Short abstract
Achilles tendon ruptures are traumatic injuries, and techniques for assessing repair outcomes rely on patient-based measures of pain and function, which do not directly assess tendon healing. Consequently, there is a need for a quantitative, in vivo measure of tendon properties. Therefore, the purpose of this study was to validate ultrasound imaging for evaluating collagen organization in tendons. In this study, we compared our novel, high-frequency ultrasound (HFUS) imaging and analysis method to a standard measure of collagen organization, crossed polarizer (CP) imaging. Eighteen mouse Achilles tendons were harvested and placed into a testing fixture where HFUS and CP imaging could be performed simultaneously in a controlled loading environment. Two experiments were conducted: (1) effect of loading on collagen alignment and (2) effect of an excisional injury on collagen alignment. As expected, it was found that both the HFUS and CP methods could reliably detect an increase in alignment with increasing load, as well as a decrease in alignment with injury. This HFUS method demonstrates that structural measures of collagen organization in tendon can be determined through ultrasound imaging. This experiment also provides a mechanistic evaluation of tissue structure that could potentially be used to develop a targeted approach to aid in rehabilitation or monitor return to activity after tendon injury.
Keywords: high-frequency ultrasound, collagen organization, Achilles tendon
Introduction
Achilles tendon ruptures are traumatic injuries that frequently occur in active individuals and result in significant medical expense. As many as 2.5 million individuals sustain Achilles tendon ruptures each year and the incidence is rising [1–3]. Healing of a ruptured Achilles tendon extends between months and years, and understanding the temporal changes in tendon properties (structural, biological, and mechanical) is essential to successful healing [4]. However, common techniques for assessing outcomes of surgical repair and rehabilitation rely heavily on patient-based measures of pain and function. These measures include functional performance tests, such as the “hop” test or heel-raise endurance, ankle range of motion, and basic descriptive scoring measures [5–9]. While these measures can provide evidence for recovery of functional performance, they do not directly assess tendon healing, which, if insufficient, can lead to rerupture. Additionally, those approaches are only semiquantitative and may be biased by confounding factors, such as strength, agility, and coordination. Consequently, there is an urgent need for a quantitative, in vivo measure of tendon properties to guide rehabilitation progression and return to activity criteria.
Tendon-collagen alignment is an important structural tissue property that changes throughout the progression of tendon healing. It is known that changes in collagen organization precede and correlate with changes in mechanical properties in tendons and are affected by both load and injury [10–14]. Therefore, the clinical evaluation of collagen organization following Achilles tendon injury may provide a more appropriate measure of healing than traditional, functional performance tests. There are currently multiple techniques for measuring collagen alignment, such as crossed polarizer imaging [14–16], quantitative polarized light microscopy [17,18], second-harmonic generation imaging [19–21,39], and infrared spectroscopy [22]. However, these methods are typically destructive to the tissue and are not easily clinically translatable. Ultrasound imaging, currently used clinically to evaluate injuries in tendon including the Achilles [23–27], has recently been investigated as a quantitative tool to analyze tendon properties. It is an attractive alternative because it has the potential to provide the same organizational information as other methods, is relatively inexpensive and portable, is noninvasive, and is not limited to tendon size or thickness. Therefore, ultrasound could be used as diagnostic clinical tool for the evaluation of tendon health. A number of studies have quantitatively evaluated tendon structural properties using ultrasound. Specifically, it has been demonstrated that changes in tendon mechanical properties correlate with changes in ultrasound echogenicity in both rat Achilles and porcine digital flexor tendons [28,29]. Additionally, it has been shown that tendinopathy in human Achilles and patellar tendons can be predicted by analyzing the degree of speckle patterning in ultrasound imaging using a Fourier analysis [30,31]. Finally, quantitative shear-wave elastography has been used for assessing the relative elasticity of injured and uninjured Achilles tendons [32].
While these current techniques correlate ultrasound properties to tendon mechanical properties and are therefore important for the field, they do not evaluate collagen alignment specifically (which directly relates to tendon strength) or establish a specific mechanism for the change in ultrasonic properties. The method described in this study advances previous research and utilizes the patterning seen in tendon ultrasound images to quantitatively describe organization, specifically the variation of collagen alignment. Collagen appears hyperechoic in ultrasound imaging, whereas the matrix between the collagen appears hypoechoic. This gives the appearance of “bands” in ultrasound images of tendons that correspond to the collagen matrix. In the method presented in this paper, these fiber elements are identified and the deviation of the fiber directions is quantified as a measure of structural organization [33]. Additionally, to further support that our results are consistent with actual changes in collagen organization, the analysis is also performed using standard organizational measure, CP imaging.
Therefore, the objective of this study was to perform an ex vivo experiment on mouse Achilles tendons to determine if HFUS imaging can capture changes in collagen alignment. We will confirm this method by using HFUS to analyze organizational changes due to applied load and injury (known cases that create changes in alignment) and then confirm these findings using the established CP-imaging method. We hypothesize that because the bands seen in tendon ultrasound images are created by collagen bundles, the changes in collagen alignment that are measurable by CP will also be measurable by HFUS. Additionally, we hypothesize that the HFUS imaging method will be capable of detecting both an increase in alignment with increased loading and a decrease in alignment with the presence of a defect.
Methods
Study Design and Sample Preparation.
Mouse Achilles tendons were selected for this proof of principle experiment because their small size enabled analysis with our CP system, which will be used for validation of our novel HFUS analysis, and because their straight and superficial anatomical position makes this system a likely first step toward direct translation to future in vivo studies. This study was performed ex vivo with a high-frequency ultrasound transducer to enhance the spatial resolution of the tendon structure in our small animal model and represents the first, necessary step toward developing a rigorous, noninvasive measure of tendon organization. Eighteen Achilles tendons were harvested and frozen (−20 °C) from C57BL/6 type mice. Tendons were thawed and fine dissected to remove all surrounding tissue, leaving the insertion site intact on the calcaneus. Tendons were tested in custom fixtures and had an initial gauge length of 5 mm. Two experimental situations that are known to cause a change in tendon organization were performed to demonstrate that collagen alignment can be quantified with our ultrasound-based technique: (1) an increase in alignment with increasing load and (2) a decrease in alignment with injury. Eleven tendons were used to evaluate collagen alignment during loading, and seven were used to analyze the effect of excisional injury on collagen alignment. To account for variability between specimens, these experiments were performed paired, so that each tendon specimen could be compared against itself.
Image Acquisition.
For HFUS- and CP-image acquisition, tendons were secured in a custom fixture, submerged in a phosphate-buffered saline bath, and aligned so that the CP and HFUS would capture coronal cross-sectional images simultaneously (Fig. 1). Ultrasound images were captured using a Vevo 770 scanner (VisualSonics, Toronto, Canada) and a 55-MHz transducer (RMV 708, VisualSonics, Toronto, Canada). A 55-MHz transducer was used in order to obtain sufficient resolution in the mouse tendon (note that a lower-frequency transducer would be more appropriate for a larger model). A motorized scanner used for 3D imaging was attached to the ultrasound transducer to allow for consecutive coronal images to be obtained. All ultrasound settings (i.e., frequency and gain) were kept constant during testing to standardize the images. The ultrasound transducer was manually positioned so that it was parallel and centered over the tendon. Consecutive cross-sectional coronal images were taken every 0.1 mm over a range of 2 mm (tendon thickness <1 mm). The CP setup consisted of a linear backlight (Dolan-Jenner, Boxborough, MA), 90 deg-offset rotating polarizer sheets (Edmund Optics, Barrington, NJ) on either side of the tendon, and a digital camera (Basler, Exton, PA). As described previously [14], images were acquired through the rotation of the polarizer sheets to obtain localized fiber-alignment data. Prior to testing, the encoder in the stepper motor (Lin Engineering, Santa Clara, CA) that rotates the polarizer sheets was set at a position corresponding to 0 deg of angular rotation. The tendon was secured in a fixture that was attached to a custom tensiometer [34] through a sliding shaft to allow for load control while the tendon is in the horizontal configuration required for ultrasound imaging (Fig. 1).
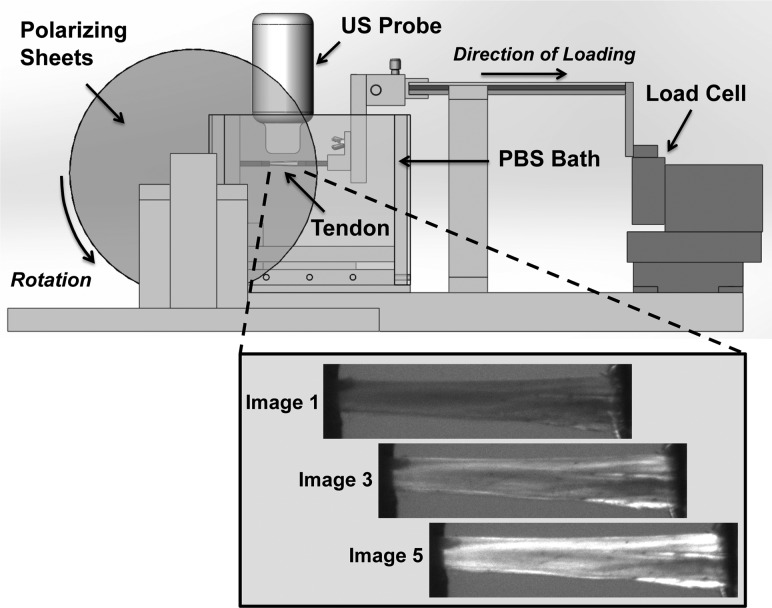
Fig. 1.
Schematic of setup for capturing CP and HFUS imaging simultaneously. The tendon is secured between two grips in a PBS tank, where the ultrasound probe can be centered over top of the tendon, submerged in the PBS. Two polarizing sheets are located on either side of the tank, with a linear backlight on one side and a camera on the other (not depicted in this image). A custom-built tensiometer is attached to a sliding shaft to allow for load control. Inset figure (below) shows representative images of a tendon as captured by the CP camera through one rotation cycle.
To examine the effect of fiber alignment during loading (n = 11), loads of 0.0, 0.5, 1.0, and 1.5 N, previously determined to show changes in alignment, were applied consecutively through control of the tensiometer, making sure the tendon had sufficient time to creep at each steady load. HFUS and CP images were captured at each load. The experimenter ensured that the load was stable before acquiring images, and this waiting period was consistent from sample to sample (∼15 seconds). Both the cross polarizer and the ultrasound only take 2–3 seconds to capture the series of images necessary for analysis, and they are captured simultaneously. Due to the speed of the image capture and the small levels of relaxation that may occur after this point, any changes in alignment from these effects would be negligible. To examine changes in collagen alignment following excisional injury (n = 7), uninjured tendons were loaded into the same custom fixture and imaged under 1.0-N load. The tendons were then injured using a 0.5-mm biopsy punch to create a standard defect and reimaged using the same protocol.
Image Analysis.
The analysis of the HFUS images was conducted using a custom matlab (Mathworks, Natick, MA) program and followed an approach based on a previously described algorithm [33]. Using a 55-MHz transducer, the effective resolution of the ultrasound images were approximately 30 μm. Tendon collagen fascicles appear hyperechoic under HFUS, where the noncollagenous matrix between the fascicles appears hypoechoic, giving rise to the appearance of bands in the images (Fig. 2) [35]. These bands are analyzed to determine a quantitative measure of tendon organization. Briefly, the ultrasound images that comprised the center of the tendon (with no edge artifacts) were chosen for analysis. The ultrasound images were filtered to remove any small particles and reduce background noise. A matrix convolution was then used to apply a linear kernel over groups of pixels in the filtered image at varying angles (0 deg–180 deg in 5 deg increments). A power series function
| (1) |
Fig. 2.

(a) HFUS image of Achilles tendon with excisional injury, (b) filtered HFUS image, (c) hyper- and hypoechoic regions that create the banded pattern that is quantified as the CSD of the fiber orientations
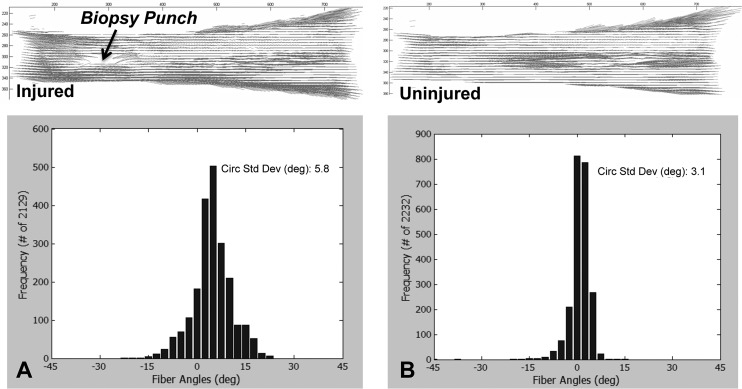
was then fit to the intensity versus angle data (least squares curve-fit) and used to determine the angle with maximum intensity and therefore the fiber direction. In addition, any regions in which the confidence intervals for the parameters of the power series fit were too high were masked. The circular standard deviation (CSD), a measure of the distribution of collagen alignment (increased CSD means increased disorganization), was calculated for the local fiber directions in each selected image segment in a specimen and averaged to obtain a value representative of the organization of the entire tendon thickness. A representative example of high- and low-CSD measures is depicted in histograms for both injured and uninjured specimens (Fig. 3). This method is novel because it translates a technology developed for identifying and quantifying properties of filaments to the collagen patterning in ultrasound images. Since this method is a post–image acquisition analysis, it can be applied to any ultrasound image, and no new equipment is necessary during the image-acquisition phase.
Fig. 3.
Alignment maps and histograms depicting the distribution of localized fiber directions throughout the tendon that were produced through CP analysis for (a) injured and (b) uninjured specimens
For the analysis of the CP images, collagen-fiber direction was determined across the tendon length and width. Collagen refracts polarized light by 90 deg; thus, the collagen-fiber direction can be determined by collecting images at varying angles of polarization (0 deg–140 deg). The images of the tendon are then divided into 2 × 2 bundles of pixels with 5 pixel spacing between bundles (effective resolution of approximately 50 μm, comparable to the HFUS), and the pixel intensities were summed in each pixel group for each angle of polarizer rotation. These intensity values were then plotted against the angle of polarizer rotation, and a sin2 wave was fit to the data to determine the angle corresponding to the minimum pixel intensity. This point represents the average localized collagen-fiber direction. A compilation of these localized directions can be represented as an alignment map over the whole tendon (Fig. 3). The CSD was calculated for each specimen in each experimental group to quantify collagen organization.
Statistics.
For analyzing the change in alignment in response to load, significance was assessed using two one-way repeated measures analysis of variance, one for each imaging modality. This was followed by paired t-test analyses with Holm–Bonferroni post hoc for multiple comparisons to determine specific differences due to load within each imaging modality. For analyzing the change in alignment in response to injury, a paired t-test was performed for each imaging modality. Finally, a regression analysis was performed on the CSD values comparing measurement techniques. In order to reflect the paired nature of the analysis, the regression was run on the change in CSD with respect to 0 N (baseline) for each load and uninjured (baseline) for the injury data. Significance was set at p < 0.05 and trends were set at p < 0.1.
Results
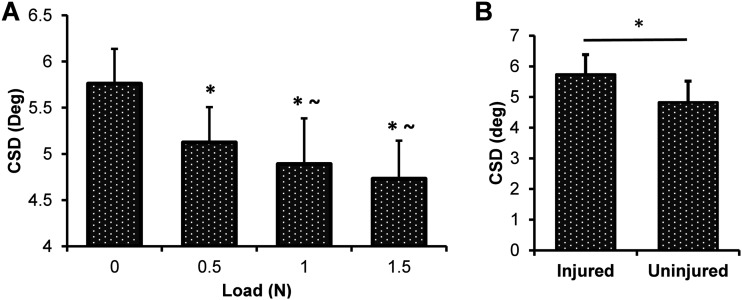
We first evaluated the HFUS results to determine if the expected changes in alignment due to load and injury were able to be detected using this novel method. It was found that the HFUS analysis detected a significant decrease in CSD in response to increasing load, meaning the tendon became more organized with increasing load as expected (Fig. 4(a)). Statistically, the 0.5, 1.0, and 1.5 N groups were all compared to the 0 N group, and additionally, the 1.0 and 1.5 N groups were compared to the 0.5 N group to demonstrate further incremental changes with load. For the injury study, CSD as measured by HFUS was significantly larger for the injured group, meaning the tendon became more disorganized with an injury also as expected (Fig. 4(b)). This demonstrates that the HFUS technique is sensitive enough to detect the expected changes in organization caused by both increases in load as well as infliction of injury.
Fig. 4.
(a) Changes in CSD in response to load for the HFUS images. There is a significant decrease in CSD (increase in organization) with increased loading. Statistics for each load compared to 0 N (*p < 0.05) and to 0.5 N (∼p < 0.05). (b) CSD measures for HFUS images before and after a biopsy punch excisional injury. There is a significant increase in CSD (decrease in organization) with injury (*p < 0.05).
To further support that our HFUS results are consistent with the actual changes in collagen alignment in these two conditions, we conducted the same experiment simultaneously on the same tendons using the established CP analysis. The CP method also was able to detect a consistent decrease in CSD with increased load (Fig. 5(a)) and an increase in CSD with injury (Fig. 5(b)), consistent with expected results and with our HFUS results.
Fig. 5.
(a) Changes in CSD in response to load for the CP images. There is a significant decrease in CSD (increase in organization) with increased loading. Statistics for each load compared to 0 N (*p < 0.05) and to 0.5 N (∼p < 0.05). (b) CSD measures for CP images before and after a biopsy punch excisional injury. There is an increase in CSD (decrease in organization) with injury (*p < 0.05).
For the comparison of the CSD measures of each imaging technique, a regression was run on the change in CSD with respect to 0 N (baseline) for each load and uninjured (baseline) for the injury data, and a correlation was determined with R2 = 0.45 and p < 0.001.
Discussion
In this study, a controlled ex vivo experiment was conducted to demonstrate the use of HFUS for detecting changes in collagen alignment, which is the first, necessary step toward developing a rigorous, noninvasive, in vivo measure of tendon organization. Our novel image-analysis algorithm identifies the hyperechoic bands seen in tendon ultrasound images, which are created by the tendon-collagen matrix, and quantifies the deviation in alignment of the collagen bands to produce a quantitative measure of collagen organization. The findings of this study support our hypothesis that, because the bands seen in ultrasound images of tendon are collagen, changes in collagen organization measurable by CP imaging, an established technique, will also be measureable by HFUS. Specifically, our HFUS method can capture the alignment of tendon-collagen fibers along the direction of loading, thereby increasing the level of organization in the tissue and decreasing the CSD of the collagen fibers. This result is consistent with previous work in our lab, which demonstrated fiber realignment during tendon tensile mechanical testing [10,11,14]. Additionally, the injury data demonstrates that our HFUS method is able to detect a disruption to the collagen fibers, which causes a decrease in organization throughout the length of the tendon (not just in the defect region), likely due to unloading of collagen fibers proximal to the injury site. Since the image-analysis algorithm filters out any nonfibrous data, including the excisional defect, the disorganization detected by these imaging modalities is a result of damage propagated through the intact regions of the tendon. Furthermore, since this is not a healing study, there is no scar formation, which would cause even further disorganization and a more drastic change in CSD in the injured tendon. Therefore, importantly, this experiment demonstrates that even this smallest possible change due to an injury can be detected through HFUS. Since consistent responses to both load and injury were observed in both the HFUS- and CP-imaging modalities, it can be concluded that HFUS is an alternative to the established CP method. Additionally, and as a potential future advantage that motivated the current work, this HFUS method has the potential to be translated both to larger ex vivo specimens, as well as to in vivo imaging, neither of which can be done with CP imaging.
The regression analysis comparing imaging methods was highly significant. However, it was only a modest correlation. This is not surprising given that the two methods are measuring different phenomena. The ultrasound is detecting the impedance difference between the collagen fibers and the surrounding matrix to obtain a striated signal of echogenicity, where the cross polarizer is using the birefringence properties of the collagen. Due to differences in the physics of the image acquisition, each method has its own biases and inaccuracies associated with the outcome measure. Since these biases are consistent within an imaging method, the same response to these different conditions was observed. However, when comparing between the two methods, the biases are not consistent and therefore manifest as differences unrelated to the overall response of the methods. Since the aim of this study is to quantify a change in organization and we have demonstrated that both methods detect the same response with significance, given these differences, this correlation is in support of our overall findings. Additionally, this is the first study to use this technique and prove its feasibility. Future studies, with larger sample sizes and a greater range of data values, could be designed to more fully characterize the correlation between the measures.
While there are a number of previous studies that demonstrate the use of ultrasound for evaluating tendon health [28–31], they are designed to provide a correlation or statistical relationship between their measured ultrasound properties and tissue properties. In contrast, our method directly measures a tissue property, collagen organization. Therefore, this method can provide a more direct evaluation of tendon health. Additionally, the correlations determined through these other methods are susceptible to confounding factors, such as density, tissue hydration, collagen fibril type, etc. Collagen organization can be measured independently of these factors, allowing for a more mechanistic evaluation that can give specific structural information about how organization contributes to healing. This mechanism also provides the opportunity to develop a targeted therapy (such as loading or rehabilitation protocols) to specifically address the changes seen in the ultrasound analysis. Finally, ultrasonic property changes are in other studies only assumed to be influenced by collagen-fiber organization. This study provides evidence that collagen organization is a contributing factor to the changes seen in ultrasound properties demonstrated in other groups.
This study is not without limitations. Only healthy tendons (from previously sacrificed animals) were evaluated, so even by applying a load or inflicting an injury, the range of achievable organization values is fairly small. Furthermore, a standardized yet simple injury model was used for this validation study. While this injury is not clinically relevant, it provides a standard defect that alters the collagen alignment. Therefore, the changes observed, while significant, only represent a small range of values that are likely larger in healing tissues with more clinically relevant defects. However, this allows demonstration of the sensitivity and capability of this experimental technique. Future studies will evaluate a more extensive injury model to further demonstrate the range of organization values that can be achieved using HFUS. Additionally, while our tensiometer allowed us to apply a range of precise loading conditions, we were not able to perform more sophisticated mechanical testing, such as preconditioning or application of a constant strain rate. However, any variability in loading speed is likely to be nominal, since it was performed by the same operator (first author). Furthermore, we are using a 55-MHz ultrasound transducer, which, because of its high resolution, is ideal for small tendons (which was needed for this study in order to perform the CP analysis). However, the depth capabilities of this frequency transducer are limited, and so in order to translate this method in vivo and to larger tendons, it must be demonstrated using a lower-frequency probe. In a rat or larger animal's Achilles tendon, since the collagen structure approximately scales with size, a lower-frequency transducer will be able to resolve the collagen structure while still penetrating through surrounding tissue layers. This can be demonstrated by looking at a clinical scan of a human Achilles tendon, where the striated pattern we are quantifying can be seen [35,36]. Clinical scanners range from 2–18 MHz, and structures such as the Achilles tendon are commonly imaged at 15–18 MHz since they are superficial. A scanner of 17.5 MHz has an axial resolution of ∼85 μm [37], and given that human-tendon fascicle structures have a diameter of approximately 300–400 μm [38], this technique would be able to resolve, and therefore quantify, the fascicle structure. Additionally, while not currently used clinically, a 25-MHz probe has an axial resolution of ∼70 μm [37] and, in most cases, may still have an appropriate focal length for imaging the human Achilles. Therefore, it could potentially be used for this application. However, further investigation is needed to determine the sensitivity of this technique using a lower-resolution scanner on a larger specimen. The current study provides evidence that such a study should be conducted, since it is shown that the banded pattern and collagen orientation are related. One limitation of this is that it may not work as well for tendons that are less superficial (such as supraspinatus) when compared to more superficial tendons (such as Achilles as used here). Finally, this new technique shows significant differences that are scientifically relevant; however, it is difficult to determine how these measures will translate to the clinical situation to determine patient-to-patient differences in organization. Further analysis would be needed to obtain a normal in vivo range, as well as the degree of change between normal and injury, in order to provide a more clinically relevant comparison. Additionally, since this technique has the potential to become noninvasive, it could be used for longitudinal studies, and therefore data would be compared within an individual over time, overcoming the potential variability between individuals in some studies.
This is the first study to directly quantify tendon-collagen alignment from ultrasound images. Specifically, it has demonstrated that HFUS is capable of detecting changes in organization due to load and injury and that these changes are consistent with both the established CP method and expected results based on previous studies [10–14]. As such, this experiment validates the use of HFUS imaging for obtaining a quantitative measure of tendon organization and provides critical data to continue to refine the technique for future in vivo applications requiring a noninvasive method. Such a method could eventually be used in clinical studies to monitor progress during treatment and ultimately improve patient outcomes.
Acknowledgment
The authors acknowledge Chandra Sehgal and Susan Schultz. This study was funded by NIH/NIAMS-supported Penn Center for Musculoskeletal Disorders and the NSF Graduate Research Fellowship.
Contributor Information
Corinne N. Riggin, McKay Orthopaedic Research Laboratory, University of Pennsylvania, 424 Stemmler Hall, 36th and Hamilton Walk, Philadelphia, PA 19104
Joseph J. Sarver, McKay Orthopaedic Research Laboratory, University of Pennsylvania, 424 Stemmler Hall, 36th and Hamilton Walk, Philadelphia, PA 19104; School of Biomedical Engineering, Science and Health Systems, Drexel University, 3141 Chestnut Street, Philadelphia PA 19104
Benjamin R. Freedman, McKay Orthopaedic Research Laboratory, University of Pennsylvania, 424 Stemmler Hall, 36th and Hamilton Walk, Philadelphia, PA 19104
Stephen J. Thomas, McKay Orthopaedic Research Laboratory, University of Pennsylvania, 424 Stemmler Hall, 36th and Hamilton Walk, Philadelphia, PA 19104; Division of Nursing and Health Sciences, Athletic Training, Neumann University, 1 Neumann Rd. Aston, PA 19014
Louis J. Soslowsky, McKay Orthopaedic Research Laboratory, University of Pennsylvania, 424 Stemmler Hall, 36th and Hamilton Walk, Philadelphia, PA 19104, e-mail: soslowsk@upenn.edu
References
- [1]. Suchak, A. A. , Bostick, G. , Reid, D. , Blitz, S. , and Jomha, N. , 2005, “The Incidence of Achilles Tendon Ruptures in Edmonton, Canada,” Foot Ankle Int., 26(11), pp. 932–936. [DOI] [PubMed] [Google Scholar]
- [2]. Houshian, S. , Tscherning, T. , and Riegels-Nielsen, P. , 1998, “The Epidemiology of Achilles Tendon Rupture in a Danish County,” Injury, 29(9), pp. 651–654. 10.1016/S0020-1383(98)00147-8 [DOI] [PubMed] [Google Scholar]
- [3]. Moller, A. , Astron, M. , and Westlin, N. , 1996, “Increasing Incidence of Achilles Tendon Rupture,” Acta Orthop. Scand., 67(5), pp. 479–481. 10.3109/17453679608996672 [DOI] [PubMed] [Google Scholar]
- [4]. Lin, T. W. , Cardenas, L. , and Soslowsky, L. J. , 2004, “Biomechanics of Tendon Injury and Repair,” J. Biomech., 37(6), pp. 865–877. 10.1016/j.jbiomech.2003.11.005 [DOI] [PubMed] [Google Scholar]
- [5]. Saxena, A. , Ewen, B. , and Maffulli, N. , 2011, “Rehabilitation of the Operated Achilles Tendon: Parameters for Predicting Return to Activity,” J. Foot Ankle Surg., 50(1), pp. 37–40. 10.1053/j.jfas.2010.10.008 [DOI] [PubMed] [Google Scholar]
- [6]. Mullaney, M. J. , McHugh, M. P. , Tyler, T. F. , Nicholas, S. J. , and Lee, S. J. , 2006, “Weakness in End-Range Plantar Flexion After Achilles Tendon Repair,” Am. J. Sports Med., 34(7), pp. 1120–1125. 10.1177/0363546505284186 [DOI] [PubMed] [Google Scholar]
- [7]. Robinson, J. M. , Cook, J. L. , Purdam, C. , Visentini, P. J. , Ross, J. , Maffulli, N. , Taunton, J. E. , and Khan, K. M. , 2001, “The VISA-A Questionnaire: A Valid and Reliable Index of the Clinical Severity of Achilles Tendinopathy,” Br. J. Sports Med., 35(5), pp. 335–341. 10.1136/bjsm.35.5.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Silbernagel, K. G. , Nilsson-Helander, K. , Thomee, R. , Eriksson, B. I. , and Karlsson, J. , 2010, “A New Measurement of Heel-Rise Endurance With the Ability to Detect Functional Deficits in Patients With Achilles Tendon Rupture,” Knee Surg. Sports Traumatol. Arthrosc., 18(2), pp. 258–264. 10.1007/s00167-009-0889-7 [DOI] [PubMed] [Google Scholar]
- [9]. Chinn, L. , and Hertel, J. , 2010, “Rehabilitation of Ankle and Foot Injuries in Athletes,” Clin. Sports Med., 29(1), pp. 157–167. 10.1016/j.csm.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Miller, K. S. , Connizzo, B. K. , Feeney, E. , and Soslowsky, L. J. , 2012, “Characterizing Local Collagen Fiber Re-Alignment and Crimp Behavior Throughout Mechanical Testing in a Mature Mouse Supraspinatus Tendon Model,” J. Biomech., 45(12), pp. 2061–2065. 10.1016/j.jbiomech.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Miller, K. S. , Edelstein, L. , Connizzo, B. K. , and Soslowsky, L. J. , 2012, “Effect of Preconditioning and Stress Relaxation on Local Collagen Fiber Re-alignment: Inhomogeneous Properties of Rat Supraspinatus Tendon,” ASME J. Biomech. Eng., 134(3), p. 031007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Gimbel, J. A. , Van Kleunen, J. P. , Mehta, S. , Perry, S. M. , Williams, G. R. , and Soslowsky, L. J. , 2004, “Supraspinatus Tendon Organizational and Mechanical Properties in a Chronic Rotator Cuff Tear Animal Model,” J. Biomech., 37(5), pp. 739–749. 10.1016/j.jbiomech.2003.09.019 [DOI] [PubMed] [Google Scholar]
- [13]. Dourte, L. M. , Perry, S. M. , Getz, C. L. , and Soslowsky, L. J. , 2010, “Tendon Properties Remain Altered in a Chronic Rat Rotator Cuff Model,” Clin. Orthop. Relat. Res., 468(6), pp. 1485–1492. 10.1007/s11999-009-1206-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Lake, S. P. , Miller, K. S. , Elliott, D. M. , and Soslowsky, L. J. , 2009, “Effect of Fiber Distribution and Realignment on the Nonlinear and Inhomogeneous Mechanical Properties of Human Supraspinatus Tendon Under Longitudinal Tensile Loading,” J. Orthop. Res., 27(12), pp. 1596–1602. 10.1002/jor.20938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Quinn, K. P. , and Winkelstein, B. A. , 2008, “Altered Collagen Fiber Kinematics Define the Onset of Localized Ligament Damage During Loading,” J. Appl. Physiol., 105(6), pp. 1881–1888. 10.1152/japplphysiol.90792.2008 [DOI] [PubMed] [Google Scholar]
- [16]. Quinn, K. P. , Bauman, J. A. , Crosby, N. D. , and Winkelstein, B. A. , 2010, “Anomalous Fiber Realignment During Tensile Loading of the Rat Facet Capsular Ligament Identifies Mechanically Induced Damage and Physiological Dysfunction,” J. Biomech., 43(10), pp. 1870–1875. 10.1016/j.jbiomech.2010.03.032 [DOI] [PubMed] [Google Scholar]
- [17]. Thomopoulos, S. , Williams, G. R. , Gimbel, J. A. , Favata, M. , and Soslowsky, L. J. , 2003, “Variation of Biomechanical, Structural, and Compositional Properties Along the Tendon to Bone Insertion Site,” J. Orthop. Res., 21(3), pp. 413–419. 10.1016/S0736-0266(03)0057-3 [DOI] [PubMed] [Google Scholar]
- [18]. Franchi, M. , Torricelli, P. , Giavaresi, G. , and Fini, M. , 2013, “Role of Moderate Exercising on Achilles Tendon Collagen Crimping Patterns and Proteoglycans,” Connect. Tissue Res., 54, pp. 267–274. 10.3109/03008207.2013.807808 [DOI] [PubMed] [Google Scholar]
- [19]. Lau, T. Y. , Ambekar, R. , and Toussaint, K. C. , 2012, “Quantification of Collagen Fiber Organization Using Three-Dimensional Fourier Transform-Second-Harmonic Generation Imaging,” Opt. Express, 20(19), pp. 21821–21832. 10.1364/OE.20.021821 [DOI] [PubMed] [Google Scholar]
- [20]. Gusachenko, I . , Tran, V . , Houssen, Y. G. , Allain, J. M. , and Schanne-Klein, M. C. , 2012, “Polarization-Resolved Second-Harmonic Generation in Tendon Upon Mechanical Stretching,” Biophys. J., 102(9), pp. 2220–2229. 10.1016/j.bpj.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Abraham, T. , Fong, G. , and Scott, A. , 2011, “Second Harmonic Generation Analysis of Early Achilles Tendinosis in Response to in vivo Mechanical Loading,” BMC Musculoskelet. Disord., 12, p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Vidal Bde, C. , and Mello, M. L. , 2011, “Collagen Type I Amide I Band Infrared Spectroscopy,” Micron, 42(3), pp. 283–289. 10.1016/j.micron.2010.09.010 [DOI] [PubMed] [Google Scholar]
- [23]. Alfredson, H. , Masci, L. , and Ohberg, L. , 2011, “Partial Mid-portion Achilles Tendon Ruptures: New Sonographic Findings Helpful for Diagnosis,” Br. J. Sports Med., 45(5), pp. 429–432. 10.1136/bjsm.2009.067298 [DOI] [PubMed] [Google Scholar]
- [24]. Maranho, D. A. , Nogueira-Barbosa, M. H. , Simao, M. N. , and Volpon, J. B. , 2009, “Ultrasonographic Evaluation of Achilles Tendon Repair After Percutaneous Sectioning for the Correction of Congenital Clubfoot Residual Equinus,” J. Pediatr. Orthop., 29(7), pp. 804–810. 10.1097/BPO.0b013e3181b76a5f [DOI] [PubMed] [Google Scholar]
- [25]. Mangat, K. S. , Kanwar, R. , Johnson, K. , Korah, G. , and Prem, H. , 2010, “Ultrasonographic Phases in Gap Healing Following Ponseti-Type Achilles Tenotomy,” J. Bone Joint Surg. Am., 92(6), pp. 1462–1467. 10.2106/JBJS.I.00188 [DOI] [PubMed] [Google Scholar]
- [26]. Vadala, A. , De Carli, A. , Vulpiani, M. C. , Iorio, R. , Vetrano, M. , Scapellato, S. , Suarez, T. , Salvo Di, F., and Ferretti , A., 2012, “Clinical, Functional and Radiological Results of Achilles Tenorraphy Surgically Treated With Mini-open Technique,” J. Sports Med. Phys. Fitness, 52(6), pp. 616–621. [PubMed] [Google Scholar]
- [27]. Poposka, A. , Georgieva, D. , and Dzoleva-Tolevska, R. , 2012, “Significance of Ultrasound in the Diagnosis and Treatment of Achilles Tendon Rupture,” Prilozi, 33(1), pp. 209–216. [PubMed] [Google Scholar]
- [28]. Chamberlain, C. S. , Duenwald-Kuehl, S. E. , Okotie, G. , Brounts, S. H. , Baer, G. S. , and Vanderby, R. , 2013, “Temporal Healing in Rat Achilles Tendon: Ultrasound Correlations,” Ann. Biomed. Eng., 41(3), pp. 477–487. 10.1007/s10439-012-0689-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Duenwald-Kuehl, S. , Lakes, R. , and Vanderby, R., Jr. , 2012, “Strain-Induced Damage Reduces Echo Intensity Changes in Tendon During Loading,” J. Biomech., 45(9), pp. 1607–1611. 10.1016/j.jbiomech.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Bashford, G. R. , Tomsen, N. , Arya, S. , Burnfield, J. M. , and Kulig, K. , 2008, “Tendinopathy Discrimination by Use of Spatial Frequency Parameters in Ultrasound B-Mode Images,” IEEE Trans. Med. Imaging, 27(5), pp. 608–615. 10.1109/TMI.2007.912389 [DOI] [PubMed] [Google Scholar]
- [31]. Kulig, K. , Landel, R. , Chang, Y. J. , Hannanvash, N. , Reischl, S. F. , Song, P. , and Bashford, G. R. , 2013, “Patellar Tendon Morphology in Volleyball Athletes With and Without Patellar Tendinopathy,” Scand. J. Med. Sci. Sports, 23(2), pp. e81–e88. 10.1111/sms.12021 [DOI] [PubMed] [Google Scholar]
- [32]. Chen, X. M. , Cui, L. G. , He, P. , Shen, W. W. , Qian, Y. J. , and Wang, J. R. , 2013, “Shear Wave Elastographic Characterization of Normal and Torn Achilles Tendons: A Pilot Study,” J. Ultrasound Med., 32(3), pp. 449–455. [DOI] [PubMed] [Google Scholar]
- [33]. Shah, S. A. , Santago, P. , and Rubin, B. K. , 2005, “Quantification of Biopolymer Filament Structure,” Ultramicroscopy, 104(3–4), pp. 244–254. 10.1016/j.ultramic.2005.04.007 [DOI] [PubMed] [Google Scholar]
- [34]. Gimbel, J. A. , Mehta, S. , Van Kleunen, J. P. , Williams, G. R. , and Soslowsky, L. J. , 2004, “The Tension Required at Repair to Reappose the Supraspinatus Tendon to Bone Rapidly Increases After Injury,” Clin. Orthop. Rela.t Res., 4(26), pp. 258–265. 10.1097/01.blo.0000136831.17696.80 [DOI] [PubMed] [Google Scholar]
- [35]. Garcia, T. , Hornof, W. J. , and Insana, M. F. , 2003, “On the Ultrasonic Properties of Tendon,” Ultrasound Med. Biol., 29(12), pp. 1787–1797. 10.1016/S0301-5629(03)01069-X [DOI] [PubMed] [Google Scholar]
- [36]. Khoury, V . , Guillin, R. , Dhanju, J. , and Cardinal, E. , 2007, “Ultrasound of Ankle and Foot: Overuse and Sports Injuries,” Semin. Musculoskelet. Radiol., 11(2), pp. 149–161. 10.1055/s-2007-1001880 [DOI] [PubMed] [Google Scholar]
- [37]. VisualSonics, 2007, VisualSonics 700-Series RMV Scanhead Selector, VisualSonics, Toronto. [Google Scholar]
- [38]. Hansen, P. , Haraldsson, B. T. , Aagaard, P. , Kovanen, V . , Avery, N. C. , Qvortrup, K. , Larsen, J. O. , Krogsgaard, M. , Kjaer, M. , and Peter Magnusson, S. , 2010, “Lower Strength of the Human Posterior Patellar Tendon Seems Unrelated to Mature Collagen Cross-Linking and Fibril Morphology,” J. Appl. Physiol., 108(1), pp. 47–52. 10.1152/japplphysiol.00944.2009 [DOI] [PubMed] [Google Scholar]
- [39]. Ros, S. J. , Andarawis-Puri, N. , and Flatow, E. L. , 2013, “Tendon extracellular matrix damage detection and quantification using automated edge detection analysis,” J. Biomech., 46(16), pp. 2844–2847. 10.1016/j.jbiomech.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]