Short abstract
Cardiac mechanical contraction is triggered by electrical activation via an intracellular calcium-dependent process known as excitation–contraction coupling. Dysregulation of cardiac myocyte intracellular calcium handling is a common feature of heart failure. At the organ scale, electrical dyssynchrony leads to mechanical alterations and exacerbates pump dysfunction in heart failure. A reverse coupling between cardiac mechanics and electrophysiology is also well established. It is commonly referred as cardiac mechanoelectric feedback and thought to be an important contributor to the increased risk of arrhythmia during pathological conditions that alter regional cardiac wall mechanics, including heart failure. At the cellular scale, most investigations of myocyte mechanoelectric feedback have focused on the roles of stretch-activated ion channels, though mechanisms that are independent of ionic currents have also been described. Here we review excitation–contraction coupling and mechanoelectric feedback at the cellular and organ scales, and we identify the need for new multicellular tissue-scale model systems and experiments that can help us to obtain a better understanding of how interactions between electrophysiological and mechanical processes at the cell scale affect ventricular electromechanical interactions at the organ scale in the normal and diseased heart.
Introduction
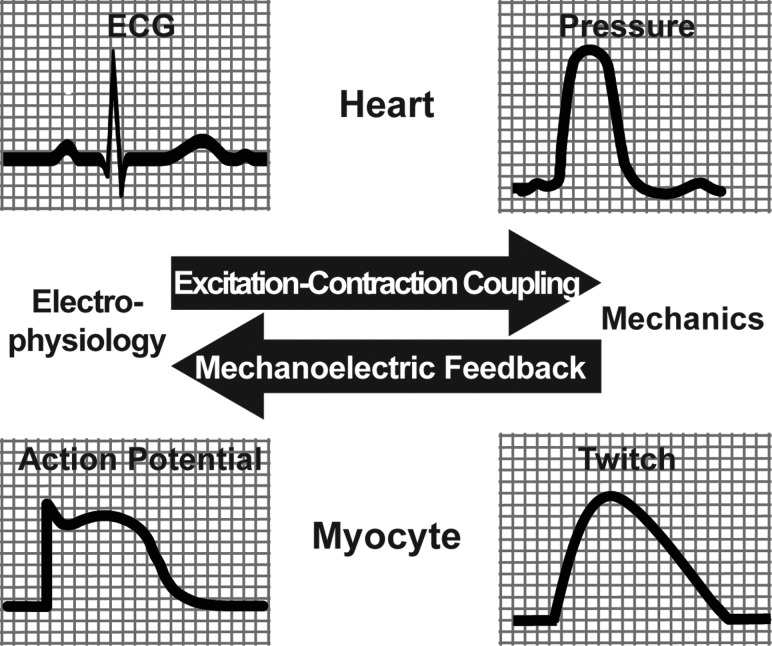
It is well known that the heart depends on electrical depolarization to trigger mechanical contraction, but mechanical perturbations have also long been seen to affect cardiac electrophysiology [1,2]. The process by which myocyte electrical activation leads to mechanical contraction is known as excitation–contraction coupling (ECC), while the process by which a mechanical alteration influences cardiac electrical activity is referred to as mechanoelectric feedback (MEF) (see Fig. 1 ). Both of these phenomena are manifested at cellular and whole heart scales.
Fig. 1.
The relationship between excitation–contraction coupling and MEF from the scale of the myocyte to the whole heart
At the scale of the whole heart, ECC and MEF have been well characterized, and are known to be altered in various diseases or states of altered contractility, such as electromechanical dyssynchrony. In the whole heart, abnormal sequences of ventricular electrical depolarization adversely affect mechanical contraction and are a major complication when pump function is compromised by heart failure [3]. Extensive studies have also been conducted to understand the cellular mechanisms of ECC and, to a lesser extent, MEF. For example, it is now well recognized that dysregulation of intracellular calcium cycling, which is central to ECC, is a major cause of contractile dysfunction in heart failure, regardless of the specific heart failure etiology [4].
Although ECC and MEF have been studied at length at the myocyte and whole heart scales, our ability to integrate and translate cellular and molecular mechanisms to in vivo physiological and pathophysiological phenotypes is still limited and the problem is inherently difficult. Ventricular mechanics in vivo are highly nonhomogeneous, and arrhythmias depend not only on cellular dynamics but on intercellular coupling, action potential propagation, and three-dimensional myocardial structure. Contributing to the paucity of integrative understanding in this field is the comparative scarcity of suitable homogeneous multicellular preparations and high-fidelity measurement techniques for investigating tissue-scale myocardial electromechanical interactions under well-controlled and readily manipulated conditions. Here we review the state of knowledge of cardiac ECC and MEF at the whole organ and single cell scales, and we conclude by surveying promising preparations and techniques for investigating these electromechanical interactions at the multicellular tissue scale.
Organ Scale: Cardiac Electromechanical Interactions
Whole heart studies of cardiac electromechanical interactions have focused both on forward ventricular excitation–contraction coupling and reverse coupling, or MEF. The former is more commonly known in this context simply as ventricular electromechanics, and studies have focused both on the relationship between electrical activation in normal sinus rhythm and the normal sequence of mechanical contraction as well as on how dyssynchronous electrical activation can alter myocardial mechanical and ventricular pump function. Mechanoelectric feedback studies in the whole heart have focused primarily on the mechanisms by which alterations in hemodynamic loading or focal mechanical stimuli can modulate atrial or ventricular electrophysiology and lead in some cases to arrhythmia [2].
Ventricular Electromechanics.
The focus of most studies on whole heart cardiac electromechanics has been on the complex spatio-temporal relationship between electrical activation sequence and the pattern of mechanical shortening. In the normal heart, the timing of mechanical activation roughly follows that of the electrical activation, giving rise to a fairly synchronous contraction pattern [5]. In the dyssynchronously activated heart, altered electrical conduction, due to ectopic ventricular pacing or conduction defects such as bundle branch block, causes asynchronous contraction in which early activated regions shorten against lower than normal loads and late-activated regions are stretched during systole due to the effects of the contraction at early activated regions. Here we review the normal electromechanics of the heart and the effects of abnormal dyssynchronous electrical activation both in the structurally normal and failing heart.
Electromechanics in the Normal Ventricles.
Myocardial contraction is initiated by electrical activation. In the normal heart, pacemaker cells are located in the sinoatrial node (SAN), and activation of the ventricles is initiated with electrical impulses originating at the atrioventricular (AV) node and travel to the His bundle, which then splits into the right and left bundle branches which leads to the Purkinje fibers, located subendocardially [1]. Conduction velocity is approximately four times faster in the Purkinje fibers than in the normal myocardium [6]. Impulse conduction in the Purkinje fibers takes place from base to apex, but exits the Purkinje system near the apex at the subendocardium [7], causing activation of the septum and LV and RV free walls to occur from apex to base and endocardium to epicardium [8,9]. Owing to the speed at which the impulse travels, normal ventricular activation is relatively synchronous and causes normal myocardial contraction during systole to be quite synchronous and efficient. The time for complete activation of the ventricles is 62–80 ms, which corresponds to a QRS duration of 70–80 ms in humans [1,10]. QRS durations in this normal range are indicative of synchronously activated ventricles, whereas prolonged intervals are usually, but not always, associated with dyssynchronous activation due to conduction block or ectopic ventricular stimulation.
When ventricular electrical activation is normal, the timing of mechanical activation roughly follows the spread of electrical depolarization and correlates well with local electrical activation time at a given site [11]. Even during normal sinus rhythm, regional gradients in depolarization times contribute to mechanical heterogeneity. The delay in the onset of myofiber shortening following local electrical depolarization is referred to as electromechanical delay (EMD), and can be on the order of tens of milliseconds [12]. One component of this delay is due to the time from membrane depolarization to myofilament activation and tension development, a process that is described in more detail in following sections and referred to as cellular ECC. It is also evident that EMD must be dependent upon loading conditions and therefore the specific activation sequence in the intact heart [12]. Experimental evidence has shown that epicardial EMD in the normal heart differs between the base and apex in sinus rhythm, and this difference can be altered with a change in activation sequence [11].
Mechanical shortening in the fiber direction occurs at the subendocardium first, and is delayed at the subepicardium, reflecting the transmural sequence of activation [13]. Transmural mechanical coupling can also dissociate depolarization from shortening with epicardial segments starting to shorten during systole slightly before the depolarization wave reaches them; as a result of endocardial contraction and tissue tethering [14]. As electrical activation spreads from the LV apex towards the base, the onset of longitudinal shortening at the subendocardium and circumferential shortening at the subepicardium occurs earlier at the apex compared with the base, reflecting differences in both activation time (regionally) and fiber orientation (transmurally). Overall, these regional heterogeneities in mechanical function are small in the normal heart, leading to comparatively synchronous myocardial contraction and efficient pressure generation simultaneously by the left and right ventricular chambers. The dependence of EMD on activation sequence is of high importance under conditions when electrical activation is dyssynchronous such as with left bundle branch block (LBBB), when EMD has been shown to be prolonged in the late-activated regions ultimately leading to even greater mechanical dyssynchrony relative to electrical dyssynchrony [15].
Electromechanics in the Dyssynchronously Activated Heart.
Proper ejection of blood from the heart requires relatively synchronous contraction. Dyssynchronous contraction compromise the coordination required to eject blood efficiently, leading to reduced pump function and ultimately heart failure. Dyssynchrony can arise from conduction system disorders such as left bundle branch block (LBBB), which is the most common conduction defect in patients with advanced heart failure [3]. The electrical activation sequence is significantly prolonged during LBBB, and can reduce left ventricular (LV) pump function substantially [16,17]. Another cause of dyssynchrony is myocardial infarction, where the infarct region has slowed or blocked conduction combined with impaired contractility. If the infarct region is large enough, it can lead to dyssynchrony and left ventricular dysfunction [18]. Discriminating the extent to which impaired mechanical function in these conditions is attributable to contractile heterogeneity versus electrical asynchrony remains a challenging clinical question.
Epicardial ventricular pacing (such as via a coronary venous pacemaker lead) significantly prolongs QRS duration and has been shown to induce differences in regional workload, which ultimately leads to regional hypertrophy [19]. QRS duration is often doubled during ventricular pacing with respect to sinus rhythm due to the slow conduction of the myocardium [1]. RV apical pacing is a good experimental model of the dyssynchrony induced due to LBBB, and the resultant alterations in QRS morphology of the surface ECG [20] and patterns of asymmetric ventricular hypertrophy [21] are similar.
Abnormal electrical activation gives rise to abnormal contraction patterns. Asynchronous activation during LBBB or ventricular pacing leads to opposite regions of the ventricles being activated too early or too late, both electrically and mechanically, relative to the timing of the pressure pulse. Contraction of early activated regions stretches remote sites that have not yet been activated [22]. The stretching of late-activated regions by early activated shortening has been previously referred to as “prestretch” [11,23,24] and can reach magnitudes of up to 20% in late-activated regions [25]. Contraction in early activated regions is also abnormal owing to interactions with the remote late-activated regions. Early activated regions undergo rapid early systolic shortening followed by minimal shortening later in systole, and sometimes even lengthening during systole [26]. These observations show that not only the timing of contraction is altered by abnormal electrical activation, but also the pattern and magnitudes of contraction.
Studying dyssynchrony and abnormal electromechanics is of particular importance in heart failure. One study showed that approximately 50% of heart failure patients have significant mechanical dyssynchrony. The prevalence increases in the presence of an electrical conduction defect, but mechanical dyssynchrony by various measures is also common in patients with normal QRS duration [27]. The use of biventricular ventricular pacing is often effective in resynchronizing contraction and improving pump function and reducing mortality in patients with dyssynchronous heart failure, but a substantial fraction of candidates for this procedure show no response to cardiac resynchronization. Hence, there is a pressing clinical need for a better mechanistic understanding of ventricular electromechanical dyssynchrony.
Cardiac Mechanoelectric Feedback.
The well-described process of electromechanical coupling can also work in reverse. In other words, acute changes in mechanics of the ventricles can affect cardiac electrophysiology. Blows to the chest, altered hemodynamic loading, or regional changes in synchrony may have the effect of altering myocardial stretch or pressure loading prior to or during electrical activation. These abnormal mechanical loading situations have been observed to disrupt or distort normal cardiac excitation, and in some cases lead to life-threatening arrhythmias.
Commotio Cordis.
Classical studies in cardiac physiology first identified MEF at the organ scale well over 100 years ago [28]. Reports of commotio cordis date to the 19th century and possibly earlier. Commotio cordis is a rare cause of ventricular fibrillation often resulting in sudden cardiac death initiated by a localized precordial impact that is not sufficiently strong to cause mechanical damage to the heart or surrounding organs. As recorded in the US Commotio Cordis Registry, the rate of survival has improved over recent decades, associated with increased workplace safety and bone health, such that incidence is now most common in youth athletics. Survival of these events has been by faster response times and availability of an on-site automated external defibrillator. There remains a greater risk of mortality when commotio cordis occurs during noncompetitive rather than competitive sports, or in African Americans [29,30]. A condition for fatality is that the chest blow occurs during a vulnerable window preceding the ECG T wave [31]. Recruitment of stretch-activated channels during this period is thought to augment repolarization, increasing dispersion of repolarization, and promoting ventricular tachycardia [29,31], a mechanism supported by computational models [32]. While perhaps the most dramatic, commotio cordis is by no means the only pathophysiological example of MEF.
Mechanically Triggered Arrhythmias.
Intrinsic mechanical pulses and perturbations can trigger arrhythmias or extrasystoles, and may be especially important in diseases where wall mechanics become altered such as ischemia and heart failure. Transiently increased hemodynamic loading of failing ventricles has been observed to give rise to extrasystolic beats and ventricular tachycardia [33,34]. In a positive counterexample, precordial thump or precordial percussion offers a rapid though low efficiency method for resuscitation of a victim of witnessed cardiac arrest, though the window for benefit versus harm is small [35–38].
The Bainbridge Response.
Changes in circulatory pressures have a well-known effect on heart rate. First described in 1915, the “Bainbridge response” describes an increase in heart rate under increased venous, but not under arterial, pressure [39]. Increases in venous return are thought to be sensed via stretch receptors in the left and right atria, and transmitted via the vagus nerve to the autonomic nervous system, which rebalances vagal and sympathetic stimulation to the SAN causing an increase in heart rate [2,40]. Unloading below normal levels during hypotension, anesthesia, or hemorrhage can result in heart rate decrease and is sometimes termed the “reverse Bainbridge response” [40]. However, the Bainbridge effect operates in counterbalance with a slowing in heart rate following a rise in arterial pressure, mediated by the baroreceptor and autonomic system. Perhaps due to this counterbalance, the Bainbridge effect is known to be strongest in the species of first discovery (dog). While rate increase is also present in humans and other primates, the net effect is reversed in small mammals [2,40]. Some reports suggest the baroreceptor response can also dominate the Bainbridge response in humans [41]. However, Bainbridge responses are thought to contribute to “physiological” respiratory sinus arrhythmias, modulating blood flow and promoting oxygen uptake [2].
Ventricular Repolarization.
The Q-T interval of the electrocardiogram, a measure of the delay between ventricular depolarization and repolarization, has been observed to lengthen, and the T-wave to flatten, when ventricular contraction occurs rapidly against a reduced afterload, in animal tissue preparations and in the human heart [42,43]. Commonly this affect is attributed to changes in action potential duration that lead to dispersion of the repolarization gradient, though the nature of these changes is unsettled and is likely dependent on heart rate [44–48].
Myocardial Electrical Properties.
Additionally, changes in ventricular conduction dependent on hemodynamic load may also contribute to electrocardiogram-perceived changes in ventricular repolarization, by delaying the timing of depolarization globally, or offsetting it within a region. Experiments in intact and perfused rabbit hearts have shown conduction slowing with ventricular volume loading, a change which would increase apparent ventricular repolarization time [48,49]. Experiments in a variety of tissue preparations have yielded acceleration, deceleration, or biphasic changes in conduction velocity under several modalities of tissue stretch [47,49]. Such electrophysiological changes may contribute to susceptibility to reentrant arrhythmia in tissue regionally affected by disease or dyssynchrony [36,45,46,49,50]. Alterations in ion currents may explain the conduction changes observed in stretched tissue, although experiments showing no effect of stretch-activated channel blockers in tissue suggest otherwise [48,49]. Simulations based on these experiments suggest that this slowing may be linked to increased cell membrane capacitance with tissue stretch [49,51].
Cell Scale: Myocyte Electromechanical Coupling
The primary origins of cardiac ECC and MEF are in the myocyte, though there is some evidence that fibroblasts are mechanosensitive and do influence action potential propagation. Myocyte ECC is the process of the depolarization of the cell membrane ultimately giving rise to a cellular contraction. Myocyte mechanoelectric coupling is the process of a cell experiencing mechanical stretch that ultimately alters its action potential. We summarize the main mechanisms of each in the following sections.
Myocyte Excitation–Contraction Coupling.
At the cellular level, the process of an electrical event leading to mechanical contraction has been termed ECC. The process is initiated by local membrane depolarization leading to calcium-induced calcium release from the sarcoplasmic reticulum, which increases the intracellular free calcium available to bind and activate the myofilaments, ultimately initiating contraction. In order to fully understand the electromechanics in the whole heart, it is important to dissect the molecular mechanisms that give rise to this phenomenon at the cellular level.
Calcium-Induced Calcium Release.
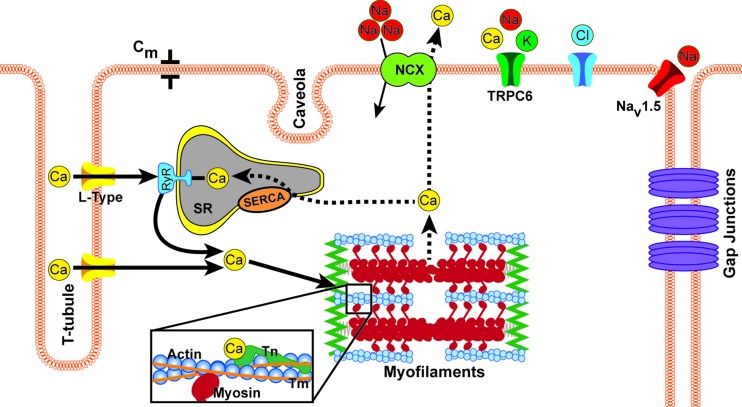
Mechanical contraction of cardiac myocytes is initiated by electrical depolarization, and the key regulator that activates contraction in the myocyte is intracellular calcium. Membrane depolarization during the action potential leads to the opening of the voltage-gated L-type calcium channels [52]. Calcium entering the myocyte via L-type channels localized primarily in the transverse-tubular membrane binds to closely apposed ryanodine receptors on the membrane of the junctional sarcoplasmic reticulum (SR), as seen in Fig. 2 [53]. This in turn opens the ryanodine receptors stimulating a release of more calcium from the SR [54]. This process is often referred to as calcium-induced calcium release, as the relatively small influx of calcium triggers the larger release of calcium from the SR. The combination of calcium influx and release from the SR leads to a rise in the intracellular calcium, which is referred to as the calcium transient. Because the L-type channels and ryanodine receptor clusters are only about 15 nm apart across a narrow dyadic cleft between the T-system membrane and the junctional SR, the magnitude of the calcium transient increases progressively with higher sarcolemmal calcium current, allowing this calcium-induced calcium-release mechanism to achieve a graded response. The ATP-dependent sarco-endoplasmic reticulum calcium ATPase (SERCA pump) resequesters cytosolic calcium into the SR to restore diastolic levels, and most of the excess calcium that entered via the L-type channels is extruded via the sodium–calcium exchanger (Fig. 2). Because of differential spatial expression of key proteins in this calcium cycling pathway, transmural heterogeneities in the dynamics of the calcium transient and the time delay between membrane depolarization and the onset of twitch tension have been described [55]. In dogs, this results in a shorter latency between depolarization and the onset of contraction in epicardial myocytes than endocardial cells, which approximately offsets the timed delay between endocardial and epicardial depolarization. Dysregulation of myocyte calcium cycling mechanisms is a common cellular phenotype of the failing ventricular myocyte, and there is intense interest not only in how these alterations impair contractile function in heart failure, but also how they may promote arrhythmias by causing cellular electrical instabilities known as afterdepolarizations [56].
Fig. 2.
The first step in the initiation of contraction begins with an influx of sodium ions, which depolarizes the membrane and opens the voltage-gated L-type calcium channels. This causes an influx of calcium into the cell, some of which binds to ryanodine receptors (RyR) located on surface of the sarcoplasmic reticulum (SR), which allows for a large scale release of calcium from inside the SR; a process referred to as calcium induced calcium release. There is then an abundance of free calcium in the cell that can bind to troponin (Tn), in particular troponin-C. This binding causes tropomyosin (Tm) to shift, exposing the myosin binding site on actin. Once the myosin head binds to actin, force is generated. At the end of the crossbridge cycle, calcium is released from troponin-C and is then either pumped out of the cell by the sodium-calcium exchanger (NCX) or resequestered into the SR via the sarcoplasmic reticulum calcium ATPase (SERCA) pump. Resulting changes in the mechanical context of the cell can alter the dynamics of conduction of electrical excitation throughout the tissue and the duration of cell action potential, by modulating channels, junctions, and cell capacitances and resistances; thus feeding back between cardiac mechanics and electrophysiology.
Myofilament Activation.
The rise in the free intracellular calcium during the calcium transient allows for calcium ions to bind to the myofilament protein regulator troponin-C [53]. Cardiac troponin-C contains two high-affinity calcium-binding sites and one low affinity binding site (different from skeletal muscle troponin-C which has two low affinity sites) [57]. The high affinity sites are always calcium bound at normal physiological levels of calcium, but it is the low affinity site that accounts for the regulation by calcium and the activation of the sarcomere [58]. The binding of calcium to this low affinity regulatory site causes the troponin-C to undergo a conformational change, which via interactions with actin, troponin-I, and troponin-T ultimately allows tropomyosin strands on the thin filaments to shift, exposing the myosin binding sites on actin, allowing the S1 region of myosin to bind to actin [59].
Myofilament Calcium Sensitivity and Cooperativity.
After strong binding of myosin to actin takes place, additional crossbridge binding is dramatically enhanced, a cooperative mechanism that probably is a function of the movement of tropomyosin into an even more favorable position [60,61]. The well-known steady state force–pCa curves for cardiac muscle show a very steep relationship between available calcium and force. This steeply nonlinear relationship indicates that there is a cooperative mechanism of myofilament activation taking place. The Hill coefficient for cooperative calcium-dependent peak tension can be as high as 5 to 9 in cardiac muscle.
Steady-state force–pCa curves show that the magnitude of contractile force developed is highly dependent on the amount of free calcium present, as well as sarcomere length. Studies have shown that sarcomere length influences the affinity of troponin-C for calcium [62]. There are many theories surrounding the length dependence of myofilament calcium sensitivity, but the true mechanism is not fully understood. Myofilament calcium sensitivity has also been shown to be dependent upon relatively quick length changes, where quick release experiments gave rise to a transient increase in intracellular calcium as some calcium is prematurely displaced from the thin filaments [63]. This is one example of a feedback mechanism in which a mechanical perturbation affects intracellular calcium, and thus contractility; more of which will be discussed in the following section.
Myocyte Mechanoelectrical Coupling.
While organ-scale examples of cardiac MEF have been documented and defined, most are not clearly linked to cellular mechanisms outside the autonomic nervous system. One source of this gap in understanding is that cardiac electrophysiology arises from the collective behavior of a population of cells situated in a dynamic mechanical and electrical tissue environment. Electrical activation is achieved through a sequence of ionic currents acting in concert, and alterations in individual currents or ion channels are not easily distinguished, or understood in relationship to interacting currents. Likewise for currents through cell–cell gap junctions and the electrical source–sink interactions surrounding electrotonic loading of passive tissues. The changing nature of cell and tissue electrical properties within a mechanically, biochemically, and electrically dynamic environment are also unclear. For these reasons, many of these effects have been initially studied at the single-cell level or within computational models in order to establish a foundational understanding of their functions. Current understanding of some of these components is summarized here.
Stretch-Activated Currents.
Various ionic currents have been proposed to be mechanosensitive in cardiomyocytes; thus producing stretch-sensitive electrophysiological responses, however the effect of these currents in healthy and diseased cardiac function is unclear. While nonspecific cationic, K+-selective, and Cl–selective mechanosensitive channels (MSCs) have been identified in various preparations, most cardiac myocyte studies to date have focused on the nonspecific cation-selective channels (nsMSCs) [2,64]. The most specific blocking agent used to study these channels is GsMTx-4, a peptide derived from tarantula venom, which is thought to embed in the membrane around nsMSCs such as TRPC6 channels, reducing transmission of membrane tension and mechanosensitive opening of the channels [65–69]. Also commonly used are the rare earth element gadolinium (Gd3+), which broadly affects cationic and anionic MSCs, and cationic antibiotics such as streptomycin, though both have technical disadvantages when compared with GsMTx-4 [2,64,67].
If TRPC6 channels are indeed mechanosensitive, their upregulation in stretch and disease, and effects on Ca2+ handling may prove to be important players in MEF [66,70,71]. Computational simulations have suggested a role for nsMSCs in heart rate acceleration and deceleration due to stretch described above, and experimental evidence in SAN tissue using GsMTx-4 is in agreement [2,72]. Computational models and experiments studies suggest a role for nsMSCs in stretch-induced ectopic ventricular contractions, repolarization shortening, and rate-dependent restitution of action potential duration [44,73–75].
Cell–Cell Coupling.
Electrochemical communication between cardiomyocytes occurs primarily through connexon channels made up of connexin proteins localized at gap junctions usually within an intercalated disk joining two cells via mechanoelectrical couplings. These gap junctions afford low resistance current flow between cells, with relative conductivity determined by the constituent connexin isoforms, which are expressed with cell type and tissue specificity varying throughout the atria, ventricles, and conduction system [76–80]. Cardiac conduction is thus dependent on the integrity of mechanical junctions between cells, and alterations in connexin expression due to disease [79,81–83] Connexon mechanosensitivity in cardiac cells is unknown, although increased conductivity with stretch has been shown in other cell types [84]. Mechanical loading increases expression of gap junction proteins through hypertrophy processes, which can lead to an increase in conduction velocity [85,86]. Alterations in cardiac mechanics via electrical stimulation and heart failure have also been observed to promote gap junction remodeling, increasing susceptibility to arrhythmia [83,87,88].
Additionally, conductivity may be enhanced by field or electrotonic coupling throughout cardiac tissue. Conduction may be fostered at the peri-nexus region surrounding gap junction plaques through field coupling boosted by Nav1.5 channel enrichment near connexins [89]. Models suggest that ephaptic or field coupling to surrounding passive tissue could be important in propagation of electrical signals through the myocardium, beyond classical cable model representations [90–92]. These findings underscore that changes in cell membrane configuration, observed to occur with stretch, could play an important role in mechanosensitivity cardiomyocyte electrophysiology [49,93–95]. An electrotonic effect of coupling between cardiomyocytes and cardiac fibroblasts has been demonstrated, in experiments and in simulations [96–99]. Both fibroblast-mediated slow conduction through myocyte-free regions, and a role in MEF for mechanosensitivity of cardiac fibroblast membrane potential have been suggested, and both often labeled as arrhythmogenic [29,100–104]. Interestingly, one of the proposed mechanisms for cardiac myofibroblast alterations of cardiac conduction is via forces exerted by the myofibroblasts on the cardiomyocytes, leaving open the possibility of MEF within the cardiomyocytes [105].
Membrane Capacitance.
Beyond affecting constituent channels and junctions, changes in cell membrane configuration with stretch may alter fundamental electrical properties such as capacitance [93]. Although there have been few studies of mechanical effects on myocyte membrane capacitance [106,107], indirect evidence in intact tissue preparations has implicated changes in membrane capacitance in stretch-induced changes in action potential conduction velocity, as described above [49]. There is some evidence for membrane capacitance increase with membrane tension in other cell types [106,108]. Increased membrane capacitance could be expected to lower excitability and slow conduction of activation through the myocardium, promoting arrhythmia as discussed above. However, a connection between this cellular property and organ-level phenomena remains to be demonstrated.
The Need for Multicellular Tissue-Scale Experiments
The previous sections have discussed ECC and MEF at the cellular and organ levels. Studying these two phenomena at the cellular scale helps elucidate the mechanisms involved, but this information does not translate well from the single cell to the whole heart, simply due to the experimental conditions of single cell preparations. Tissue level preparations can help translate the ECC and MEF discoveries made in single cell experiments to the whole heart.
Understanding ECC at the cellular level provides functional and molecular insight into muscle cell contraction, but the ultimate goal is to relate these cellular mechanisms to observations at the organ level. Experimental techniques have been designed in an attempt to study the role of altered ECC in heart failure and disease at the level of the single cell [109]. While cellular studies like these lead to important new information as to the role of ECC in heart failure, we cannot directly apply the mechanical correlates at the single cell level to understand mechanical function in the whole heart. There is no doubt that single cell studies provide valuable insight to ECC, but the cellular environment in vivo is very different. In the myocardium, cardiac myocytes are mechanically and electrically coupled with surrounding cells. There is a hierarchical three-dimensional extracellular matrix, and myocytes interact with several other cell types in the myocardium. Cell metabolism is certainly affected by the three-dimensional environment in vivo. When a cardiac myocyte is removed from its normal environment, cell electrical and mechanical functions are significantly affected. This fact makes it very difficult to take ECC mechanisms observed at the cellular level and apply them to the electromechanics observed at the organ level. Tissue level investigations of these mechanisms are needed to bridge this gap. Such preparations allow the myocytes to be studied in an environment that more closely mimics how they are found in the heart, but also allows for collection of data that is not possible in the intact heart, in which tissue structure and regional stresses and strains are highly nonhomogeneous.
Many physiologically interesting phenomenological observations of MEF have been made through studies of clinical and in vivo responses, Langendorff-perfused intact hearts, and whole muscle preparations, while in parallel, complex mechanisms are gradually becoming known at the subcellular and single cell levels. However, the specific cellular mechanisms for many organ-level observations remain elusive, as do the role of particular cellular mechanisms when in concert with the organ. Though there is extensive knowledge and detailed mathematical models of the electrophysiology of single cardiac myocytes in a wide variety of species, arrhythmias are complex, whole-organ, spatio-temporally dynamic phenomena that depend on multicellular interactions and cannot be understood based solely on single cell data. Conversely, the whole heart is three dimensional and inhomogeneous, and it is not yet possible to simultaneously map three-dimensional mechanics and electrophysiology throughout the intact heart, much less to perturb them in a well-controlled manner.
The following sections summarize several different multicellular preparations that have been used for studying ECC and MEF. These approaches promise to help better integrate the cellular and molecular mechanisms of excitation–contraction coupling and mechanoelectric feedback with the whole organ physiology of ventricular electromechanical interactions.
Cardiomyocyte Tissue Cultures.
One concern regarding single cell experiments is that techniques for stretching isolated adult cardiomyocytes are technically quite difficult, and coupling these techniques with electrophysiological study present a difficult hurdle [110]. Depending on the scientific question, there is interest in testing isolated adult cells as singles, doublets, or as slices or strips of tissue [88,111–113] Still, precise but population-level electrophysiological studies of electrically coupled cells under precise and physiological mechanical strain conditions remains challenging.
Over the years, improved techniques for microfabrication of patterned surfaces and polymer chemistry have been put to work to engineer culture conditions that closely resemble native myocardium. While classical tissue culture presents a cell body/substrate stiffness mismatch that typically has deleterious effects on cell development and morphology, softer polymer substrates have been found to optimize cardiomyocyte maturation in culture [114–116]. However, soft substrates often preclude experiments imposing prescribed stretch. Freestanding gel or polymer strip platforms have been designed to study patterned or biologically manipulated cardiomyocyte force generation [117–120]. Though early cardiomyocyte culture work focused on larger and more robust rat cardiomyocytes, transgenic mouse models of human arrhythmia phenotypes have motivated a shift to murine cell culture [121,122]. Nonmammalian models of human cardiac disease are also employed to study small numbers of cardiomyocytes under near-native mechanoelectric conditions [123,124].
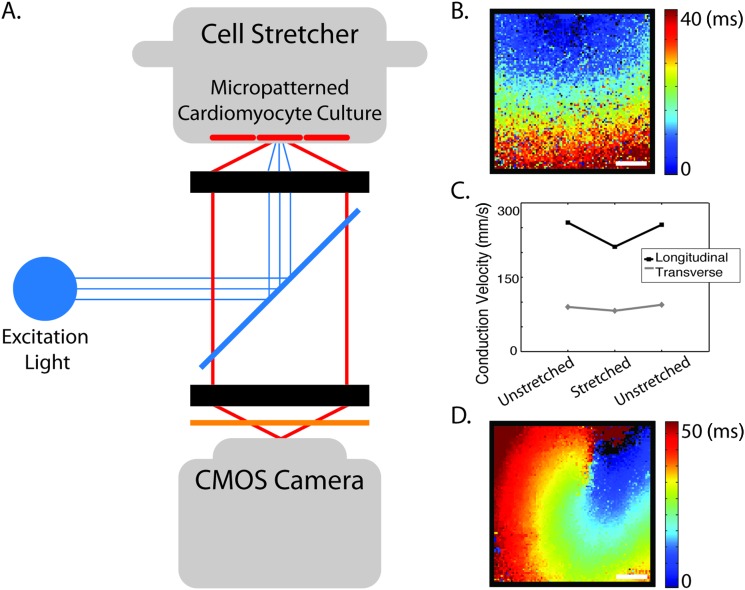
Working with neonatal cardiomyocytes, which are capable of forming mechanoelectric junctions in culture, geometrical cues presenting extracellular matrix protein have been found to guide cells into anisotropic and aligned morphology similar to that of developing myocardium [125–130]. Stretch devices have been designed which give options for studying micropatterned cardiomyocytes under physiological biaxial loading conditions [85,131–133]. When combined with optical methods for mapping changes in membrane potential as in Fig. 3, these techniques permit precise measurement with the capability to observe changes in conduction (slowing) across aligned, uniformly and precisely stretched, multicellular preparations [134]. The length scale of the micropatterned cell culture permits study of arrhythmogenic mechanisms, including those associated with proarrhythmic substrate such as reentrant spiral waves [Fig. 3(d)]. These experimental setups allow for direct evaluation of mechanoelectric mechanisms at the multicellular level, giving insight into cell-level function and its role in overall cardiac MEF.
Fig. 3.
Combined apparatus for biaxial stretch of micropatterned neonatal cardiomyocytes and optical mapping of cell membrane potential permits study of conduction through multicellular preparations. (a) Diagram of optical mapping and micropatterned stretch equipment; (b) representative map of electrical activation, spatial scale 2 mm; (c) example stretch experiment result, showing that conduction in the longitudinal and transverse directions of the micropatterned cell culture slows with biaxial stretch, scale 2 mm; and (d) Example activation map in a transgenic mouse model of arrhythmia associated with mechanoelectric junctions, in collaboration with Dr. Farah Sheikh, UCSD.
Engineered Cardiac Tissue.
Engineered cardiac tissue (ECT) is a relatively new and emerging technology that can be used to study both the mechanical and electrical properties of the myocardium at the tissue scale. This method allows for the study of neonatal cardiomyocytes at the tissue scale, which was previously not feasible in intact preparations such as trabeculae or papillary muscles, due to the small size of neonatal hearts. ECTs initially utilized cardiomyocytes from embryonic chick hearts [135,136] and neonatal rat hearts [125]. Recently, the source of cardiomyocytes was expanded to include neonatal mouse hearts, which allows access to the multitude of gene-targeted mouse models of heart disease [137]. Mechanics studies performed using ECTs have shown that they exhibit the Frank–Starling mechanism [136] as well as a positive force–frequency relation [135], both of which are observed in more traditional trabeculae and papillary preparations. These preparations have also been shown to propagate electrical impulse and produce calcium transients that are very representative of what is measured in traditional intact and whole heart preparations [125,138]. However, a key disadvantage to using ECT preparations to study mechanics is that they still produce comparatively low active tension values, generally less than 1 mN/mm2 [136], whereas trabeculae and papillary muscles easily produce in excess of 20 mN/mm2 [139,140]. These low active tension values raise some questions regarding how closely these preparations mimic healthy intact tissue. There are also concerns with the extracellular matrix structure, cell architecture, and cell–cell junctions that need to be addressed in these engineered tissues.
Isolated Muscle Preparations.
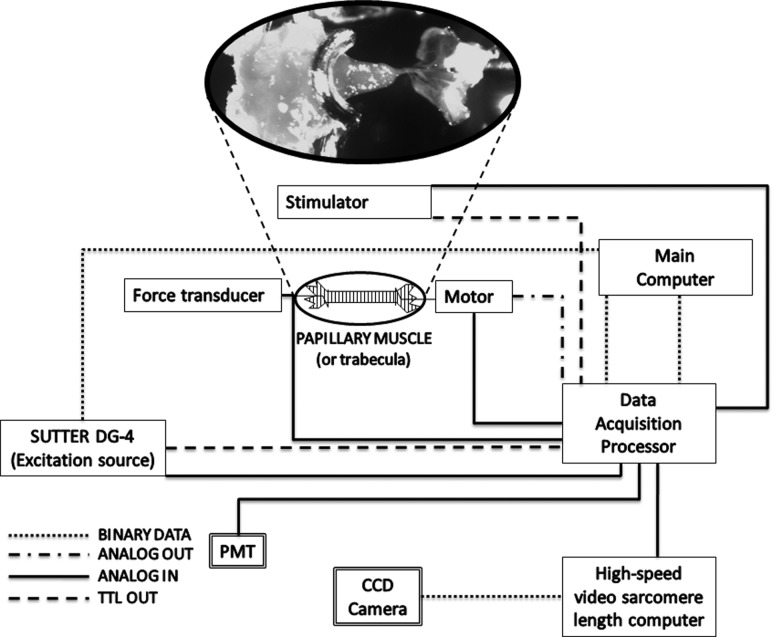
The best preparations for detailed measurements of cardiac muscle mechanics are isolated trabeculae and small papillary muscles, frequently from rodents [141]. Experimental setups designed to focus on isolated muscle mechanics usually consist of five main components: (1) A muscle chamber that allows for perfusion of the tissue with the desired solution; (2) a transducer to measure force produced by the muscle; (3) a servomotor to control the length of the muscle; (4) a laser directed at the muscle to measure sarcomere length; and (5) a photodiode array to measure the diffraction pattern produced by the laser. Figure 4 shows an example layout of a computer controlled muscle mechanics system. These setups measure force directly via the force transducer, which can be converted to tension by measuring the cross-sectional area of the muscle. Overall muscle length and length change are monitored via the servomotor, which comes equipped with a displacement transducer. The sarcomeres in the isolated tissue act as a grating and diffract the incident laser light into bands, which can be analyzed to determine sarcomere length [141]. The size and uniformity of the preparation are critical so that strains in the central portion of the preparation are relatively homogeneous, oxygen delivery is not limited by high diffusion distances and sarcomeres can be detected by laser diffraction.
Fig. 4.
Layout of a computer-controlled system designed for measuring cardiac muscle mechanics. The system is capable of measuring force, calcium transients, sarcomere length (in trabeculae), muscle length, and local muscle strain. The high-speed servomotor performs very precise stretches.
Setups often include components to allow for the measurement of free intracellular calcium. The change in free intracellular calcium after activation is referred to as a calcium transient. To measure calcium transient in intact muscle preparations, two additional pieces of equipment are needed: (1) An excitation light source that emits light at the desired wavelength to excite the calcium indicator, and (2) a photomultiplier tube (PMT) or a CCD camera to measure the fluorescent intensity emitted by the fluorophore [142]. Fura-2 is a popular calcium indicator which allows for quantification of free intracellular calcium in a ratiometric fashion [143]. With the ability to measure calcium transients simultaneously with force and sarcomere length, a whole new category of experiments is possible that can bridge the gap between cellular ECC experiments and observations of electromechanics in the whole heart [144].
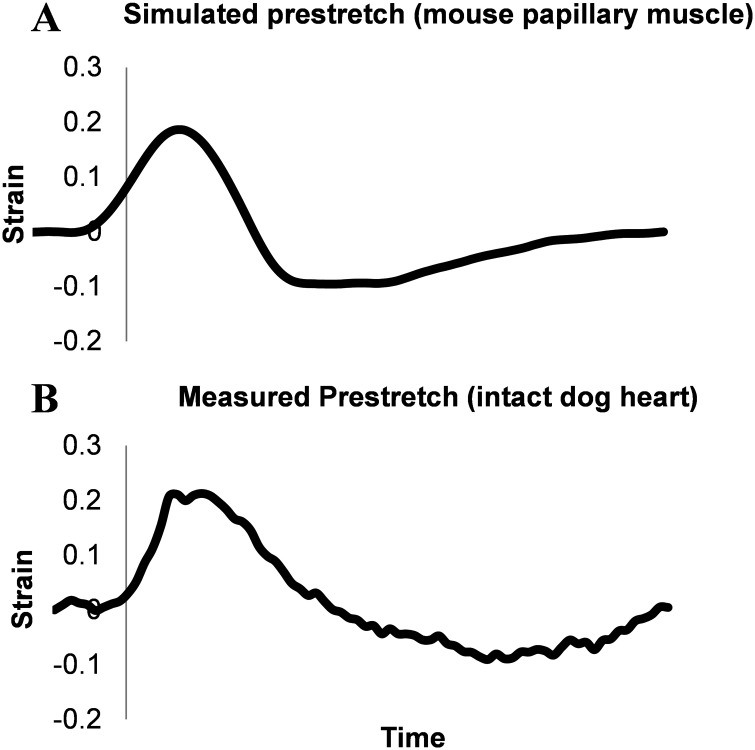
To investigate the effects of altered electrical activation relative to the timing of passive mechanical state of the local tissue, the altered strain pattern that is observed in dyssynchronous hearts can be imposed on papillary muscles or trabeculae preparations. Studies in the past have put two papillary muscles in series and electrically activated them in a dyssynchronous manner to mimic dyssynchrony [145]. This study was a good way to roughly mimic dyssynchrony, but did not provide precise control over the strains imposed on the muscles. A computer controlled setup with a high speed servomotor to precisely control strains imposed on the muscle allows for more control over the timing and magnitude of stretch in muscle relative to the time of electrical activation (Fig. 4). The prestretch that is observed in late-activated regions of dyssynchronous hearts (either due to LBBB or ventricular pacing) can be imposed on papillary muscles to determine the effects of this stretch, as seen in Fig. 5. This experiment utilized topical markers on the papillary muscles to measure and control regional strain in order to realistically simulate local prestretch in late-activated regions on the whole heart. These measured muscle strains were combined with force and calcium measurements using the system outlined in Fig. 4. The results from these experiments showed that the prestretch induced by dyssynchrony alters force production in the muscle, as well as the mechanical work performed, and this difference is amplified depending upon the timing of stretch. Calcium transients can be then analyzed to give insight into the role of alterations to cellular ECC mechanisms. Experimental setups like these allow for electromechanics observed in the whole heart to be studied in much more intricate detail with measures and control that are not available in whole heart preparations. For example, they will allow the mechanisms contributing to the deactivating effects of prestretch on myofilament force development to be dissected. It is likely that different mechanisms govern the responses to prestretches that are early with respect to the stimulus timing compared with later ones.
Fig. 5.
(a) Measured strain in a mouse papillary muscle due to a 20% prestretch with a timing in relation to activation (vertical line) that is similar to (b) measured strain in the late activated region of a ventricularly paced dog heart
Ventricular Slice and Wedge Preparations.
Other preparations that are used to study the myocardium at the tissue level are the ventricular slice and wedge. Ventricular wedge preparations have provided a wealth of knowledge regarding electrophysiology and arrythmogenesis, and have been shown to be a valid preclinical model [146]. These preparations keep the entire ventricular wall intact, which allows for determination of transmural electrophysiological heterogeneities that is not possible in many other preparations [147]. Studies were originally performed using canine ventricles, but have more recently been expanded to include human tissue [111,148]. Ventricular slice preparations have also been performed using human tissue, and consist of a very thin slice of tissue that is suitable for multicellular studies. Slice preparations provide stable electrical activity and have shown conduction velocities similar to that of the whole heart [111]. While this novel and highly promising approach provides an ideal framework for studying electrophysiology, using both optical and electrical methods, it has not yet been used for investigating mechanics or electromechanics, though this should be feasible.
Conclusions
The sequence of electrical activation in the whole heart has been shown to give rise to a relatively uniform contraction wavefront in the normal heart. This uniform contraction can be disrupted due to conduction disturbances such as LBBB or MEF, ultimately leading to dyssynchronous activation and contraction. It is known that dyssynchronous activation alters the elctromechanics of the heart, but the mechanisms involved in this alteration are not completely understood. At the level of the single cell, ECC has been studied as a means to give insight to the electromechanics taking place at the whole heart scale, and MEF has been investigated in the context of component ion channels response to cell membrane tension. At this point in time, it is fairly well understood how electrical excitation gives rise to mechanical contraction via calcium induced calcium release. However, studies at the cellular level lack the important cell–cell and cell–ECM mechanoelectrical interactions that are present in intact tissue. The lack of the ability to simulate dyssynchrony to study how it alters ECC, and the ability to apply precise mechanical loads to cell populations in order to understand MEF hinders progress toward identifying cell-scale mechanisms for organ-scale phenomena. Engineered cardiac tissue and ventricular wedge preparations are extremely useful for studying the electrophysiology of the myocardium, but require the ability to reliably study the mechanics to draw insight into MEF. Multicellular preparations with capability for biaxial stretch can provide this level of precision and show promise in identifying mechanisms of MEF. Tissue level preparations are the ideal model for studying dyssynchrony and its effect on ECC. Intact tissue preparations such as trabeculae and papillary muscles have been used for decades, but provide the ideal model for studying ECC and in particular the effects of dyssynchrony on ECC, due to their ability to produce valid mechanics and calcium data. These preparations in modern computer controlled tissue level systems are also rugged enough to withstand the physiological stretch seen in dyssynchrony. These and other novel approaches to measuring cardiac electromechanics will help bridge the gap in knowledge between what is well known at the cellular level, and how this information can be translated to the whole heart.
Acknowledgment
We gratefully acknowledge the collaborative contributions of Dr. Farah Sheikh and Dr. Valeria Mezzano, the scientific input of Stuart Campbell, Barbara Murienne, and Adam Wright, and the technical assistance of Jennifer Stowe, Kyle Buchholz, Katie McNall, Justin Tan, Tammy Soo-hoo, Jenny Cheng, and William Auyeung. This work and the authors has been supported by grants from the NHLBI and NIGMS.
Contributor Information
Emily R. Pfeiffer, Department of Bioengineering, Cardiac Biomedical Science and, Engineering Center, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093-0412
Jared R. Tangney, Department of Bioengineering, Cardiac Biomedical Science and, Engineering Center, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093-0412
Jeffrey H. Omens, Department of Bioengineering and Department of Medicine, Cardiac Biomedical, Science and Engineering Center, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093-0412
Andrew D. McCulloch, Department of Bioengineering and Department of Medicine, Cardiac Biomedical, Science and Engineering Center, University of California, San Diego 9500 Gilman Drive, La Jolla, CA 92093-0412, e-mail: amcculloch@ucsd.edu.
References
- [1]. Prinzen, F. W. , and Peschar, M. , 2002, “Relation Between the Pacing Induced Sequence of Activation and Left Ventricular Pump Function in Animals,” Pacing Clin. Electrophysiol., 25(4 Pt 1), pp. 484–498. 10.1046/j.1460-9592.2002.00484.x [DOI] [PubMed] [Google Scholar]
- [2]. Quinn, T. A. , Bayliss, R. A. , and Kohl, P. , 2011, “Mechano-electric Feedback in the Heart: Effects on Heart Rate and Rhythm,” Heart Rate and Rhythm, Tripathi O. N., Ravens U., and Sanguinetti M. C., Eds., Springer, Berlin, pp. 133–151. [Google Scholar]
- [3]. Leclercq, C. , and Kass, D. A. , 2002, “Retiming the Failing Heart: Principles and Current Clinical Status of Cardiac Resynchronization,” J. Am. Coll. Cardiol., 39(2), pp. 194–201. 10.1016/S0735-1097(01)01747-8 [DOI] [PubMed] [Google Scholar]
- [4]. Pogwizd, S. M. , and Bers, D. M. , 2002, “Calcium Cycling in Heart Failure: The Arrhythmia Connection,” J. Cardiovasc. Electrophysiol., 13(1), pp. 88–91. 10.1046/j.1540-8167.2002.00088.x [DOI] [PubMed] [Google Scholar]
- [5]. Prinzen, F. W. , Augustijn, C. H. , Allessie M. a., Arts, T. , Delhaas, T. , and Reneman, R. S. , 1992, “The Time Sequence of Electrical and Mechanical Activation During Spontaneous Beating and Ectopic Stimulation,” Eur. Heart J., 13(4), pp. 535–543. [DOI] [PubMed] [Google Scholar]
- [6]. Hoffman, B. , Cranefield, P. , Stuckey, J. , Amer, N. , Cappelletti, R. , and Domingo, R. , 1959, “Direct Measurement of Conduction Velocity in In Situ Specialized Conducting System of Mammalian Heart,” Proc. Soc. Exp. Biol. Med., 102, pp. 55–57. 10.3181/00379727-102-25141 [DOI] [PubMed] [Google Scholar]
- [7]. Myerburg, R. J. , Nilsson, K. , and Gelband, H. , 1972, “Physiology of Canine Intraventricular Conduction and Endocardial Excitation,” Circ. Res., 30(2), pp. 217–243. 10.1161/01.RES.30.2.217 [DOI] [PubMed] [Google Scholar]
- [8]. Scher, A. M. , Young, A. C. , Malmgren, A. L. , and Erickson, R. V. , 1955, “Activation of the Interventricular Septum,” Circ. Res., 3(1), pp. 56–64. 10.1161/01.RES.3.1.56 [DOI] [PubMed] [Google Scholar]
- [9]. Sodi-Pallares, D. , Bisteni, A. , Medrano, G. , and Cisneros, F. , 1955, “The Activation of the Free Left Ventricular Wall in the Dog's Heart; In Normal Conditions and in Left Bundle Branch Block,” Am. Heart J., 49(4), pp. 587–602. 10.1016/0002-8703(55)90077-6 [DOI] [PubMed] [Google Scholar]
- [10]. Durrer, D. , Van Dam, R. T. , Freud, G. E. , Janse, M. J. , Meijler, F. L. , and Arzbaecher, R. C. , 1970, “Total Excitation of the Isolated Human Heart,” Circulation, 41(6), pp. 899–912. 10.1161/01.CIR.41.6.899 [DOI] [PubMed] [Google Scholar]
- [11]. Badke, F. , Boinay, P. , and Covell, J. W. , 1980, “Effects of Ventricular Pacing on Regional Left Ventricular Performance in the Dog,” Am. J. Physiol., 238, pp. H858–867. [DOI] [PubMed] [Google Scholar]
- [12]. Gurev, V. , Constantino, J. , Rice, J. J. , and Trayanova, N. A. , 2010, “Distribution of Electromechanical Delay in the Heart: Insights From a Three-Dimensional Electromechanical Model,” Biophys. J., 99(3), pp. 745–754. 10.1016/j.bpj.2010.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Ashikaga, H. , Coppola, B. A. , Hopenfeld, B. , Leifer, E. S. , McVeigh, E. R. , and Omens, J. H. , 2007, “Transmural Dispersion of Myofiber Mechanics: Implications for Electrical Heterogeneity in vivo,” J. Am. Coll. Cardiol., 49(8), pp. 909–916. 10.1016/j.jacc.2006.07.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Usyk, T. , and McCulloch, A. D. , 2003, “Relationship Between Regional Shortening and Asynchronous Electrical Activation in a Three-Dimensional Model of Ventricular Electromechanics,” J. Cardiovasc. Electrophysiol., 14(10 Suppl), pp. S196–S202. 10.1046/j.1540.8167.90311.x [DOI] [PubMed] [Google Scholar]
- [15]. Russell, K. , Smiseth O. a., Gjesdal, O. , Qvigstad, E. , Norseng, P. A. , Sjaastad, I. , Opdahl, A. , Skulstad, H. , Edvardsen, T. , and Remme, E. W. , 2011, “Mechanism of Prolonged Electromechanical Delay in Late Activated Myocardium During Left Bundle Branch Block,” Am. J. Physiol. Heart Circ. Physiol., 301(6), pp. H2334–H243. 10.1152/ajpheart.00644.2011 [DOI] [PubMed] [Google Scholar]
- [16]. Wyndham, C. R. , Smith, T. , Meeran, M. K. , Mammana, R. , Levitsky, S. , and Rosen, K. M. , 1980, “Epicardial Activation in Patients With Left Bundle Branch Block,” Circulation, 61(4), pp. 696–703. 10.1161/01.CIR.61.4.696 [DOI] [PubMed] [Google Scholar]
- [17]. Grines, C. L. , Bashore, T. M. , Boudoulas, H. , Olson, S. , Shafer, P. , and Wooley, C. F. , 1989, “Functional Abnormalities in Isolated Left Bundle Branch Block. The Effect of Interventricular Asynchrony,” Circulation, 79(4), pp. 845–853. 10.1161/01.CIR.79.4.845 [DOI] [PubMed] [Google Scholar]
- [18]. Uusimaa, P. , Risteli, J. , Niemelä, M. , Lumme, J. , Ikäheimo, M. , Jounela, A. , and Peukhurinen, K. , 1997, “Collagen Scar Formation After Acute Myocardial Infarction: Relationships to Infarct Size, Left Ventricular Function, and Coronary Artery Patency,” Circulation, 96, pp. 2565–2572. 10.1161/01.CIR.96.8.2565 [DOI] [PubMed] [Google Scholar]
- [19]. van Oosterhout, M. F. M. , Prinzen, F. W. , Arts, T. , Schreuder, J. J. , Vanagt, W. Y. R. , Cleutjens, J. P. M. , Reneman, R. S. , and Ward, Y. R. , 1998, “Asynchronous Electrical Activation Induces Asymmetrical Hypertrophy of the Left Ventricular Wall,” Circulation, 98(6), pp. 588–595. 10.1161/01.CIR.98.6.588 [DOI] [PubMed] [Google Scholar]
- [20]. Vassallo, J. a. , Cassidy, D. M. , Marchlinski, F. E. , Buxton, a. E. , Waxman, H. L. , Doherty, J. U. , and Josephson, M. E. , 1984, “Endocardial Activation of Left Bundle Branch Block,” Circulation, 69(5), pp. 914–923. 10.1161/01.CIR.69.5.914 [DOI] [PubMed] [Google Scholar]
- [21]. Prinzen, F. , Cheriex, E. , Delhaas, T. , van Oosterhout, M. , Arts, T. , Wellens, H. , and Reneman, R. , 1995, “Asymmetric Thickness of the Left Ventricular Wall Resulting From Asynchronous Electric Activation: A Study in Dogs With Ventricular Pacing and in Patients With Left Bundle Branch Block,” Am. Heart J., 130(5), pp. 1045–1053. 10.1016/0002-8703(95)90207-4 [DOI] [PubMed] [Google Scholar]
- [22]. Sweeney, M. O. , and Prinzen, F. W. , 2006, “A New Paradigm for Physiologic Ventricular Pacing,” J. Am. Coll. Cardiol., 47(2), pp. 282–288. 10.1016/j.jacc.2005.09.029 [DOI] [PubMed] [Google Scholar]
- [23]. Delhaas, T. , Arts, T. , Prinzen, F. , and Reneman, R. , 1993, “Relation Between Regional Electrical Activation Time and Subepicardial Fiber Strain in the Canine Left Ventricle,” Pflügers Arch., 423(1–2), pp. 78–87. 10.1007/BF00374964 [DOI] [PubMed] [Google Scholar]
- [24]. Prinzen, F. W. , Augustijn, C. H. , Arts, T. , Allessie, M. a. , and Reneman, R. S. , 1990, “Redistribution of Myocardial Fiber Strain and Blood Flow by Asynchronous Activation,” Am. J. Physiol., 259(2 Pt 2), pp. H300–H308. [DOI] [PubMed] [Google Scholar]
- [25]. Wyman, B. T. , Hunter, W. C. , Prinzen, F. W. , Faris, O. P. , and McVeigh, E. R. , 2002, “Effects of Single- and Biventricular Pacing on Temporal and Spatial Dynamics of Ventricular Contraction,” Am. J. Physiol. Heart Circ. Physiol., 282(1), pp. H372–H379. [DOI] [PubMed] [Google Scholar]
- [26]. Prinzen, F. , and Hunter, W. , 1999, “Mapping of Regional Myocardial Strain and Work During Ventricular Pacing: Experimental Study Using Magnetic Resonance Imaging Tagging,” J. Am. Coll. Cardiol., 33(6), pp. 1735–1742. 10.1016/S0735-1097(99)00068-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Ghio, S. , Constantin, C. , Klersy, C. , Serio, A. , Fontana, A. , Campana, C. , and Tavazzi, L. , 2004, “Interventricular and Intraventricular Dyssynchrony Are Common in Heart Failure Patients, Regardless of QRS Duration,” Eur. Heart J., 25(7), pp. 571–578. 10.1016/j.ehj.2003.09.030 [DOI] [PubMed] [Google Scholar]
- [28]. Maron, B. J. , Doerer, J. J. , Haas, T. S. , Estes, N. A. M. , and Link, M. S. , 2006, “Historical Observation on Commotio Cordis,” Heart Rhythm, 3(5), pp. 605–606. 10.1016/j.hrthm.2005.12.011 [DOI] [PubMed] [Google Scholar]
- [29]. Kohl, P. , Hunter, P. , and Noble, D. , 1999, “Stretch-Induced Changes in Heart Rate and Rhythm: Clinical Observations, Experiments and Mathematical Models,” Prog. Biophys. Mol. Biol., 71, pp. 91–138. 10.1016/S0079-6107(98)00038-8 [DOI] [PubMed] [Google Scholar]
- [30]. Maron, B. J. , Haas, T. S. , Ahluwalia, A. , Garberich, R. F. , Estes, N. A. M. , and Link, M. S. , 2013, “Increasing Survival Rate From Commotio Cordis,” Heart Rhythm, 10(2), pp. 219–223. 10.1016/j.hrthm.2012.10.034 [DOI] [PubMed] [Google Scholar]
- [31]. Madias, C. , Maron, B. J. , Alsheikh-ali, A. A. , Estes, N. A. M. , and Link, M. S. , 2007, “Commotio Cordis,” Indian Pacing Electrophysiol. J., 7(4), pp. 235–245. [PMC free article] [PubMed] [Google Scholar]
- [32]. Trayanova N. a., Constantino, J. , and Gurev, V. , 2010, “Models of Stretch-Activated Ventricular Arrhythmias,” J. Electrocardiol., 43(6), pp. 479–485. 10.1016/j.jelectrocard.2010.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Hansen, D. E. , Craig, C. S. , and Hondeghem, L. M. , 1990, “Stretch-Induced Arrhythmias in the Isolated Canine Ventricle. Evidence for the Importance of Mechanoelectrical Feedback,” Circulation, 81(3), pp. 1094–1105. 10.1161/01.CIR.81.3.1094 [DOI] [PubMed] [Google Scholar]
- [34]. Wang, Z. , Taylor, L. K. , Denney, W. D. , and Hansen, D. E. , 1994, “Initiation of Ventricular Extrasystoles by Myocardial Stretch in Chronically Dilated and Failing Canine Left Ventricle,” Circulation, 90(4), pp. 2022–2031. 10.1161/01.CIR.90.4.2022 [DOI] [PubMed] [Google Scholar]
- [35]. Berdowski, J. , Tijssen, J. G. P. , and Koster, R. W. , 2010, “Chest Compressions Cause Recurrence of Ventricular Fibrillation After the First Successful Conversion by Defibrillation in Out-of-Hospital Cardiac Arrest,” Circ. Arrhythmia Electrophysiol., 3(1), pp. 72–78. 10.1161/CIRCEP.109.902114 [DOI] [PubMed] [Google Scholar]
- [36]. Janse, M. J. , Coronel, R. , Wilms-Schopman, F. J. , and de Groot, J. R. , 2003, “Mechanical Effects on Arrhythmogenesis: From Pipette to Patient,” Prog. Biophys. Mol. Biol., 82(1–3), pp. 187–195. 10.1016/S0079-6107(03)00015-4 [DOI] [PubMed] [Google Scholar]
- [37]. Pellis, T. , Kette, F. , Lovisa, D. , Franceschino, E. , Magagnin, L. , Mercante, W. P. , and Kohl, P. , 2009, “Utility of Pre-cordial Thump for Treatment of Out of Hospital Cardiac Arrest: A Prospective Study,” Resuscitation, 80(1), pp. 17–23. 10.1016/j.resuscitation.2008.10.018 [DOI] [PubMed] [Google Scholar]
- [38]. Pellis, T. , and Kohl, P. , 2010, “Extracorporeal Cardiac Mechanical Stimulation: Precordial Thump and Precordial Percussion,” Br. Med. Bull., 93, pp. 161–177. 10.1093/bmb/ldp045 [DOI] [PubMed] [Google Scholar]
- [39]. Bainbridge, F. A. , 1915, “The Influence of Venous Filling Upon the Rate of the Heart,” J. Physiol., 50(2), pp. 65–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Crystal, G. J. , and Salem, M. R. , 2012, “The Bainbridge and the ‘Reverse’ Bainbridge Reflexes: History, Physiology, and Clinical Relevance,” Anesth. Analg., 114(3), pp. 520–532. 10.1213/ANE.0b013e3182312e21 [DOI] [PubMed] [Google Scholar]
- [41]. Cui, J. , Gao, Z. , Blaha C. a., Herr, M. D. , Mast, J. L. , and Sinoway, L. I. , 2013, “Distension of Central Great Vein Decreases Sympathetic Outflow in Humans,” Am. J. Physiol. Heart Circ. Physiol., 305(3), pp. H378–H385. 10.1152/ajpheart.00019.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Lab, M. J. , 1982, “Contraction-Excitation Feedback in Myocardium. Physiological Basis and Clinical Relevance,” Circ. Res., 50(6), pp. 757–766. 10.1161/01.RES.50.6.757 [DOI] [PubMed] [Google Scholar]
- [43]. Marrus, S. B. , and Nerbonne, J. M. , 2008, “Mechanisms Linking Short- and Long-Term Electrical Remodeling in the Heart… Is It a Stretch?,” Channels, 2(2), pp. 117–124. 10.4161/chan.2.2.6104 [DOI] [PubMed] [Google Scholar]
- [44]. Eckardt, L. , Kirchhof, P. , Monnig, G. , Breithardt, G. , Borggrefe, M. , and Haverkamp, W. , 2000, “Modification of Stretch-Induced Shortening of Repolarization by Streptomycin in the Isolated Rabbit Heart,” J. Cardiovasc. Pharmacol., 36(6), pp. 1–17. [DOI] [PubMed] [Google Scholar]
- [45]. Franz, M. R. , and Bode, F. , 2003, “Mechano-electrical Feedback Underlying Arrhythmias: The Atrial Fibrillation Case,” Prog. Biophys. Mol. Biol., 82(1–3), pp. 163–174. 10.1016/S0079-6107(03)00013-0 [DOI] [PubMed] [Google Scholar]
- [46]. Kiseleva, I. , Kamkin, A. , Wagner, K.-D. , Theres, H. , Ladhoff, A. , Scholz, H. , Günther, J. , and Lab, M. J. , 2000, “Mechanoelectric Feedback After Left Ventricular Infarction in Rats,” Cardiovasc. Res., 45(2), pp. 370–378. 10.1016/S0008-6363(99)00361-2 [DOI] [PubMed] [Google Scholar]
- [47]. McNary, T. G. , Sohn, K. , Taccardi, B. , and Sachse, F. B. , 2008, “Experimental and Computational Studies of Strain-Conduction Velocity Relationships in Cardiac Tissue,” Prog. Biophys. Mol. Biol., 97(2–3), pp. 383–400. 10.1016/j.pbiomolbio.2008.02.023 [DOI] [PubMed] [Google Scholar]
- [48]. Sung, D. , Mills, R. W. , Schettler, J. , Narayan, S. M. , Omens, J. H. , and McCulloch, A. D. , 2003, “Ventricular Filling Slows Epicardial Conduction and Increases Action Potential Duration in an Optical Mapping Study of the Isolated Rabbit Heart,” J. Cardiovasc. Electrophysiol., 14(7), pp. 739–749. 10.1046/j.1540-8167.2003.03072.x [DOI] [PubMed] [Google Scholar]
- [49]. Mills, R. W. , Narayan, S. M. , and McCulloch, A. D. , 2008, “Mechanisms of Conduction Slowing During Myocardial Stretch by Ventricular Volume Loading in the Rabbit,” Am. J. Physiol. Heart Circ. Physiol., 295(3), pp. H1270–H1278. 10.1152/ajpheart.00350.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Satoh, T. , and Zipes, D. P. , 1996, “Unequal Atrial Stretch in Dogs Increases Dispersion of Refractoriness Conducive to Developing Atrial Fibrillation,” J. Cardiovasc. Electrophysiol., 7(9), pp. 833–842. 10.1111/j.1540-8167.1996.tb00596.x [DOI] [PubMed] [Google Scholar]
- [51]. Mills, R. W. , Wright, A. T. , Narayan, S. M. , and McCulloch, A. D. , 2011, “The Effects of Wall Stretch on Ventricular Conduction and Refractoriness in the Whole Heart,” Cardiac Mechano-Electric Coupling and Arrhythmias, Kohl P., Sachs F., and Franz M. R., Eds., Oxford University Press, Oxford, pp. 180–186. [Google Scholar]
- [52]. López-López, J. , Shacklock, P. , Balke, C. , and Wier, W. , 1995, “Local Calcium Transients Triggered by Single L-Type Calcium Channel Currents in Cardiac Cells,” Science, 268(5213), pp. 1042–1045. 10.1126/science.7754383 [DOI] [PubMed] [Google Scholar]
- [53]. Bers, D. , 2002, “Cardiac Excitation-Contraction Coupling,” Nature, 415(6868), pp. 198–205. 10.1038/415198a [DOI] [PubMed] [Google Scholar]
- [54]. Endo, M. , Tanaka, M. , and Ogawa, Y. , 1970, “Calcium Induced Release of Calcium From the Sarcoplasmic Reticulum of Skinned Skeletal Muscle Fibres,” Nature, 228(5266), pp. 34–36. 10.1038/228034a0 [DOI] [PubMed] [Google Scholar]
- [55]. Cordeiro, J. M. , Greene, L. , Heilmann, C. , Antzelevitch, D. , and Antzelevitch, C. , 2004, “Transmural Heterogeneity of Calcium Activity and Mechanical Function in the Canine Left Ventricle,” Am. J. Physiol. Heart Circ. Physiol., 286(4), pp. H1471–H1479. 10.1152/ajpheart.00748.2003 [DOI] [PubMed] [Google Scholar]
- [56]. Bers, D. M. , 2008, “Calcium Cycling and Signaling in Cardiac Myocytes,” Annu. Rev. Physiol., 70, pp. 23–49. 10.1146/annurev.physiol.70.113006.100455 [DOI] [PubMed] [Google Scholar]
- [57]. Holroydes, M. J. , Robertson, S. P. , Johnsong, J. D. , Solarosgl, R. J. , James, D. , Solaro, R. J. , and Potter, J. D. , 1980, “The Calcium and Magnesium Binding Sites on Cardiac Troponin and Their Role in the Regulation of Myofibrillar Adenosine Triphosphatase,” J. Biol. Chem., 255(24), pp. 11688–11693. [PubMed] [Google Scholar]
- [58]. Kobayashi, T. , and Solaro, R. J. , 2005, “Calcium, Thin Filaments, and the Integrative Biology of Cardiac Contractility,” Annu. Rev. Physiol., 67, pp. 39–67. 10.1146/annurev.physiol.67.040403.114025 [DOI] [PubMed] [Google Scholar]
- [59]. Gordon, A. M. , Homsher, E. , and Regnier, M. , 2000, “Regulation of Contraction in Striated Muscle,” Physiol. Rev., 80(2), pp. 853–924. [DOI] [PubMed] [Google Scholar]
- [60]. de Tombe, P. P. , Mateja, R. D. , Tachampa, K. , Ait Mou, Y. , Farman, G. P. , and Irving, T. C. , 2010, “Myofilament Length Dependent Activation.,” J. Mol. Cell. Cardiol., 48(5), pp. 851–858. 10.1016/j.yjmcc.2009.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Campbell, S. G. , Lionetti, F. V. , Campbell, K. S. , and McCulloch, A. D. , 2010, “Coupling of Adjacent Tropomyosins Enhances Cross-Bridge-Mediated Cooperative Activation in a Markov Model of the Cardiac Thin Filament,” Biophys. J., 98(10), pp. 2254–2264. 10.1016/j.bpj.2010.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Hofmann, P. A. , and Fuchs, F. , 1988, “Bound Calcium and Force Development in Skinned Cardiac Muscle Bundles: Effect of Sarcomere Length,” J. Mol. Cell. Cardiol., 20(8), pp. 667–677. 10.1016/S0022-2828(88)80012-9 [DOI] [PubMed] [Google Scholar]
- [63]. Allen, D. G. , Kurihara, S. , and Allen, B. Y. D. G. , 1982, “The Effects of Muscle Length on Intracellular Calcium Transients in Mammalian Cardiac Muscle,” J. Physiol., 327, pp. 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Bett, G. C. , and Sachse, F. B. , 1997, “Cardiac Mechanosensitivity and Stretch-Activated Ion Channels,” Trends Cardiovasc. Med., 7(1), pp. 4–8. 10.1016/S1050-1738(96)00119-3 [DOI] [PubMed] [Google Scholar]
- [65]. Bowman, C. L. , Gottlieb, P. a. , Suchyna, T. M. , Murphy, Y. K. , and Sachs, F. , 2007, “Mechanosensitive Ion Channels and the Peptide Inhibitor GsMTx-4: History, Properties, Mechanisms and Pharmacology,” Toxicon, 49(2), pp. 249–270. 10.1016/j.toxicon.2006.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Dyachenko, V. , Husse, B. , Rueckschloss, U. , and Isenberg, G. , 2009, “Mechanical Deformation of Ventricular Myocytes Modulates Both TRPC6 and Kir2.3 Channels,” Cell Calcium, 45(1), pp. 38–54. 10.1016/j.ceca.2008.06.003 [DOI] [PubMed] [Google Scholar]
- [67]. Hu, H. , and Sachs, F. , 1997, “Stretch-Activated Ion Channels in the Heart,” J. Mol. Cell. Cardiol., 29, pp. 1511–1523. 10.1006/jmcc.1997.0392 [DOI] [PubMed] [Google Scholar]
- [68]. Spassova, M. a. , Hewavitharana, T. , Xu, W. , Soboloff, J. , and Gill, D. L. , 2006, “A Common Mechanism Underlies Stretch Activation and Receptor Activation of TRPC6 Channels,” Proc. Natl. Acad. Sci. U.S.A., 103(44), pp. 16586–16591. 10.1073/pnas.0606894103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Suchyna, T. M. , Johnson, J. H. , Hamer, K. , Leykam, J. F. , Gage, D. a. , Clemo, H. F. , Baumgarten, C. M. , and Sachs, F. , 2000, “Identification of a Peptide Toxin From Grammostola Spatulata Spider Venom That Blocks Cation-Selective Stretch-Activated Channels,” J. Gen. Physiol., 115(5), pp. 583–598. 10.1085/jgp.115.5.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Eder, P. , and Molkentin, J. D. , 2011, “TRPC Channels As Effectors of Cardiac Hypertrophy,” Circ. Res., 108(2), pp. 265–272. 10.1161/CIRCRESAHA.110.225888 [DOI] [PubMed] [Google Scholar]
- [71]. Friedrich, O. , Wagner, S. , Battle, A. R. , Schürmann, S. , and Martinac, B. , 2012, “Mechano-regulation of the Beating Heart at the Cellular Level—Mechanosensitive Channels in Normal and Diseased Heart,” Prog. Biophys. Mol. Biol., 110(2–3), pp. 226–238. 10.1016/j.pbiomolbio.2012.08.009 [DOI] [PubMed] [Google Scholar]
- [72]. Quinn, T. A. , and Kohl, P. , 2012, “Mechano-sensitivity of Cardiac Pacemaker Function: Pathophysiological Relevance, Experimental Implications, and Conceptual Integration With Other Mechanisms of Rhythmicity,” Prog. Biophys. Mol. Biol., 110(2–3), pp. 257–268. 10.1016/j.pbiomolbio.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Healy, S. N. , and McCulloch, A. D. , 2005, “An Ionic Model of Stretch-Activated and Stretch-Modulated Currents in Rabbit Ventricular Myocytes,” Europace, 7, pp. S128–S134. 10.1016/j.eupc.2005.03.019 [DOI] [PubMed] [Google Scholar]
- [74]. Kong, C.-R. , Bursac, N. , and Tung, L. , 2005, “Mechanoelectrical Excitation by Fluid Jets in Monolayers of Cultured Cardiac Myocytes,” J. Appl. Physiol., 98(6), pp. 2328–2336. 10.1152/japplphysiol.01084.2004 [DOI] [PubMed] [Google Scholar]
- [75]. Weise, L. D. , and Panfilov, A. V. , 2013, “A Discrete Electromechanical Model for Human Cardiac Tissue: Effects of Stretch-Activated Currents and Stretch Conditions on Restitution Properties and Spiral Wave Dynamics,” PLoS One, 8(3), p. e59317. 10.1371/journal.pone.0059317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Clapham, D. E. , Shrier, A. , and DeHaan, R. L. , 1980, “Junctional Resistance and Action Potential Delay Between Embryonic Heart Cell Aggregates,” J. Gen. Physiol., 75(6), pp. 633–654. 10.1085/jgp.75.6.633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. Elfgang, C. , Eckert, R. , Lichtenberg-Fraté, H. , Butterweck, A. , Traub, O. , Klein, R. a. , Hülser, D. F. , and Willecke, K. , 1995, “Specific Permeability and Selective Formation of Gap Junction Channels in Connexin-Transfected HeLa Cells,” J. Cell Biol., 129(3), pp. 805–817. 10.1083/jcb.129.3.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78]. Gourdie, R. G. , 1995, “A Map of the Heart: Gap Junctions, Connexin Diversity and Retroviral Studies of Conduction Myocyte Lineage,” Clin. Sci., 88, pp. 257–262. [DOI] [PubMed] [Google Scholar]
- [79]. Severs, N. J. , Bruce, A. F. , Dupont, E. , and Rothery, S. , 2008, “Remodelling of Gap Junctions and Connexin Expression in Diseased Myocardium,” Cardiovasc. Res., 80(1), pp. 9–19. 10.1093/cvr/cvn133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Weingart, R. , 1986, “Electrical Properties of the Nexal Membrane Studied in Rat Ventricular Cell Pairs,” J. Physiol., 370, pp. 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Fontes, M. S. C. , van Veen, T. a., B. , de Bakker, J. M. T. , and van Rijen, H. V. M. , 2011, “Functional Consequences of Abnormal Cx43 Expression in the Heart,” Biochim. Biophys. Acta, 1818(8), pp. 2020–202. 10.1016/j.bbamem.2011.07.039 [DOI] [PubMed] [Google Scholar]
- [82]. Saffitz, J. E. , Laing, J. G. , and Yamada, K. a. , 2000, “Connexin Expression and Turnover: Implications for Cardiac Excitability,” Circ. Res., 86, pp. 723–728. 10.1161/01.RES.86.7.723 [DOI] [PubMed] [Google Scholar]
- [83]. Saffitz, J. E. , 2005, “Dependence of Electrical Coupling on Mechanical Coupling in Cardiac Myocytes: Insights Gained From Cardiomyopathies Caused by Defects in Cell-Cell Connections,” Ann. N.Y. Acad. Sci., 1047, pp. 336–344. 10.1196/annals.1341.030 [DOI] [PubMed] [Google Scholar]
- [84]. Cherian, P. P. , Siller-Jackson, A. J. , Gu, S. , Wang, X. , Bonewald, L. F. , Sprague, E. , and Jiang, J. X. , 2005, “Mechanical Strain Opens Connexin 43 Hemichannels in Osteocytes: A Novel Mechanism for the Release of Prostaglandin,” Mol. Biol. Cell, 16(7), pp. 3100–3106. 10.1091/mbc.E04-10-0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Gopalan, S. M. , Flaim, C. , Bhatia, S. N. , Hoshijima, M. , Knoell, R. , Chien, K. R. , Omens, J. H. , and McCulloch, A. D. , 2003, “Anisotropic Stretch-Induced Hypertrophy in Neonatal Ventricular Myocytes Micropatterned on Deformable Elastomers,” Biotechnol. Bioeng., 81(5), pp. 578–587. 10.1002/bit.10506 [DOI] [PubMed] [Google Scholar]
- [86]. Zhuang, J. , Yamada, K. a. , Saffitz, J. E. , and Kléber, A. G. , 2000, “Pulsatile Stretch Remodels Cell-to-Cell Communication in Cultured Myocytes,” Circ. Res., 87(4), pp. 316–322. 10.1161/01.RES.87.4.316 [DOI] [PubMed] [Google Scholar]
- [87]. Kontogeorgis, A. , Kaba, R. a. , Kang, E. , Feig, J. E. , Gupta, P. P. , Ponzio, M. , Liu, F. , Rindler, M. J. , Wit, A. L. , Fisher, E. a. , Peters, N. S. , and Gutstein, D. E. , 2008, “Short-Term Pacing in the Mouse Alters Cardiac Expression of Connexin43,” BioMed Cent. Physiol., 8, p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Poelzing, S. , and Rosenbaum, D. S. , 2004, “Altered Connexin43 Expression Produces Arrhythmia Substrate in Heart Failure,” Am. J. Physiol. Heart Circ. Physiol., 287(4), pp. H1762–H1770. 10.1152/ajpheart.00346.2004 [DOI] [PubMed] [Google Scholar]
- [89]. Rhett, J. M. , Veeraraghavan, R. , Poelzing, S. , and Gourdie, R. G. , 2013, “The Perinexus: Sign-Post on the Path to a New Model of Cardiac Conduction?,” Trends Cardiovasc. Med., 23(6), pp. 222–228. 10.1016/j.tcm.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Hand, P. E. , and Peskin, C. S. , 2010, “Homogenization of an Electrophysiological Model for a Strand of Cardiac Myocytes With Gap-Junctional and Electric-Field Coupling,” Bull. Math. Biol., 72(6), pp. 1408–1424. 10.1007/s11538-009-9499-2 [DOI] [PubMed] [Google Scholar]
- [91]. Lin, J. , and Keener, J. P. , 2013, “Ephaptic Coupling in Cardiac Myocytes,” IEEE Trans. Biomed. Eng., 60(2), pp. 576–582. 10.1109/TBME.2012.2226720 [DOI] [PubMed] [Google Scholar]
- [92]. Roberts, S. F. , Stinstra, J. G. , and Henriquez, C. S. , 2008, “Effect of Nonuniform Interstitial Space Properties on Impulse Propagation: A Discrete Multidomain Model,” Biophys. J., 95(8), pp. 3724–3737. 10.1529/biophysj.108.137349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93]. Kohl, P. , Cooper, P. J. , and Holloway, H. , 2003, “Effects of Acute Ventricular Volume Manipulation on In Situ Cardiomyocyte Cell Membrane Configuration,” Prog. Biophys. Mol. Biol., 82(1–3), pp. 221–227. 10.1016/S0079-6107(03)00024-5 [DOI] [PubMed] [Google Scholar]
- [94]. McNary, T. G. , Spitzer, K. W. , Holloway, H. , Bridge, J. H. B. , Kohl, P. , and Sachse, F. B. , 2012, “Mechanical Modulation of the Transverse Tubular System of Ventricular Cardiomyocytes,” Prog. Biophys. Mol. Biol., 110(2–3), pp. 218–225. 10.1016/j.pbiomolbio.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Suchyna, T. M. , Tape, S. E. , Koeppe, R. E. , Andersen, O. S. , Sachs, F. , and Gottlieb, P. A. , 2004, “Bilayer-Dependent Inhibition of Mechanosensitive Channels by Neuroactive Peptide Enantiomers,” Nature, 430(6996), pp. 235–240. 10.1038/nature02743 [DOI] [PubMed] [Google Scholar]
- [96]. Maleckar, M. M. , Greenstein, J. L. , Giles, W. R. , and Trayanova, N. A. , 2009, “Electrotonic Coupling Between Human Atrial Myocytes and Fibroblasts Alters Myocyte Excitability and Repolarization,” Biophys. J., 97(8), pp. 2179–2190. 10.1016/j.bpj.2009.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97]. Miragoli, M. , Gaudesius, G. , and Rohr, S. , 2006, “Electrotonic Modulation of Cardiac Impulse Conduction by Myofibroblasts,” Circ. Res., 98(6), pp. 801–810. 10.1161/01.RES.0000214537.44195.a3 [DOI] [PubMed] [Google Scholar]
- [98]. Rook, M. , Jongsma, H. , and Jonge, B. , 1989, “Single Channel Currents of Homo- and Heterologous Gap Junctions Between Cardiac Fibroblasts and Myocytes,” Pflügers Arch. Eur. J. Physiol., 414(1), pp. 95–98. 10.1007/BF00585633 [DOI] [PubMed] [Google Scholar]
- [99]. Rudy, Y. , 2005, “Electrotonic Cell-Cell Interactions in Cardiac Tissue: Effects on Action Potential Propagation and Repolarization,” Ann. N.Y. Acad. Sci., 1047, pp. 308–313. 10.1196/annals.1341.027 [DOI] [PubMed] [Google Scholar]
- [100]. Kohl, P. , Kamkin, A. , Kiseleva, I. S. , and Streubel, T. , 1992, “Mechanosensitive Cells in the Atrium of Frog Heart,” Exp. Physiol., 77(1), pp. 213–216. [DOI] [PubMed] [Google Scholar]
- [101]. Kohl, P. , Kamkin, A. , Kiseleva, I. S. , and Noble, D. , 1994, “Mechanosensitive Fibroblasts in the Sino-Atrial Node Region of Rat Heart: Interaction With Cardiomyocytes and Possible Role,” Exp. Physiol., 79(6), pp. 943–956. [DOI] [PubMed] [Google Scholar]
- [102]. Haraguchi, Y. , Shimizu, T. , Yamato, M. , and Okano, T. , 2010, “Electrical Interaction Between Cardiomyocyte Sheets Separated by Non-cardiomyocyte Sheets in Heterogeneous Tissues,” Tissue Eng., 4, pp. 291–299. [DOI] [PubMed] [Google Scholar]
- [103]. Rohr, S. , 2009, “Myofibroblasts in Diseased Hearts: New Players in Cardiac Arrhythmias?,” Heart Rhythm, 6(6), pp. 848–856. 10.1016/j.hrthm.2009.02.038 [DOI] [PubMed] [Google Scholar]
- [104]. Vasquez, C. , Benamer, N. , and Morley, G. E. , 2011, “The Cardiac Fibroblast: Functional and Electrophysiological Considerations in Healthy and Diseased Hearts,” J. Cardiovasc. Pharmacol., 57(4), pp. 380–388. 10.1097/FJC.0b013e31820cda19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Thompson, S. a. , Copeland, C. R. , Reich, D. H. , and Tung, L. , 2011, “Mechanical Coupling Between Myofibroblasts and Cardiomyocytes Slows Electric Conduction in Fibrotic Cell Monolayers,” Circulation, 123(19), pp. 2083–2093. 10.1161/CIRCULATIONAHA.110.015057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. Suchyna, T. M. , and Sachs, F. , 2007, “Mechanosensitive Channel Properties and Membrane Mechanics in Mouse Dystrophic Myotubes,” J. Physiol., 581(1), pp. 369–387. 10.1113/jphysiol.2006.125021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Sokabe, M. , Sachs, F. , and Jing, Z. Q. , 1991, “Quantitative Video Microscopy of Patch Clamped Membranes Stress, Strain, Capacitance, and Stretch Channel Activation,” Biophys. J., 59(3), pp. 722–728. 10.1016/S0006-3495(91)82285-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108]. Morris, C. , and Homann, U. , 2001, “Cell Surface Area Regulation and Membrane Tension,” J. Membr. Biol., 179, pp. 79–102. [DOI] [PubMed] [Google Scholar]
- [109]. Gómez, A. M. , Guatimosim, S. , Dilly, K. W. , Vassort, G. , Lederer, W. J. , and Gómez, A. M. , 2001, “Heart Failure After Myocardial Infarction: Altered Excitation-Contraction Coupling,” Circulation, 104(6), pp. 688–693. 10.1161/hc3201.092285 [DOI] [PubMed] [Google Scholar]
- [110]. Isenberg, G. , Kondratev, D. , Dyachenko, V. , Kazanski, V. , and Gallitelli, M. F. , 2005, “Isolated Cardiomyocytes: Mechanosensitivity of Action Potential, Membrane Current and Ion Concentration,” Mechanosensitivity in Cells and Tissues, Kamkin A., and Kiseleva I., Eds., Academia, Moscow. [PubMed] [Google Scholar]
- [111]. Camelliti, P. , Al-Saud, S. A. , Smolenski, R. T. , Al-Ayoubi, S. , Bussek, A. , Wettwer, E. , Banner, N. R. , Bowles, C. T. , Yacoub, M. H. , and Terracciano, C. M. , 2011, “Adult Human Heart Slices Are a Multicellular System Suitable for Electrophysiological and Pharmacological Studies,” J. Mol. Cell. Cardiol., 51(3), pp. 390–398. 10.1016/j.yjmcc.2011.06.018 [DOI] [PubMed] [Google Scholar]
- [112]. Seo, K. , Inagaki, M. , Nishimura, S. , Hidaka, I. , Sugimachi, M. , Hisada, T. , and Sugiura, S. , 2010, “Structural Heterogeneity in the Ventricular Wall Plays a Significant Role in the Initiation of Stretch-Induced Arrhythmias in Perfused Rabbit Right Ventricular Tissues and Whole Heart Preparations,” Circ. Res., 106, pp. 176–184. 10.1161/CIRCRESAHA.109.203828 [DOI] [PubMed] [Google Scholar]
- [113]. de Boer, T. P. , Camelliti, P. , Ravens, U. , and Kohl, P. , 2009, “Myocardial Tissue Slices: Organotypic Pseudo-2D Models for Cardiac Research & Development,” Future Cardiol., 5(5), pp. 425–430. 10.2217/fca.09.32 [DOI] [PubMed] [Google Scholar]
- [114]. Engler, A. J. , Carag-Krieger, C. , Johnson, C. P. , Raab, M. , Tang, H.-Y. , Speicher, D. W. , Sanger, J. W. , Sanger, J. M. , and Discher, D. E. , 2008, “Embryonic Cardiomyocytes Beat Best on a Matrix With Heart-Like Elasticity: Scar-Like Rigidity Inhibits Beating,” J. Cell Sci., 121(Pt 22), pp. 3794–802. 10.1242/jcs.029678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Jacot, J. G. , McCulloch, A. D. , and Omens, J. H. , 2008, “Substrate Stiffness Affects the Functional Maturation of Neonatal Rat Ventricular Myocytes,” Biophys. J., 95(7), pp. 3479–3487. 10.1529/biophysj.107.124545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116]. Yeung, T. , Georges, P. C. , Flanagan, L. A. , Marg, B. , Ortiz, M. , Funaki, M. , Zahir, N. , Ming, W. , Weaver, V. , and Janmey, P. A. , 2005, “Effects of Substrate Stiffness on Cell Morphology, Cytoskeletal Structure, and Adhesion,” Cell Motil. Cytoskeleton, 60(1), pp. 24–34. 10.1002/cm.20041 [DOI] [PubMed] [Google Scholar]
- [117]. Alford, P. W. , Feinberg, A. W. , Sheehy, S. P. , and Parker, K. K. , 2010, “Biohybrid Thin Films for Measuring Contractility in Engineered Cardiovascular Muscle,” Biomaterials, 31(13), pp. 3613–3621. 10.1016/j.biomaterials.2010.01.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118]. Aubin, H. , Nichol, J. W. , Hutson, C. B. , Bae, H. , Sieminski, A. L. , Cropek, D. M. , Akhyari, P. , and Khademhosseini, A. , 2010, “Directed 3D Cell Alignment and Elongation in Microengineered Hydrogels,” Biomaterials, 31(27), pp. 6941–6951. 10.1016/j.biomaterials.2010.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119]. Sarig, U. , and Machluf, M. , 2011, “Engineering Cell Platforms for Myocardial Regeneration,” Expert Opin. Biol. Ther., 11(8), pp. 1055–1077. 10.1517/14712598.2011.578574 [DOI] [PubMed] [Google Scholar]
- [120]. Nawroth, J. C. , Lee, H. , Feinberg, A. W. , Ripplinger, C. M. , McCain, M. L. , Grosberg, A. , Dabiri, J. O. , and Parker, K. K. , 2012, “A Tissue-Engineered Jellyfish With Biomimetic Propulsion,” Nat. Biotechnol., 30, pp. 792–797. 10.1038/nbt.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. Davis, J. , Westfall, M. V. , Townsend, D. , Blankinship, M. , Herron, T. J. , Guerrero-serna, G. , Wang, W. , Devaney, E. , and Metzger, J. M. , 2008, “Designing Heart Performance by Gene Transfer,” Physiol. Rev., 88, pp. 1567–1651. 10.1152/physrev.00039.2007 [DOI] [PubMed] [Google Scholar]
- [122]. Houser, S. R. , Margulies, K. B. , Murphy, A. M. , Spinale, F. G. , Francis, G. S. , Prabhu, S. D. , Rockman, H. A. , Kass, D. A. , Molkentin, J. D. , Sussman, M. A. , and Koch, W. J. , 2012, “Animal Models of Heart Failure: A Scientific Statement From the American Heart Association,” Circ. Res., 111, pp. 131–150. 10.1161/RES.0b013e3182582523 [DOI] [PubMed] [Google Scholar]
- [123]. Arrenberg, A. B. , Stainier, D. Y. , Baier, H. , and Huisken, J. , 2010, “Optogenetic Control of Cardiac Function,” Science, 330(6006), pp. 971–974. 10.1126/science.1195929 [DOI] [PubMed] [Google Scholar]
- [124]. Kaushik, G. , Fuhrmann, A. , Cammarato, A. , and Engler, A. J. , 2011, “In Situ Mechanical Analysis of Myofibrillar Perturbation and Aging on Soft, Bilayered Drosophila Myocardium,” Biophys. J., 101(11), pp. 2629–2637. 10.1016/j.bpj.2011.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125]. Rohr, S. , Scholly, D. , and Kleber, A. G. , 1991, “Patterned Growth of Neonatal Rat Heart Cells in Culture. Morphological and Electrophysiological Characterization,” Circ. Res., 68, pp. 114–130. 10.1161/01.RES.68.1.114 [DOI] [PubMed] [Google Scholar]
- [126]. Bursac, N. , Parker, K. K. , Iravanian, S. , and Tung, L. , 2002, “Cardiomyocyte Cultures With Controlled Macroscopic Anisotropy: A Model for Functional Electrophysiological Studies of Cardiac Muscle,” Circ. Res., 91(12), p. e45–e54. 10.1161/01.RES.0000047530.88338.EB [DOI] [PubMed] [Google Scholar]
- [127]. Camelliti, P. , McCulloch, A. D. , and Kohl, P. , 2005, “Microstructured Cocultures of Cardiac Myocytes and Fibroblasts: A Two-Dimensional In Vitro Model of Cardiac Tissue,” Microsc. Microanal., 11(3), pp. 249–259. 10.1017/S1431927605050506 [DOI] [PubMed] [Google Scholar]
- [128]. Costa, K. D. , Lee, E. J. , and Holmes, J. W. , 2003, “Creating Alignment and Anisotropy in Engineered Heart Tissue: Role of Boundary Conditions in a Model Three-Dimensional Culture System,” Tissue Eng., 9(4), pp. 567–577. 10.1089/107632703768247278 [DOI] [PubMed] [Google Scholar]
- [129]. Kuo, P.-L. , Lee, H. , Bray, M.-A. , Geisse, N. a. , Huang, Y.-T. , Adams, W. J. , Sheehy, S. P. , and Parker, K. K. , 2012, “Myocyte Shape Regulates Lateral Registry of Sarcomeres and Contractility,” Am. J. Pathol., 181(6), pp. 2030–2037. 10.1016/j.ajpath.2012.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130]. McDevitt, T. , Angello, J. , Whitney, M. , Reinecke, H. , Hauschka, S. , Murry, C. , and Stayton, P. , 2002, “In Vitro Generation of Differentiated Cardiac Myofibers on Micropatterned Laminin Surfaces,” J. Biomed. Mater. Res., 60(3), pp. 472–479. 10.1002/jbm.1292 [DOI] [PubMed] [Google Scholar]
- [131]. Barbee, K. A. , Macarak, E. J. , and Thibault, L. E. , 1994, “Strain Measurements in Cultured Vascular Smooth Muscle Cells Subjected to Mechanical Deformation,” Ann. Biomed. Eng., 22(1), pp. 14–22. 10.1007/BF02368218 [DOI] [PubMed] [Google Scholar]
- [132]. Lee, A. A. , Delhaas, T. , McCulloch, A. D. , and Villarreal, F. J. , 1999, “Differential Responses of Adult Cardiac Fibroblasts to In Vitro Biaxial Strain Patterns,” J. Mol. Cell. Cardiol., 31(10), pp. 1833–183. 10.1006/jmcc.1999.1017 [DOI] [PubMed] [Google Scholar]
- [133]. Camelliti, P. , Gallagher, J. O. , Kohl, P. , and McCulloch, A. D. , 2006, “Micropatterned Cell Cultures on Elastic Membranes As an In Vitro Model of Myocardium,” Nat. Protoc., 1(3), pp. 1379–1391. 10.1038/nprot.2006.203 [DOI] [PubMed] [Google Scholar]
- [134]. Zhang, Y. , Sekar, R. B. , McCulloch, A. D. , and Tung, L. , 2008, “Cell Cultures As Models of Cardiac Mechanoelectric Feedback,” Prog. Biophys. Mol. Biol., 97(2–3), pp. 367–382. 10.1016/j.pbiomolbio.2008.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135]. Eschenhagen, T. , Fink, C., Remmers, U., Scholz, H., Watfchow, J., Well, J., Zimmermann, W., Dohmen, H. H., Schafer, H., Bishopric, N., Wakatsuki, T., and Elson, E., 1997, “Three-Dimensional Reconstitution of Embryonic Cardiomyocytes in a Collagen Matrix: A New Heart Muscle Model System,” FASEB J., 11(8), pp. 683–694. [DOI] [PubMed] [Google Scholar]
- [136]. Asnes, C. F. , Marquez, J. P., Elson, E. L., and Wakatsuki, T., 2006, “Reconstitution of the Frank-Starling Mechanism in Engineered Heart Tissues,” Biophys. J., 91(5), pp. 1800–1810. 10.1529/biophysj.105.065961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137]. de Lange, W. J. , et al. , 2011, “Neonatal Mouse-Derived Engineered Cardiac Tissue: A Novel Model System for Studying Genetic Heart Disease,” Circ Res, 109(1), pp. 8–19. 10.1161/CIRCRESAHA.111.242354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138]. Ralphe, J. C. , and de Lange, W. J. , 2013, “3D Engineered Cardiac Tissue Models of Human Heart Disease: Learning More From Our Mice,” Trends Cardiovasc Med, 23(2), pp. 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139]. Raman, S. , Kelley, M. A. , and Janssen, P. M. , 2006, “Effect of Muscle Dimensions on Trabecular Contractile Performance Under Physiological Conditions,” Pflugers Arch, 451(5), pp. 625–630. 10.1007/s00424-005-1500-9 [DOI] [PubMed] [Google Scholar]
- [140]. Tong, C. W. , Gaffin, R. D., Zawieja, D., and Muthuchamy, M., 2004, “Roles of Phosphorylation of Myosin Binding Protein-C and Troponin I in Mouse Cardiac Muscle Twitch Dynamics,” J. Physiol., 558(Pt 3), pp. 927–41. 10.1113/jphysiol.2004.062539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141]. ter Keurs, H. E. , Rijnsburger, W. H. , van Heuningen, R. , and Nagelsmit, M. J. , 1980, “Tension Development and Sarcomere Length in Rat Cardiac Trabeculae. Evidence of Length-Dependent Activation,” Circ. Res., 46(5), pp. 703–714. 10.1161/01.RES.46.5.703 [DOI] [PubMed] [Google Scholar]
- [142]. Backx, P. H. , and Ter Keurs, H. E. , 1993, “Fluorescent Properties of Rat Cardiac Trabeculae Microinjected With Fura-2 Salt,” Am. J. Physiol., 264(4 Pt 2), pp. H1098–H1110. [DOI] [PubMed] [Google Scholar]
- [143]. Stull, L. B. , Leppo, M. K. , Marbán, E. , and Janssen, P. M. L. , 2002, “Physiological Determinants of Contractile Force Generation and Calcium Handling in Mouse Myocardium,” J. Mol. Cell. Cardiol., 34(10), pp. 1367–1376. 10.1006/jmcc.2002.2065 [DOI] [PubMed] [Google Scholar]
- [143]. ter Keurs, H. , Schouten, V. , Bucx, J. , Mulder, B. , and de Tombe, P. , 1987, “Excitation-Contraction Coupling in Myocardium: Implications of Calcium Release and Na+-Ca2+ Exchange,” Can. J. Physiol. Pharmacol., 65(4), pp. 619–626. 10.1139/y87-104 [DOI] [PubMed] [Google Scholar]
- [144]. Tyberg, J. V. , Parmley, W. W. , and Sonnenblick, E. H. , 1969, “In-Vitro Studies of Myocardial Asynchrony and Regional Hypoxia,” Circ. Res., 25(5), pp. 569–579. 10.1161/01.RES.25.5.569 [DOI] [PubMed] [Google Scholar]
- [145]. Liu, T. , Brown, B. S. , Wu, Y. , Antzelevitch, C. , Kowey, P. R. , and Yan, G. X. , 2006, “Blinded Validation of the Isolated Arterially Perfused Rabbit Ventricular Wedge in Preclinical Assessment of Drug-Induced Proarrhythmias,” Heart Rhythm, 3(8), pp. 948–956. 10.1016/j.hrthm.2006.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146]. Di Diego, J. M. , Sicouri, S., Myles, R. C., Burton, F. L., Smith, G. L., and Antzelevitch, C., 2013, “Optical and Electrical Recordings From Isolated Coronary-Perfused Ventricular Wedge Preparations,” J. Mol. Cell Cardiol., 54, pp. 53–64. 10.1016/j.yjmcc.2012.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147]. Glukhov, A. V. , Fedorov, V. V. , Lou, Q. , Ravikumar, V. K. , Kalish, P. W. , Schuessler, R. B. , Moazami, N., and Efimov, I. R., 2010, “Transmural Dispersion of Repolarization in Failing and Nonfailing Human Ventricle,” Circ. Res., 106, pp. 981–991. 10.1161/CIRCRESAHA.109.204891 [DOI] [PMC free article] [PubMed] [Google Scholar]