Abstract.
Mirror therapy is a therapy to treat patients with pain syndromes or hemiparesis after stroke. However, the underlying neurophysiologic mechanisms are not clearly understood. In order to determine the effect of a mirror-like illusion (MIR) on brain activity using functional near-infrared spectroscopy, 20 healthy right-handed subjects were examined. A MIR was induced by a digital horizontal inversion of the subjects’ filmed hand. Optodes were placed on the primary motor cortex (M1) and the occipito-parietal cortex (precuneus, PC). Regions of interest (ROI) were defined a priori based on previous results of similar studies and confirmed by the analysis of effect sizes. Analysis of variance of the ROI signal revealed a dissociated pattern: at the PC, the MIR caused a significant inversion of a hemispheric lateralization opposite to the perceived hand, independent of the moving hand. In contrast, activity in M1 showed lateralization opposite to the moving hand, but revealed no mirror effect. These findings extend our understanding on interhemispheric rivalry and indicate that a MIR is integrated into visuomotor coordination similar to normal view, irrespective of the hand that is actually performing the task.
Keywords: functional near-infrared spectroscopy, optical imaging, mirror illusion, mirror therapy, precuneus
1. Introduction
The mirror illusion describes a phenomenon where movements of one limb are perceived as movements of the opposite one. In recent years, evidence for a therapeutic use of the mirror illusion (“mirror therapy”) has increased significantly,1,2 especially for the treatment of limbs affected by pain syndromes3–5 or hemiparesis after stroke.6 In spite of its increasing clinical popularity, the presumed cortical mechanisms of the mirror illusion and mirror therapy remain not satisfactorily understood.7,8
In normal subjects, inversion of the visual feedback has already been shown to lead to an activation of the hemisphere ipsilateral to the moving hand.9–13 However, in these studies, a change of activity in the ipsilateral primary motor cortex (M1) could not been established consistently. Similarly, studies using transcranial magnetic stimulation did not show consistent increase of cortico-muscular excitability due to the mirror illusion.14–16 Apart from M1, Dohle and coworkers suggested an involvement of the precuneus (PC) of either hemisphere.10,11 This area was also found to be activated in stroke patients under mirrored visual feedback only during bimanual movements.17
One of the reasons for this heterogeneity of these results might be the heterogeneity in the chosen methodological approach to implement the mirror illusion. Some studies employed a real mirror and instructed participants to change their direction of gaze.14,18 Other studies employed the so-called “mirror box” which provides the image of two hands moving simultaneously.12 We, thus, decided to investigate this issue in a systematic fashion. In accordance with previous fMRI studies,10,19 we made use of a video setup in order to establish a mirror-like illusion (MIR), allowing a systematic, randomized variation of the visual feedback. In order to allow subsequent studies for comparison of these effects with that of a real mirror, functional near-infrared spectroscopy (fNIRS) was selected for registration of brain activity. fNIRS allows the registration of the hemodynamic response in predefined brain regions. Because of its ease of application and portability, the method is a promising alternative to fMRI in clinical trials.20–24
In accordance with the above-mentioned studies, it was expected that this MIR would increase activation of the PC ipsilateral to the moving hand, i.e., contralateral to the perceived hand. In addition, we expected that—as in the majority of other studies—we would not detect any differences in the activity of M1 due to the MIR.
2. Material and Methods
2.1. Subjects
Twenty right-handed healthy subjects (five females, 21 to 40 years old; mean age 27.7) without any history of neurological or psychiatric disorders were investigated. Handedness was assessed by the German version of the Edinburgh Handedness Inventory.25 The participants were employees or students of the Charité and participated voluntarily in the experiment with financial compensation. The study was approved by the Ethics Committee of the Charité—Universitätsmedizin Berlin, and all subjects gave informed consent prior to the experiment.
2.2. Experimental Design
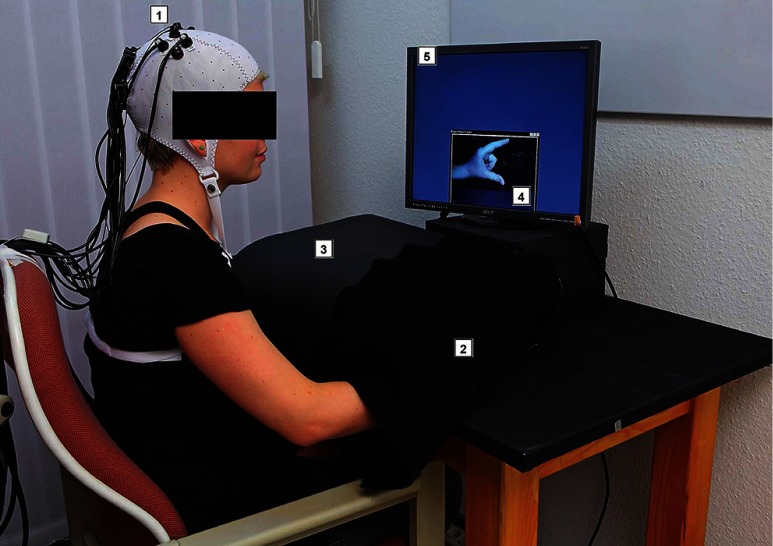
Mirror therapy has been applied using different setups. Originally a “mirror box” was used, which provides the visual image of two hands moving simultaneously.3 Subsequent studies mainly employed a real mirror located at the patients’ midsagittal plane.15 In order to allow a systematic and randomized variation of the seen and moved hand without changing other cofactors, we implemented a mirror-like paradigm in accordance with the previous study of Dohle et al.10 by means of a video chain (Fig. 1). The lateralized cerebral activation elicited by this setup was not shown to be confounded by hemifield stimulation.19 Subjects had to either hold their hand static or perform a finger-thumb opposition movement sequence under visual control. Subjects could not see their hand directly, as it was covered by a black paperboard in front of them, but viewed it via a video chain. The subject’s hand was filmed online with a video webcam (Logitech Webcam Pro 9000). By means of a software package (Logitech Webcam Software v1.1, frame rate: 50 Hz), the image was displayed in real time on a screen (Acer, pixel, frame rate: 60 Hz) in front of the subjects with 0-deg eccentricity. Thus, the delay was maximal 20 ms which is well below the threshold of subjective awareness.26
Fig. 1.
Photography of the experimental setup: (1) Subject wearing an EEG cap with functional near-infrared spectroscopy (fNIRS) detectors and sources; (2) subject’s hand inside the black paperboard covered by a black drapery being filmed by (3) a web-cam inside the black paperboard; (4) visual feedback the subject gets of his hand, in this case mirror-like illusion (MIR) on (5) a precuneus (PC) monitor. Not in the picture: control PCs.
The distance between subject and screen was approximately 55 cm. The size of the outer frame was ( pixel). The size of the online film of the hand was individually different, because we adopted this to the real size of the subject’s hand, but for all subjects it fitted into the outer frame, which means the stimulus was not bigger than , i.e., not bigger than 21-deg horizontal and 16-deg vertical visual angle.
There were two movement tasks: The first one (“movement”) consisted of moving the thumb and the index finger sequentially at three different opposition distances. The opposition distances reached from just not touching both fingers to maximal comfortable distance and movement sequences were performed at an individually chosen rate of approximately 1 Hz. The second task (“static”) consisted of holding the hand in a static posture with the thumb and index finger opposing each other at a distance of a few millimeters. Both finger movement tasks were demonstrated and explained verbally by the investigator. During these tasks, the filmed image of the hand could be inverted horizontally in such a way that the subjects’ right hand appeared as if it was their left hand and vice versa (“mirror”). With the horizontal inversion of the video image, either normal visual feedback (NOR) or a MIR could be induced. This results in the following four randomized presented conditions:
-
•
Finger movements, normal (movement, NOR)
-
•
Finger movements, mirrored (movement, MIR)
-
•
No movements, normal (static, NOR)
-
•
No movements, mirrored (static, MIR).
As previous studies showed no effect of the MIR neither on eye movements10 nor on muscle activity, as assessed with electromyography,18,27 these parameters were not recorded in this experiment.
For each hand, a total number of 80 trials under these four conditions were arranged in randomized sequences. Each trial was initiated by an acoustic cue (“start” or “stop”) and lasted 10 s followed by a rest period with an average duration of 15 s (jittered from 10 to 20 s), where only a black screen was shown. This rest period served as a baseline for the correction in the analysis. Each condition was presented 20 times, yielding in a total experimental time of 38 min per hand. Each hand was examined separately, and the whole experiment lasted approximately an hour and a half. During the whole experiment, the subjects were instructed to watch the screen, even during the rest period and to focus the hand during the active tasks (“static” and “movement”). The subjects’ adherence to the task was ensured by the investigator, who was present during the entire experiment.
By comparing corresponding channels in both hemispheres, a study design with the four factors movement (movement/static), mirror (MIR/NOR), hand (left/right), and hemisphere (ipsilateral/contralateral to the moving hand) was applied, resulting in 16 different conditions.
2.3. fNIRS Data Acquisition
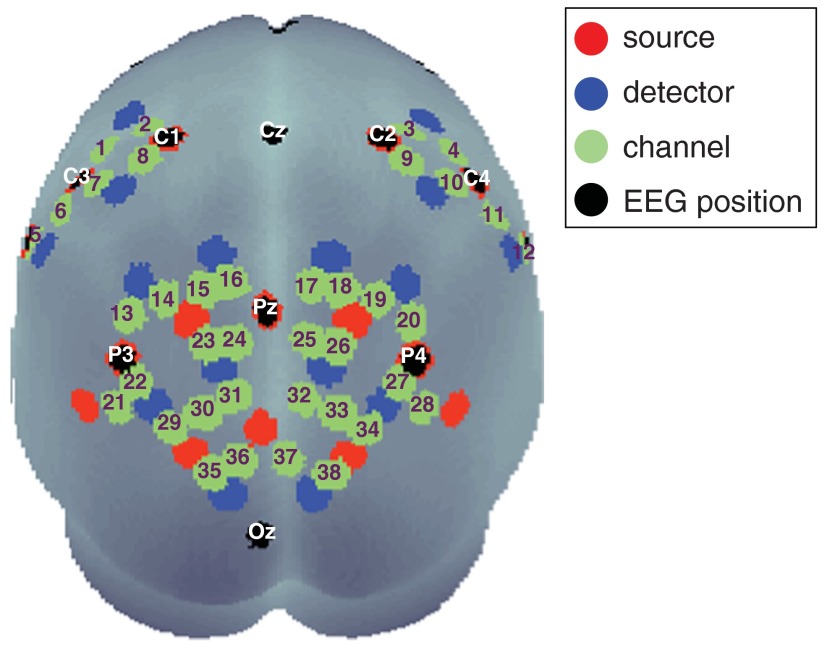
During the experiment, the blood oxygenation at the surface of the subjects’ brain was measured with a fNIRS system which offers up to 16 detectors and 16 emitters (NIRScout 16-16, NIRx Medizintechnik GmbH, Berlin, Germany) at two wavelengths (850 and 760 nm). Based on previous findings on the role of the PC during movement mirroring,10,11 we chose fiber optode positions to cover the bilateral occipito-parietal and precentral areas of the subject’s head, providing a total of 38 useful channels where source and detector were placed at a distance of between 2.5 and 3 cm from each other. This arrangement is shown in Fig. 2. To guarantee optimal safety in optode localization and convenience for the subjects, the emitters and detectors were integrated into a commercially available EEG cap (www.easycap.de) with 128 possible positions. fNIRS data were continuously sampled at 3.13 Hz.
Fig. 2.
Display of optode positions, measurement channels (numbered in green), including main EEG positions from the standard 10–20 system (white labels). A number of fNIRS sources are at the same location as main standard EEG position.
2.4. Preprocessing of fNIRS Data
Data analysis was performed using software routines employing the software packet Matlab (Mathworks Inc., Natick, Massachusetts, version 7.5.0.342 R2007b). fNIRS data were corrected for movement artifacts by a semiautomated approach, which replaces contaminated data segments by linear interpolation.28 Subsequently, attenuation changes of both wavelengths were transformed to concentration changes of oxy- and deoxygenated hemoglobin (HbO and HbR) using a modified Beer–Lambert law [differential pathlength factors: 5.98 (higher wavelength: 850 nm), 7.15 (lower wavelength: 760 nm), extinction coefficients for HbO 2.53/1.49 (higher/lower wavelength) and HbR 1.80/3.84 (higher/lower wavelength), and an interoptode-distance of 3 cm].29 Data were then band-pass filtered between 0.2 and 0.016 Hz (using a 3rd order Butterworth filter) to attenuate for heartbeat, breathing-related changes, and drifts.
For effect estimation, a general linear model (GLM) was run in all subjects. Regressors were composed by convolution of stimulus onset and length with a hemodynamic response function.30 For each subject, condition and channel, the GLM provided two beta values (for HbO and HbR, respectively), indicating the strength of the modulation of the hemodynamic response. It should be noted that the stronger activity is indicated by positive beta values for HbO and by negative beta values for HbR.31,32
2.5. Definition of the Regions of Interest
For visual inspection and demonstration, we employed the freeware MATLAB toolbox NFRI (http://brain.job.affrc.go.jp/tools/) described by Singh et al.,33 which takes EEG 10 to 20 positions as references to estimate the brain regions underlying the channel locations. This toolbox also enables statistical results for each channel to be plotted on the surface of a schematic brain (already used for Fig. 2).
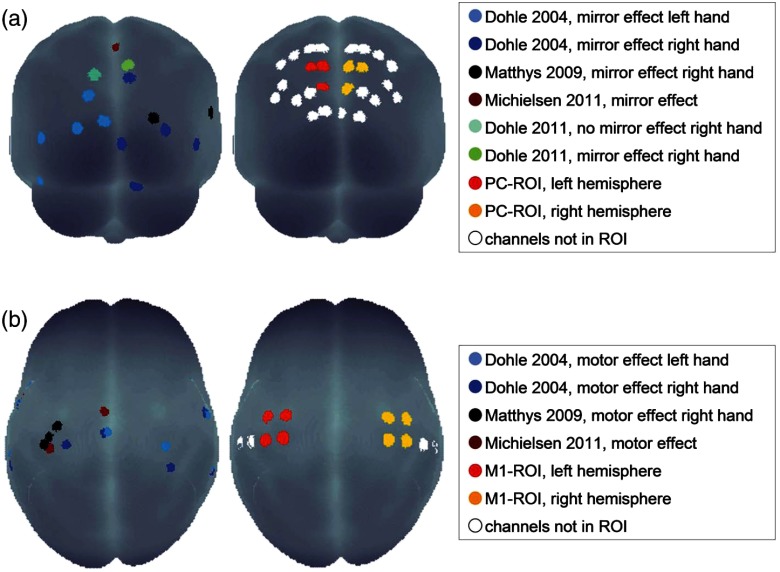
Regions of interest (ROI) were defined corresponding to the activation foci of the above-mentioned studies: in the occipito-parietal cortex (PC), the three channels displayed in Fig. 3(a), right sides were chosen (further referred to as “PC-ROI”) for both hemispheres [i.e., for the left hemisphere (LH): channel numbers 23, 24, and 31; for the right hemisphere (RH): channel numbers 25, 26, and 32; see also Fig. 2]. The M1-ROI was defined as channels around EEG positions C3 and C4, known to cover the precentral regions of the brain.34 Therefore, the four midsagittal channels on either hemisphere were selected (i.e., for the LH: channel numbers 1, 2, 7, and 8; for the RH: channel numbers 3, 4, 9, and 10; see also Fig. 2). This selection was in agreement with the analysis of statistical effect sizes (see Appendix).
Fig. 3.
Left: Activation foci from previous imaging studies projected onto the surface of a standardized brain for (a) mirror effect in occipito-parietal area, and (b) movement effect in precentral area. Right: Channels chosen as regions of interest (ROI) for further statistical analysis in the area of the PC (a) and precentral areas (b).
2.6. Time Courses
For both ROIs, baseline-corrected time courses were calculated averaged across subjects and smoothed (moving window of 10 s) for the MIR and NOR conditions separately.
2.7. Statistical Analysis
Statistical analysis was performed with the software packet PASW Statistics (SPSS Inc., Chicago, Illinois, version 18.0.0). For statistical group analysis, a repeated measure four-way analysis of variance (ANOVA) with the factors movement (movement/static), mirror (MIR/NOR), hand (left/right), and hemisphere (ipsilateral/contralateral) was conducted for the mean beta values of the ROIs. As M1 activation is much greater in the movement condition compared with the static condition,10 a further three-way repeated measures ANOVA with the factors mirror (MIR/NOR), hand (left/right), and hemisphere (ipsilateral/contralateral) was applied on the mean beta values of the M1-ROI for the trials with movement only. Level of significance was always set at . The ANOVA analysis was not corrected for multiple-comparison. Post-hoc analysis was performed using paired -tests for all significant interactions of the ANOVAs. As the ANOVA is classified as a so called Omnibus test, the post-hoc tests were not Bonferroni corrected.35
3. Results
3.1. Effects at the PC-ROI
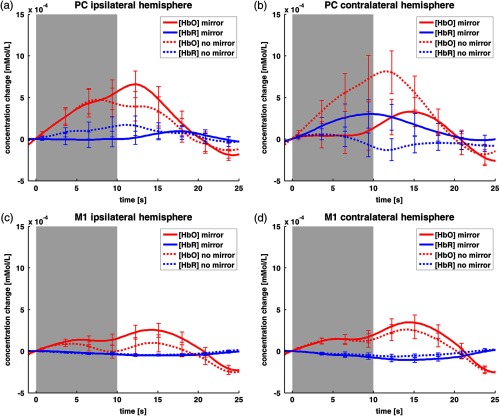
Baseline-corrected time courses for the PC-ROI are shown in Fig. 4, upper panel. In the hemisphere ipsilateral to the moving hand, we observed a stronger change in concentration of both HbO and HbR (Fig. 4, left side) in the MIR condition compared with the NOR condition. This difference peaks at about 10 s after stimulus onset. In contrast, in the hemisphere contralateral to the moving hand (Fig. 4, right side), there is a less concentration change in the MIR compared with the NOR condition for both wavelengths.
Fig. 4.
Time courses averaged over all subjects () of the PC- (a, b) and M1-ROIs (c, d), contra- (a, c) and ipsilateral (b, d) to the moving hand. The duration of stimulus presentation is shaded in gray. HbO is presented in red, while HbR is coded in blue. Time courses have error bars with standard error of the mean.
Mean beta values for the PC-ROI are shown in Fig. 5(a)–5(c), upper panel and in Table 1. Mean beta values indicated a stronger activation in the MIR than in the NOR condition ipsilateral. In contrast, activation was reduced in the MIR as compared with the NOR condition contralateral. Mean beta values for HbR indicate the same as for HbO, respectively. Figure 5(c) also displays the mean interhemispheric difference between the ipsi- and contralateral hemisphere for MIR and NOR conditions.
Fig. 5.
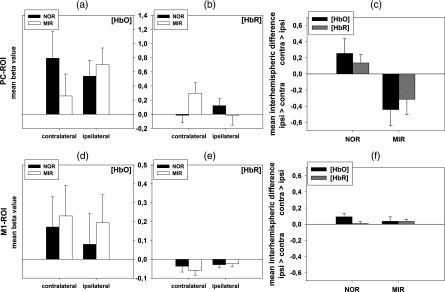
Mean beta values and differences, and their corresponding standard errors at the PC-ROI (top row, a–c) and M1-ROI (bottom row, d–f). Left column (): mean beta values for HbO of the ipsi- and contralateral hemisphere for the MIR and NOR conditions; middle column (): mean beta values for HbR of the ipsi- and contralateral hemisphere for the MIR and NOR condition; right column (): mean interhemispheric differences in the MIR and NOR conditions for HbO and HbR (multiplied by ). Note that, in the figures of the right column, values indicate activation in the contralateral ipsilateral hemisphere, and values indicate activation ipsilateral contralateral.
Table 1.
Mean beta values and standard errors (in brackets) for PC-ROI (top rows) and M1-ROI (bottom rows). Left column: mean beta values for HbO. Right column: mean beta values for HbR.
| HbO | HbR | ||
|---|---|---|---|
| PC-ROI | |||
| Movement | 0.80 (0.18) | 0.07 (0.09) | |
| Static | 0.35 (0.12) | 0.13 (0.08) | |
| MIR ipsilateral | 0.70 (0.24) | (0.14) | |
| NOR ipsilateral | 0.54 (0.23) | 0.12 (0.10) | |
| MIR contralateral | 0.26 (0.31) | 0.30 (0.15) | |
| NOR contralateral | 0.79 (0.37) | (0.11) | |
| M1-ROI | |||
| Movement | 0.26 (0.18) | (0.02) | |
| Static | 0.08 (0.13) | 0.003 (0.03) | |
| Movement trials only | contralateral | 0.33 (0.19) | (0.03) |
| ipsilateral | 0.19 (0.18) | (0.02) | |
The repeated measures four-way ANOVA over the mean beta values of the PC-ROI showed a significant main effect of the factor movement [; , ] for HbO. The mean beta values indicate more activation in movement conditions as compared with static conditions (Table 1).
Furthermore, the ANOVA showed a significant two-way interaction of the factors [; , ] for HbO. Post-hoc analysis of this two-way interaction revealed no significant effect in the paired -tests. This two-way interaction of the factors was similar for HbR, but failed to reach significance [; , ].
No other effects reached significance in the PC-ROI.
3.2. Effects at the M1-ROI
Baseline-corrected time courses for the M1-ROI are shown in Fig. 4, lower panel. There is only a slight difference in the change of concentration in both hemispheres for the MIR compared with the NOR condition for HbO and almost no difference between both conditions for HbR (Fig. 4, lower panel, left and right).
Mean beta values for the M1-ROI are shown in Fig. 5(d)–5(f), lower panel and in Table 1. As expected, the mean beta values for static trials were not significantly different from zero, whereas mean beta values for movement trials were clearly increased. Correspondingly, the four-way ANOVA over the mean beta values showed a significant main effect of the factor movement both for HbO [; , ] and for HbR [; , ].
The three-way ANOVA of the movement trials only (discarding the static trials) showed a main effect of hemisphere [, , ] for HbR. The mean beta values indicate more activation contralateral compared with ipsilateral for the movement trials. The same effect was observed for HbO, but failed to reach significance [, , ]. No other effects reached significance for the three-way ANOVA.
The three-way ANOVA of the static trials (discarding the movement trials) showed no significant effects.
4. Discussion
The aim of the present study was to systematically examine the neural correlates of a MIR with fNIRS. As described in previous studies with a similar setup10,11 or a real mirror,17 we found an increase of activation of the hemisphere ipsilateral to the moving hand for the PC-ROI for HbO. In extension to the above-mentioned studies, however, we also found a decrease of activation of the hemisphere contralateral to the moving hand. The effect for the PC-ROI for HbR was similar, but failed to reach statistical significance. In contrast to the activation pattern of the PC, activity in M1 was only modulated by the side of the hand that is actually moved, but not by the visually perceived hand.
In extension to previous findings, our results show that a MIR does not simply induce increased activation ipsilateral to the moving hand as expected, but also decreased activation contralateral to the moving hand. Furthermore, our results show that in the NOR condition there is more activation in the contralateral hemisphere compared with the ipsilateral. This indicates that the presentation of a right or a left hand elicits a hemispheric lateralization in the PC opposite to the seen hand—irrespective of the hand that is actually moved. This lateralization is inverted by the MIR: in the MIR condition, there is more activation in the ipsilateral hemisphere compared with the contralateral.
In the present study, the ANOVA of the PC-ROI revealed an interaction of the factor mirror and hemisphere, but no significant difference in the post-hoc testing. This indicates that activation differences in the ipsilateral and contralateral hemisphere do not occur independently, but in parallel: as an effect of the MIR, activation in the ipsilateral hemisphere increases as the activation in the contralateral hemisphere decreases. These results extend previous results of a higher activation level in the ipsilateral hemisphere due to a mirror.10–12,19 In those studies, no significant change in activation was found in the contralateral hemisphere during the inverse comparison (NOR versus MIR). This might be due to a threshold effect: as Fig. 5(c) (right side) suggests, the interhemispheric difference is slightly higher for the MIR condition as compared with the NOR condition. Thus, possibly, the reverse comparison might simply not have surpassed the threshold of significance in the studies of Dohle and Matthys.10–12 In extension of these studies, our results indicate that the processing of the visual image of a moving hand relies on a balance between both hemispheres, just similar to that reported for primary motor cortices.36
In summary, the present study using near-infrared spectroscopy demonstrated bilateral activation in M1- and PC-ROI with dissociated lateralization patterns: while motor cortex activity is lateralized opposite to the moving hand, the PC activity is lateralized opposite to the visually perceived hand. These patterns seem to be independent of each other. These findings indicate that the MIR in our paradigm should not be regarded as an isolated phenomenon. Rather, at least at the level of the PC, inversion of the visual feedback seems to be integrated into visuomotor behavior in the same way as processing of regular nonmirrored movements.
Nevertheless, there are some limitations of the present study. One might argue that the use of a video chain might weaken the strength of the mirror illusion compared with the use of a real mirror. However, the use of a real mirror introduces two variations at the same time: first, a right hand is perceived as a left one and vice versa; second, a movement to the right appears as a movement to the left and vice versa.37,38 There is a good evidence that these two transformations are processed independently.11 Thus, we decided to use the video setup, allowing isolated and randomized variation of visual feedback of the moving hand. By this approach, the possible confounder was present in all conditions. Admittedly, however, our setup lacks an overlap between visual feedback and proprioceptive knowledge of the actual hand position, which is considered as crucial for the effect of mirror therapy by some authors.39,40 In spite of this incongruence, however, the subjects in our study reported to have experienced a mirror illusion. Further studies are necessary in order to compare the effect on brain activation of this setup with that of a similar one with a more congruent position of the subject’s own hand and video screen or even with a real mirror. However, it should be noted that this comparison is only possible with a fNIRS system, which has hardly any limitation of head and body position.
Besides, our study cannot exclude that no other regions or structures of the brain are involved in the processing of the mirror illusion, as only a limited region of the brain could be covered with optodes. Especially, some authors claim the mirror neuron system (MNS) to be involved, i.e., neural structures that are involved both in movement execution and observation of other’s movements.12,41 Our results cannot exclude these theories. However, an involvement of the MNS in processing of the mirror illusion has never been shown explicitly. Contrary, our study is now the fourth one demonstrating change of activity in the PC by inversion of the visual image of a moving limb.10,11,19 Furthermore, a recent study could show distinct neural mechanisms of movement observation and movement mirroring, finding no distinct activation of the MNS by the mirror illusion.19
Furthermore, we cannot fully exclude the possibility that the negative effect on M1 might be at least in part due to insufficient coverage of M1. However, the clear movement effect at the M1-ROI demonstrates that relevant parts of the primary cortex were registered. Ultimately, a further experiment with broader coverage of M1 might be suitable to resolve this question.
Although we carefully chose the paradigm (e.g., long and jittered interstimulus intervals) to prevent extracerebral contamination42,43 of our data and corrected for movement artifacts, this issue could be enhanced by using a multidistance approach.44–47 Such an approach needs dense fiber arrangements, and thereby would cause a further limitation of the researched brain areas or an increased number of fibers, which is accompanied by longer preparation times and not suitable for patients.
Our findings are not only important for the understanding of visuomotor behavior, but they might also help to understand and optimize the effect of the mirror therapy. Even considering the fact that our video setup bears some confounders (as discussed above), our findings can be interpreted such that activity in the PC does not depend on the hand that is actually used. This would imply that, if active movements are possible with both limbs, the effect of mirror therapy would not differ from training with direct view on the affected limb. This was confirmed by a recent systematic review using Cochrane methodology, which found a significant effect of mirror therapy only compared with therapies without view, but not with unrestricted view on the affected limb.6 For rehabilitation of a plegic limb, however, mirror therapy provides a real advantage.48
Furthermore, the lack of direct increase of activation at the primary motor cortex supports the clinical finding that mirror therapy is only effective for rehabilitation of motor function when applied over a rather long time, stimulating reorganization processes (presumably mediated via the PC). This seems to be different for the effect of mirror therapy for rehabilitation of pain syndromes, where effects are observed on a much shorter time range.3
Finally, apart from its relevance for understanding the effect of the mirror illusion, the findings of the study might lead to new applications of fNIRS in the field of neurological rehabilitation. In our study, we successfully established a mirror-like paradigm during brain activity measurement, comparable with previous fMRI studies. As detailed above, further studies are now possible to compare these findings to those setups with more congruent anatomical positions or even application of a real mirror. Ultimately, this type of experiments might help to validate findings of rather restricted fMRI studies in comparison with real-world settings.
Acknowledgments
We would like to thank C. Fritzsch and L. dos Santos for assistance with data acquisition and data management. We also want to thank NIRx Medizintechnik GmbH for provision of the fNIRS system and C. Schmitz for his support. Finally, we would like to thank the anonymous reviewers for helpful comments on a previous version of the manuscript. The study was supported by grants from the Center for Stroke Research Berlin (Flexfunds CS-2009-10), the “Gesellschaft zur Förderung der Neurologischen Rehabilitation” and in part under the following grants: NIH Grant Nos. R42NS050007 and R44NS049734, the “Bernstein Focus Neurotechnology” (BMBF-Fkz 01GQ0850) funded by the German Federal Ministry of Education and Research, and the “World Class University Program” through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology, under Grant R31-10008.
Appendix: Calculation and Mapping of Statistical Effect Sizes
To further validate the defined ROIs in the PC and primary motor area, we calculated statistical effect sizes using Cohen’s as defined by Cohen49 for each channel. Our previous studies indicated no differences in activation regarding the hemispheres when comparing left and right hands.10,11 Thus, for the calculation of the statistical effect sizes, beta values (as achieved by the GLM) of the ipsilateral and contralateral hemisphere, respectively, were pooled over the left and right hands.
Effect sizes were defined according to our hypothesis for the ipsilateral hemisphere: positive effect sizes indicate a higher beta value in the MIR condition compared with the NOR condition, whereas negative values indicate a higher beta value in the NOR condition as compared with the MIR condition. As described above, this relation is inverted for the hemodynamic of HbR.
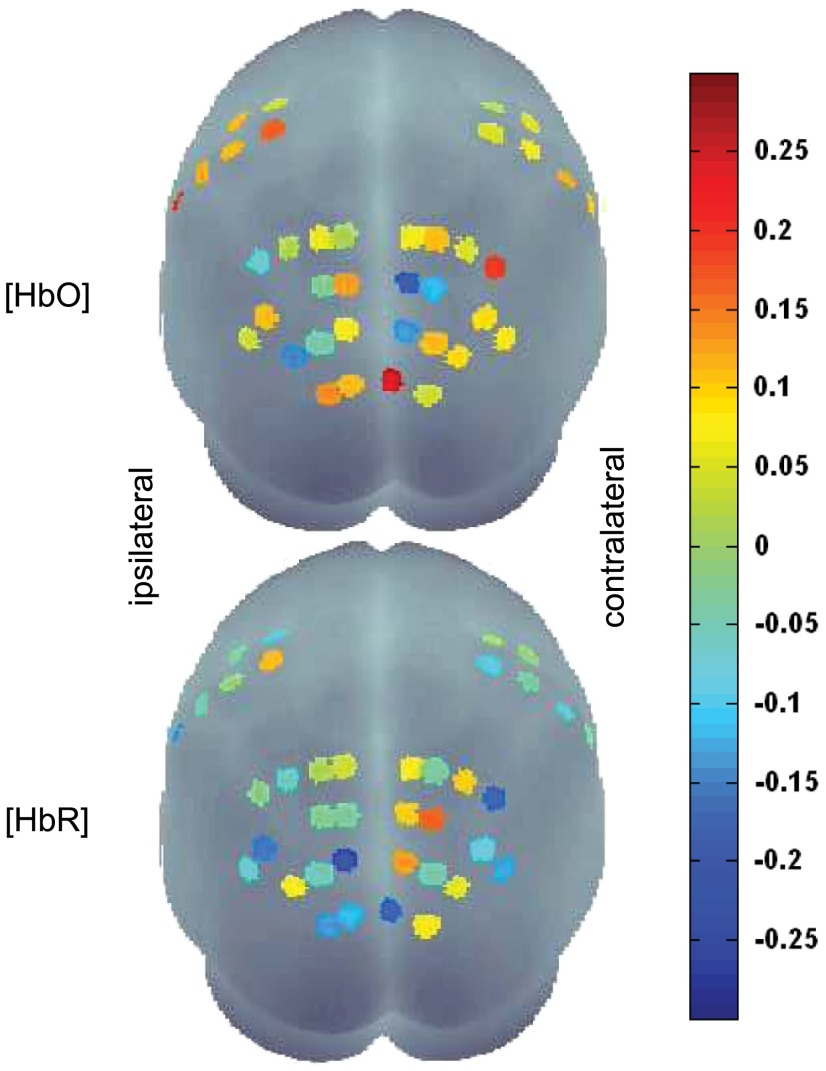
Statistical effect sizes are shown in Fig. 6. Generally, the effect sizes were rather small, ranging from to 0.26 for HbO and to 0.18 for HbR. For the hemisphere contralateral to the moving hand, it is noteworthy that for HbO, only three of all occipito-parietal channels in the contralateral hemisphere show negative effect sizes. These are the same channels as in the a priori defined PC-ROI [LH: channel numbers 23, 24, and 31; RH: channel numbers 25, 26, and 32; Figs. 2 and 3(a), right side]. For HbR in the contralateral hemisphere, two of these three channels have the largest effect sizes ( and 0.14) of all the channels. In the ipsilateral hemisphere, for HbO, one out of these three ROI channels has the second largest positive effect size () in the entire occipito-parietal area (LH: channel number 24; RH: channel number 25), and for HbR, one out of these three ROI channels has the largest negative effect size () of all the channels (LH: channel number 31; RH: channel number 32; Figs. 2 and 6). These results strongly support the choice of PC-ROI based on functional data of previous studies.
Fig. 6.
Effect sizes for the MIR for channels in the hemispheres ipsilateral (left hemisphere) and contralateral (right hemisphere) to the moving hand (top row: HbO, bottom row: HbR, view from occipital).
Statistical effect sizes for the MIR in the M1-ROI were not as prominent as for the PC-ROI. All effect sizes of the M1-ROI are near to zero, except one channel in the ipsilateral hemisphere for HbO (; LH: channel number 5; RH: channel number 12). Based on these results, no channels could be identified challenging the preselected M1-ROI.
References
- 1.Ezendam D., et al. , “Systematic review of the effectiveness of mirror therapy in upper extremity function,” Disabil. Rehabil. 31(26), 2135–2149 (2009). 10.3109/09638280902887768 [DOI] [PubMed] [Google Scholar]
- 2.Rothgangel A. S., et al. , “The clinical aspects of mirror therapy in rehabilitation: a systematic review of the literature,” Int. J. Rehabil. Res. 34(1), 1–13 (2011). 10.1097/MRR.0b013e3283441e98 [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran V. S., et al. , “Touching the phantom limb,” Nature 377, 489–490 (1995). 10.1038/377489a0 [DOI] [PubMed] [Google Scholar]
- 4.Chan B. L., et al. , “Mirror therapy for phantom limb pain,” N. Engl. J. Med. 357, 2206–2207 (2007). 10.1056/NEJMc071927 [DOI] [PubMed] [Google Scholar]
- 5.Bowering K. J., et al. , “The effects of graded motor imagery and its components on chronic pain: a systematic review and meta-analysis,” J. Pain 14(1), 3–13 (2013). 10.1016/j.jpain.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 6.Thieme H., et al. , “Mirror therapy for improving motor function after stroke,” Cochrane Database Syst. Rev. 3, CD008449 (2012). 10.1002/14651858.CD008449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moseley G. L., et al. , “Is mirror therapy all it is cracked up to be? Current evidence and future directions,” Pain 138(1), 7–10 (2008). 10.1016/j.pain.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 8.Ramachandran V. S., Altschuler E. L., “The use of visual feedback, in particular mirror visual feedback, in restoring brain function,” Brain 132(7), 1693–1710 (2009). 10.1093/brain/awp135 [DOI] [PubMed] [Google Scholar]
- 9.Diers M., et al. , “Mirrored, imagined and executed movements differentially activate sensorimotor cortex in amputees with and without phantom limb pain,” Pain 149(2), 296–304 (2010). 10.1016/j.pain.2010.02.020 [DOI] [PubMed] [Google Scholar]
- 10.Dohle C., et al. , “Body scheme gates visual processing,” J. Neurophysiol. 91(5), 2376–2379 (2004). 10.1152/jn.00929.2003 [DOI] [PubMed] [Google Scholar]
- 11.Dohle C., et al. , “Representation of virtual arm movements in precuneus,” Exp. Brain Res. 208(4), 543–555 (2011). 10.1007/s00221-010-2503-0 [DOI] [PubMed] [Google Scholar]
- 12.Matthys K., et al. , “Mirror-induced visual illusion of hand movements: a functional magnetic resonance imaging study,” Arch. Phys. Med. Rehab. 90(4), 675–681 (2009). 10.1016/j.apmr.2008.09.571 [DOI] [PubMed] [Google Scholar]
- 13.Shinoura N., et al. , “Mirror therapy activates outside of cerebellum and ipsilateral M1,” Neurorehabilitation 23(3), 245–252 (2008). [PubMed] [Google Scholar]
- 14.Garry M. I., et al. , “Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability,” Exp. Brain Res. 163(1), 118–122 (2005). 10.1007/s00221-005-2226-9 [DOI] [PubMed] [Google Scholar]
- 15.Fukumura K., et al. , “Influence of mirror therapy on human motor cortex,” Int. J. Neurosci. 117(7), 1039–1048 (2007). 10.1080/00207450600936841 [DOI] [PubMed] [Google Scholar]
- 16.Funase K., et al. , “Increased corticospinal excitability during direct observation of self-movement and indirect observation with a mirror box,” Neurosci. Lett. 419(2), 108–112 (2007). 10.1016/j.neulet.2007.04.025 [DOI] [PubMed] [Google Scholar]
- 17.Michielsen M. E., et al. , “The neuronal correlates of mirror therapy: an fMRI study on mirror induced visual illusions in patients with stroke,” J. Neurol. Neurosurg. Psychiatry 82(4), 393–398 (2011). 10.1136/jnnp.2009.194134 [DOI] [PubMed] [Google Scholar]
- 18.Touzalin-Chretien P., Dufour A., “Motor cortex activation induced by a mirror: evidence from lateralized readiness potentials,” J. Neurophysiol. 100(1), 19–23 (2008). 10.1152/jn.90260.2008 [DOI] [PubMed] [Google Scholar]
- 19.Wang J., et al. , “A comparison of neural mechanisms in mirror therapy and movement observation therapy,” J. Rehabil. Med. 45(4), 410–413 (2013). 10.2340/16501977-1127 [DOI] [PubMed] [Google Scholar]
- 20.Kato H., et al. , “Near-infrared spectroscopic topography as a tool to monitor motor reorganization after hemiparetic stroke: a comparison with functional MRI,” Stroke 33, 2032–2036 (2002). 10.1161/01.STR.0000021903.52901.97 [DOI] [PubMed] [Google Scholar]
- 21.Liebert A., et al. , “Bed-side assessment of cerebral perfusion in stroke patients based on optical monitoring of a dye bolus by time-resolved diffuse reflectance,” Neuroimage 24(2), 426–435 (2005). 10.1016/j.neuroimage.2004.08.046 [DOI] [PubMed] [Google Scholar]
- 22.Muehlschlegel S., et al. , “Feasibility of NIRS in the neurointensive care unit: a pilot study in stroke using physiological oscillations,” Neurocrit. Care 11(2), 288–295 (2009). 10.1007/s12028-009-9254-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strangman G., et al. , “Near-infrared spectroscopy and imaging for investigating stroke rehabilitation: test-retest reliability and review of the literature,” Arch. Phys. Med. Rehab. 87(12), 12–19 (2006). 10.1016/j.apmr.2006.07.269 [DOI] [PubMed] [Google Scholar]
- 24.Villringer A., et al. , “Editorial comment--cerebral near-infrared spectroscopy: how far away from a routine diagnostic tool?,” Stroke 35, 70–72 (2004). 10.1161/01.STR.0000110122.57772.C3 [DOI] [PubMed] [Google Scholar]
- 25.Oldfield R. C., et al. , “The assessment and analysis of handedness: the Edinburgh inventory,” Neuropsychologia 9(1), 97–113 (1971). 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- 26.Franck N., et al. , “Defective recognition of one’s own actions in patients with schizophrenia,” Am. J. Psychiatry 158(3), 454–459 (2001). 10.1176/appi.ajp.158.3.454 [DOI] [PubMed] [Google Scholar]
- 27.Tominaga W., et al. , “A mirror reflection of a hand modulates stimulus-induced 20-Hz activity,” Neuroimage 46(2), 500–504 (2009). 10.1016/j.neuroimage.2009.02.021 [DOI] [PubMed] [Google Scholar]
- 28.Koch S. P., et al. , “Stimulus-induced and state-dependent sustained gamma activity is tightly coupled to the hemodynamic response in humans,” J. Neurosci. 29(44), 13962–13970 (2009). 10.1523/JNEUROSCI.1402-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cope M., Delpy D. T., “System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination,” Med. Biol. Eng. Comput. 26(3), 289–294 (1988). 10.1007/BF02447083 [DOI] [PubMed] [Google Scholar]
- 30.Boynton G. M., et al. , “Linear systems analysis of functional magnetic resonance imaging in human V1,” J. Neurosci. 16(13), 4207–4221 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox P. T., Raichle M.E., “Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects,” Proc. Natl. Acad. Sci. U. S. A. 83(4), 1140–1144 (1986). 10.1073/pnas.83.4.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logothetis N. K., Wandell B. A., “Interpreting the BOLD signal,” Annu. Rev. Physiol. 66, 735–769 (2004). 10.1146/annurev.physiol.66.082602.092845 [DOI] [PubMed] [Google Scholar]
- 33.Singh A. K., et al. , “Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI,” Neuroimage 27(4), 842–851 (2005). 10.1016/j.neuroimage.2005.05.019 [DOI] [PubMed] [Google Scholar]
- 34.Koessler L., et al. , “Automated cortical projection of EEG sensors: anatomical correlation via the international 10–10 system,” Neuroimage 46(1), 64–72r (2009). 10.1016/j.neuroimage.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 35.Bortz J., Schuster C., Statistik für Human- und Sozialwissenschaftler, Springer, Berlin: (2010). [Google Scholar]
- 36.Grefkes C., Fink G. R., “Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches,” Brain 134(5), 1264–1276 (2011). 10.1093/brain/awr033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmes N. P., et al. , “When mirrors lie: “visual capture” of arm position impairs reaching performance,” Cogn. Affect. Behav. Neurosci. 4(2), 193–200 (2004). 10.3758/CABN.4.2.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmes N. P., Spence C., “Visual bias of unseen hand position with a mirror: spatial and temporal factors,” Exp. Brain Res. 166(3–4), 489–497 (2005). 10.1007/s00221-005-2389-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramachandran V. S., Rogers-Ramachandran D., “Synaesthesia in phantom limbs induced with mirrors,” Proc. Biol. Sci. 263(1369), 377–386 (1996). 10.1098/rspb.1996.0058 [DOI] [PubMed] [Google Scholar]
- 40.Ramachandran V. S., Altschuler E. L., “The use of visual feedback, in particular mirror visual feedback, in restoring brain function,” Brain 132(7), 1693–1710 (2009). 10.1093/brain/awp135 [DOI] [PubMed] [Google Scholar]
- 41.Sutbeyaz S., et al. , “Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: a randomized controlled trial,” Arch. Phys. Med. Rehabil. 88(5), 555–559 (2007). 10.1016/j.apmr.2007.02.034 [DOI] [PubMed] [Google Scholar]
- 42.Takahashi T., et al. , “Influence of skin blood flow on near-infrared spectroscopy signals measured on the forehead during a verbal fluency task,” Neuroimage 57(3), 991–1002 (2011). 10.1016/j.neuroimage.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 43.Kirilina E., et al. , “The physiological origin of task-evoked systemic artefacts in functional near infrared spectroscopy,” Neuroimage 61(1), 70–81 (2012). 10.1016/j.neuroimage.2012.02.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeff B. W., et al. , “Retinotopic mapping of adult human visual cortex with high-density diffuse optical tomography,” Proc. Natl. Acad. Sci. U. S. A. 104(29), 12169–12174 (2007). 10.1073/pnas.0611266104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamada T., et al. , “Multidistance probe arrangement to eliminate artifacts in functional near-infrared spectroscopy,” J. Biomed. Opt. 14(6), 064034 (2009). 10.1117/1.3275469 [DOI] [PubMed] [Google Scholar]
- 46.Koch S. P., et al. , “High-resolution optical functional mapping of the human somatosensory cortex,” Front. Neuroenergetics 2, 1–8 (2010). 10.3389/fnene.2010.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habermehl C., et al. , “Somatosensory activation of two fingers can be discriminated with ultrahigh-density diffuse optical tomography,” Neuroimage 59(4), 3201–3211 (2012). 10.1016/j.neuroimage.2011.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dohle C., et al. , “Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial,” Neurorehabil. Neural. Repair 23(3), 209–217 (2009). 10.1177/1545968308324786 [DOI] [PubMed] [Google Scholar]
- 49.Cohen J., Statistical Power Analysis for the Behavioral Sciences, Erlbaum Associates, Hillsdale, New Jersey: (1988). [Google Scholar]