Abstract
Leishmania amazonensis infection promotes alteration of host cellular signaling and intracellular parasite survival, but specific mechanisms are poorly understood. We previously demonstrated that L. amazonensis infection of dendritic cells (DC) activated extracellular signal-regulated kinase (ERK), a MAP-kinase kinase kinase, leading to altered DC maturation and non-healing cutaneous leishmaniasis. Studies using growth factors and cell lines have shown that targeted, robust, intracellular phosphorylation of ERK1/2 from phagolysosomes required recruitment and association with scaffolding proteins, including p14/MP1 and MORG1, on the surface of late endosomes. Based on the intracellular localization of L. amazonensis within a parasitophorous vacuole with late endosome characteristics, we speculated that scaffolding proteins would be important for intracellular parasite-mediated ERK signaling. Our findings demonstrate that MP1, MORG1, and ERK all co-localized on the surface of parasite-containing LAMP2-positive phagolysosomes. Infection of MEK1 mutant fibroblasts unable to bind MP1 demonstrated dramatically reduced ERK1/2 phosphorylation following L. amazonensis infection but not following positive control EGF treatment. This novel mechanism for localization of intracellular L. amazonensis-mediated ERK1/2 phosphorylation required the endosomal scaffold protein MP1 and localized to L. amazonensis parasitophorous vacuoles. Understanding how L. amazonensis parasites hijack host cell scaffold proteins to modulate signaling cascades provides targets for antiprotozoal drug development.
Keywords: Leishmania, Dendritic cell, MAPK, Phagosome, MP1
1. Introduction
Leishmania spp. cause an array of clinical disease from localized cutaneous lesions to fatal, visceralizing disease. These parasites reside within parasitophorous vacuoles (PV) or late endosomal/lysosomal compartments, which usually serve as a site of pathogen degradation. L. amazonensis amastigotes are able to persist within these compartments and to date, the mechanisms of how this occurs have not been well-defined. Most studies have focused on understanding Leishmania parasite-host interactions and the alteration in host signaling pathways upon initial entry. However, little is known regarding modulation of host signaling pathways by established parasites within PV.
Endocytic-pathway internalization of receptors upon ligand binding is a common regulatory mechanism for receptor desensitization used by receptor tyrosine kinases (RTK) and G-protein coupled receptors [1]. Although this process was initially thought to promote signal attenuation, presence of activated epidermal factor receptors (EGFR) and downstream effectors on endosome surfaces suggested the possibility of signaling from the endocytic pathway [2]. The concept of endosomes as signaling platforms for a variety of receptors has since been strengthened by subsequent studies showing signal attenuation during inhibition of endocytosis or subcellular mislocalization of endosomes [3–5]. Scaffold proteins provide sites for the formation of signaling complexes, enhance signal transduction, and provide specific sub-cellular localization [6].
Signaling cascades rely on scaffolding complexes to provide a structure that ensures substrate interaction with their cognate signaling molecules in a temporally and geographically controlled manner [7]. Activation of the mitogen activated protein (MAP) extracellular regulated kinase (ERK) pathway from subcellular locations, including endosomal organelles, has been previously described [8]. Mediating this process are two scaffolding proteins, MEK Partner (MP) 1 and MAPK organizer (MORG) 1, [9, 10]. MP1 contains a proline-rich motif, which has a docking site for MEK1/2 [9]. Once scaffold bound, MEK1/2 phosphorylation of ERK1/2 is augmented. Limited overexpression of MP1 leads to increased ERK1/2 activity, while down-regulation of MP1 leads to reduced ERK1/2 activity [6], demonstrating the importance of MP1 in the magnitude of ERK1/2 phosphorylation.
We have previously shown that L. amazonensis promastigotes mediate ERK1/2 activation hours after infection once the parasite was internalized and within a phagosome [11]. We sought to determine how L. amazonensis triggers ERK1/2 phosphorylation from within the parasitophorous vacuole, a phagosome-derived compartment. Based on the role of MP1 and MORG1 in regulating ERK1/2 phosphorylation from late endosomal compartments, we tested the hypothesis that these scaffolding molecules were involved in L. amazonensis-mediated ERK1/2 phosphorylation. L. amazonensis-dependent ERK1/2 phosphorylation was actively mediated by the parasite, as heat-killed organisms were unable to mediate ERK1/2 activation. Moreover, parasites were found within late endosomal compartments, characterized by association with LAMP2. These L. amazonensis-containing organelles co-localized with scaffold proteins MP1 and MORG1, previously demonstrated to serve as ERK1/2 scaffolds for endosomal activation. This did not occur in BMDC infected with L. major. Through the use of a mutant fibroblast cell line in which MEK cannot bind to MP1, we demonstrated that L. amazonensis-meditated ERK activation was completely abrogated in cells unable to bind MEK to MP1. MP1 and MEK co-localization did not occur in L. amazonensis-infected mutant fibroblasts. Here we describe a novel Leishmania-dependent mechanism of host signal modulation from the phagolysosomal compartment, mediated by host cell MAP kinase ERK1/2 scaffolding proteins MORG1 and MP1.
2. Materials and methods
2.1. Bone marrow-derived dendritic cells (BMDC)
BMDC were cultured from C3H/HeJ mice in the presence of 10 ng/ml of murine granulocyte-macrophage colony-stimulating factor (PeproTech Inc., Rocky Hill, NJ) as previously described (Lutz et al., 1999). At day 10 of culture BMDC were harvested for use and approximately 90% of the cells were positive for the DC marker CD11c as analyzed via flow cytometry.
2.2. Mouse embryonic fibroblasts (MEF)
Fibroblasts derived from MEK1−/− mouse embryos from 129Sv/Ev mice (provided by Jean Charron, Université Laval, Quebec, Canada, [12]) were reconstituted with wild type or L274S MEK1 [13] rat MEK1 using the Flp-In system (Invitrogen). Clones expressing exogenous MEK1 at levels comparable to endogenous MEK1 in fibroblasts derived from wild type littermate animals were chosen for further study (supplemental Fig. 1). The L274S MEK1 mutant contains a leucine to serine change in the proline-rich sequence of MEK resulting in abrogation of MEK1 binding to MP1 [13]. These cells are hereafter referred to as “WT” and “LS” cells. WT and LS cells were cultured in vitro with 0.1% Hygromycin in DMEM (Gibco) supplemented with 10% Fetal Bovine Serum (Atlanta Biologicals, Lawrenceville, GA).
2.3. Parasite culture, infection and treatments
Culture of L. amazonensis (MHOM/BR/00/LTB0016) parasites was performed as previously described [14]. For in vitro promastigote experiments, stationary phase L. amazonensis promastigotes were used. Where indicated, parasites were labeled with CellTracker Orange CMRA (Invitrogen, Carlsbad, CA). Parasites in PBS were incubated with 0.1 uM solution of CellTracker Orange CMRA dye for 15 minutes at room temperature, washed, and re-suspended in PBS. For heat-killed experiments, L. amazonensis promastigotes were diluted to the desired concentration and then incubated at 65 °C for 1 hour and placed on ice for at least 20 minutes prior to use. Where indicated, BMDC were treated with Pertussis toxin (100 ng/ml) (Calbiochem, San Diego, CA), and washed immediately prior to infection. After infection, coverslips seeded into parallel cultures were removed and counted for PTX treated vs. mock treated cells infected with L. amazonensis, indicating that PXT treatment of cells in this way did not interfere with parasite infection. BMDC, WT and LS cells were incubated at 34 °C, 5% CO2 and infected with L. amazonensis promastigotes at a parasite to cell ratio of 3:1.
2.4. Immunoblot
To make whole cell lysates, 3 × 106 BMDC or fibroblasts were re-suspended in 400 μl of 1x cell lysis buffer (Cell Signaling Technologies, Beverly, MA), supplemented with 1mM phenylmethylsulphonyl fluoride (PMSF) and a protease inhibitor cocktail (Roche, Indianapolis, IN) immediately prior to use. Samples were incubated on ice for 15 min and then centrifuged at 16,000 × g for 10 min at 4 °C. Supernatants were collected as whole cell lysates and stored at −80 °C until further use. Protein content of all cell extracts was determined via BCA protein assay (Pierce, Rockford, IL) according to manufacturer’s recommendations, and all samples were normalized to 1 mg/ml using distilled water. Samples (20–30 μg of protein) were denatured in 1x loading buffer and electrophoresis was performed on a 12% SDS- PAGE gel. Gels were blotted onto polyvinylidene fluoride membranes (PVDF), blocked with 5% bovine serum albumin, and probed with antibodies specific for phospho-ERK, total-ERK1/2 (1:1000) (Cell Signaling, Beverly, MA) and β-actin (1:5,000) (Sigma, St. Louis, MO). Signal was detected with horseradish-peroxidase (HRP)-conjugated goat anti rabbit antibody (1:20,000) (Jackson, West Grove, PA) using West chemiluminescent substrate (Pierce, Rockford, IL) and exposed to autoradiography film (MidSci, St. Louis, MO).
2.5. Immunofluorescence analysis
1×106 BMDC were plated onto glass tissue cover slips. BMDC were infected with CellTracker Orange CMRA-labeled L. amazonensis promastigotes at a MOI of 3:1 and incubated at 34 °C with 5% CO2. Coverslips were harvested and fixed with 4% paraformaldehyde in phosphate buffered saline (PBS). BMDC were permeabilized with 0.1% saponin in PBS. Coverslips were incubated with rabbit anti-MP1 (Santa Cruz Biotechnology), rabbit anti-MORG1 (Serotec), rabbit anti-pERK1/2 (Cell Signaling, Beverly, MA), and rabbit anti-LAMP2 (eBiosciences) at a 1:100 dilution in 0.1% saponin. After incubation, cover slips were washed and incubated with rabbit anti-mouse Cy2-conjugated antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) at a 1:200 dilution in 0.1% saponin. BMDC were counter-stained with DAPI according to manufacturer’s instructions (Molecular Probes, Eugene, OR), mounted using MOWIOL (Calbiochem, La Jolla, CA) and viewed using an Olympus IX71 inverted epifluorescence scope (Olympus America Inc., Center Valley, PA). Co-localization analyses were carried out using MacBiophotonics ImageJ software and confirmed by sequential scanning confocal microscopy using an Olympus IX81 inverted scope. For WT and LS MEF cell experiments, cells were infected and processed similar to BMDC. After incubation with primary antibodies as above, coverslips were washed and incubated with anti-donkey AlexaFluor 488, 594, and 680 (Life Technologies, CA) at 1:200. Fibroblasts were counterstained and mounted with hardmount DAPI, Vectashield, (Vector Laboratories, Burlingame, CA) and analyzed using a Leica Confocal Scope (TCS SP5 X (Leica Microsystems, Exton, PA) and analyzed via the Leica Application Suite Software (Leica Microsystems, Exton, PA).
2.6. Acquisition and processing of images
In addition to primary acquisition by the Leica and Olympus software, images were converted to Adobe Photoshop CS3. Brightness and contrast were improved for visualization in print form and figures arranged in GraphPad Prism 5 as appropriate and Photoshop CS3, exported as tiff files.
2.7. Ethics Statement
All animal experiments were approved by the Iowa State University IACUC (log number 3-06-6071-M).
2.8. Statistical analysis
Statistical significances were analyzed using Prism4 (Graph Pad Software Inc., La Jolla, CA). Differences between groups were determined using Student’s t-tests or one-way ANOVA. P-values below 0.05 were considered statistically significant.
3. Results
3.1. Live L. amazonensis promastigotes required for ERK phosphorylation
Previous studies have shown that multiple signaling events appear to be triggered by Leishmania internalization, but are not necessarily triggered by the parasite itself [15, 16]. We wanted to demonstrate that ERK1/2 phosphorylation observed after L. amazonensis infection was an active, parasite-mediated, response and not a normal response to particle internalization. To test this we treated bone marrow-derived dendritic cells (BMDC) with either live or heat-killed L. amazonensis promastigotes and collected lysates at the indicated time points (Fig. 1) and measured ERK1/2 phosphorylation using western blot. Live L. amazonensis promastigotes resulted in significantly increased ERK1/2 phosphorylation as compared to heat-killed parasites (Fig. 1), supporting our hypothesis of a parasite-driven event rather than a response to internalization of parasite material.
Figure 1. Live L. amazonensis promastigotes are required to promote phosphorylation of the MAP kinase ERK1/2.
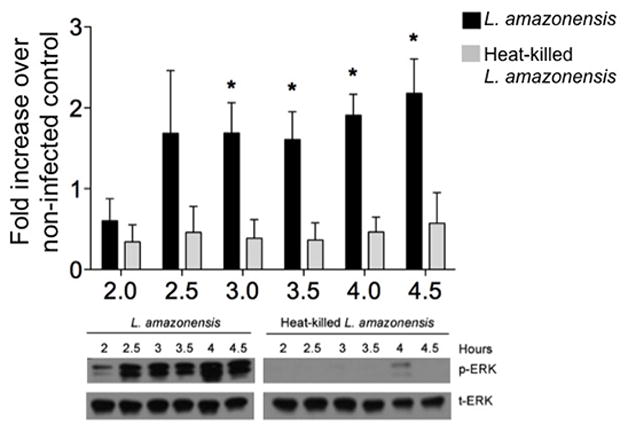
BMDC were treated with live or heat-killed L. amazonensis promastigotes and incubated at 34 °C with 5% CO2. BMDC were harvested at the indicted time points and total cell lysates were made. Samples were analyzed via western blot for phosphorylated and total ERK1/2. Densitometry values were normalized to total ERK and then to non-infected controls. Densitometry analysis of at least three different experiments and representative blot are shown; error bars denote ±SEM.
3.2. L. amazonensis in late endosomal compartments and developmental progression to amastigotes
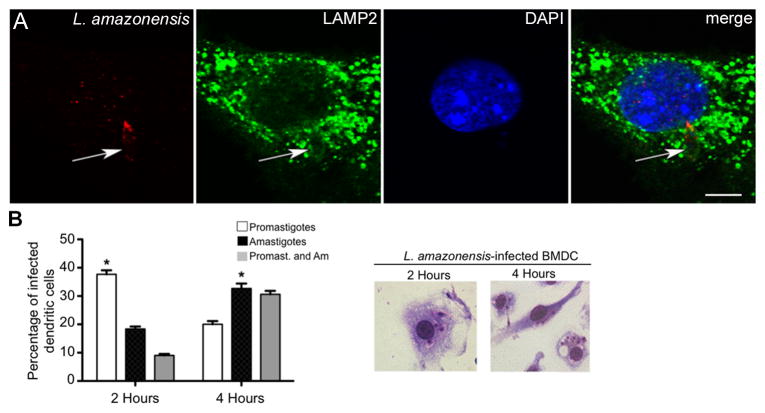
It has previously been shown that upon phagocytosis, Leishmania promastigotes were contained within a double membrane-bound parasitophorous vacuole (PV) [17]. In DC, newly-formed, Leishmania-containing phagosomes rapidly acquired late-endosomal markers including LAMP-1 and -2 [18]. During this time promastigotes transformed into amastigotes. Based on this information, we wanted to determine the compartment and parasite developmental stage that corresponded with ERK1/2 phosphorylation. We infected BMDC with fluorescently labeled L. amazonensis promastigotes and harvested cells on coverslips at 2, 3 and 4 hours post-infection. We evaluated the phagosomal compartment via LAMP-2 immunolabeling and epifluorescent microscopy. L. amazonensis parasites were found within compartments that tightly delineated the shape of the parasite (Fig. 2A, first panel). Green, punctate staining indicated presence of LAMP-2 (Fig. 2A, second panel) on the surface of this compartment. These data confirm previous findings indicating that PV maturation occurs within 1 hour of infection into a LAMP-2 positive, late endosomal/phagosomal compartment [18].
Figure 2. L. amazonensis parasites found within LAMP2-positive compartments where amastigotes predominate four hours post-infection.
BMDC in 24 well plates containing glass coverslips were infected with CellTracker Orange CMRA-labeled or unlabeled L. amazonensis promastigotes. Coverslips were recovered at indicated time points, fixed and stained. (A) Sequential scanning confocal microscopy (x60, oil) analysis of BMDC infected with L. amazonensis (red, arrow), and intracellular LAMP2 (green) at 3 hours post-infection. Nuclear material was stained with DAPI (blue). Detailed image of one cell from overlay. Control labeling for LAMP2 with secondary antibody only in supplemental data. Data representative of at least three experiments. (B) Coverslips analyzed via light microscopy (x100, oil) to stage parasites. Stage was determined by presence or absence of flagellum and parasite diameter. Graph indicates the percentage of cells infected by either or both forms. Representative images for both time points are shown. Data from at least three different experiments; error bars denote ±SEM; *p≤0.05.
Previously we described that although ERK activation in DC occurred rapidly after exposure to L. amazonensis amastigotes (seconds to minutes), we found peak ERK activation after exposure of L. amazonensis promastigotes to DC occurred three to five hours post-exposure [11]. Although New World species of Leishmania take several days to transform from promastigotes to amastigotes, others have found that L. amazonensis transforms relatively quickly [15]. To further characterize parasite development within infected BMDC, we sought to determine the percentage of promastigotes and amastigotes before (2hr post-infection) and during ERK1/2 activation (4hr post-infection). L. amazonensis-infected BMDC cultured on coverslips were harvested 2 and 4 hours post-infection and analyzed via light microscopy. Promastigotes and amastigotes were differentiated based on morphology, presence or absence of a flagellum, and size. As expected, while promastigotes predominated within infected cells at 2 hours post-infection, there was a significant increase in the number of intracellular amastigotes by 4 hours post-infection (Fig. 2B). These data suggest that at the time of observed ERK1/2 phosphorylation, L. amazonensis parasites were found within late endosomal/lysosomal compartments, and were undergoing expected transformation into amastigotes.
3.3. Intracellular-phosphorylated ERK co-localizes with L. amazonensis
We have previously shown that L. amazonensis promastigote-infected BMDC had significant and robust ERK1/2 phosphorylation 3–4hr post-infection as compared to mock-treated or L. major-infected BMDC [11]. In addition, we demonstrated that at this time, parasites were within late endosomal compartments (Fig. 2A). Based on these findings, we hypothesized that L. amazonensis-mediated ERK1/2 activation may localize to this compartment. To test this hypothesis we infected BMDC with fluorescently-labeled L. amazonensis promastigotes and identified the location of phosphorylated ERK1/2 using intracellular immunofluorescence analysis and sequential scanning confocal microscopy. Green cytosolic staining indicated phospho-ERK1/2 in close apposition with parasites within BMDC (Fig. 3A). Immunofluorescence particle co-localization analysis showed that phospho-ERK1/2 co-localized with L. amazonensis promastigotes (red) at three hours post-infection (Fig. 3A, fourth panel), this also occurs at two and four hours post-infection (data not shown). These data corroborate our previous hypotheses indicating L. amazonensis-mediated ERK1/2 phosphorylation occurred from intracellular, parasite-containing, compartments.
Figure 3. Co-localization of L. amazonensis and intracellular phosphorylated-ERK1/2.
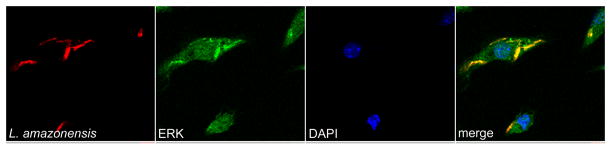
BMDC in 24 well plates containing glass coverslips were infected with CellTracker Orange CMRA-labeled L. amazonensis promastigotes. Sequential scanning confocal microscopy (x60, oil) analysis of L. amazonensis (red) and intracellular phospho-ERK1/2 (green) at 3 hours post-infection. Co-localization of L. amazonensis and phospho-ERK1/2 is observed (fourth column, areas of yellow). Data representative of images from at least three different experiments.
3.4. Scaffold proteins MP1 and MORG1 associated with L. amazonensis-containing organelles
MP1 and MORG1 are structural proteins shown to serve as a scaffold for ERK1/2 phosphorylation on the surface of late endosomal compartments [9, 10]. While MP1 directly connects to late endosomes via heterodimerization with p14, MORG1 can only join the scaffold complex on late endosomes through a direct interaction with MP1 [10, 19]. As phospho-ERK1/2 co-localized with intracellular L. amazonensis parasites within late endosomal compartments, we hypothesized that these scaffold proteins may help tether ERK1/2 to parasite-containing late-endosomes for activation by MEK1/2. We again utilized BMDC infection with fluorescently labeled L. amazonensis and sequential scanning confocal microscopy to determine association between MORG1, MP1 and parasite-containing compartments. We found a cytosolic distribution for MORG1 co-localized with intracellular parasites (Fig. 4A). We similarly found that MP1 co-localized with L. amazonensis (Fig. 4B). This immunofluorescent labeling was shown to be host-MP1 or MORG1 specific and not due to specific or non-specific labeling of the parasite (supplemental Fig. 1) MORG1 and MP1 were associated with intracellular L. amazonensis and may mediate observed L. amazonensis-dependent activation of ERK1/2.
Figure 4. Co-localization of the scaffold proteins MORG1 and MP1 with L. amazonensis within infected BMDC.
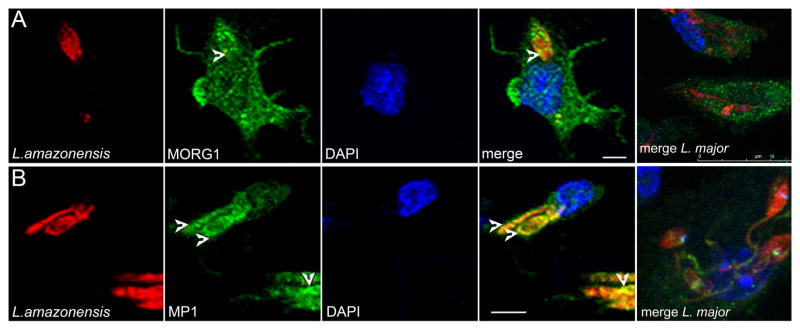
BMDC were infected with CellTracker Orange CMRA-labeled L. amazonensis promastigotes. Sequential scanning confocal microscopy (x60, oil) analysis of L. amazonensis (red) and intracellular (A) MORG1 or (B) MP1 (green) at 3 hours post-infection. Detailed images of one infected cell. Comparison overlay image of L. major-infected BMDC 3 hrs post-infection shown on far right. Images indicate areas of co-localization between parasite (arrows) and MORG1 and MP1, indicated by areas of yellow.
3.5. G protein-coupled receptor upstream of L. amazonensis-dependent ERK1/2 phosphorylation
Little is known regarding regulation and recruitment of MP1 or MORG1 to late endosomal compartments. However, of the two, MORG1 appears to show more selectivity as it is only recruited to endosomal ERK1/2-phosphorylation scaffolds after G protein-coupled receptor (GPCR) activation [1]. To determine whether GPCR activation was required for L. amazonensis-mediated ERK1/2 phosphorylation, perhaps correlating with MORG1 localization to the signaling scaffold, we pre-treated BMDC with Pertussis toxin (PTX), which binds to the alpha subunit of Gα-i/o subfamily of G proteins and prevents their activation. When BMDC were pre-treated with PTX and then infected with L. amazonensis promastigotes, we observed a significant decrease in ERK1/2 phosphorylation as compared to untreated, infected cells (Fig. 5). PTX treatment did not decrease parasite infection in these cells (data not shown). These data suggest that activation of the Gα-i/o subfamily was involved in mediating enhanced L. amazonensis-dependent ERK1/2 activation, possibly through recruitment of MORG1 to late endosomal compartments.
Figure 5. Pertussis toxin treatment reduces L. amazonensis-dependent ERK1/2 phosphorylation and MORG1 co-localization.
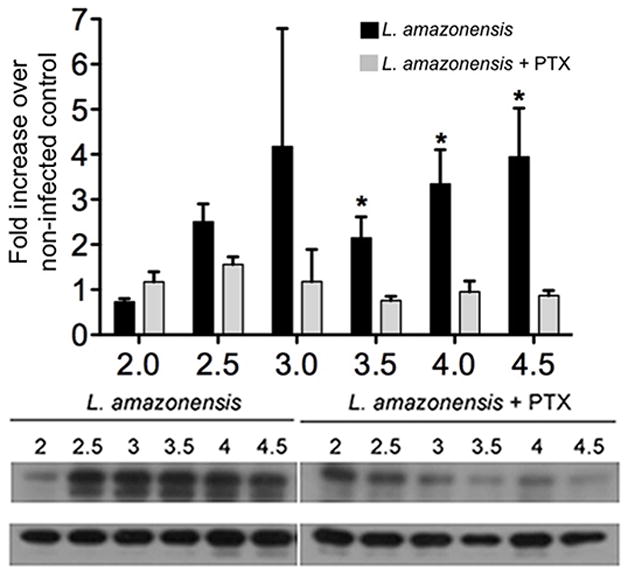
BMDC were infected with L. amazonensis promastigotes and left untreated or treated with Pertussis toxin (100 ng/ml) and incubated at 34 °C with 5% CO2. BMDC were harvested at the indicted time points and total cell lysates were made. Samples were analyzed via western blot for phosphorylated and total ERK1/2. Densitometry values were normalized to total ERK and then to non-infected controls. Densitometry analysis of at least three different experiments and representative blot are shown; error bars denote ±SEM, *p≤0.05.
3.6. L. amazonensis-mediated ERK activation requires MP1-MEK binding
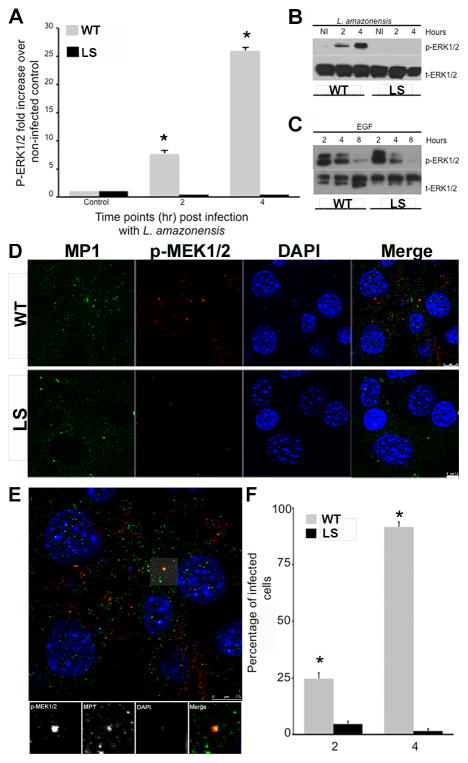
Co-localization of MORG1 and MP1 at the surface of L. amazonensis PV corresponding to ERK1/2 phosphorylation suggested that this scaffold was needed for L. amazonensis-mediated ERK phosphorylation from the PV. We wanted to determine if MP1-MEK binding was required for parasite-mediated ERK phosphorylation. We infected LS cells, in which MEK1 lacks the ability to interact and bind to MP1 (supplemental Fig. 2) and assessed the level of ERK phosphorylation at 2 and 4 hours post-L. amazonensis infection. The absence of MP1-MEK interaction resulted in significantly reduced ERK1/2 phosphorylation following L. amazonensis infection of LS cells as measured via western blot (Fig. 6A and B) when compared to WT cells. To confirm that abrogation of ERK1/2 phosphorylation was specific to endosomal, MP1-mediated signaling and did not affect ERK1/2 signaling from other cellular locations, we stimulated LS and WT cells with epidermal growth factor (EGF). EGF triggers plasma membrane-localized ERK1/2 phosphorylation, independent of MP1-based scaffolds [20]. EGF treatment resulted in similar ERK phosphorylation between LS and WT cells (Fig. 6C). These findings indicate that MP1-MEK interaction is required for L. amazonensis-mediated ERK1/2 phosphorylation
Figure 6. L. amazonensis mediated ERK1/2 phosphorylation requires the MP1 scaffold complex located specifically at the endosome, and co-localization of MP1 and p-MEK1/2 in WT and LS cells.
Wildtype (WT) and MEK mutant fibroblasts (LS cells) were infected with L. amazonensis parasites at the indicated time points. Whole cell lysates were collected and analyzed via western blot for phosphorylated-ERK1/2 as well as for total ERK1/2. (A) Densitometry analysis for at least four independent experiments. (B) Representative western blot showing robust increase in ERK1/2 phosphorylation in L. amazonensis-infected WT cells as compared to LS cells. (C) Representative western blot showing similar ERK phosphorylation in LS and WT MEF cells following EGF stimulation. Error bars denote ±SD, *p≤0.05. (D) WT and LS MEF cells infected with L. amazonensis. Confocal microscopy (x60, oil) analysis of MP1 (green), p-MEK1/2 (red), L. amazonensis and fibroblast nuclei (DAPI, blue) at four hours post-infection. Co-localization of MP1 and p-MEK1/2 is observed (top panel, fourth column, areas of bright orange/yellow). (E) Co-localization of MP1 and p-MEK in WT cells. Inset is a zoom magnification showing co-localization of MP1, p-MEK, and close proximity of L. amazonensis (blue nuclei). (F) Counts of number of cells with co- localization of MP1 and p-MEK1/2. Values in indicate number of cells containing co- localization events of MP1/pMEK1/2. Data is from three different experiments, 300 cells counted per coverslip, for a total of 900 cells counted per timepoint.
3.7. L. amazonensis/MP1/p-MEK1/2 colocalization lacking in LS Mutant cells
Cellular scaffolds are critical for bolstering both signal amplitude and signal location. We have demonstrated that the MP1-scaffold is important for the presence and amplitude of L. amazonensis-mediated ERK1/2 phosphorylation. We additionally wanted to assess the level of MP1/MEK/L. amazonensis interaction following L. amazonensis promastigote infection of LS cells. Immunofluorescence and confocal microscopy were performed to identify the location of MP1, MEK, and parasite nuclei. At four hours post-infection we observed significantly more phospho-MEK in WT cells compared to LS cells (Fig. 6D, second panel). Co-localization of MP1 and phospho-MEK was observed in WT and not in LS (mutant) cells (Fig. 6E). Number of cells with red and green signal in the same space (p-MEK and MP1) were counted at 100x magnification for both WT and LS cells, using three coverslips, 300 cells per coverslip, for a total of 900 cells counted per time post-infection, for both 2 and 4 hours post L. amazonensis infection. Significantly more WT cells had overlapping signal as compared to LS cells at both times points, although more pronounced by 4h post-infection (Fig. 6F). Z-stack analysis showed colocalization of parasite nuclei with MP1 and p-MEK activation (Supplemental fig. 3,4). Together these findings demonstrate that following L. amazonensis infection, MP1-MEK interaction is required for endosomal MEK phosphorylation and parasite localization to the MP1 scaffold with subsequent downstream robust phosphorylation of ERK1/2.
Discussion
L. amazonensis modulates DC maturation through activation of ERK1/2 [11], altering a critical step for promotion of a T helper 1 immune response and clearance of L. amazonensis infection [21–23]. It was previously unknown if L. amazonensis-mediated ERK1/2 phosphorylation was an active process by the parasite or produced by cell entry via phagocytosis. Treatment of BMDC with heat-killed L. amazonensis promastigotes resulted in significantly decreased ERK1/2 phosphorylation compared to BMDC treated with live parasites (Fig. 1), indicating that ERK1/2 phosphorylation was dependent on viable, intracellular L. amazonensis parasites. Due to the delay in ERK1/2 phosphorylation, and the predominance of amastigotes at the time points corresponding to ERK1/2 phosphorylation (Fig. 2), we propose that it is the amastigote form of the parasite that is responsible for initiating this signaling cascade. Amastigotes have been previously shown to alter host cell processes, including signaling, to establish infection and persist within the phagolysosome [24]. Further studies are required to determine the identity of putative amastigote factors that may meditate ERK1/2 phosphorylation.
We and others have previously reported activation of MAP kinase ERK1/2 during L. amazonensis infection [11, 25], which we showed did not occur after L. major infection of BMDC. In this manuscript we show co-localization of phosphorylated ERK1/2 with intracellular L. amazonensis (Fig. 3A and B), suggesting activation of this signaling cascade from the membrane of an intracellular vesicle. While the plasma membrane is the canonical site for signal transduction initiation, signaling from intracellular organelles has been described for the MAP kinase pathway [8]. Scaffold proteins have emerged as critical mediators in the regulation of ERK1/2 signaling from subcellular compartments. These molecules act by binding one or more kinases and regulating association with other kinases or substrates, enhancing their activity and specificity as well as determining specific subcellular localization for that signaling complex [6]. MP1 and MORG1 are two such scaffold proteins shown to mediate ERK1/2 activation from late endosomal compartments. We show that MP1, MORG1, or both are recruited to parasite-containing organelles and mediate phosphorylation of ERK1/2. Previous studies have shown that MORG1 is recruited to endosomal compartments under specific conditions, specifically when G-protein coupled receptors (GPCR) are stimulated [10]. Inhibition of MORG1 recruitment results in decreased ERK1/2 phosphorylation following receptor stimulation, as MORG1 functions to enhance activation of this kinase from late endosomal compartments [10]. Treatment of L. amazonensis-infected BMDC with Pertussis toxin (PTX), a known Gαi/o subunit inhibitor, led to a decrease in ERK1/2 phosphorylation as compared to non-treated cells (Fig. 5). This observation suggests a role for Gαi/o signaling and MORG1/MP1 scaffold-dependent modulation of ERK1/2 phosphorylation following L. amazonensis infection (Fig. 7).
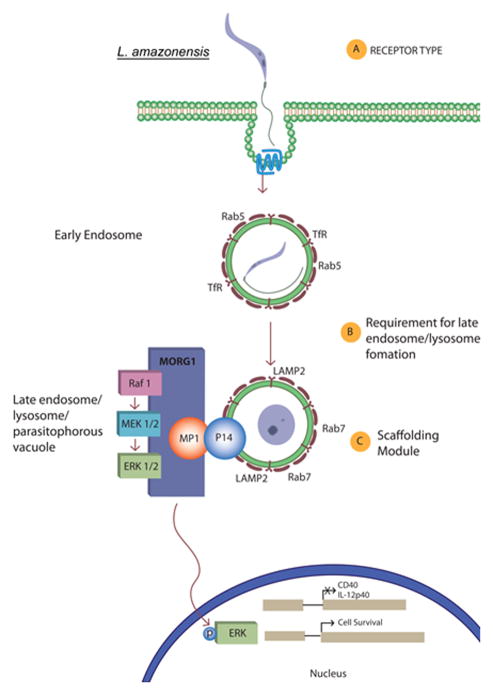
Figure 7. Model. Proposed Leishmania entry into BMDC with MORG1/MP1 scaffolding complex assembly at the phagolysosome.
(A) Upon uptake of L. amazonensis into a BMDC, in conjunction with Gα-i/o GPCR signaling (blue), the parasite is housed initially within an early phagosome, typically characterized by markers such as Rab5 and transferrin receptor (TfR). (B) As maturation into a phagolysosome occurs, there is an increase of late endosomal markers such as LAMP2 and Rab7. (C) At this stage, when the parasite has begun transformation into the amastigote form, that downstream of with Gα-i/o GPCR signaling, proposed host and parasite factors stimulate interaction between MORG1, MP1 and MEK1/2 at the phagolysosome surface. This interaction augments L. amazonensis-mediated MEK phosphorylation of ERK at the phagolysosome surface. This in turn allows p-ERK to translocate to the nucleus and further activate downstream substrates which prevent dendritic cell surface expression of CD40, production of IL-12 p40 and modulates DC maturation, while promoting DC survival.
Given these findings, we sought to assess the role of another scaffold protein required for endosomal ERK1/2 signaling, MP1. MEK-mutant MEF LS cells showed nearly complete abrogation of ERK1/2 phosphorylation, indicating that interaction of MP1 with MEK1 is essential for parasite-mediated ERK1/2 phosphorylation (Fig. 6). However, when these cells were stimulated with epidermal growth factor (EGF), which triggers ERK1/2 signaling from the plasma membrane, ERK1/2 phosphorylation remained intact. These findings indicate that there is no deficiency in pan-ERK1/2 signaling (Fig. 6C) in these mutant cells. Confocal microscopy demonstrated that in LS cells neither MP1 and phospho-MEK1/2 nor MP1 and L. amazonensis co-localize (Fig. 6D). These data demonstrate that L. amazonensis-mediated ERK1/2 phosphorylation requires a functional endosomal MEK1/2-MP1 scaffold for both signal strength and location of signal to the parasitophorous vacuole.
The p14/MP1 scaffold complex was recently been shown as important for host-pathogen interaction in a Salmonella infection model, as lack of function of this complex induced increased bacterial replication [26]. These findings suggest that the p14/MP1 complex has a positive role in clearing Salmonella infection. In contrast, our current and previous findings [11], suggest that the MP1 scaffolding complex enhances maintenance of L. amazonensis intracellular infection. It is likely, therefore, that the p14/MP1 complex may play different roles in determining the course of infection depending on pathogen virulence. While Salmonella results in sub-acute to acute infection, L. amazonensis persists within the host, and must alter its environment in order to do so [27].
The p14/MP1 complex, which is present in the endosome, serves in anchoring MEK1/2 to the late endosomal surface and augmenting the activation of ERK1/2, which is then free to activate further downstream substrates that inhibit BMDC maturation (Fig. 7). This complex has also has recently been shown to be part of the “ragulator” complex, comprised of p14, p18 and MP1, necessary for activation and targeting of mTORC1 to the lysosomal surface [28]. Activation of mTORC1 is important for regulation of autophagy during nutrient-rich conditions [29]. It has been previously demonstrated that L. amazonensis-mediated induction of autophagy in macrophages increases parasite load, suggesting perhaps that L. amazonensis utilizes this pathway in other ways as well to promote its survival [30]. Understanding whether autophagy is altered through this signaling scaffold complex during L. amazonensis infection ia a potential additional area of study. Inhibition of the p14/MP1 scaffolding complex could lead to both reduced autophagy and reduced ERK1/2 phosphorylation in L. amazonensis-infected macrophages with subsequent increased parasite clearance.
L. amazonensis-mediated ERK1/2 phosphorylation utilized a novel means of spatial targeting to the endosomal surface, a scaffold formed by the modular proteins MP1, MORG1 and likely others. Moreover, MP1/MEK1/2 interaction was required for parasite-dependent ERK1/2 signaling at an endosomal location. We previously demonstrated that ERK1/2 phosphorylation following L. amazonensis infection leads to impaired DC maturation and decreased parasite removal [11, 31]. Inhibition of ERK1/2 phosphorylation restores DC maturation in vitro and partial recovery in vivo [11]. Our findings provide a specific, scaffold-mediated, mechanism of L. amazonensis-induced ERK1/2 signaling. This level of target and location specificity would allow for development of drug targets that specifically modify parasite mediated-ERK1/2 phosphorylation and enhance host immune function and parasite clearance during L. amazonensis infection.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Guglielmo GM, Baass PC, Ou WJ, Posner BI, Bergeron JJ. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. Embo J. 1994;13:4269–4277. doi: 10.1002/j.1460-2075.1994.tb06747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SSG, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- 4.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 5.Taub N, Teis D, Ebner HL, Hess MW, Huber LA. Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling. Mol Biol Cell. 2007;18:4698–4710. doi: 10.1091/mbc.E07-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolch W, Calder M, Gilbert D. When kinases meet mathematics: the systems biology of MAPK signalling. FEBS Lett. 2005;579:1891–1895. doi: 10.1016/j.febslet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Scott JD, Pawson T. Cell Signaling in Space and Time: Where Proteins Come Together and When They’re Apart. Science. 2009;326:1220–1224. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP. Scaffold proteins of MAP-kinase modules. Oncogene. 2007;26:3185–3202. doi: 10.1038/sj.onc.1210411. [DOI] [PubMed] [Google Scholar]
- 9.Schaeffer HJ, Catling AD, Eblen ST, Collier LS, Krauss A, Weber MJ. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science. 1998;281:1668–1671. doi: 10.1126/science.281.5383.1668. [DOI] [PubMed] [Google Scholar]
- 10.Vomastek T, Schaeffer HJ, Tarcsafalvi A, Smolkin ME, Bissonette EA, Weber MJ. Modular construction of a signaling scaffold: MORG1 interacts with components of the ERK cascade and links ERK signaling to specific agonists. Proc Natl Acad Sci U S A. 2004;101:6981–6986. doi: 10.1073/pnas.0305894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boggiatto PM, Jie F, Ghosh M, Gibson-Corley KN, Ramer-Tait AE, Jones DE, Petersen CA. Altered dendritic cell phenotype in response to Leishmania amazonensis amastigote infection is mediated by MAP kinase, ERK. Am J Pathol. 2009;174:1818–1826. doi: 10.2353/ajpath.2009.080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giroux S, Tremblay M, Bernard D, Cardin-Girard JF, Aubry S, Larouche L, Rousseau S, Huot J, Landry J, Jeannotte L, Charron J. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr Biol. 1999;9:369–372. doi: 10.1016/s0960-9822(99)80164-x. [DOI] [PubMed] [Google Scholar]
- 13.Pullikuth A, McKinnon E, Schaeffer HJ, Catling AD. The MEK1 scaffolding protein MP1 regulates cell spreading by integrating PAK1 and Rho signals. Mol Cell Biol. 2005;25:5119–5133. doi: 10.1128/MCB.25.12.5119-5133.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones DE, Ackermann MR, Wille U, Hunter CA, Scott P. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect Immun. 2002;70:2151–2158. doi: 10.1128/IAI.70.4.2151-2158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortez M, Huynh C, Fernandes MC, Kennedy KA, Aderem A, Andrews NW. Leishmania promotes its own virulence by inducing expression of the host immune inhibitory ligand CD200. Cell Host Microbe. 2011;9:463–471. doi: 10.1016/j.chom.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morehead J, Coppens I, Andrews NW. Opsonization modulates Rac-1 activation during cell entry by Leishmania amazonensis. Infect Immun. 2002;70:4571–4580. doi: 10.1128/IAI.70.8.4571-4580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kima PE, Burleigh B, Andrews NW. Surface-targeted lysosomal membrane glycoprotein-1 (Lamp-1) enhances lysosome exocytosis and cell invasion by Trypanosoma cruzi. Cell Microbiol. 2000;2:477–486. doi: 10.1046/j.1462-5822.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- 18.Korner U, Fuss V, Steigerwald J, Moll H. Biogenesis of Leishmania major-harboring vacuoles in murine dendritic cells. Infect Immun. 2006;74:1305–1312. doi: 10.1128/IAI.74.2.1305-1312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teis D, Wunderlich W, Huber LA. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev Cell. 2002;3:803–814. doi: 10.1016/s1534-5807(02)00364-7. [DOI] [PubMed] [Google Scholar]
- 20.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 21.Ramer AE, Vanloubbeeck YF, Jones DE. Antigen-Responsive CD4+ T Cells from C3H Mice Chronically Infected with Leishmania amazonensis Are Impaired in the Transition to an Effector Phenotype. Infect Immun. 2006;74:1547–1554. doi: 10.1128/IAI.74.3.1547-1554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marovich MA, McDowell MA, Thomas EK, Nutman TB. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J Immunol. 2000;164:5858–5865. doi: 10.4049/jimmunol.164.11.5858. [DOI] [PubMed] [Google Scholar]
- 23.McDowell MA, Sacks DL. Inhibition of host cell signal transduction by Leishmania: observations relevant to the selective impairment of IL-12 responses. Curr Opin Microbiol. 1999;2:438–443. doi: 10.1016/S1369-5274(99)80077-0. [DOI] [PubMed] [Google Scholar]
- 24.Kima PE. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int J Parasitol. 2007;37:1087–1096. doi: 10.1016/j.ijpara.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Mosser DM, Zhang X. Activation of the MAPK, ERK, following Leishmania amazonensis infection of macrophages. J Immunol. 2007;178:1077–1085. doi: 10.4049/jimmunol.178.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taub N, Nairz M, Hilber D, Hess MW, Weiss G, Huber LA. The late endosomal adaptor p14 is a macrophage host-defense factor against Salmonella infection. J Cell Sci. 2012;125:2698–2708. doi: 10.1242/jcs.100073. [DOI] [PubMed] [Google Scholar]
- 27.Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag Complex Targets mTORC1 to the Lysosomal Surface and Is Necessary for Its Activation by Amino Acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–914. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinheiro RO, Nunes MP, Pinheiro CS, D’Avila H, Bozza PT, Takiya CM, Corte-Real S, Freire-De-Lima CG, DosReis GA. Induction of autophagy correlates with increased parasite load of Leishmania amazonensis in BALB/c but not C57BL/6 macrophages. Microbes Infect. 2009;11:181–190. doi: 10.1016/j.micinf.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Mukbel RM, Patten C, Jr, Gibson K, Ghosh M, Petersen C, Jones DE. Macrophage killing of Leishmania amazonensis amastigotes requires both nitric oxide and superoxide. Am J Trop Med Hyg. 2007;76:669–675. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.