline in physical activity exerted an influence on this rising trend.
Keywords: Prediabetes, Prevalence, Type 2 diabetes
Introduction
The number of individuals with type 2 diabetes has been rapidly growing worldwide, especially in Asia, probably owing to economic development, population growth, aging and a Westernized lifestyle1. The burden of type 2 diabetes and its complications, including macro‐ and microvascular diseases, are increasingly recognized as a global health priority. Reliable estimates of secular trends in the prevalence of type 2 diabetes are required to develop effective strategies for prevention and management of type 2 diabetes. Several epidemiological studies have examined trends in the prevalence of type 2 diabetes and prediabetes, which were defined by a 75‐g oral glucose tolerance test (OGTT) in Asian populations3, as well as in Western populations7, but there are no reliable data on this issue in Japan, where the number of patients with type 2 diabetes has increased steeply9. Meanwhile, some epidemiological studies in Western populations have shown that the rise in the prevalence of type 2 diabetes was mainly driven by increasing levels of obesity7. In the general Asian community, however, there are limited data assessing factors that contribute to the trends in the prevalence of type 2 diabetes13.
The purpose of the present study was to investigate secular trends in the prevalence of type 2 diabetes and prediabetes, defined by the OGTT, and their risk factors over a 14‐year period from 1988 to 2002 in community‐dwelling Japanese subjects.
Materials and Methods
Study Population
A population‐based prospective study of cardiovascular disease and its risk factors has been underway since 1961 in the town of Hisayama, a suburb of the Fukuoka metropolitan area on Japan's Kyushu Island. The population of the town has been stable for 50 years, and was approximately 8,000 in 2010. The age and occupational distributions, and nutritional intake of the population were almost similar to those of Japan as a whole based on data from the national census and nutrition survey15. As a part of the study, two cross‐sectional diabetes surveys have been carried out on Hisayama residents in a similar manner in 1988 and 2002. A detailed description of these surveys was published previously15. Briefly, of a total of 3,227 residents in 1988 aged 40–79 years based on the town registry, 2,587 (participation rate, 80.2%) consented to taking part in a comprehensive assessment, including the 75‐g OGTT. After excluding 82 subjects who had already had breakfast, 10 who were on insulin therapy and 15 because of complaints of nausea or general fatigue during the ingestion of glucose, a total of 2,480 subjects completed the 75‐g OGTT. Among the excluded subjects, 10 who were on insulin therapy for type 2 diabetes, who had been diagnosed by their attending physicians, were included in the analysis; thus, the final 1988 study group comprised 2,490 participants (1,077 men and 1,413 women). In 2002, of a total of 3,896 residents aged 40–79 years, 3,000 (participation rate, 77.0%) consented to participating in the examination, and underwent a comprehensive assessment. Of these, a total of 2,822 participants completed the OGTT after excluding 46 who had already eaten breakfast, 32 who were on insulin therapy and 100 who refused the OGTT. Among the participants who received insulin therapy, two with a clinical diagnosis of type 1 diabetes were excluded, and the remaining 30 subjects who were on insulin therapy were included in the analysis. Consequently, 2,852 participants (1,257 men and 1,595 women) made up the final 2002 study group.
Clinical Evaluation and Laboratory Measurements
In both the 1988 and 2002 surveys, clinical evaluation and laboratory measurements were carried out in a similar manner. The study participants underwent the OGTT between 08.00 h and 10.30 h after an overnight fast of at least 12 h. Blood for the glucose assay was obtained by venipuncture into tubes containing sodium fluoride at fasting and at 2‐h postload, and was separated into plasma and blood cells within 20 min. Plasma glucose concentrations were determined by the glucose‐oxidase method. Glucose tolerance status was defined by the 1998 World Health Organization criteria17; namely, for normal glucose tolerance, fasting plasma glucose (FPG) <6.1 and 2‐h postload glucose (PG) <7.8; for impaired fasting glycemia (IFG), FPG 6.1–6.9 and 2‐h PG <7.8; for impaired glucose tolerance (IGT), FPG <7.0 and 2‐h PG 7.8–11.0; and for diabetes, FPG ≥7.0 mmol/L or 2‐h PG ≥11.1 mmol/L or both, or the use of antidiabetic medications. Prediabetes was defined as either IFG or IGT. Total and high‐density lipoprotein (HDL) cholesterols and triglycerides were determined enzymatically.
The height and weight were measured with the participant in light clothes without shoes, and the body mass index (BMI; kg/m2) was calculated. Overall obesity was defined as a BMI ≥25.0 kg/m2. Waist circumference was measured at the umbilical level with the participant standing by a trained staff member, and central obesity was defined as a waist circumference ≥90 cm in men and ≥80 cm in women. Blood pressure was obtained three times using a mercury sphygmomanometer in 1988 and an automated sphygmomanometer (BP‐203RV III; Colin, Tokyo, Japan) in 2002 with the participant in a sitting position after rest for at least 5 min; the average values were used in the analyses. Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg or current treatment with antihypertensive agents.
Each participant completed a self‐administered questionnaire covering medical history, antidiabetic and antihypertensive treatments, alcohol intake, smoking habits, and physical activity. Alcohol intake and smoking habits were classified as either current use or not. Participants engaging in sports at least three times per week during their leisure time were defined as the regular exercise group.
Statistical Analysis
The sas software package version 9.3 (SAS Institute, Cary, NC, USA) was used to carry out all statistical analyses. The prevalences of type 2 diabetes and each diabetes‐related factor were adjusted for the age distribution of the world standard population18 by using the direct method with 10‐year age groupings. The age‐adjusted mean values of diabetes‐related factors were calculated using the analysis of covariance method with age included as a continuous variable. The statistical significance of the difference in the prevalence or mean of each factor between the surveys was assessed using the logistic or linear regression model fit by the generalized estimating equations, respectively, to take into account the individuals who participated in the two surveys19. Serum triglyceride values were transformed into logarithms to improve the skewed distribution. A value of P < 0.05 was considered statistically significant in all analyses.
Ethical Considerations
The present study was carried out with the approval of the Kyushu University Institutional Review Board for Clinical Research, and written informed consent was obtained from the participants.
Results
The age‐adjusted mean values or frequencies of diabetes‐related factors in 1988 and 2002 are shown by sex in Table 1. The mean values of age, FPG, 2‐h PG and HDL cholesterol, and the frequency of alcohol intake significantly increased from 1988 to 2002 in both sexes. In men, the mean values of BMI, waist circumference and diastolic blood pressure, and the prevalence of overall and central obesity significantly rose over time, whereas the frequency of smoking habits declined. In women, the mean values of total cholesterol, triglycerides and systolic blood pressure, and the prevalence of central obesity significantly decreased with time. The frequencies of hypertension and regular exercise did not differ over the study period for either sex.
Table 1. Age‐adjusted mean values or frequencies of diabetes‐related factors in 1988 and 2002 by sex.
| Variable | Men | Women | ||||
|---|---|---|---|---|---|---|
| 1988 (n = 1,077) | 2002 (n = 1,257) | P‐value | 1988 (n = 1,413) | 2002 (n = 1,595) | P‐value | |
| Age (years) | 57 (10) | 59 (10) | <0.001 | 58 (10) | 59 (11) | <0.001 |
| Fasting plasma glucose (mmol/L) | 5.9 (1.3) | 6.3 (1.4) | <0.001 | 5.7 (1.3) | 5.9 (1.1) | <0.001 |
| 2‐h postload glucose (mmol/L) | 7.8 (3.9) | 8.5 (4.2) | <0.001 | 7.5 (3.3) | 7.7 (3.5) | 0.003 |
| Body mass index (kg/m2) | 22.8 (2.9) | 23.6 (3.0) | <0.001 | 23.0 (3.2) | 23.1 (3.5) | 0.40 |
| Overall obesity (%) | 25.2 | 30.8 | <0.001 | 24.6 | 24.2 | 0.27 |
| Waist circumference (cm) | 82.1 (8.1) | 84.0 (8.1) | <0.001 | 81.5 (10.0) | 81.0 (9.9) | 0.07 |
| Central obesity (%) | 17.6 | 23.2 | <0.001 | 56.4 | 48.5 | 0.007 |
| Total cholesterol (mmol/L) | 5.10 (1.07) | 5.08 (0.88) | 0.33 | 5.57 (1.05) | 5.45 (0.90) | <0.001 |
| HDL cholesterol (mmol/L) | 1.26 (0.31) | 1.48 (0.38) | <0.001 | 1.34 (0.29) | 1.73 (0.41) | <0.001 |
| Triglycerides (mmol/L) | 1.33 (0.41–4.30) | 1.35 (0.43–4.31) | 0.33 | 1.07 (0.42–2.77) | 1.03 (0.39–2.70) | <0.001 |
| Systolic blood pressure (mmHg) | 135 (20) | 134 (20) | 0.27 | 132 (21) | 128 (21) | <0.001 |
| Diastolic blood pressure (mmHg) | 81 (11) | 82 (11) | 0.008 | 76 (11) | 76 (12) | 0.15 |
| Hypertension (%) | 42.9 | 42.0 | 0.60 | 32.7 | 30.0 | 0.87 |
| Current drinking (%) | 63.4 | 73.1 | <0.001 | 9.7 | 30.3 | <0.001 |
| Current smoking (%) | 51.0 | 48.0 | 0.009 | 6.7 | 8.4 | 0.19 |
| Regular exercise (%) | 11.2 | 10.7 | 0.32 | 8.6 | 8.4 | 0.81 |
Age is not age‐adjusted. Triglycerides are shown by geometric means and 95% confidence intervals due to the skewed distribution. All other values are given as the mean (standard deviations) or as a percentage. Overall obesity was defined as a body mass index ≥25.0 kg/m2.Central obesity was defined as a waist circumference ≥90 cm in men and ≥80 cm in women. Hypertension was defined as blood pressure ≥140/90 mmHg and/or current use of antihypertensive agents. Regular exercise was defined as engaging in sports at least three times per week during leisure time. HDL, high‐density lipoprotein.
The secular trends in the crude and age‐adjusted prevalence of type 2 diabetes, IGT and IFG are shown by sex in Figure 1. The crude prevalence of type 2 diabetes and IFG increased significantly, and that of IGT tended to increase in both sexes from 1988 to 2002. The increasing trends in the prevalence of type 2 diabetes remained significant even after adjustment for age in both sexes (14.6% in 1988 to 20.8% in 2002, P < 0.001 for men; 9.1% in 1988 to 11.2% in 2002, P = 0.002 for women). The age‐adjusted prevalence of IFG increased significantly in both men and women (7.9% in 1988 to 15.1% in 2002, P < 0.001 for men; 4.6% in 1988 to 6.3% in 2002, P = 0.049 for women), whereas the increase in that of IGT was not significant in either sex (18.3% in 1988 to 20.2% in 2002, P = 0.30 for men; 17.9% in 1988 to 18.8% in 2002, P = 0.26 for women). When IFG and IGT were grouped together as prediabetes, a significant rise in the age‐adjusted prevalence of prediabetes was found over time in both sexes (26.2% in 1988 to 35.3% in 2002, P < 0.001 for men; 22.5% in 1988 to 25.1% in 2002, P = 0.04 for women).
Figure 1.
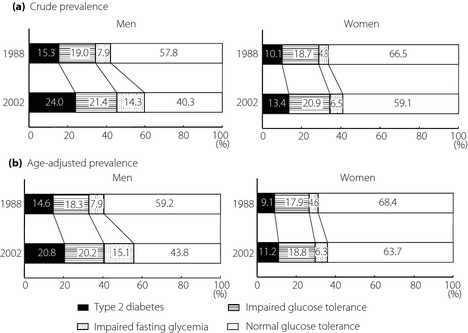
Secular trends in the (a) crude and (b) age‐adjusted prevalence of type 2 diabetes, impaired glucose tolerance, and impaired fasting glycemia in 1988 and 2002 by sex.
Figure 2 shows the prevalence of type 2 diabetes according to age groups in the two surveys by sex. In 1988, the prevalence of type 2 diabetes increased with age and reached a peak at 50–59 years‐of‐age for men and at 60–69 years‐of‐age for women, and then it decreased thereafter. In contrast, in 2002, it increased consistently with age and peaked in the oldest age group in both sexes (both P for trend <0.001). Compared with that in 1988, the prevalence of type 2 diabetes in 2002 rose markedly in men aged 60–69 and 70–79 years (both P < 0.001), and in women aged 70–79 years (P = 0.02).
Figure 2.
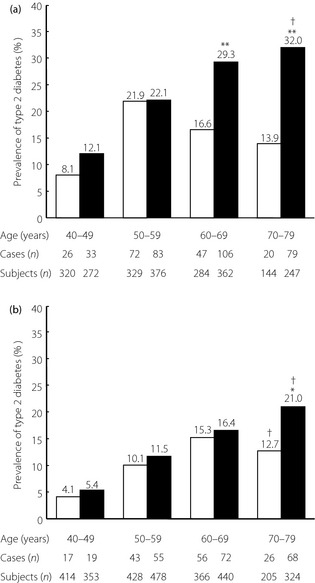
Secular trends in the prevalence of type 2 diabetes according to age groups in 1988 and 2002 by sex. (a) Men. (b) Women. *P < 0.05, **P < 0.001 vs 1988, †P for trend <0.001. □, 1988; ■, 2002.
To investigate factors contributing to the increased prevalence of type 2 diabetes, the mean values or frequencies of risk factors for type 2 diabetes were estimated according to age groups in 1988 and 2002 by sex (Table 2). During the study period, the mean values of BMI clearly increased in men aged 60–69 and 70–79 years and in women aged 70–79 years (all P < 0.001). Similar trends were observed for the mean value of waist circumference, and the prevalence of overall and central obesity in the same age groups. Meanwhile, in men aged 40–49 and 50–59 years and women aged 60–69 years, these parameters of adiposity were almost unchanged between the two surveys, whereas women aged 40–49 and 50–59 years in 2002 had lower levels of adiposity than in 1988. The frequency of regular exercise decreased only in men aged 70–79 years in 2002 compared with that in 1988 (P = 0.02).
Table 2. Mean values or frequencies of risk factors for type 2 diabetes according to age groups in 1988 and 2002 by sex.
| Variable | Age (years) | Men | Women | ||||
|---|---|---|---|---|---|---|---|
| 1988 | 2002 | P‐value | 1988 | 2002 | P‐value | ||
| Body mass index (kg/m2) | 40–49 | 23.5 (2.9) | 23.9 (3.3) | 0.05 | 23.2 (3.1) | 22.5 (3.7) | 0.008 |
| 50–59 | 23.4 (2.9) | 23.9 (3.0) | 0.04 | 23.3 (3.2) | 23.0 (3.5) | 0.13 | |
| 60–69 | 22.4 (2.9) | 23.6 (2.9) | <0.001 | 23.0 (3.2) | 23.4 (3.3) | 0.06 | |
| 70–79 | 21.3 (2.6) | 22.8 (2.9) | <0.001 | 22.0 (3.3) | 23.3 (3.4) | <0.001 | |
| Overall obesity (%) | 40–49 | 29.1 | 33.1 | 0.29 | 24.6 | 18.7 | 0.048 |
| 50–59 | 29.2 | 31.4 | 0.53 | 25.7 | 24.7 | 0.73 | |
| 60–69 | 21.8 | 30.7 | 0.01 | 26.2 | 30.7 | 0.16 | |
| 70–79 | 7.6 | 21.9 | <0.001 | 18.5 | 30.3 | 0.003 | |
| Waist circumference (cm) | 40–49 | 83.1 (8.1) | 83.6 (8.4) | 0.45 | 79.3 (9.5) | 76.8 (9.7) | <0.001 |
| 50–59 | 83.3 (7.7) | 84.2 (7.9) | 0.11 | 82.1 (9.9) | 79.8 (9.4) | <0.001 | |
| 60–69 | 81.5 (8.4) | 84.1 (7.8) | <0.001 | 83.3 (9.8) | 83.2 (9.2) | 0.87 | |
| 70–79 | 78.8 (7.7) | 83.3 (8.5) | <0.001 | 80.3 (10.6) | 84.8 (9.5) | <0.001 | |
| Central obesity (%) | 40–49 | 18.5 | 23.5 | 0.13 | 49.4 | 32.6 | <0.001 |
| 50–59 | 20.6 | 23.9 | 0.28 | 60.0 | 47.6 | <0.001 | |
| 60–69 | 15.5 | 21.6 | 0.052 | 67.5 | 68.4 | 0.79 | |
| 70–79 | 10.6 | 23.5 | 0.002 | 50.8 | 68.8 | <0.001 | |
| Regular exercise (%) | 40–49 | 10.0 | 11.0 | 0.68 | 6.5 | 5.1 | 0.40 |
| 50–59 | 6.1 | 8.2 | 0.28 | 7.0 | 9.8 | 0.13 | |
| 60–69 | 12.7 | 10.8 | 0.45 | 11.8 | 10.7 | 0.63 | |
| 70–79 | 26.4 | 16.2 | 0.02 | 14.2 | 11.4 | 0.36 | |
All values are given as the mean (standard deviations) or as a percentage. Overall obesity was defined as a body mass index ≥25.0 kg/m2. Central obesity was defined as a waist circumference ≥90 cm in men and ≥80 cm in women. Regular exercise was defined as engaging in sports at least three times per week during leisure time.
Discussion
Using data from two cross‐sectional surveys in a Japanese community, we showed that the age‐adjusted prevalence of type 2 diabetes and prediabetes defined by the OGTT increased significantly in both sexes from 1988 to 2002. In 2002, the age‐adjusted prevalence of type 2 diabetes was 20.8% in men and 11.2% in women, and that of prediabetes was 35.3 and 25.1%, respectively, indicating that, in recent years, the prevalence rate of hyperglycemia has been approximately 60% in men and 40% in women in community‐dwelling Japanese subjects aged 40–79 years. To our knowledge, the present study is the first report to show that the prevalence of type 2 diabetes and prediabetes, determined by the OGTT, increased significantly over time in Japanese. In the age‐stratified analysis, a marked rise in the prevalence of type 2 diabetes with time was observed in the elderly population for both sexes. These findings suggest that the increasing prevalence of type 2 diabetes is a serious concern, especially among older adults in Japan.
The prevalence of type 2 diabetes and prediabetes in our 2002 survey was higher than the data from the National Health and Nutrition Survey of Japan (individuals strongly suspected of having diabetes, 15.7% for men and 7.6% for women; individuals in whom diabetes cannot be ruled out, 17.3% for men and 15.4% for women, in 2011)21 and another epidemiological study in a rural area of Japan (diabetes, 11.5%; prediabetes, 18.6%, in 2000–2002)22. This diversity might be attributable to a difference in the definition of diabetes and prediabetes among the studies. The National Health and Nutrition Survey of Japan used a measurement of glycated hemoglobin (HbA1c) and medical history, not of glucose, for determining glucose tolerance status, whereas the OGTT was used in the other study and in the present study. Recent epidemiological studies have shown that the prevalence of diabetes and prediabetes defined by HbA1c levels alone are lower than those defined by the OGTT in different populations23. In addition, the participation rate in our 2002 survey (77.0%) was higher than those in the other studies (nearly 50–60%). These might be reasons for the relatively high prevalence of diabetes and prediabetes in the present study. Several population‐based studies in other Asian populations have investigated the prevalence of diabetes defined by the OGTT in the 2000s. The prevalence of diabetes was 10.6% for men and 8.8% for women in a nationwide survey in China in 2007–20084, 12.5% for men and 11.9% for women in a study carried out in urban India in 200025, and 12.3% for men and 10.4% for women in a national study in Singapore in 20106. Considering the findings in our 2002 survey (20.8% for men and 11.2% for women), the prevalence of type 2 diabetes in the Japanese population was much higher in men, and similar or higher in women compared with those in other Asians. Furthermore, based on our prevalence estimates, if the OGTT is used for determining glucose tolerance status, it was calculated that among middle‐aged and old‐aged adults in Japan, there were at least 11.7 million persons with type 2 diabetes and 20.0 million persons with prediabetes, and these figures were higher than those noted in the latest data from the International Diabetes Federation in 2011 (10.7 million persons and 13.6 million persons, respectively)10. These findings suggest that type 2 diabetes and prediabetes might actually be more prevalent in Japan than was previously thought.
In the present study, the prevalence of type 2 diabetes increased 1.4‐fold in men and 1.2‐fold in women from 1988 to 2002, and that of prediabetes rose 1.3‐fold in men and 1.1‐fold in women; all these changes were statistically significant. Other Asian population studies, which used the OGTT for determining glucose tolerance status, showed that the prevalence of diabetes increased 3.9‐fold (2.5% in 1994 to 9.7% in 2007–2008) in a Chinese population3, 1.7‐fold (8.3% in 1988–1989 to 14.3% in 2003–2004) in Asian Indians5 and 2.4‐fold (4.7% in 1984–1985 to 11.3% in 2010) in a Singapore population6. Furthermore, in these studies, a rising trend in the prevalence of prediabetes was also observed during the study period (1.2–16.0‐fold)3. In contrast, in a national survey in the USA8, there was a 1.2‐fold increase (14.4 to 17.4%) in the prevalence of diabetes, but no change (35.9 to 35.4%) in that of prediabetes between 1988–1994 and 2005–2006. Taken together, these findings imply that the Japanese had a similar rate of increase in the prevalence of type 2 diabetes compared with the Americans, whereas other Asians showed a more rapid increase. Currently, at the start of the 2000s, China and India are experiencing an economic boom, whereas Japan and the USA are facing an era of slow growth. Thus, the pace of increase in the prevalence of type 2 diabetes by each country or area might be correlated with the economic growth rate.
In our age‐stratified analysis, in 1988, the prevalence of type 2 diabetes increased with age, and reached its peak at 50–59 years‐of‐age for men and at 60–69 years‐of‐age for women, followed by a decline in older ages; whereas in 2002, it rose with age, reaching peak levels in the oldest age‐groups in both sexes. These patterns were nearly mirrored by the patterns of the parameters of adiposity according to age groups. We speculate that changes from traditional to Westernized lifestyles occurred in the younger age group earlier, and then, as this group advanced in age, the Westernized lifestyle spread to the older age groups. In addition, a sedentary lifestyle was significantly more prevalent in men aged 70–79 years. Overall obesity, central obesity and the decline in physical activity have been shown to be associated with elevated risk of incident type 2 diabetes, independent of one another16, and therefore, it is reasonable to suppose that the increase in overall and central obesity, and decreased frequency of physical activity contributed to the steep increment in the prevalence of type 2 diabetes among the elderly in the present study. Another possible explanation is that higher intake of animal fat is related to the rise in the prevalence of type 2 diabetes1. Our previous study reported that the percentage of energy intake from fat among Hisayama residents was unchanged at approximately 25% between 1985 and 2004, but the proportion of animal fat to total fat intake tended to increase from 42.3 to 47.5% during this period30. Other possible factors, such as an increase in the incidence of type 2 diabetes and improved survival in individuals with type 2 diabetes, might also be linked to the increasing prevalence of type 2 diabetes.
The strengths of the present study include the high participation rates and the use of an OGTT for determining glucose tolerance status in both surveys. However, some limitations of the present study should be discussed. First, the diagnosis of glucose tolerance status was based on a single measurement of glucose levels, as was the case in most other epidemiological studies. This limitation might have led to misclassification of glucose tolerance categories. However, we believe that the extent of misclassification of glucose tolerance categories would be similar across the surveys, and therefore such misclassification would not have substantially altered our conclusions. Second, because the present study was carried out in a suburban population, the generalizability of our results to the entire population of Japan is limited. Third, because of issues of overlap and uniformity with the 1988 survey data, we did not use medical history of diabetes in the estimation of prevalence of diabetes. This might have resulted in the underestimation of the prevalence of diabetes. Fourth, there might have been a selection bias resulting from the exclusion of participants who did not have the OGTT. However, the study participants had a similar age distribution and proportion of men (43.3 vs 45.8% in 1988, and 44.1 vs 46.3% in 2002) compared with the original population. Furthermore, the present study had high participation rates in both surveys (approximately 80%). Therefore, we believe that the findings of the present study reflect the actual secular trends in the prevalence of glucose intolerance in our population. Finally, the use of HbA1c has now been recommended for the diagnosis of diabetes by the international expert committee31, but in the present study, the values of HbA1c were not used for diagnosing diabetes because of the non‐standardized HbA1c assay in our 1988 survey. However, HbA1c measurement has been found to be less sensitive for detecting subjects with diabetes compared with the OGTT32. Furthermore, glucose tolerance status was evaluated in the same way across the two surveys. Thus, this limitation is not likely to distort the prevalence trends in the present study.
In conclusion, the present analysis showed that the prevalence of type 2 diabetes and prediabetes increased significantly in both sexes from the 1980s to the 2000s in a Japanese population. The increasing prevalence of overall and central obesity, and the decline in physical activity seemed to have an influence on this rising trend. More intense efforts for the prevention of type 2 diabetes by modification of lifestyle are required to reduce the burden of type 2 diabetes in Japan.
Acknowledgements
The authors thank the staff of the Division of Health and Welfare of Hisayama for their cooperation in this study. No potential conflicts of interest relevant to this article were reported. This study was supported in part by Grants‐in‐Aid for Scientific Research on Innovative Areas (22116010) and for Scientific Research (A) (25253048 and 22240073), (B) (25293428), and (C) (23590797, 23590798, 23500842, 24590796, 24590797, and 25460758) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare of Japan (Comprehensive Research on Life‐Style Related Diseases including Cardiovascular Diseases and Diabetes Mellitus: H22‐Junkankitou [Seishuu]‐Ippan‐005, H23‐Junkankitou [Seishuu]‐Ippan‐005, H25‐Junkankitou [Seishuu]‐Ippan‐005, H25‐Junkankitou [Seishuu]‐Ippan‐009, and H25‐Junkankitou [Seishuu]‐Sitei‐022; and Comprehensive Research on Dementia: H25‐Ninchisho‐Ippan‐004), and by a research grant from the Japan Diabetes Society.
J Diabetes Invest 2014; 5: 162–169
References
- 1.Chan JC, Malik V, Jia W, et al Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009; 301: 2129–2140 [DOI] [PubMed] [Google Scholar]
- 2.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet 2009; 375: 408–418 [DOI] [PubMed] [Google Scholar]
- 3.Pan XR, Yang WY, Li GW, et al Prevalence of diabetes and its risk factors in China, 1994. National Diabetes Prevention and Control Cooperative Group. Diabetes Care 1997; 20:1664–1669 [DOI] [PubMed] [Google Scholar]
- 4.Yang W, Lu J, Weng J, et al Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101 [DOI] [PubMed] [Google Scholar]
- 5.Mohan V, Deepa M, Deepa R, et al Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban South India‐the Chennai Urban Rural Epidemiology Study (CURES‐17). Diabetologia 2006; 49: 1175–1178 [DOI] [PubMed] [Google Scholar]
- 6.Epidemiology and Disease Control Division, Ministry of Health, Singapore: National health survey 2010. 2011
- 7.Dunstan DW, Zimmet PZ, Welborn TA, et al The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes. Obesity and Lifestyle Study. Diabetes Care 2002; 25: 829–834 [DOI] [PubMed] [Google Scholar]
- 8.Cowie CC, Rust KF, Ford ES, et al Full accounting of diabetes and pre‐diabetes in the U.S. population in 1988‐1994 and 2005‐2006. Diabetes Care 2009; 32: 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Diabetes Federation. IDF Atlas 4th edition. 2009
- 10.International Diabetes Federation. IDF Atlas 5th edition. 2011
- 11.Gregg EW, Cadwell BL, Cheng YJ, et al Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care 2004; 27: 2806–2812 [DOI] [PubMed] [Google Scholar]
- 12.Hardoon SL, Morris RW, Thomas MC, et al Is the recent rise in type 2 diabetes incidence from 1984 to 2007 explained by the trend in increasing BMI?: evidence from a prospective study of British men. Diabetes Care 2010; 33: 1494–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran A, Snehalatha C, Baskar AD, et al Temporal changes in prevalence of diabetes and impaired glucose tolerance associated with lifestyle transition occurring in the rural population in India. Diabetologia 2004; 47: 860–865 [DOI] [PubMed] [Google Scholar]
- 14.Ning F, Pang ZC, Dong YH, et al Risk factors associated with the dramatic increase in the prevalence of diabetes in the adult Chinese population in Qingdao. China. Diabet Med 2009; 26: 855–863 [DOI] [PubMed] [Google Scholar]
- 15.Ohmura T, Ueda K, Kiyohara Y, et al Prevalence of type 2 (non‐insulin‐dependent) diabetes mellitus and impaired glucose tolerance in the Japanese general population: the Hisayama Study. Diabetologia 1993; 36: 1198–1203 [DOI] [PubMed] [Google Scholar]
- 16.Doi Y, Ninomiya T, Hata J, et al Two risk score models for predicting incident Type 2 diabetes in Japan. Diabet Med 2012; 29:107–114 [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553 [DOI] [PubMed] [Google Scholar]
- 18.Ahmad OB, Boschi‐Pinto C, Lopez AD, et al Age standardization of rates: a new WHO standard. World Health Organization, Geneva, 2001 [Google Scholar]
- 19.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrics 1986; 78: 13–22 [Google Scholar]
- 20.Wolf PA, Benjamin EJ, Belanger AJ, et al Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J 1996; 131: 790–795 [DOI] [PubMed] [Google Scholar]
- 21.Ministry of Health, Labour and Welfare. Outline of the National Health and Nutrition Survey Japan. 2011. (Japanese).
- 22.Nakagami T, Tominaga M, Nishimura R, et al Is the measurement of glycated hemoglobin A1c alone an efficient screening test for undiagnosed diabetes? Japan National Diabetes Survey. Diabetes Res Clin Pract 2007; 76: 251–256 [DOI] [PubMed] [Google Scholar]
- 23.Cowie CC, Rust KF, Byrd‐Holt DD, et al Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988‐2006. Diabetes Care 2010; 33: 562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen DL, Witte DR, Kaduka L, et al Moving to an A1C‐based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care 2010; 33: 580–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramachandran A, Snehalatha C, Kapur A, et al High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia 2001; 44: 1094–1101 [DOI] [PubMed] [Google Scholar]
- 26.Thai AC, Yeo PP, Lun KC, et al Diabetes mellitus and its chronic complications in Singapore: an increasing healthcare problem. Ann Acad Med Singapore 1990; 19: 517–523 [PubMed] [Google Scholar]
- 27.Ohnishi H, Saitoh S, Takagi S, et al Incidence of type 2 diabetes in individuals with central obesity in a rural Japanese population: the Tanno and Sobetsu Study. Diabetes Care 2006; 29: 1128–1129 [DOI] [PubMed] [Google Scholar]
- 28.Lee DC, Sui X, Church TS, et al Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care 2009; 32: 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindström J, Peltonen M, Eriksson JG, et al High‐fibre, low‐fat diet predicts long‐term weight loss and decreased type 2 diabetes risk: the Finnish Diabetes Prevention Study. Diabetologia 2006; 49: 912–920 [DOI] [PubMed] [Google Scholar]
- 30.Tomonou M, Shirota T, Uchida K, et al Changes of nutritional intakes and food group intakes over a 40‐year period in Hisayama. Bull Nakamura Gakuen 2007; 39: 255–262 (Japanese). [Google Scholar]
- 31.The International Expert Committee . International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32: 1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson DE, Rhee MK, Herrick K, et al Screening for diabetes and pre‐diabetes with proposed A1C‐based diagnostic criteria. Diabetes Care 2010; 33: 2184–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araneta MR, Grandinetti A, Chang HK. A1C and diabetes diagnosis among Filipino Americans, Japanese Americans, and Native Hawaiians. Diabetes Care 2010; 33: 2626–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Picón MJ, Murri M, Muñoz A, et al Hemoglobin A1c versus oral glucose tolerance test in postpartum diabetes screening. Diabetes Care 2012; 35: 1648–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]


