Abstract
Aims/Introduction
Little is known about the long‐term effects of Roux‐en‐Y gastric bypass (RYGB) in severely obese Asian individuals.
Methods and Materials
A total of 33 severely obese patients with type 2 diabetes underwent RYGB. All patients were followed up for 2 years. Visceral and abdominal subcutaneous fat areas were assessed using computed tomography (CT) before, and 12 and 24 months after RYGB. The muscle attenuation (MA) of paraspinous muscles observed by CT were used as indices of intramuscular fat.
Results
The mean percentage weight loss was 22.2 ± 5.3% at 12 months, and 21.3 ± 5.1% at 24 months after surgery. Compared with the baseline values, the visceral fat area was 53.6 ± 17.1% lower 24 months after surgery, and the abdominal subcutaneous fat area was 32.7 ± 16.1% lower 24 months after surgery. The MA increased from 48.7 ± 10.0 at baseline to 52.2 ± 8.9 (P = 0.009) 12 months after surgery. The MA after the first 12 months maintained changes until 24 months. Triglycerides and free fatty acids were reduced after surgery, whereas the high‐density lipoprotein cholesterol levels were increased significantly after surgery. At the last follow‐up visit, 18 patients (55%) had diabetes remission. The percentage of iron and vitamin D deficiency was 30% and 52%, respectively.
Conclusions
We found that patients subjected to RYGB had significant sustained reductions in visceral and intramuscular fat. There were durable improvements in the cardiometabolic abnormalities without any significant comorbidities. However, there were mild nutritional deficiencies in these patients despite daily supplementation with multivitamins and minerals.
Keywords: Bariatric surgery, Visceral fat, Intramuscular fat
Introduction
Bariatric surgery is an appropriate treatment for people with body mass index (BMI) ≥35 kg/m2 and type 2 diabetes, especially if the diabetes or associated comorbidities are difficult to control with lifestyle modifications and pharmacological therapy1. The recent International Diabetes Federation guidelines have decreased the cut‐off for BMI as an indication for bariatric surgery by 2.5 kg/m2 for Asian subjects2. Caution is required in extrapolating the benefits of surgery to diabetic patients who have lower BMI. Before bariatric surgery is introduced clinically to large populations, we require more studies with longer follow‐up to validate its risk‐to‐benefit ratio, and confirm that the effects are sustainable and not fleeting. The durability of any improvement of type 2 diabetes after bariatric surgery has not been well characterized, especially among Asian populations. Regarding safety, patients undergoing Roux‐en‐Y gastric bypass (RYGB) are at risk of developing nutritional deficiencies in the long term because of a reduction in the absorptive capacity of the small bowel3. However, there are no previous reports that assessed the incidence of nutritional deficiencies after RYGB in Asian individuals.
Bariatric surgery, especially RYGB, causes significant rapid weight loss, including loss of a substantial amount of fat4. The quality and site of fat loss are as important as the amount of fat gained or lost5. Our previous study showed a greater reduction in visceral fat than in subcutaneous fat at 1 year after RYGB6. Visceral adiposity is linked to the presence of insulin resistance, and is significantly related to the severity of coronary artery disease5. Intramuscular fat is of particular interest because of the important role of muscle, particularly skeletal muscle, in insulin‐mediated glucose uptake7. As a long‐term outcome, reduction in visceral and intramuscular fat after surgery might be associated with the decrease in cardiovascular mortality in patients undergoing bariatric surgery. However, the long‐term effects of bariatric surgery on visceral and intramuscular fat have been barely investigated, and are controversial.
The aim of the present was to investigate the long‐term impact of RYGB on abdominal and intramuscular fat after surgery, and to analyze the changes in metabolic parameters after RYGB. Second, we aimed to evaluate the incidence of long‐term complications, especially micronutrient deficiencies, in patients undergoing RYGB.
Methods
Study Participants
We carried out a prospective cohort study in severely obese Korean patients with type 2 diabetes who received laparoscopic RYGB at the Yeouido St. Mary's Hospital from July 2009 to December 2009. The inclusion criteria for patients in the present study were: age 19–64 years, BMI >30 kg/m2 (the recommended cut‐off for bariatric surgery in Asian patients) and the presence of type 2 diabetes that was controlled inadequately by conventional treatments8. The exclusion criteria included a history of type 1 or secondary diabetes, a positive antiglutamic acid decarboxylase (GAD) antibody titer, a lack of normal pancreatic β‐cell function (fasting C‐peptide concentration <1 ng/mL or glucagon‐stimulated C‐peptide concentration <1.5 ng/mL) and the presence of severe complications of diabetes (proliferative retinopathy, serum creatinine >2 mg/dL, vascular complications or severe neuropathy). Bypass surgery was carried out by just two surgeons, using the same laparoscopic technique in all patients. The operative technique produced a 15 to 20‐cm3 gastric pouch, a 30 to 50‐cm long biliopancreatic limb and a 100‐cm long Roux limb. Patients were seen at the diabetes/obesity clinic before, and at 6, 12 and 24 months after surgery. A total of 33 patients were enrolled. Our institutional ethics committee approved the study, and all patients provided written informed consent.
After surgery, all patients received a daily multivitamin and mineral supplement. Daily supplementation with 500 mg of calcium and 1,000 IU of vitamin D was prescribed. No additional fat‐soluble vitamin supplementation was given other than that included in the multivitamin supplement prescribed to all patients. Vitamin supplements are uniformly prescribed at a dose of one tablet a day of Centrum® (Wyeth Consumer Healthcare Inc., Ontario, Canada). Each tablet contains 4000 IU of vitamin A, 200 IU of vitamin D, 300 μg of folic acid, 3 μg cobalamin and 3.5 mg of iron among others. An oral iron supplement was prescribed for all premenopausal women at a dose of 80 mg/day. We recommended that, to avoid protein malnutrition, protein intake should be at least 60 g daily. The recommendation for energy intake was determined on an individual basis. Nutritional parameters including the circulating levels of hemoglobin (Hb), iron, ferritin, vitamin B12, folic acid, homocysteine, vitamin A and vitamin D were measured at 24 months after surgery. Anemia was defined as Hb <13 g/dL in men and <12 g/dL in women. Iron deficiency was defined as a serum ferritin level <15 ng/mL. Folate deficiency was defined as serum folate levels <2 ng/mL, and vitamin B12 deficiency was assumed at <200 pg/mL9. Vitamin D deficiency was defined as <20 ng/mL, and <10 ng/mL was considered to indicate severe vitamin D deficiency10. Secondary hyperparathyroidism was defined as a parathyroid hormone (PTH) level >90 pg/mL.
Anthropometrics
Bodyweight and height were measured with the participant barefoot and wearing light clothing, and these measures were used to calculate the BMI in kg/m2. The percentage weight loss was used to examine the influence of weight loss as actual weight loss and percentage of excess weight loss are distorted by a large BMI range.
A computed tomography (CT) scan at L4–5 level was carried out to measure the cross‐sectional areas of total abdominal fat, visceral abdominal fat and subcutaneous abdominal fat as described11. First, the total area of abdominal adipose tissue was measured at −190 to −30 Hounsfield units. The visceral fat area (VFA) was distinguished from the subcutaneous fat area (SFA) by manually tracing the abdominal muscular wall separating the two adipose tissue compartments. The VFA was measured, and the SFA was then calculated by subtracting the VFA area from the total abdominal fat area. The abdominal fat CT scan was evaluated before, and 12 and 24 months after bariatric surgery.
The muscle attenuation (MA) of paraspinous muscles observed by CT were used as indices of intramuscular fat7. The MA of paraspinous muscles observed by CT at the mid‐abdomen level were used as indices of intramuscular fat. A ~1‐cm region of interest was placed on both the left and right paraspinous muscles at mid‐abdomen level. The MA of these two areas were averaged in order to calculate mean MA; the correlation between the two reads was 0.8. We focused on paraspinous muscle because of their slow‐twitch characteristics, which tend to have more intramuscular fat than fast‐twitch muscles7.
Definition of the Remission of Diabetes
Remission was defined as a glycated hemoglobin (A1c) level <6.5%, and a fasting glucose concentration of <126 mg/dL for 1 year or more without active pharmacological therapy12.
Laboratory Analyses
Fasting glucose (8 h minimum fast) and A1c levels were measured in all patients before, and at 6, 12 and 24 months after surgery. Glucose concentration was measured using the glucose oxidase method. The A1c level was measured using an automated high‐performance liquid chromatography (HPLC) analyzer (HLC‐723 G7; Tosoh Corporation, Tokyo, Japan). Serum 25‐hydroxy‐vitamin D (25(OH)D) concentration was measured using a radioimmunoassay (RIA; 25OH‐VIT.D3‐RIA‐CT; BioSource Europe S.A., Nivelles, Belgium). Folate and vitamin B12 concentrations were measured with commercial, competitive chemiluminescence immunoassays on an ADVIA Centaur automated analyzer (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). Serum free fatty acids (FFAs) were measured by an enzymatic method (Daiichi Pharmaceutical Co., Tokyo, Japan). Plasminogen activator inhibitor‐1 (PAI‐1) levels were measured using an enzyme‐linked immunoassay (Diagnostica Stago, Asnières, France).
Statistical Analyses
All data were analyzed using the spss statistical package (SPSS Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation unless stated otherwise. If necessary, logarithmic transformation was carried out to achieve a normal distribution. Longitudinal changes after surgery were tested with the use of repeated‐measures analysis of variance, and Bonferroni's correction was used to adjust for multiple comparisons. Student's t‐test and the χ2‐test were used to compare values between the remission and non‐remission groups. P < 0.05 was accepted as significant.
Results
Clinical Characteristics of the Study Participants
The data from 33 patients in total were analyzed. All patients were treated with oral hypoglycemic agents or insulin preoperatively. The mean age was 45.8 ± 9.9 years, and the mean BMI was 32.9 ± 4.3 kg/m2. The mean duration of diabetes was 6.3 ± 4.6 years, and the mean A1c level was 8.5 ± 1.6%. The mean percentage weight loss at the end of 12 months was 22.2 ± 5.3%. The mean percentage weight loss at 24 months after surgery was 21.3 ± 5.1%.
Changes in Abdominal Fat Depots Before and at 12 and 24 Months After RYGB
A total of 12 months after the operation, the mean VFA had decreased by 58.7 ± 16.3% from the baseline value, and the mean SFA had decreased by 39.5 ± 19.7%. Compared with the baseline values, the mean VFA was 53.6 ± 17.1% lower, and the mean SFA was 32.7 ± 16.1% lower 24 months after surgery. The visceral‐to‐subcutaneous fat area ratio decreased from 0.59 ± 0.28 at baseline to 0.49 ± 0.25 6 months after surgery, and then decreased further to 0.36 ± 0.11 12 months after surgery (P < 0.001; Figure 1). However, the visceral‐to‐subcutaneous fat area ratio after the first 12 months was maintained without any significant changes until 24 months (0.36 ± 0.11 vs 0.39 ± 0.16; P = 0.375; Figure 1).
Figure 1.
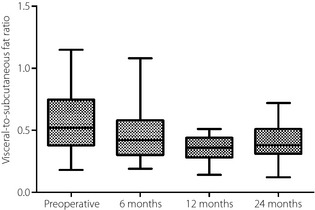
The visceral‐to‐subcutaneous fat ratio before, and 6, 12 and 24 months after Roux‐en‐Y gastric bypass in severely obese Korean patients with type 2 diabetes. Boxes, values from lower to upper quartiles; central lines, medians; whiskers extend from minimal to maximal values.
Changes in Intramuscular Fat After RYGB
The MA increased from 48.7 ± 10.0 at baseline to 52.2 ± 8.9 (P = 0.027) 12 months after surgery. The MA after the first 12 months was maintained without any significant changes until 24 months (52.2 ± 8.9 vs 53.3 ± 5.5; P = 1.00; Figure 2).
Figure 2.
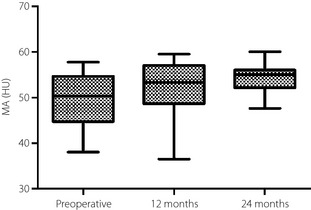
The muscle attenuation (Hounsfield Units; HU) changes after Roux‐en‐Y gastric bypass. Boxes, values from lower to upper quartiles; central lines, medians; whiskers extend from minimal to maximal values.
Changes in Cardiovascular Risk Profiles After RYGB
There were remarkable improvements in lipid profiles. The mean levels of high‐density lipoprotein (HDL) cholesterol after surgery were significantly higher than the corresponding levels at baseline (42.8 ± 8.4 vs 50.6 ± 9.9 mg/dL; P < 0.001). Mean triglyceride levels were reduced after surgery (188.4 ± 127.1 vs 104.1 ± 50.8 mg/dL; P < 0.001), whereas the total cholesterol levels were not significantly changed. A total of 17 (52%) patients were taking statins in the preoperative period for dyslipidemia, but just four of them were on lipid‐modifying medications at the last follow up. The mean serum FFA level decreased markedly from 804 to 452 μmol/L at 12 months, and then to 550 μmol/L at 24 months (Table 1). A total of 10 participants underwent repeated evaluation of PAI‐1 (preoperative, 12 months and 24 months after surgery). PAI‐1 decreased levels markedly at 12 months, and then slightly increased to levels lower than the baseline at 24 months. Antihypertensive medications were taken by 20 (61%) of these patients preoperatively. Nine (45%) of these patients were normotensive at 2 years after surgery without any medication.
Table 1. Patient characteristics before, and 12 and 24 months after Roux‐en‐Y gastric bypass.
| Baseline | 12 months | 24 months | P‐value | |
|---|---|---|---|---|
| n | 33 | – | ||
| Age (years) | 45.8 ± 9.9 | – | ||
| Male/female | 10/23 | – | ||
| BMI (kg/m2) | 32.9 ± 4.3 | 25.5 ± 4.0 | 26.5 ± 4.3 | <0.001 |
| Bodyweight (kg) | 88.3 ± 16.4 | 68.6 ± 13.9 | 69.6 ± 14.6 | <0.001 |
| Percentage weight loss (%) | – | 22.2 ± 5.3 | 21.3 ± 5.1 | 0.088 |
| Total cholesterol (mg/dL) | 166.9 ± 28.5 | 167.4 ± 26.0 | 168.6 ± 23.3 | 0.907 |
| Triglyceride (mg/dL) | 188.4 ± 127.1 | 98.7 ± 36.8 | 104.1 ± 50.8 | <0.001 |
| HDL cholesterol (mg/dL) | 42.8 ± 8.4 | 51.8 ± 10.1 | 50.6 ± 9.9 | <0.001 |
| FFA (μmol/L) | 804.4 ± 328.1 | 452.5 ± 116.6 | 550.6 ± 220.6 | <0.001 |
| PAI‐1 (n = 10) | 46.3 ± 21.5 | 19.8 ± 13.5 | 27.3 ± 13.0 | 0.005 |
| Fasting glucose (mg/dL) | 185.2 ± 45.5 | 117.0 ± 48.2 | 120.2 ± 27.5 | <0.001 |
| A1c (%) | 8.4 ± 1.6 | 6.3 ± 0.9 | 6.5 ± 1.0 | <0.001 |
| Visceral fat area (cm2) | 161.4 ± 49.0 | 70.3 ± 40.1 | 83.2 ± 49.6 | <0.001 |
| Subcutaneous fat area (cm2) | 312.5 ± 110.8 | 206.6 ± 119.3 | 228.1 ± 121.6 | <0.001 |
| Visceral‐to‐subcutaneous fat area ratio | 0.59 ± 0.28 | 0.36 ± 0.11 | 0.39 ± 0.16 | <0.001 |
A1c, glycated hemoglobin; BMI, body mass index; FFA, free fatty acids; HDL, high‐density lipoprotein; PAI‐I, plasminogen activator inhibitor‐1.
Durable Remission of Type 2 Diabetes
Of the 33 patients, 21 (64%) had diabetes remission at 1 year after surgery. Type 2 diabetes recurred in three patients after an initial period of remission. At the last follow‐up visit, 18 (55%) had undergone remission. A total of 6 months after surgery, patients with remission had significantly lower levels of fasting glucose (101.0 ± 13.3 vs 137.0 ± 32.0 mg/dL; P < 0.001) and A1c (5.79 ± 0.44 vs 7.18 ± 0.94%; P < 0.001) compared with those with no remission. These differences in A1c levels persisted during the follow‐up period (5.85 ± 0.53 vs 7.42 ± 0.88%; P < 0.001). Of the 15 patients in the non‐remission group, all of them continued to require oral hypoglycemic agents postoperatively. The requirement for antidiabetic medications decreased after RYGB in all patients, and no patients needed to take insulin injections postoperatively.
At baseline, compared with patients in the non‐remission group, patients in the remission group had a shorter duration of diabetes (5.0 ± 4.4 vs 9.4 ± 3.3 years, P = 0.008; Table 2) and a higher stimulated C‐peptide level (9.4 ± 5.5 vs 6.4 ± 2.9 ng/mL, P = 0.048), and were less likely to use insulin preoperatively (33 vs 73%, P = 0.025). The visceral‐to‐subcutaneous fat area ratio at baseline was significantly lower in the remission group than that in the non‐remission group (0.47 ± 0.20 vs 0.71 ± 0.30, P = 0.027). The MA tended to be higher in the remission group than in the non‐remission group (51.3 ± 8.9 vs 45.0 ± 10.0 HU, P = 0.159).
Table 2. Comparison of clinical characteristics of severely obese Korean patients with type 2 diabetes at baseline between remission and non‐remission groups.
| Remission of diabetes | Non‐remission of diabetes | P‐value | |
|---|---|---|---|
| n | 18 | 15 | |
| Age (years) | 43.9 ± 12.2 | 49.9 ± 7.3 | 0.129 |
| Male/female | 5/13 | 5/10 | 0.567 |
| BMI (kg/m2) | 32.8 ± 3.5 | 32.5 ± 4.9 | 0.837 |
| Duration of diabetes (years) | 5.0 ± 4.4 | 9.4 ± 3.3 | 0.008 |
| Treatment (n) | |||
| OHA (oral hypoglycemic agents) | 12 | 4 | 0.025 |
| Insulin | 6 | 11 | |
| Fasting C‐peptide (ng/mL) | 3.7 ± 2.0 | 2.7 ± 1.0 | 0.094 |
| Stimulated C‐peptide (ng/mL) | 9.4 ± 5.5 | 6.4 ± 2.9 | 0.048 |
| A1c (%) | 8.2 ± 1.6 | 8.8 ± 1.5 | 0.311 |
| Visceral fat area (cm2) | 149.3 ± 45.8 | 187.2 ± 64.9 | 0.094 |
| Subcutaneous fat area (cm2) | 345.5 ± 110.5 | 292.9 ± 114.9 | 0.247 |
| Visceral‐to‐subcutaneous fat area ratio | 0.47 ± 0.20 | 0.71 ± 0.30 | 0.027 |
| Muscle attenuation (HU) | 51.3 ± 8.9 | 45.0 ± 10.0 | 0.159 |
| Percentage weight loss at 2 years (%) | 22.8 ± 5.5 | 19.0 ± 5.8 | 0.148 |
A1c, glycated hemoglobin; BMI, body mass index; OHA, oral hypoglycemic agents.
Late Postoperative Complications
Hemoglobin dropped from 14.1 ± 1.4 g/dL at preoperative to 12.2 ± 1.8 g/dL at 2 years' follow up (Table 3). Iron deficiency was present in 30%, and anemia was present in 21% of the patients. All anemic patients were premenopausal women and had iron deficiency. The mean vitamin B12 levels decreased from 707.1 ± 186.2 pg/mL at baseline to 336.2 ± 110.4 pg/mL 24 months after surgery (Table 3). Vitamin B12 deficiency occurred in 13% of the patients (<200 pg/mL and/or elevated homocysteine >15 μmol/L). Mean 25(OH)D levels increased from 14.8 ± 7.1 ng/mL at baseline to 20.0 ± 8.8 ng/mL (P = 0.001) 24 months after surgery. However, 52% of the patients had vitamin D deficiency (<20 ng/mL). Four patients had a severe vitamin D deficiency with a level of <10 ng/mL, and two of these had secondary hyperparathyroidism. However, levels of calcium and phosphorus remained within the normal range throughout the study in all patients. No spontaneous bone fractures occurred, nor were there any signs or symptoms of metabolic bone disease based on clinical observations and the parameters studied. Hypoalbuminemia did not occur during the postoperative follow up. There were no patients developing folate deficiency at 2 years of follow up. Vitamin A deficiency was present in 8% of the patients. Two patients experienced small bowel obstruction during the follow‐up period, and they successfully underwent laparotomy for reduction of the obstruction. There was no mortality in the study group.
Table 3. Changes in nutritional parameters after Roux‐en‐Y gastric bypass.
| Baseline | 24 months | P‐value | |
|---|---|---|---|
| Hemoglobin (g/dL) | 14.1 ± 1.4 | 12.2 ± 1.8 | <0.001 |
| Iron (μg/dL) | 66.8 ± 20.7 | 62.8 ± 30.9 | 0.678 |
| Ferritin (ng/mL) | – | 29.3 ± 30.0 | – |
| Vitamin B12 (pg/mL)a | 707.1 ± 186.2 | 336.2 ± 110.4 | <0.001 |
| Folate (ng/mL)a | – | 18.7 ± 5.5 | – |
| Albumin (g/dL) | 4.3 ± 0.3 | 4.4 ± 0.3 | 0.250 |
| Calcium (mg/dL) | 9.2 ± 0.5 | 9.1 ± 0.5 | 0.532 |
| 25(OH)D (ng/mL) | 14.8 ± 7.1 | 20.0 ± 8.8 | 0.001 |
| Vitamin A (mg/L)a | – | 0.41 ± 0.13 | – |
Vitamin B12, folate and vitamin A levels were available for 24 patients.
Discussion
We found in the present 2‐year prospective study that these severely obese patients with type 2 diabetes had a significant sustained reduction in visceral and intramuscular fat after RYGB. There was a preferential loss of visceral fat, and a significant decrease in basal circulating FFA levels after surgery. There were durable improvements in the cardiometabolic abnormalities without significant comorbidities.
The effects on fat mass, intra‐abdominal adipose tissue volume and intrahepatic triglyceride contents were not different between patients undergoing RYGB, and those undergoing laparoscopic adjustable gastric banding surgery when surgery‐induced loss of a targeted 20% of bodyweight was matched13. Weight loss itself, regardless of surgery type, caused marked changes in body composition. However, there was a greater weight loss after RYGB than after gastric banding, attributable to a larger reduction in total body fat, where reduction of truncal fat contributed the most14. The massive weight loss after RYGB comprised a 50% reduction in whole‐body fat mass including a 60% decrease in visceral adipose tissue as assessed at 12 months. During the same time, lean body mass was reduced by 15–20%15. We also observed preferential loss of visceral fat (54%) from baseline to 24 months, with an overall weight loss of nearly 21%. A reduction in dietary fat after RYGB was the single most pronounced factor contributing to this14. A low proportion of dietary fat is associated with lowered formation of body fat, and thereby leads to a lower bodyweight.
Elevated circulating free fatty acid (FFA) concentrations caused by increased adipose tissue lipolysis are thought to mediate insulin resistance in both obesity and type 2 diabetes16. Enlarged visceral adipocytes seen in individuals with visceral obesity are inherently resistant to insulin. Weight loss through diet and exercise improves insulin regulation of lipolysis, which reduces adipocyte size, whereas surgical removal of abdominal subcutaneous fat by liposuction – with no decrease in fat cell size – does not16. Gastric bypass surgery is associated with near‐normal insulin suppression of lipolysis in non‐diabetic individuals17. In the present study, we found a preferential loss of visceral fat, and a significant decrease in the mean levels of basal circulating FFAs 24 months after RYGB. Improvements in adipose tissue insulin sensitivity after surgery might arise from a preferential reduction in visceral fat and decreased FFA availability17.
A reduced MA was interpreted to reflect an increased lipid component within muscle18. The findings of a reduced MA are consistent with an increased muscle lipid content determined histochemically. Increasing a solution's lipid content by 1% results in a predictable 1‐HU decrease in attenuation18. We found that patients subjected to RYGB had a significant increase in the mean MA. Skeletal MA, as a marker of an increased muscle fat, is strongly associated with insulin resistance independent of visceral adipose19. Reduction in visceral and intramuscular fat after surgery might contribute to the improvements in metabolic abnormalities.
DiGiorgi et al.20 reported a remission rate of 64% in a cohort of 42 participants who had undergone RYGB at least 3 years before evaluation. They reported a recurrence of type 2 diabetes in 26%, with a mean follow‐up period of 5 years. Chikunguwo et al.21 reported a 43.4% recurrence rate in a series of 177 participants who underwent RYGB with a follow up of 5–16 years. Consideration should be given to the possibility of a worsening in diabetes remission rates over time. According to the 5‐year results among Indian patients with type 2 diabetes and a low BMI (30–35 kg/m2)22, 84.6% of the patients had achieved euglycemia (defined as A1c <7.0%) without medication at the end of 1 year. No hyperglycemia recurred in any of the patients showing remission, despite regaining weight. In our cohort, 55% of the participants undergoing RYGB surgery presented with remission of their type 2 diabetes at 2 years after surgery. Type 2 diabetes also recurred in three patients after an initial period of remission. This finding is low compared with previous reports on the occurrence of type 2 diabetes remission after RYGB surgery at more than 2 years of follow up. By comparison, the present study participants had a lower preoperative BMI (mean 32.9 kg/m2), a more severe form of type 2 diabetes (preoperative A1c 8.4%, and all patients required oral hypoglycemic agents and/or insulin, preoperatively), and relatively long‐standing type 2 diabetes (mean duration 6.3 years). In the present study, patients with less visceral adiposity and intramuscular fat (less ectopic fat accumulation) at baseline were more likely to achieve remission after RYGB. These results are consistent with the conclusions of a previous study that the severity of diabetes is an important predictor of remission6.
There is a high prevalence of vitamin D deficiency in morbidly obese women seeking fat‐reducing surgery. In the present study, preoperative vitamin D levels were significantly lower than the postoperative levels. Many obese patients have suboptimal levels of vitamin D, and it is important to normalize these levels preoperatively when the procedure contemplated is likely to result in vitamin D malabsorption23. Overall, after a malabsorptive bariatric procedure, 17–52% of patients develop a vitamin D deficiency by 2 years, and 50–63% by 4 years. In the present study, half of the patients had a moderate deficiency in vitamin D after surgery, and four of them had a severe deficiency; two of these had secondary hyperparathyroidism. Vitamin D deficiency, a major clinical concern after bariatric surgery, must be treated aggressively with sufficient supplementation to prevent the development of metabolic bone disease3.
Common micronutrient deficiencies after bariatric surgery include deficiencies of iron and vitamin B12, which are known causes of anemia24. Iron deficiency in patients with RYGB is related to a reduced intake of organic (heme) iron, and the bypass of the acid environment of the stomach and absorptive surface of the duodenum, and proximal jejunum. The iron deficiency is more evident if there is chronic blood loss or among women of reproductive age24. In the present study, anemia was caused by iron deficiency, not vitamin B12 deficiency. Vitamin B12 deficiencies can occur after bariatric surgery procedures that bypass the lower stomach3. Given that hepatic and kidney vitamin B12 stores last up to 3 years in humans, vitamin B12 deficiency might only become clinically relevant several years after surgery. The reported prevalence of deficiency is 3.6% 12 months after RYGB, but rises to 61.8% at ≥5 years3. The clinical manifestations include depression, pernicious anemia and the development of a potentially irreversible peripheral neuropathy, as well as neuropsychiatric symptoms or ataxia3. In the present study, vitamin B12 deficiency was recognized in 13% of patients at the 2‐year follow up. Furthermore, the vitamin B12 levels at 2 years after surgery were significantly lower than the preoperative levels. There was mild vitamin B12 deficiency, but no patients developed anemia from this. For long‐term follow up after RYGB, it is necessary to pay attention to the potential problems of vitamin B12 deficiency. Some authors have found that the amount of intrinsic factor is sufficient for the absorption of crystalline vitamin B12 oral supplements24. Nevertheless, multivitamin supplements with low amounts of vitamin B12 are insufficient to prevent deficiency. Although micronutrient deficiencies were encountered, most were mild and there was no clinical symptomatology or need for hospitalization.
The present study was limited by the relatively insufficient follow‐up period (2 years) to confirm the long‐term effects of bariatric surgery. Another weakness of the present study was the relatively small sample size.
The strength of the present study was that favorable results, including decreases in visceral and intramuscular fat, were sustained for a 2‐year period in these Asian patients with a mean initial BMI of 32.9 kg/m2. There were durable improvements in the cardiometabolic abnormalities (blood glucose, lipid profiles including FFAs and blood pressure) without significant comorbidities. Amelioration of cardiometabolic abnormalities is imperative for reducing the potential micro‐ and macrovascular complications, and cardio‐vascular risks. Therefore, further studies are necessary to establish the role of bariatric surgery in protecting against death from cardiovascular causes.
Acknowledgements
There are no conflicts of interest. The authors have nothing to disclose.
J Diabetes Invest 2014; 5: 221–227
References
- 1.American Diabetes Association . Standards of medical care in diabetes‐2013. Diabetes Care 2013; 36: S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon JB, Zimmet P, Alberti KG, et al Bariatric surgery: an IDF statement for obese Type 2 diabetes. Diabet Med 2011; 28: 628–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bal BS, Finelli FC, Shope TR, et al Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol 2012; 8: 544–556 [DOI] [PubMed] [Google Scholar]
- 4.Vetter ML, Cardillo S, Rickels MR, et al Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med 2009; 150: 94–103 [DOI] [PubMed] [Google Scholar]
- 5.Ferrannini E, Mingrone G. Impact of different bariatric surgical procedures on insulin action and beta‐cell function in type 2 diabetes. Diabetes Care 2009; 32: 514–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim MK, Lee HC, Kwon HS, et al Visceral obesity is a negative predictor of remission of diabetes 1 year after bariatric surgery. Obesity (Silver Spring) 2011; 19: 1835–1839 [DOI] [PubMed] [Google Scholar]
- 7.Therkelsen KE, Pedley A, Speliotes EK, et al Intramuscular fat and associations with metabolic risk factors in the Framingham heart study. Arterioscler Thromb Vasc Biol 2013; 33: 863–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakdawala M, Bhasker A; Asian Consensus Meeting on Metabolic Surgery (ACMOMS) . Report: Asian Consensus Meeting on Metabolic Surgery. Recommendations for the use of Bariatric and Gastrointestinal Metabolic Surgery for Treatment of Obesity and Type II Diabetes Mellitus in the Asian Population: August 9th and 10th, 2008, Trivandrum, India. Obes Surg 2010; 20: 929–936 [DOI] [PubMed] [Google Scholar]
- 9.Mazokopakis EE, Starakis IK. Recommendations for diagnosis and management of metformin‐induced vitamin B12 (Cbl) deficiency. Diabetes Res Clin Pract 2012; 97: 359–367 [DOI] [PubMed] [Google Scholar]
- 10.Holick MF, Binkley NC, Bischoff‐Ferrari HA, et al Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 1911–1930 [DOI] [PubMed] [Google Scholar]
- 11.van der Kooy K, Seidell JC. Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord 1993; 17: 187–196 [PubMed] [Google Scholar]
- 12.Buse JB, Caprio S, Cefalu WT, et al How do we define cure of diabetes? Diabetes Care 2009; 32: 2133–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley D, Conte C, Mittendorfer B, et al Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest 2012; 122: 4667–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olbers T, Björkman S, Lindroos A, et al Body composition, dietary intake, and energy expenditure after laparoscopic Roux‐en‐Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg 2006; 244: 715–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dirksen C, Jørgensen NB, Bojsen‐Møller KN, et al Mechanisms of improved glycaemic control after Roux‐en‐Y gastric bypass. Diabetologia 2012; 55: 1890–1901 [DOI] [PubMed] [Google Scholar]
- 16.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 2008; 93: S57–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curry TB, Roberts SK, Basu R, et al Gastric bypass surgery is associated with near‐normal insulin suppression of lipolysis in nondiabetic individuals. Am J Physiol Endocrinol Metab 2011; 300: E746–E751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci 2000; 904: 18–24 [DOI] [PubMed] [Google Scholar]
- 19.Komiya H, Mori Y, Yokose T, et al Effect of intramuscular fat difference on glucose and insulin reaction in oral glucose tolerance test. J Atheroscler Thromb 2006; 13: 136–142 [DOI] [PubMed] [Google Scholar]
- 20.DiGiorgi M, Rosen DJ, Choi JJ, et al Re‐emergence of diabetes after gastric bypass in patients with mid‐ to long‐term follow‐up. Surg Obes Relat Dis 2010; 6: 249–253 [DOI] [PubMed] [Google Scholar]
- 21.Chikunguwo SM, Wolfe LG, Dodson P, et al Analysis of factors associated with durable remission of diabetes after Roux‐en‐Y gastric bypass. Surg Obes Relat Dis 2010; 6: 254–259 [DOI] [PubMed] [Google Scholar]
- 22.Lakdawala M, Shaikh S, Bandukwala S, et al Roux‐en‐Y gastric bypass stands the test of time: 5‐year results in low body mass index (30‐35 kg/m2) Indian patients with type 2 diabetes mellitus. Surg Obes Relat Dis 2013; 9: 370–378 [DOI] [PubMed] [Google Scholar]
- 23.Heber D, Greenway FL, Kaplan LM, et al Endocrine and nutritional management of the post‐bariatric surgery patient: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95: 4823–4843 [DOI] [PubMed] [Google Scholar]
- 24.Vargas‐Ruiz AG, Hernández‐Rivera G, Herrera MF. Prevalence of iron, folate, and vitamin B12 deficiency anemia after laparoscopic Roux‐en‐Y gastric bypass. Obes Surg 2008; 18: 288–293 [DOI] [PubMed] [Google Scholar]


