Short abstract
Tumor progression depends critically upon the interactions between the tumor cells and their microenvironment. The tumor microenvironment is heterogeneous and dynamic; it consists of extracellular matrix, stromal cells, immune cells, progenitor cells, and blood and lymphatic vessels. The emerging fields of tissue engineering and microtechnologies have opened up new possibilities for engineering physiologically relevant and spatially well-defined microenvironments. These in vitro models allow specific manipulation of biophysical and biochemical parameters, such as chemical gradients, biomatrix stiffness, metabolic stress, and fluid flows; thus providing a means to study their roles in certain aspects of tumor progression such as cell proliferation, invasion, and crosstalk with other cell types. Challenges and perspectives for deconvolving the complexity of tumor microenvironments will be discussed. Emphasis will be given to in vitro models of tumor cell migration and invasion.
Keywords: mechanical stress, 3D culture; extracellular matrix; invasion; interstitial flow
Introduction
The tumor microenvironment plays critical roles in tumor progression as it controls the ease at which tumors can grow and invade, the chemical signaling between cells, and the host immune response to the tumor, among others. Evidence is mounting that the molecular gradients and mechanical stresses around tumor cells influence tumor progression in significant ways [1,2]; for example, chemokines abundantly expressed in chronic inflammatory conditions are implicated in tumor progression [3] and matrix stiffness promotes tumor malignant phenotypes [4]. Clinically, “occult” tumors (i.e., benign tumors that do not invade or become malignant) are far more common than cancer, and it has been argued that this is largely due to the microenvironment; e.g., normal tissue environments can suppress malignancy, while certain pathogenic tissue features may induce malignancy [5]. Furthermore, the tumor microenvironment can also suppress any host immune responses against the tumor, even those induced by immunotherapies [6–8]. Thus, understanding what defines malignancy-promoting tumor microenvironments, and teasing out the contributions and crosstalk between various cellular and molecular players, are critical for eventual development of therapeutic strategies that target the microenvironment.
The tumor microenvironment is known for its complexity, both in its content as well as its dynamic nature. It consists of various immune cells, stromal fibroblasts, extracellular matrix (ECM) components often associated with wound healing and inflammation, blood and lymph vessels, and various other cell types like endothelial progenitor cells [9]. Tumor cells interact and evolve with this complex community in multiple ways. Aside from its composition, two key biophysical parameters controlling tumor interactions with its microenvironment are molecular gradients and the mechanical stresses. Molecular gradients within the tumor microenvironment typically originate from cell–cell signaling (e.g., chemokines and growth factors), metabolic stress (e.g., pH, oxygen, glucose, and other nutrients) [10,11], and physical features of the ECM like composition, stiffness, hydraulic conductivity, and growth factor binding that affect transport properties [12,13]. Mechanical stress within a tumor microenvironment can arise from fluid shear and drag forces on the ECM and cells from interstitial flows or the mechanical tension generated by either the expanding tumor on its surroundings or matrix contraction from the stromal fibroblasts [14–17]. Because mechanical stresses can cause pressure gradients that drive interstitial fluid flow, mechanical stresses are also inherently coupled to chemical gradients [11,18–22].
In vitro models have the potential to explore mechanistically the individual contributions of various components of the microenvironment on tumor cell behavior, and the ability to modulate the biophysical and biomechanical aspects. At the same time, in vitro models cannot recapitulate every aspect of the tumor microenvironment, and thus care must be taken to identify their limitations and assumptions. For example, while a three-dimensional (3D) culture of tumor cells in a collagen gel under interstitial flow might be used to explore direct effects of interstitial flow on tumor cell invasion [23–25], it ignores other factors present at an invasive edge of the tumor such as the architecture and composition of the ECM (which is highly heterogeneous), the diversity of cell types present in the tumor stroma and their physiological interactions, and gradients of oxygen, pH, metabolites, and nutrient that are formed from the tumor mass. As such, one can only infer from such studies the direct effects of interstitial flow on uniformly distributed tumor cells within a homogeneous collagen ECM (which itself is important), but not necessarily translate those findings directly to the tumor invasive edge, since one does not know the relative importance of interstitial flow versus the other factors that were excluded from the in vitro model. The field has often suffered because of erroneous interpretations drawn from oversimplified in vitro models, starting with the earliest two-dimensional (2D) monocultures; even as more complex models are built, the questions they can be used to address, and how data they generate can be interpreted, require careful consideration.
Several advances in tissue engineering have allowed more physiologically relevant in vitro tumor microenvironment models to be developed. These include better control of 3D matrices with defined compositions and mechanical properties, and improved microtechnology advances at the “mesoscale” (∼102–103 μm, which is needed for recapitulating most multicellular 3D microenvironments) for developing culture chambers that can be perfused, that can maintain well-defined and controlled chemical and mechanical environments for cells, and that allow real-time imaging [26–28]. Additionally, great advances in intravital imaging using animal models has revealed much of what we know about tumor microenvironments [29] and thus helped to guide development.
We describe in vitro modeling efforts of the tumor microenvironment, comparing the biological events during tumor progression with the corresponding tissue engineering considerations (see Table 1).
Table 1.
Engineering design considerations and models used for various steps in tumor initiation, progression, and metastasis
| Biological event | Engineering considerations | Models used |
|---|---|---|
| Tumor initiation and growth | • ECM composition and stiffness | • 3D spheroid assay |
| • Compressive stress | • Hanging drop assay | |
| • Adhesion molecule density | ||
| Blood and lymphangiogenesis | • Endothelial sprouting into 3D ECM | • Bead assay |
| • Growth factor gradients | • Tubulogenesis assays | |
| • Luminal and interstitial flow | • Microfluidic devices | |
| • Role of macrophages, stromal fibroblasts, and other cells | • 3D flow chambers | |
| Cell invasion and chemotaxis | • Cell contraction and motility | • Modified Boyden chamber |
| • Interstitial flow and ECM stress | • 3D cell tracking | |
| • Co-culture with stromal cells | • Microfluidic devices | |
| • 3D matrix, stiffness, adhesion molecule density | • Vertical layered assay | |
| • Oxygen gradients/hypoxia | ||
| • Chemokine gradients | ||
| Intravasation into blood or lymphatic vessels | • Interactions between tumor cells and endothelial cells | • Modified Boyden chamber |
| • ECM composition; presence of basement membrane | • Microfluidic devices | |
| • Transendothelial flow (e.g., into lymphatic vessels) | ||
| • Luminal flow on endothelial cells | ||
| Tumor cell circulation | • Luminal shear stresses | • Microfluidic flow chamber |
| • Complex geometry (e.g., branching, narrowing capillaries) | ||
| • Platelets, blood cells, clotting factors | ||
| Adhesion and extravasation | • Co-culture with endothelial cells | • Parallel plate flow chamber |
| • Luminal flow | ||
| • Chemokines and adhesion molecules | ||
| Growth in ectopic site | • Tissue-specific features of ectopic site (e.g., lymph node, liver, brain, bone) including chemokines and ECM components | • 3D cultures |
Tumor Initiation and Growth
3D spheroid tumor cell cultures serve as powerful tools for dissecting the roles of various biochemical and biophysical cues in carcinoma initiation and progression [30]. Pioneering work from the Bissell lab [31] demonstrated that normal mammary epithelial cells and carcinoma cells display distinctly different phenotypes both in structure and function when cultured in a reconstituted basement membrane matrix (matrigel); e.g., normal mammary epithelial cells self-organize and form acinar structures with lumen, while carcinoma cells form cell-filled spheroids. This model has been used extensively to elucidate the roles of mechanical forces (both tensional and compressive stress), ECM components, and other factors in tumorigenesis [2,30,32,33].
Blood and Lymphangiogenesis
The size of prevascularized tumors is limited mainly by oxygen diffusion and consumption rates, such that tumors cannot grow beyond hundreds of microns before requiring new blood vessels [12,34,35]. Thus, angiogenesis is required for tumor growth beyond this size, and inhibitors of angiogenesis are used clinically to slow tumor progression [9]. Because of the ongoing development of therapeutic targets that block tumor angiogenesis, there is a need for physiologically relevant in vitro models of tumor angiogenesis. Furthermore, although much less studied, tumor lymphangiogenesis is thought to be important for metastasis of many solid tumors, and thus models of lymphangiogenesis are also included here.
Previous studies comparing the transport properties within tumor and normal tissues found that tumor tissues were more heterogeneous in their perfusion [36–39], which may need to be considered in model systems. Microfluidic models that incorporate on-chip blood vessels have been developed to provide well defined microenvironments for studies of blood and lymphatic angiogenesis, and their roles in tumor cell invasion [40–43] For more detailed review on this subject, please refer to other review articles [17,34].
Tumor Cell Migration and Invasion
Tumor cell migration and invasion are important first steps in cancer metastasis [44,45]. During epithelial to mesenchymal transition, tumor cells downregulate cell–cell adhesions like E-cadherin, upregulate matrix metalloproteinases (MMPs), and invade the ECM. Often, tumor-associated macrophages or stromal fibroblasts also help guide tumor cells.
Modes of Cell Migration in 3D.
Two types of cell migration behaviors within a 3D matrix have been described: amoeboid and mesenchymal [29]. Amoeboid migration, commonly seen in dendritic cells and macrophages, occurs primarily by squeezing through pores of the fibrous ECM network via a series of well-orchestrated front actin filament polymerization and back cell cortex contraction [46–48]. Since it is independent of firm adhesion to the ECM, amoeboid migration is fast—up ∼4 μm/min in animal models [29]—and has been observed in breast, prostate, and skin cancers. Mesenchymal migration; on the other hand, involves integrin-mediated cell adhesion [49] and tension generation on the ECM fibers [50,51]. Furthermore, it may also involve proteolytic degradation of the ECM via MMPs as cells invade [52,53], leading to much slower migration speeds (∼0.1 to 1 μm/min) than amoeboid. Zaman et al. observed DU-145 prostate carcinoma cells migrating in ECMs with varying stiffness and adhesion molecule density, and concluded that cell migration speed in this system was dependent on the balance between ECM stiffness and adhesion. Both types of migration have been observed in most tumor types, including breast, prostate, and skin cancers [1]; tumor cells are not committed to a specific migration phenotype, but rather switches between the two depending on microenvironmental factors [54,55]. For example, total MMP inhibition can transform tumor cells from mesenchymal into amoeboid phenotypes in both 3D culture and animal models [53]. Such plasticity of tumor cell invasive behavior can pose challenges for therapeutic strategies targeting invading tumor cells [56].
Mechanical Forces Involved in Cell Migration.
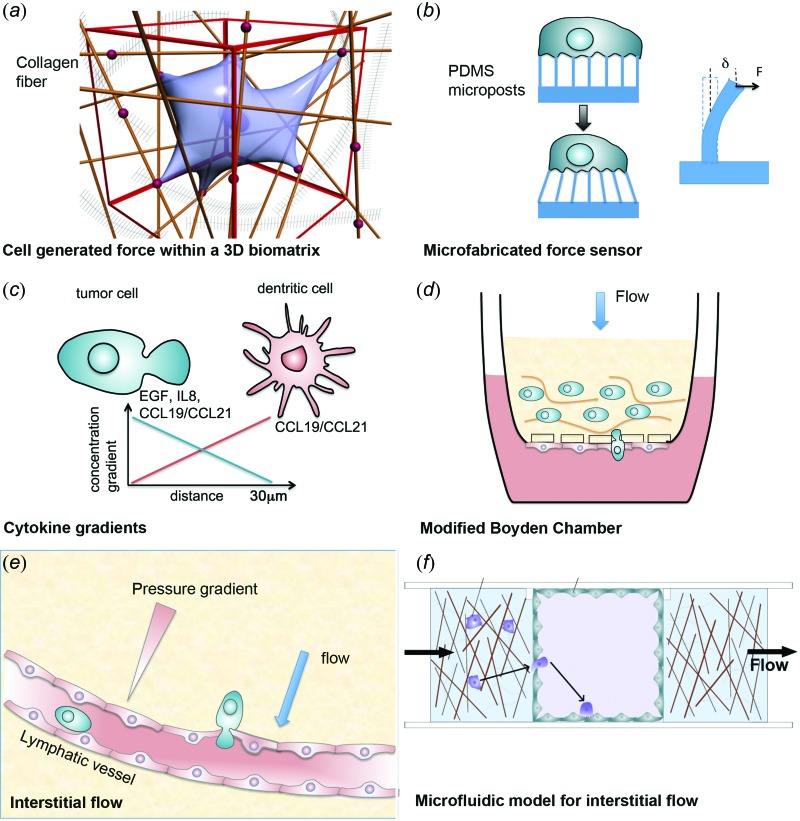
Mechanical forces generated by cells are important during cell migration, as cells squeeze through the fibrillar ECM network and pull on ECM fibers [see Fig. 1]. Matrix stiffness determines the extent to which cells can deform the ECM, and stiffness has been associated with the invasion of breast cancer [4] and glioma [57], among others. Compressive stresses have been found to promote phenotypic changes in cancer cells cultured in either 2D or 3D conditions toward more invasive phenotypes [58,59]. Microfabricated devices have been useful for measuring forces generated by single cells [60,61] as well as populations of cells [62]. Figure 1(b) illustrates one such example, where cells are cultured on a bed of flexible microposts that deflect as the cells pull on them; these deflections can be measured optically to infer cellular traction forces [63]. A number of recent work estimated traction forces of single cells moving through 3D biomatrices by the movement of beads embedded within the gel [64,65]. A challenge here is the modeling of the natively derived biomatrices, which often are nonlinear in nature [66].
Fig. 1.
Tumor microenvironments and the corresponding in vitro models. (a) Single cell embedded in a 3D biomatrix. The cell is pulling on the collagen fiber. Dots represent adhesion molecules. (b) A microfabricated cell force sensor. The bending of the micropillars is used to report the cellular force. Graph is adapted from [60] and copyright of the Proceedings of National Academy of Sciences. (c) Molecular gradients via paracrine signaling. (d) A modified Boyden Chamber assay for tumor cell invasion and transendothelial migration under interstitial flow conditions. Graph is adapted from [22] with permission from American Association for Cancer Research. (e) Illustration of interstitial flow near a lymphatic vessel and tumor cell intravasation. (f) Microfluidic model for studies of tumor cell intravasation in the presence of a flow. (Graph is adapted from [41]. with permission from the Royal Society of Chemistry.)
Chemokine and Growth Factor Gradients.
Molecular gradients of oxygen and metabolites, growth factors, cytokines, and pH within a tumor microenvironment arise from numerous factors including cell–cell signaling, cell and ECM heterogeneity, metabolic stress, abnormal vasculature and thus perfusion, and mechanical forces like interstitial flow [1,10,12,14,15,23,67–70] [see also Fig. 1(c)]. Gradients are particularly important in tumor invasion; for example, hypoxia in the tumor core due to rapid tumor growth, aberrant angiogenesis, and heterogeneous perfusion can drive tumor cell invasion up oxygen gradients in the tumor margin. Indeed, this may contribute to the increased metastasis that has been observed after antiangiogenic therapy [9,71]. Hypoxia can also drive the expression of growth factors and cytokines such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and interleukin-8 (IL-8) [34,72], contributing further to their heterogeneity.
Chemokine gradients can play multiple roles in shaping the tumor microenvironment, as they can attract various subsets of immune cells, drive angiogenesis, and matrix remodeling, and directly affect tumor cell survival and proliferation [3]. In the context of chemokine-mediated tumor cell invasion, specific chemokine-receptor pairs have associated with the invasion and metastasis of specific tumor cells. The chemokine (C-X-C motif) ligand 12 and its receptor CXCR4 are well documented to help drive metastasis of breast cancer, glioblastoma, and others, and CXCR4 expression has been associated with cancer stem cells [3,55,73]. The most widely used model system to study tumor cell response to chemokine gradients (i.e., chemotaxis) is the Boyden chamber [1,74], which is typically in the form of porous culture inserts with pore sizes that allow cell transmigration (∼8 um) and a chamber beneath containing other cells or chemokines [see Fig. 1(d)]. Modifications to the Boyden chamber include 3D gels in the upper or lower chambers and the addition of transmembrane flow due to a pressure head [21,22], described below.
Chemokine gradients can also arise from co-cultured cells. Condeelis and colleagues used a vertically layered co-culture platform to explore how macrophages affect in tumor cell invasion [75,76]; using this setup, they identified a paracrine signaling loop between colony stimulating factor from tumor cells and epidermal growth factor from macrophages that promoted tumor cell invasion. In co-cultures of tumor cells and lymphatic endothelial cells (LECs), Issa and colleagues demonstrated that tumor-secreted factors caused LECs to secrete tumor-attracting chemokines in a positive feedback loop [77]. Fibroblasts are another important cell type in the tumor stroma that signal to tumor cells and have been studied in 3D co-culture [21,78,79].
Interstitial Flow.
Interstitial flow exists in the interstitial space between the blood and lymphatic vessels [12] and is driven by hydrostatic pressure differences [Fig. 1(e)]. Interstitial flow is directed toward draining lymphatic vessels, which carry cells, fluid, and tumor-secreted factors to the sentinel or tumor-draining lymph node (LN). Since the sentinel LN is a first site of metastasis for many tumor types, the role of interstitial flow on tumor cell invasion has recently gained some research attention [17].
Using a modified Boyden chamber [Fig. 1(d)] with a pressure difference driving flow through an ECM containing various types of tumor cells, and with a layer of LECs lining the underside of the insert, the first reported effect of interstitial flow (∼1 μm/s) was to enhance tumor cell invasion and migration of CCR7+ CCL21-expressing mammary carcinoma cells [22]. The hypothesis tested was that cells with autocrine chemokine signaling loops—that is, both secreting a chemokine and expressing its receptor—could use such a system to sense fluid flow and migrate towards a draining lymphatic vessel. Using computational models [18], it was shown that pericellular gradients would form around the cell with higher concentrations in the direction of flow. In the modified Boyden chamber, flow-enhanced migration or “autologous chemotaxis” could be blocked by blocking the receptor CCR7 [22]. Such a mechanism was also demonstrated with CXCR4+CXCL12+ glioblastoma cells [20]. Recently, microfluidic devices allowing interstitial flow have been developed to directly visualize autologous chemotaxis [23] and study tensional effects between the ECM and tumor cells [24,25] [see also Fig. 1(f)].
One challenge of 3D cell culture, particularly when using tension-generating or contractile cells such as fibroblasts, is maintaining counteracting tension in the 3D matrix to avoid overall gel contraction. To overcome this limitation, a radial flow chamber was developed in which a thin annulus of porous polyethylene membrane was sandwiched between two glass slides to host the cell-embedded collagen [80]. The porous polyethylene, with pores of ∼100 μm, served to anchor the collagen that permeated it before gelation, while the glass was pretreated with glutaraldehyde for collagen attachment. Flow entered from a polyethylene rod in the center and flowed outward such that a tenfold velocity range existed from inlet to outlet. Using this model, Shieh et al. demonstrated interstitial flow can also drive TGF-β-dependent autologous chemotaxis of fibroblasts that guided tumor cells in the flow direction [21].
The advantage of the modified Boyden chamber for chemotaxis studies is its ease of use, commercial availability, and high throughput nature. However, it does not provide a well-defined chemical gradient, does not allow for live cell imaging, and generally gives only endpoint results, making it difficult to differentiate increased (random) motility from directed migration, for example. Microfluidic models have been developed to overcome these limitations and allow more quantitative analysis of tumor cell migration under well-defined molecular gradients, interstitial flows, and with various co-culture possibilities [26,81].
Tumor Cell Intravasation Across Endothelium
Once a tumor cell approaches a blood or lymphatic vessel, it must cross the endothelial barrier. Here, again, modifications to the Boyden chamber has been used most widely [Fig. 1(d)]. In this case, a monolayer of endothelial cells are plated on the underside of the insert, and transmigration of the tumor cells from 3D matrix, across the porous insert, and through the endothelial layer is assessed at an endpoint. Using this model, it was found that LECs enhance human mammary carcinoma cell transmigration [22]. In another modification of this model, human mammary carcinoma (MCF-7) spheroids were placed atop LEC monolayers plated on insert membranes. This resulted in highly reproducible formation of circular discontinuities, mediated by tumor-secreted lipoxygenase, in the endothelial monolayers that correlated with tumor invasiveness [82]. To directly observe tumor cell interactions and transmigration across endothelial monolayers, however, microfluidic models have been developed in various configurations allowing a perfused, endothelial-lined channel in contact with a 3D ECM embedded with tumor cells [43,83–88].
Circulating Tumor Cells
Circulating tumor cells (CTCs) are considered indicative of tumor dissemination to distant organs and represent an important diagnostic marker due to access to a patient's blood. The challenge, however, is the relative rarity of CTCs in a blood sample (as few as one cell in 109 hematologic cells). Microfluidic devices provide unique opportunities for capturing rare tumor cells, particularly in that they can be easily designed and fabricated to contain geometric barriers for blood cell filtration, large surface-to-volume ratios, and modified surfaces for CTC capturing [89–91]. Briefly, the CTC chip contains a microfluidic channel with embedded microposts for enhancing the surface-to-volume ratio, and the posts are coated with antibodies that recognize tumor cells. Recent work from the Toner lab using an antibody against EpCAM demonstrated that a CTC chip can capture spiked rare tumor cells with a 99% confidence level. Furthermore, they could successfully identify CTCs in the peripheral blood of patients with metastatic lung, prostate, pancreatic, breast, and colon cancer in 115 of 116 samples. The Kirby lab used an antibody against prostate-specific membrane antigen to coat the microposts and demonstrated capture of CTCs from peripheral whole blood samples of castration-resistant prostate cancer patients [91].
Tumor Cell Adhesion, Extravasation, and Growth in Ectopic Sites
For blood-borne CTCs to form successful metastases in a secondary site, adhesion to the endothelium followed by extravasation, in a manner similar to that of leukocytes, is thought to be required [92]. Adhesion molecules that are common to leukocyte-endothelial interactions [93], such as selectins, have been implicated in cancer metastasis [94–99]. The most widely used in vitro models that mimic this process are standard parallel-plate flow chambers in which tumor cells introduced into the medium flowing over a monolayer of endothelial cells and imaged [100,101]. For example, using this model, E- P-, and L-selectins have been found to mediate adhesion events [94,101,102]. However, numerous clinical observations have demonstrated that different tumor types have distinct organ-specific metastatic potential [44,103,104], and organ-specific chemokines are known to promote relevant tumor cell adhesion to endothelium and facilitate processes of extravasation into the target tissue [105,106], To this end, the Fischbach lab developed a mineralized 3D tumor model to investigate the roles of mineral hydroxyapatite (HA) in breast tumor cell progression and found that tumor cell adhesion, proliferation, and secretion of pro-osteoclastic IL-8 was increased in mineralized tumor models as compared to nonmineralized tumor models [107]. However, our understanding of how the physical and chemical microenvironments at the ectopic site impact the initiation and growth of a new tumor remain limited, and more physiologically relevant in vitro models are needed that incorporate target tissue cells underneath the endothelial cells.
Perspectives and Future Development
Looking ahead, a major challenge is to develop the types of aforementioned in vitro tumor models into devices that are easy to use, high-throughput, and highly reproducible so that they can be used more readily for biological experiments and thus have more impact in cancer biology. Furthermore, they must contain enough complexity to faithfully recapitulate the problem at hand, while maintaining straightforward analysis. As discussed above, simpler devices like the Boyden chamber allow for more high-throughput experimentation, while the more recent microfluidic models typically require engineering knowledge to use, are lower throughput, and suffer from difficult reproducibility. Another major challenge is that developing and using such models requires interdisciplinary cooperation between biologists and engineers, although more bioengineers are being trained in tumor biology labs. A third challenge is to create a set of data analysis tools that can be used easily by biologists, clinicians, and engineers needed to understand large data sets generated by such models. The long-term hope is that human in vitro models will one day replace mouse models in recreating tumor microenvironments for the purpose of diagnostics, preclinical testing, and basic cancer biology research.
Acknowledgment
M.W. is supported by grants from the National Center for Research Resources (5R21RR025801-03), and the NIH (8R21 GM103388-03, R21CA138366, and U54CA143876). M.A.S. is supported by grants from the European Research Council (AdG – 323053), the Swiss National Science Foundation (31003AB_135756 and 143754/1), the Swiss Cancer League (2696-08-2010), the Leenaards Foundation, and the NIH (RO1 HL096539).
Contributor Information
Mingming Wu, Department of Biological, and Environmental Engineering, Cornell University, Ithaca, NY 14853.
Melody A. Swartz, Institute of Bioengineering and, Institute for Experimental, Cancer Research (ISREC), School of Life Sciences, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne 1015, Switzerland
References
- [1]. Roussos, E. T. , Condeelis, J. S. , and Patsialou, A. , 2011, “Chemotaxis in Cancer,” Nat. Rev. Cancer, 11, pp. 573–587. 10.1038/nrc3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Butcher, D. T. , Alliston, T. , and Weaver, V. M. , 2009, “A Tense Situation: Forcing Tumour Progression,” Nat. Rev. Cancer, 9, pp. 108–122. 10.1038/nrc2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Allavena, P. , Germano, G. , Marchesi, F. , and Mantovani, A. , 2011, “Chemokines in Cancer Related Inflammation,” Exp. Cell Res., 317, pp. 664–673. 10.1016/j.yexcr.2010.11.013 [DOI] [PubMed] [Google Scholar]
- [4]. Paszek, M. J. , Zahir, N. , Johnson, K. R. , Lakins, J. N. , Rozenberg, G. I. , Gefen, A. , Reinhart-King, C. A. , Margulies, S. S. , Dembo, M. , Boettiger, D. , Hammer, D. A. , and Weaver, V. M. , 2005, “Tensional Homeostasis and the Malignant Phenotype,” Cancer Cell, 8, pp. 241–254. 10.1016/j.ccr.2005.08.010 [DOI] [PubMed] [Google Scholar]
- [5]. Bissell, M. J. , and Hines, W. C. , 2011, “Why Don't We Get More Cancer? A Proposed Role of the Microenvironment in Restraining Cancer Progression,” Nat. Med., 17, pp. 320–329. 10.1038/nm.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Gajewski, T. F. , 2012, “Cancer Immunotherapy,” Mol. Oncol., 6, pp. 242–250. 10.1016/j.molonc.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Mellman, I. , Coukos, G. , and Dranoff, G. , 2011, “Cancer Immunotherapy Comes of Age,” Nature, 480, pp. 480–489. 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Zitvogel, L. , Tesniere, A. , and Kroemer, G. , 2006, “Cancer Despite Immunosurveillance: Immunoselection and Immunosubversion,” Nat. Rev. Immunol., 6, pp. 715–727. 10.1038/nri1936 [DOI] [PubMed] [Google Scholar]
- [9]. Hanahan, D. , and Weinberg, R. A. , 2011, “Hallmarks of Cancer: The Next Generation,” Cell, 144, pp. 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- [10]. Helmlinger, G. , Yuan, F. , Dellian, M. , and Jain, R. K. , 1997, “Interstitial pH and pO(2) Gradients in Solid Tumors In Vivo: High-Resolution Measurements Reveal a Lack of Correlation,” Nat. Med., 3, pp. 177–182. 10.1038/nm0297-177 [DOI] [PubMed] [Google Scholar]
- [11]. Netti, P. A. , Berk, D. A. , Swartz, M. A. , Grodzinsky, A. J. , and Jain, R. K. , 2000, “Role of Extracellular Matrix Assembly in Interstitial Transport in Solid Tumors,” Cancer Res., 60, pp. 2497–2503. [PubMed] [Google Scholar]
- [12]. Jain, R. K. , 1987, “Transport of Molecules in the Tumor Interstitium: A Review,” Cancer Res., 47, pp. 3038–3050. [PubMed] [Google Scholar]
- [13]. Swartz, M. A. , and Fleury, M. E. , 2007, “Interstitial Flow and Its Effects in Soft Tissues,” Annu. Rev. Biomed. Eng., 9, pp. 229–256. 10.1146/annurev.bioeng.9.060906.151850 [DOI] [PubMed] [Google Scholar]
- [14]. Baxter, L. T. , and Jain, R. K. , 1989, “Transport of Fluid and Macromolecules in Tumors. I. Role of Interstitial Pressure and Convection,” Microvasc. Res., 37, pp. 77–104. 10.1016/0026-2862(89)90074-5 [DOI] [PubMed] [Google Scholar]
- [15]. Boucher, Y. , Baxter, L. T. , and Jain, R. K. , 1990, “Interstitial Pressure Gradients in Tissue-Isolated and Subcutaneous Tumors: Implications for Therapy,” Cancer Res., 50, pp. 4478–4484. [PubMed] [Google Scholar]
- [16]. Helmlinger, G. , Netti, P. A. , Lichtenbeld, H. C. , Melder, R. J. , and Jain, R. K. , 1997, “Solid Stress Inhibits the Growth of Multicellular Tumor Spheroids,” Nat. Biotechnol., 15, pp. 778–783. 10.1038/nbt0897-778 [DOI] [PubMed] [Google Scholar]
- [17]. Swartz, M. A. , and Lund, A. W. , 2012, “Lymphatic and Interstitial Flow in the Tumour Microenvironment: Linking Mechanobiology With Immunity,” Nat. Rev. Cancer, 12, pp. 210–219. 10.1038/nrc3186 [DOI] [PubMed] [Google Scholar]
- [18]. Fleury, M. E. , Boardman, K. C. , and Swartz, M. A. , 2006, “Autologous Morphogen Gradients by Subtle Interstitial Flow and Matrix Interactions,” Biophys. J., 91, pp. 113–121. 10.1529/biophysj.105.080192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Helm, C. L. E. , Fleury, M. E. , Zisch, A. H. , Boschetti, F. , and Swartz, M. A. , 2005, “Synergy Between Interstitial Flow and VEGF Directs Capillary Morphogenesis In Vitro Through a Gradient Amplification Mechanism,” Proc. Natl. Acad. Sci. U.S.A., 102, pp. 15779–15784. 10.1073/pnas.0503681102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Munson, J. M. , Bellamkonda, R. V. , and Swartz, M. A. , 2013, “Interstitial Flow in a 3D Microenvironment Increases Glioma Invasion by a CXCR4-Dependent Mechanism,” Cancer Res., 73, pp. 1536–1546. 10.1158/0008-5472.CAN-12-2838 [DOI] [PubMed] [Google Scholar]
- [21]. Shieh, A. C. , Rozansky, H. A. , Hinz, B. , and Swartz, M. A. , 2011, “Tumor Cell Invasion Is Promoted by Interstitial Flow-Induced Matrix Priming by Stromal Fibroblasts,” Cancer Res., 71, pp. 790–800. 10.1158/0008-5472.CAN-10-1513 [DOI] [PubMed] [Google Scholar]
- [22]. Shields, J. D. , Fleury, M. E. , Yong, C. , Tomei, A. A. , Randolph, G. J. , and Swartz, M. A. , 2007, “Autologous Chemotaxis As a Mechanism of Tumor Cell Homing to Lymphatics Via Interstitial Flow and Autocrine CCR7 Signaling,” Cancer Cell, 11, pp. 526–538. 10.1016/j.ccr.2007.04.020 [DOI] [PubMed] [Google Scholar]
- [23]. Haessler, U. , Teo, J. C. M. , Foretay, D. , Renaud, P. , and Swartz, M. A. , 2012, “Migration Dynamics of Breast Cancer Cells in a Tunable 3D Interstitial Flow Chamber,” Integr. Biol., 4, pp. 401–409. 10.1039/c1ib00128k [DOI] [PubMed] [Google Scholar]
- [24]. Polacheck, W. J. , Charest, J. L. , and Kamm, R. D. , 2011, “Interstitial Flow Influences Direction of Tumor Cell Migration Through Competing Mechanisms,” Proc. Natl. Acad. Sci. U.S.A., 108, pp. 11115–11120. 10.1073/pnas.1103581108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Qazi, H. , Shi, Z. D. , and Tarbell, J. M. , 2011, “Fluid Shear Stress Regulates the Invasive Potential of Glioma Cells Via Modulation of Migratory Activity and Matrix Metalloproteinase Expression,” PLoS One, 6(5), p. e20348. 10.1371/journal.pone.0020348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Kim, S. , Kim, H. J. , and Jeon, N. L. , 2010, “Biological Applications of Microfluidic Gradient Devices,” Integr. Biol., 2, pp. 584–603. 10.1039/c0ib00055h [DOI] [PubMed] [Google Scholar]
- [27]. Wlodkowic, D. , and Cooper, J. M. , 2010, “Tumors on Chips: Oncology Meets Microfluidics,” Curr. Opin. Chem. Biol., 14, pp. 556–567. 10.1016/j.cbpa.2010.08.016 [DOI] [PubMed] [Google Scholar]
- [28]. Meyvantsson, I. , and Beebe, D. J. , 2008, “Cell Culture Models in Microfluidic Systems,” Annu. Rev. Anal. Chem., 1, pp. 423–449. 10.1146/annurev.anchem.1.031207.113042 [DOI] [PubMed] [Google Scholar]
- [29]. Condeelis, J. , and Segall, J. E. , 2003, “Intravital Imaging of Cell Movement in Tumours,” Nat. Rev. Cancer, 3, pp. 921–930. 10.1038/nrc1231 [DOI] [PubMed] [Google Scholar]
- [30]. Hebner, C. , Weaver, V. M. , and Debnath, J. , 2008, “Modeling Morphogenesis and Oncogenesis in Three-Dimensional Breast Epithelial Cultures,” Annu. Rev. Pathol., 3, pp. 313–339. 10.1146/annurev.pathmechdis.3.121806.151526 [DOI] [PubMed] [Google Scholar]
- [31]. Petersen, O. W. , Ronnovjessen, L. , Howlett, A. R. , and Bissell, M. J. , 1992, “Interaction With Basement Membrane Serves to Rapidly Distinguish Growth and Differentiation Patterns of Normal and Malignant Human Breast Epithelial Cells,” Proc. Natl. Acad. Sci. U.S.A., 89, pp. 9064–9068. 10.1073/pnas.89.19.9064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Yu, H. M. , Mouw, J. K. , and Weaver, V. M. , 2011, “Forcing Form and Function: Biomechanical Regulation of Tumor Evolution,” Trends Cell Biol., 21, pp. 47–56. 10.1016/j.tcb.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Kaufman, L. J. , Brangwynne, C. P. , Kasza, K. E. , Filippidi, E. , Gordon, V. D. , Deisboeck, T. S. , and Weitz, D. A. , 2005, “Glioma Expansion in Collagen I Matrices: Analyzing Collagen Concentration-Dependent Growth and Motility Patterns,” Biophys. J., 89, pp. 635–650. 10.1529/biophysj.105.061994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Carmeliet, P. , and Jain, R. K. , 2000, “Angiogenesis in Cancer and Other Diseases,” Nature, 407, pp. 249–257. 10.1038/35025220 [DOI] [PubMed] [Google Scholar]
- [35]. Jain, R. K. , 2005, “Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy,” Science, 307, pp. 58–62. 10.1126/science.1104819 [DOI] [PubMed] [Google Scholar]
- [36]. Clauss, M. A. , and Jain, R. K. , 1990, “Interstitial Transport of Rabbit and Sheep Antibodies in Normal and Neoplastic Tissues,” Cancer Res., 50, pp. 3487–3492. [PubMed] [Google Scholar]
- [37]. Berk, D. A. , Yuan, F. , Leunig, M. , and Jain, R. K. , 1997, “Direct In Vivo Measurement of Targeted Binding in a Human Tumor Xenograft,” Proc. Natl. Acad. Sci. U.S.A., 94, pp. 1785–1790. 10.1073/pnas.94.5.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Banerjee, R. K. , van Osdol, W. W. , Bungay, P. M. , Sung, C. , and Dedrick, R. L. , 2001, “Finite Element Model of Antibody Penetration in a Prevascular Tumor Nodule Embedded in Normal Tissue,” J. Control Rel., 74, pp. 193–202. 10.1016/S0168-3659(01)00317-0 [DOI] [PubMed] [Google Scholar]
- [39]. Banerjee, R. K. , Sung, C. , Bungay, P. M. , Dedrick, R. L. , and van Osdol, W. W. , 2002, “Antibody Penetration Into a Spherical Prevascular Tumor Nodule Embedded in Normal Tissue,” Ann. Biomed. Eng., 30, pp. 828–839. 10.1114/1.1496087 [DOI] [PubMed] [Google Scholar]
- [40]. Zheng, Y. , Chen, J. , Craven, M. , Choi, N. W. , Totorica, S. , Diaz-Santana, A. , Kermani, P. , Hempstead, B. , Fischbach-Teschl, C. , Lopez, J. A. , and Stroock, A. D. , 2012, “In Vitro Microvessels for the Study of Angiogenesis and Thrombosis,” Proc. Natl. Acad. Sci. U.S.A., 109, pp. 9342–9347. 10.1073/pnas.1201240109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Tung, C. K. , Krupa, O. , Apaydin, E. , Liou, J. J. , Diaz-Santana, A. , Kim, B. J. , and Wu, M. , 2013, “A Contact Line Pinning Based Microfluidic Platform for Modelling Physiological Flows,” Lab Chip, 13, pp. 3876–3885. 10.1039/c3lc50489a [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Song, J. W. , Cavnar, S. P. , Walker, A. C. , Luker, K. E. , Gupta, M. , Tung, Y. C. , Luker, G. D. , and Takayama, S. , 2009, “Microfluidic Endothelium for Studying the Intravascular Adhesion of Metastatic Breast Cancer Cells,” PLoS One, 4, p. e5756. 10.1371/journal.pone.0005756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Zervantonakis, I. K. , Hughes-Alford, S. K. , Charest, J. L. , Condeelis, J. S. , Gertler, F. B. , and Kamm, R. D. , 2012, “Three-Dimensional Microfluidic Model for Tumor Cell Intravasation and Endothelial Barrier Function,” Proc. Natl. Acad. Sci. U.S.A., 109, pp. 13515–13520. 10.1073/pnas.1210182109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Chambers, A. F. , Groom, A. C. , and MacDonald, I. C. , 2002, “Dissemination and Growth of Cancer Cells in Metastatic Sites,” Nat. Rev. Cancer, 2, pp. 563–572. 10.1038/nrc865 [DOI] [PubMed] [Google Scholar]
- [45]. Steeg, P. S. , 2006, “Tumor Metastasis: Mechanistic Insights and Clinical Challenges,” Nat. Med., 12, pp. 895–904. 10.1038/nm1469 [DOI] [PubMed] [Google Scholar]
- [46]. Renkawitz, J. , and Sixt, M. , 2010, “Mechanisms of Force Generation and Force Transmission During Interstitial Leukocyte Migration,” EMBO Rep., 11, pp. 744–750. 10.1038/embor.2010.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Guck, J. , Lautenschlager, F. , Paschke, S. , and Beil, M. , 2010, “Critical Review: Cellular Mechanobiology and Amoeboid Migration,” Integ. Biol., 2, pp. 575–583. 10.1039/c0ib00050g [DOI] [PubMed] [Google Scholar]
- [48]. Friedl, P. , and Weigelin, B. , 2008, “Interstitial Leukocyte Migration and Immune Function,” Nat. Immunol., 9, pp. 960–969. 10.1038/ni.f.212 [DOI] [PubMed] [Google Scholar]
- [49]. Zaman, M. H. , Trapani, L. M. , Siemeski, A. , MacKellar, D. , Gong, H. Y. , Kamm, R. D. , Wells, A. , Lauffenburger, D. A. , and Matsudaira, P. , 2006, “Migration of Tumor Cells in 3D Matrices Is Governed by Matrix Stiffness Along With Cell-Matrix Adhesion and Proteolysis,” Proc. Natl. Acad. Sci. U.S.A., 103, pp. 10889–10894. 10.1073/pnas.0604460103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Lauffenburger, D. A. , and Horwitz, A. F. , 1996, “Cell Migration: A Physically Integrated Molecular Process,” Cell, 84, pp. 359–369. 10.1016/S0092-8674(00)81280-5 [DOI] [PubMed] [Google Scholar]
- [51]. Galbraith, C. G. , Yamada, K. M. , and Sheetz, M. P. , 2002, “The Relationship Between Force and Focal Complex Development,” J. Cell Biol., 159, pp. 695–705. 10.1083/jcb.200204153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Stetler-Stevenson, W. G. , Aznavoorian, S. , and Liotta, L. A. , 1993, “Tumor Cell Interactions With the Extracellular Matrix During Invasion and Metastasis,” Annu. Rev. Cell Biol., 9, pp. 541–573. 10.1146/annurev.cb.09.110193.002545 [DOI] [PubMed] [Google Scholar]
- [53]. Wolf, K. , Mazo, I. , Leung, H. , Engelke, K. , von Andrian, U. H. , Deryugina, E. I. , Strongin, A. Y. , Brocker, E. B. , and Friedl, P. , 2003, “Compensation Mechanism in Tumor Cell Migration: Mesenchymal-Amoeboid Transition After Blocking of Pericellular Proteolysis,” J. Cell Biol., 160, pp. 267–277. 10.1083/jcb.200209006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Wells, A. , Grahovac, J. , Wheeler, S. , Ma, B. , and Lauffenburger, D. , 2013, “Targeting Tumor Cell Motility As a Strategy Against Invasion and Metastasis,” Trends Pharmacol. Sci., 34, pp. 283–289. 10.1016/j.tips.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Kim, B. J. , Hannanta-anan, P. , Chau, M. , Kim, Y. S. , Swartz, M. A. , and Wu, M. , 2013, “Cooperative Roles of SDF-1alpha and EGF Gradients on Tumor Cell Migration Revealed by a Robust 3D Microfluidic Model,” PloS One, 8, p. e68422. 10.1371/journal.pone.0068422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Coussens, L. M. , Fingleton, B. , and Matrisian, L. M. , 2002, “Matrix Metalloproteinase Inhibitors and Cancer: Trials and Tribulations,” Science, 295, pp. 2387–2392. 10.1126/science.1067100 [DOI] [PubMed] [Google Scholar]
- [57]. Ulrich, T. A. , Pardo, E. M. D. , and Kumar, S. , 2009, “The Mechanical Rigidity of the Extracellular Matrix Regulates the Structure, Motility, and Proliferation of Glioma Cells,” Cancer Res., 69, pp. 4167–4174. 10.1158/0008-5472.CAN-08-4859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Tse, J. M. , Cheng, G. , Tyrrell, J. A. , Wilcox-Adelman, S. A. , Boucher, Y. , Jain, R. K. , and Munn, L. L. , 2012, “Mechanical Compression Drives Cancer Cells Toward Invasive Phenotype,” Proc. Natl. Acad. Sci. U.S.A., 109, pp. 911–916. 10.1073/pnas.1118910109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Demou, Z. N. , 2010, “Gene Expression Profiles in 3D Tumor Analogs Indicate Compressive Strain Differentially Enhances Metastatic Potential,” Ann. Biomed. Eng., 38, pp. 3509–3520. 10.1007/s10439-010-0097-0 [DOI] [PubMed] [Google Scholar]
- [60]. Tan, J. L. , Tien, J. , Pirone, D. M. , Gray, D. S. , Bhadriraju, K. , and Chen, C. S. , 2003, “Cells Lying on a Bed of Microneedles: An Approach to Isolate Mechanical Force,” Proc. Natl. Acad. Sci. U.S.A., 100, pp. 1484–1489. 10.1073/pnas.0235407100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Zheng, X. R. , and Zhang, X. , 2011, “Microsystems for Cellular Force Measurement: A Review,” J. Micromech. Microeng., 21, p. 054003. 10.1088/0960-1317/21/5/054003 [DOI] [Google Scholar]
- [62]. Legant, W. R. , Pathak, A. , Yang, M. T. , Deshpande, V. S. , McMeeking, R. M. , and Chen, C. S. , 2009, “Microfabricated Tissue Gauges to Measure and Manipulate Forces From 3D Microtissues,” Proc. Natl. Acad. Sci. U.S.A., 106, pp. 10097–10102. 10.1073/pnas.0900174106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Sochol, R. D. , Higa, A. T. , Janairo, R. R. R. , Li, S. , and Lin, L. W. , 2011, “Unidirectional Mechanical Cellular Stimuli Via Micropost Array Gradients,” Soft Matter, 7, pp. 4606–4609. 10.1039/c1sm05163f [DOI] [Google Scholar]
- [64]. Polackwich, R. J. , Koch, D. , Arevalo, R. , Miermont, A. M. , Jee, K. J. , Lazar, J. , Urbach, J. , Mueller, S. C. , and McAllister, R. G. , 2013, “A Novel 3D Fibril Force Assay Implicates SRC in Tumor Cell Force Generation in Collagen Networks,” PLoS One, 8, p. e58138. 10.1371/journal.pone.0058138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Legant, W. R. , Miller, J. S. , Blakely, B. L. , Cohen, D. M. , Genin, G. M. , and Chen, C. S. , 2010, “Measurement of Mechanical Tractions Exerted by Cells in Three-Dimensional Matrices,” Nat. Methods, 7, pp. 969–U113. 10.1038/nmeth.1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Storm, C. , Pastore, J. J. , MacKintosh, F. C. , Lubensky, T. C. , and Janmey, P. A. , 2005, “Nonlinear Elasticity in Biological Gels,” Nature, 435, pp. 191–194. 10.1038/nature03521 [DOI] [PubMed] [Google Scholar]
- [67]. Mocellin, S. , Wang, E. , and Marincola, F. M. , 2001, “Cytokines and Immune Response in the Tumor Microenvironment,” J. Immunother., 24, pp. 392–407. 10.1097/00002371-200109000-00002 [DOI] [PubMed] [Google Scholar]
- [68]. Sautes-Fridman, C. , Cherfils-Vicini, J. , Damotte, D. , Fisson, S. , Fridman, W. H. , Cremer, I. , and Dieu-Nosjean, M. C. , 2011, “Tumor Microenvironment Is Multifaceted,” Cancer Met. Rev., 30, pp. 13–25. 10.1007/s10555-011-9279-y [DOI] [PubMed] [Google Scholar]
- [69]. Kim, B. J. , and Wu, M. , 2012, “Microfluidics for Mammalian Cell Chemotaxis,” Ann. Biomed. Eng., 40, pp. 1316–1327. 10.1007/s10439-011-0489-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Pluen, A. , Netti, P. A. , Jain, R. K. , and Berk, D. A. , 1999, “Diffusion of Macromolecules in Agarose Gels: Comparison of Linear and Globular Configurations,” Biophys. J., 77, pp. 542–552. 10.1016/S0006-3495(99)76911-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Jain, R. K. , 2008, “Lessons From Multidisciplinary Translational Trials on Anti-Angiogenic Therapy of Cancer,” Nat. Rev. Cancer, 8, pp. 309–316. 10.1038/nrc2346 [DOI] [PubMed] [Google Scholar]
- [72]. Fischbach, C. , Kong, H. J. , Hsiong, S. X. , Evangelista, M. B. , Yuen, W. , and Mooney, D. J. , 2009, “Cancer Cell Angiogenic Capability Is Regulated by 3D Culture and Integrin Engagement,” Proc. Natl. Acad. Sci. U.S.A., 106, pp. 399–404. 10.1073/pnas.0808932106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Muller, A. , Homey, B. , Soto, H. , Ge, N. F. , Catron, D. , Buchanan, M. E. , McClanahan, T. , Murphy, E. , Yuan, W. , Wagner, S. N. , Barrera, J. L. , Mohar, A. , Verástegui, E. , and Zlotnick, A. , 2001, “Involvement of Chemokine Receptors in Breast Cancer Metastasis,” Nature, 410, pp. 50–56. 10.1038/35065016 [DOI] [PubMed] [Google Scholar]
- [74]. Boyden, S. , 1962, “The Chemotactic Effect of Mixtures of Antibody and Antigen on Polymorphonuclear Leucocytes,” J. Exp. Med., 115, pp. 453–466. 10.1084/jem.115.3.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Wyckoff, J. , Wang, W. , Lin, E. Y. , Wang, Y. , Pixley, F. , Stanley, E. R. , Graf, T. , Pollard, J. W. , Segall, J. , and Condeelis, J. , 2004, “A Paracrine Loop Between Tumor Cells and Macrophages Is Required for Tumor Cell Migration in Mammary Tumors,” Cancer Res., 64, pp. 7022–7029. 10.1158/0008-5472.CAN-04-1449 [DOI] [PubMed] [Google Scholar]
- [76]. Goswami, S. , Sahai, E. , Wyckoff, J. B. , Cammer, M. , Cox, D. , Pixley, F. J. , Stanley, E. R. , Segall, J. E. , and Condeelis, J. S. , 2005, “Macrophages Promote the Invasion of Breast Carcinoma Cells Via a Colony-Stimulating Factor-1/Epidermal Growth Factor Paracrine Loop,” Cancer Res., 65, pp. 5278–5283. 10.1158/0008-5472.CAN-04-1853 [DOI] [PubMed] [Google Scholar]
- [77]. Issa, A. , Le, T. X. , Shoushtari, A. N. , Shields, J. D. , and Swartz, M. A. , 2009, “Vascular Endothelial Growth Factor-C and C-C Chemokine Receptor 7 in Tumor Cell-Lymphatic Cross-Talk Promote Invasive Phenotype,” Cancer Res., 69, pp. 349–357. 10.1158/0008-5472.CAN-08-1875 [DOI] [PubMed] [Google Scholar]
- [78]. Mishra, P. , Banerjee, D. , and Ben-Baruch, A. , 2011, “Chemokines at the Crossroads of Tumor-Fibroblast Interactions That Promote Malignancy,” J. Leuk Biol., 89, pp. 31–39. 10.1189/jlb.0310182 [DOI] [PubMed] [Google Scholar]
- [79]. Dolznig, H. , Rupp, C. , Puri, C. , Haslinger, C. , Schweifer, N. , Wieser, E. , Kerjaschki, D. , and Garin-Chesa, P. , 2011, “Modeling Colon Adenocarcinomas In Vitro a 3D Co-Culture System Induces Cancer-Relevant Pathways Upon Tumor Cell and Stromal Fibroblast Interaction,” Am. J. Pathol., 179, pp. 487–501. 10.1016/j.ajpath.2011.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Ng, C. P. , and Swartz, M. A. , 2005, “Fibroblast Alignment Under Interstitial Fluid Flow Using a Novel 3-D Tissue Culture Model,” Am. J. Physiol. Heart Circ. Physiol., 288, pp. H3016–H3016. 10.1152/ajpheart.00270.2005 [DOI] [PubMed] [Google Scholar]
- [81]. Huang, Y. , Agrawal, B. , Sun, D. , Kuo, J. S. , and Williams, J. C. , 2011, “Microfluidics-Based Devices: New Tools for Studying Cancer and Cancer Stem Cell Migration,” Biomicrofluidics, 5, p. 13412. 10.1063/1.3555195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82]. Kerjaschki, D. , Bago-Horvath, Z. , Rudas, M. , Sexl, V. , Schneckenleithner, C. , Wolbank, S. , Bartel, G. , Krieger, S. , Kalt, R. , Hantusch, B. , Keller, T. , Nagy-Bojarszky, K. , Huttary, N. , Raab, I. , Lackner, K. , Krautgasser, K. , Schachner, H. , Kaserer, K. , Rezar, S. , Madlener, S. , Vonach, C. , Davidovits, A. , Nosaka, H. , Hämmerle, M. , Viola, K. , Dolznig, H. , Schreiber, M. , Nader, A. , Mikulits, W. , Gnant, M. , Hirakawa, S. , Detmar, M. , Alitalo, K. , Nijman, S. , Offner, F. , Maier, T. J. , Steinhilber, D. , and Krupitza, G. , 2011, “Lipoxygenase Mediates Invasion of Intrametastatic Lymphatic Vessels and Propagates Lymph Node Metastasis of Human Mammary Carcinoma Xenografts in Mouse,” J. Clin. Invest., 121, pp. 2000–2012. 10.1172/JCI44751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. Zheng, C. H. , Zhao, L. , Chen, G. E. , Zhou, Y. , Pang, Y. H. , and Huang, Y. Y. , 2012, “Quantitative Study of the Dynamic Tumor-Endothelial Cell Interactions Through an Integrated Microfluidic Coculture System,” Anal. Chem., 84, pp. 2088–2093. 10.1021/ac2032029 [DOI] [PubMed] [Google Scholar]
- [84]. Chan, J. M. , Zervantonakis, I. K. , Rimchala, T. , Polacheck, W. J. , Whisler, J. , and Kamm, R. D. , 2012, “Engineering of In Vitro 3D Capillary Beds by Self-Directed Angiogenic Sprouting,” PLoS One, 7(12), p. e50582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Hielscher, A. C. , and Gerecht, S. , 2012, “Engineering Approaches for Investigating Tumor Angiogenesis: Exploiting the Role of the Extracellular Matrix,” Cancer Res., 72, pp. 6089–6096. 10.1158/0008-5472.CAN-12-2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86]. Kim, S. , Lee, H. , Chung, M. , and Jeon, N. L. , 2013, “Engineering of Functional, Perfusable 3D Microvascular Networks on a Chip,” Lab Chip, 13, pp. 1489–1500. 10.1039/c3lc41320a [DOI] [PubMed] [Google Scholar]
- [87]. Jeon, J. S. , Zervantonakis, I. K. , Chung, S. , Kamm, R. D. , and Charest, J. L. , 2013, “In Vitro Model of Tumor Cell Extravasation,” PLoS One, 8, p. e56910. 10.1371/journal.pone.0056910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Moya, M. L. , Hsu, Y. H. , Lee, A. P. , Hughes, C. C. W. , and George, S. C. , 2013, “In Vitro Perfused Human Capillary Networks,” Tissue Eng. Part C, 19, pp. 730–737. 10.1089/ten.tec.2012.0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89]. Yu, M. , Stott, S. , Toner, M. , Maheswaran, S. , and Haber, D. A. , 2011, “Circulating Tumor Cells: Approaches to Isolation and Characterization,” J. Cell Biol., 192, pp. 373–382. 10.1083/jcb.201010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Nagrath, S. , Sequist, L. V. , Maheswaran, S. , Bell, D. W. , Irimia, D. , Ulkus, L. , Smith, M. R. , Kwak, E. L. , Digumarthy, S. , Muzikansky, A. , Ryan, P. , Balis, U. J. , Tompkins, R. G. , Haber, D. A. , and Toner, M. , 2007, “Isolation of Rare Circulating Tumour Cells in Cancer Patients by Microchip Technology,” Nature, 450, pp. 1235–U1210. 10.1038/nature06385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. Gleghorn, J. P. , Pratt, E. D. , Denning, D. , Liu, H. , Bander, N. H. , Tagawa, S. T. , Nanus, D. M. , Giannakakou, P. A. , and Kirby, B. J. , 2010, “Capture of Circulating Tumor Cells From Whole Blood of Prostate Cancer Patients Using Geometrically Enhanced Differential Immunocapture (GEDI) and a Prostate-Specific Antibody,” Lab Chip, 10, pp. 27–29. 10.1039/b917959c [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92]. Konstantopoulos, K. , and Thomas, S. N. , 2009, “Cancer Cells in Transit: The Vascular Interactions of Tumor Cells” Annu. Rev. Biomed. Eng., 11, pp. 177–202. 10.1146/annurev-bioeng-061008-124949 [DOI] [PubMed] [Google Scholar]
- [93]. Bevilacqua, M. P. , 1993, “Endothelial-Leukocyte Adhesion Molecules,” Annu. Rev. Immunol., 11, pp. 767–804. 10.1146/annurev.iy.11.040193.004003 [DOI] [PubMed] [Google Scholar]
- [94]. Laubli, H. , and Borsig, L. , 2010, “Selectins Promote Tumor Metastasis,” Semin. Cancer Biol., 20, pp. 169–177. 10.1016/j.semcancer.2010.04.005 [DOI] [PubMed] [Google Scholar]
- [95]. Shibue, T. , and Weinberg, R. A. , 2011, “Metastatic Colonization: Settlement, Adaptation and Propagation of Tumor Cells in a Foreign Tissue Environment,” Semin. Cancer Biol., 21, pp. 99–106. 10.1016/j.semcancer.2010.12.003 [DOI] [PubMed] [Google Scholar]
- [96]. Barthel, S. R. , Gavino, J. D. , Descheny, L. , and Dimitroff, C. J. , 2007, “Targeting Selectins and Selectin Ligands in Inflammation and Cancer,” Expert Opin. Therap. Targets, 11, pp. 1473–1491. 10.1517/14728222.11.11.1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97]. Witz, I. P. , 2008, “The Selectin-Selectin Ligand Axis in Tumor Progression,” Cancer Metas. Rev., 27, pp. 19–30. 10.1007/s10555-007-9101-z [DOI] [PubMed] [Google Scholar]
- [98]. Kobayashi, H. , Boelte, K. C. , and Lin, P. C. , 2007, “Endothelial Cell Adhesion Molecules and Cancer Progression,” Curr. Med. Chem. 14, pp. 377–386. 10.2174/092986707779941032 [DOI] [PubMed] [Google Scholar]
- [99]. Reyes-Reyes, M. E. , George, M. D. , Roberts, J. D. , and Akiyama, S. K. , 2006, “P-Selectin Activates Integrin-Mediated Colon Carcinoma Cell Adhesion to Fibronectin,” Exp. Cell Res., 312, pp. 4056–4069. 10.1016/j.yexcr.2006.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]. McCarty, O. J. T. , Jadhav, S. , Burdick, M. M. , Bell, W. R. , and Konstantopoulos, K. , 2002, “Fluid Shear Regulates the Kinetics and Molecular Mechanisms of Activation-Dependent Platelet Binding to Colon Carcinoma Cells” Biophys. J., 83, pp. 836–848. 10.1016/S0006-3495(02)75212-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Burdick, M. M. , Bochner, B. S. , Collins, B. E. , Schnaar, R. L. , and Konstantopoulos, K. , 2001, “Glycolipids Support E-Selectin-Specific Strong Cell Tethering Under Flow,” Biochem. Biophys. Res. Commun., 284, pp. 42–49. 10.1006/bbrc.2001.4899 [DOI] [PubMed] [Google Scholar]
- [102]. Burdick, M. M. , Bochner, B. S. , and Konstantopoulos, K. , 2002, “Relative Contributions of Glycolipids, Integrins, and Other Glycoproteins in LS174T Colon Carcinoma Cell Adhesion Under Dynamic Flow Conditions,” FASEB J., 16, pp. A1053–A1053. [Google Scholar]
- [103]. Minn, A. J. , Kang, Y. B. , Serganova, I. , Gupta, G. P. , Giri, D. D. , Doubrovin, M. , Ponomarev, V. , Gerald, W. L. , Blasberg, R. , and Massague, J. , 2005, “Distinct Organ-Specific Metastatic Potential of Individual Breast Cancer Cells and Primary Tumors,” J. Clin. Invest., 115, pp. 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104]. Mareel, M. M. , Vanroy, F. M. , and Bracke, M. E. , 1993, “How and When Do Tumor Cells Metastasize?,” Crit. Rev. Oncogen., 4, pp. 559–594. [PubMed] [Google Scholar]
- [105]. Ben-Baruch, A. , 2008, “Organ Selectivity in Metastasis: Regulation by Chemokines and Their Receptors,” Clin. Exp. Metas., 25, pp. 345–356. 10.1007/s10585-007-9097-3 [DOI] [PubMed] [Google Scholar]
- [106]. Dittma, T. , Heyder, C. , Gloria-Maercker, E. , Hatzmann, W. , and Zanker, K. S. , 2008, “Adhesion Molecules and Chemokines: The Navigation System for Circulating Tumor (Stem) Cells to Metastasize in an Organ-Specific Manner,” Clin. Exp. Metas, 25, pp. 11–32. 10.1007/s10585-007-9095-5 [DOI] [PubMed] [Google Scholar]
- [107]. Pathi, S. P. , Kowalczewski, C. , Tadipatri, R. , and Fischbach, C. , 2010, “A Novel 3-D Mineralized Tumor Model to Study Breast Cancer Bone Metastasis,” PLoS One, 5, p. e8849. 10.1371/journal.pone.0008849 [DOI] [PMC free article] [PubMed] [Google Scholar]