Synopsis
Although the majority of patients with Hodgkin lymphoma (HL) are cured with primary therapy, patients with primary refractory disease or relapse after initial treatment have poor outcomes and represent an unmet medical need. Recent advances in unraveling the biology of HL have yielded a plethora of novel targeted therapies. This review provides an overview of the data behind the hype generated by these advances and addresses the question of whether or not clinically these targeted therapies offer hope for patients with HL.
Keywords: Hodgkin lymphoma, relapsed disease, targeted therapies, brentuximab vedotin
Introduction
Classical Hodgkin lymphoma (HL) represents ~ 10% of all lymphomas diagnosed annually in the developing world. In 2013, approximately 9000 cases of HL were diagnosed in the US 1. With a median age of 38 years, and at least 40% of patients under age 35 at the time of diagnosis, it is the most common lymphoma affecting young patients 2. Over the past 30 years valuable lessons learnt about late effects of therapy, specifically cardiovascular and second cancer risk, have led to treatment modifications of radiation dose and field size as well as alkylator exposure, which have led to significant risk reduction of competing causes of death 3–5. As a result of these advances more than 75% of patients are cured with contemporary frontline therapy 6,7.
For patients who relapse after attaining an initial complete remission (CR), or have primary refractory disease, the standard treatment approach is salvage chemotherapy followed by autologous stem cell transplant (ASCT) with ~ 50% cure rate 8. Several studies show that achieving a CR prior to ASCT is one of the most important factors in determining long-term outcome post ASCT 9,10. Other pre-transplant prognostic factors include: duration of initial remission, extent of disease at relapse, and constitutional symptoms 11–14. In an international collaborative effort from 5 countries, data on 756 patients with relapsed HL with a minimum of 1 year follow-up post-transplant were pooled 15. The overall median post-progression survival (PPS) for patients relapsing after ASCT was 1.3 years. Seventy-one percent of relapses occurred within 1 year after ASCT, and were roughly equally distributed in the periods: <3 months (22%), >3 and <6 months (22%), and >6 and <12 months (27%). The median PPS for these periods were 0.55, 1.6, 1.68, and 2.26 years for time to relapse after ASCT respectively (p<0.0001). 15. Allogeneic stem cell transplantation (alloSCT) can induce durable remissions in some of these patients; however its utility is limited by the challenges of finding an available stem cell donor, and achieving adequate disease control prior to transplantation 16. Therefore novel treatments to increase the CR rate pre SCT, or significantly prolong remission duration post SCT, have been sought.
The recent approval in 2011 of brentuximab vedotin, an antibody drug conjugate (ADC) targeting CD30, has been the first major advance in the management of HL after several decades and offers considerable hope to patients with refractory disease or relapse after SCT 17. Better understanding of the biology of HL has led to exploration of several other potential targets as therapeutic options. This review provides an overview of HL tumor biology in the context of the development of novel targeted therapies. We discuss four broad categories of targeted therapies either approved or under investigation: 1) therapies targeting HRS cell surface receptors, 2) therapies targeting reactive immune cells in the tumor microenvironment, 3) adoptive immunotherapy, and 4) therapies targeting signaling and intracellular survival pathways (Tables 1 and 2). While some of the agents discussed below are highly active as single agents, many others demonstrate modest single agent activity. Moving forward the challenge will be how to develop rational combinations of these novel agents within the context of current paradigms of care to achieve enhanced efficacy with minimal toxicity.
Table 1.
Current Results in Selected Targeted Therapies in HL
| DRUG/PHASE | MAIN TARGET | CLINICAL TRIAL NUMBER | FAILED ASCT (%) | CLINICAL RESULTS | REFERENCE |
|---|---|---|---|---|---|
| RECEPTOR TARGETED THERAPIES | |||||
| 1) SGN-30 (I) 2) SGN-30 (II) |
CD 30+ HRS Cells | 1) NCT00051597 2) NCT00337194 |
83a 68 |
No significant response | 35–37 |
| MDX-060 (1/2) | CD 30+ HRS cells | NCT00284804 | 87a | No significant response | 35–37 |
| Brentuximab Vedotin (BV) 1) BV/(I) 2) BV/(I) 3) BV/(II) |
CD 30+ HRS cells | 1) NCT00947856 2) NCT01100502 3) NCT01060904 |
73a 68a 100 |
PIVOTAL Trial: ORR 75%, CR 34%, median PFS 5.6 mos, median DOR 20.5 mos | 17,38,39 |
| HCD122 (II) | CD40+ HRS cells; Th2/Treg signaling | NCT00670592 | NR | ORR 16% (all PR) | 49 |
| Galiximab (II) | CD80+ HRS cells | NCT00516217 | 83 | ORR of 6.9%, TTP 1.6 months | 46,47 |
| MICROENVIRONMENT TARGETING | |||||
| Lenalidomide (II) Lenalidomide (II) |
Immunomodulation, anti- angiogenesis | 1) NCT00540007 2) NCT00478959 |
76 67 |
1) ORR 19% (N = 32) 2) ORR 13% (N = 15) |
54,55 |
| AFM 13 (I) | CD 16/30+ HRS cells | NCT01221571 | NR | 7% PR / 50% SD | 56 |
| 1) Rituximab single agent (I Pilot) 2) Rituximab + Gemcitibine (II) 3) A) Rituximab + ABVD frontline (I) B) Rituximab + ABVD frontline (II) |
CD20+ peritumoral B lymphocytes; CD20+ HRS cells | 3A) NCT00504504 3B) NCT00369681 |
82 55 0 0 |
1) ORR 22%, median DOR 8.7 mos 2) ORR 48%, median FFS 2.7 mos 3A) EFS 83% and OS 96% 3B) EFS 83% and OS 98% |
61,62,63,64 |
| PLX3397 (II) | CSF1R inhibitor | NCT01217229 | NR | ORR 5% | 66 |
| ADOPTIVE IMMUNOTHERAPY | |||||
| Epstein-Bar virus positive specific cytotoxic T-cells | EBV+ HRS cells | NCT00058617a | 40 | 83% of 28 patients with EBV+ HL had a clinical response, including 4 CRs sustained >9 mos | 67,68 |
| DOWNSTREAM SIGNALING PATHWAY | |||||
| Panobinostat (I) | Histone modification | NCT00742027 | 100 | ORR 27% including 4% CR, median PFS was 6.1 mos | 78 |
| Vorinostat (I) | Histone modification, STAT signaling (pSTAT6) | NCT00132028 | 44 | ORR 4% | 77 |
| Mocetinostat (I) | Histone modification, STAT signaling | NCT00358982 | 84 | ORR 21% | 80 |
| Everolimus (I) | PI3K signaling, mTOR, TNFR signaling | NCT01022996 | 84% | ORR 47%. 8 PR, 1 CR. Median TTP 7.2 months, 4 responders remained progression free at 12 mos | 88 |
| SB1518 | JAK/STAT pathway | NCT01263899 | NR | No significant clinical activity | 85 |
All Trials are in Relapsed/Refractory Patients Unless Otherwise Indicated
Includes patients with HL and NHL;
NR: not reported; NA: not available, ORR: overall response rate; CR: complete response; PR: partial response, PFS: progression free survival; TTP: time to progression; DOR: duration of response ASCT: autologous stem cell transplant; mos: months
Table 2.
Selected Ongoing Clinical Trials of Novel Agents
| DRUG | MAIN TARGET | CLINICAL TRIAL NUMBER |
|---|---|---|
|
| ||
| RECEPTOR TARGETED THERAPIES | ||
|
| ||
| Brentuximab vedotin (BV) combinations | ||
| Frontline | CD 30+ HRS cells | |
| 1) Phase 3 frontline with AVD versus brentuximab/AVD | 1) NCT01712490 | |
| 2) ECAPPB vs. ECADD B (frontline) | 2) NCT01569204 | |
| Relapsed/Refractory | ||
| 3) ABVD → BV (relapsed) | 3) NCT01578967 | |
| 4) BV+ Bendamustine (relapsed) | 4) NCT01874054 | |
| 5) BV+ Ipilimumab (relapsed) | 5) NCT01896999 | |
| 6) BV vs. ICE pre ASCT (relapsed) | 6) NCT01393717 | |
| 7) BV → ICE (relapsed) | 7) NCT01508312 | |
| 8) BV + Rituximab (relapsed) | 8) NCT01900496 | |
| Maintenance | ||
| 9) BV Maintenance after ASCT (ATHERA) (maintenance) | 9) NCT01620229 | |
|
| ||
| TNX-650 | IL-13 | NCT00441818 |
|
| ||
| MICROENVIRONMENT TARGETING | ||
|
| ||
| Lenalidomide Combinations (relapsed) | ||
| 1) AVD | Immunomodulation, anti-angiogenesis | 1) NCT0105667 |
| 2) Bendamustine, | 2) NCT01412307 | |
| 3) Romidepsin | 3) NCT01742793 | |
| 4) Everolimus | 4) NCT01075321 | |
|
| ||
| Rituximab Combinations | ||
| Frontline | CD20+ peritumoral B lymphocytes; CD20+ HRS cells | |
| 1) Rituximab ABVD vs. ABVD Phase 2 | 1) NCT00654732 | |
| 2) Rituximab + BEACOPP (HD18) | 2) NCT00515554 | |
| Relapsed | ||
| 3) Rituximab + Bendamustine | 3) NCT01900496 | |
|
| ||
| Ipilimumab (relapsed) | Immunomodulation of tumor microenvironment | NCT01896999 |
|
| ||
| Nivolumumaba (relapsed) | PD-1 expressing peritumoral lymphocytes | NCT01592370 |
|
| ||
| CDX1127 (relapsed) | anti-CD27 antibody | NCT0146013 |
|
| ||
| ADOPTIVE IMMUNOTHERAPY | ||
|
| ||
| Autologous CAR.CD30 EBV specific-cytotoxic T-lymphocytes (relapsed) | EBV+ CD30+ HRS cells; CD30+ HRS cells | NCT01192464 |
|
| ||
| DOWNSTREAM SIGNALING PATHWAYS | ||
|
| ||
| MLN4924 (relapsed) | NFκB via inhibition of Ned8 | NCT00722488 |
|
| ||
| Everolimus (relapsed) | PI3K signaling, mTOR, TNFR signaling | |
| 1) Everolimus and Panobinostat | 1) NCT00918333 | |
| 2) Everolimus and Lenalidomide | 2) NCT01075321 | |
Includes patients with HL and NHL;
NR: not reported; NA: not available, ORR: overall response rate; CR: complete response; PR: partial response, PFS: progression free survival; TTP: time to progression; DOR: duration of response; ASCT: autologous stem cell transplant; BV: brentuximab vedotin; AVD: adriamycin, vinblastine, dacarbazine; ECAPP B: brentuximab vedotin in combination with etoposide, cyclophosphamide, adriamcyin, procarbazine, prednisone and brentuximab; ECADD B: etoposide, cyclophosphamide, adriamcyin, doxorubicin, dacarbazine and brentuximab; ABVD: adriamycin, bleomycin, vinblastine, and dacarbazine; BEACOPP: bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone.
Emerging Targets in the Biology of Hodgkin Lymphoma
Classical HL is a B cell lymphoid neoplasm, characterized by Hodgkin Reed Sternberg (HRS) cells. The malignant HRS cells represent only a small fraction (0.1–1,0%) of the total cellular population and exist within an inflammatory microenvironment that supports tumor growth and suppresses immune surveillance 18–22. HRS cells grow poorly both in vitro and in vivo murine models without microenvironment support, underscoring its role in HL growth and survival 19,23. The cross talk between the HRS cells, the peritumoral cells in the tumor microenvironment, and secreted cytokines, propagates HRS cell growth, proliferation, and evasion of immune regulation.
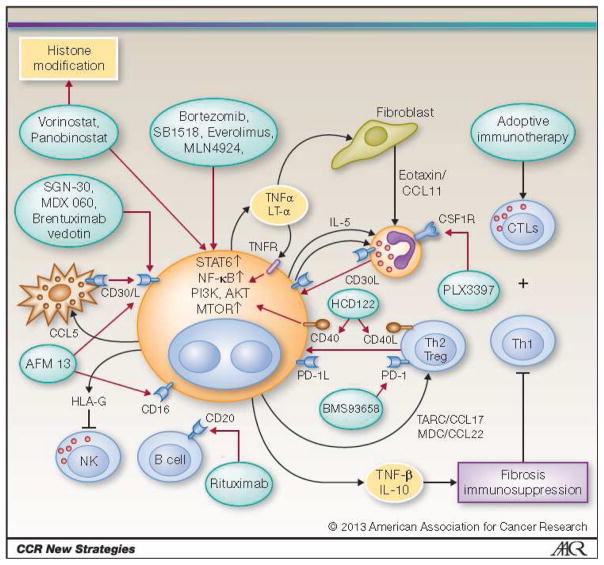
HRS cells express many surface receptors including CD15, CD30 CD40, CD80, and CD25 (the alpha chain of the IL-2 receptor)24. Additionally up-regulation of the programmed death ligand-1 (PDL-1) on HRS cells induces anergy in peritumoral T cells, which themselves express PD-1. High expression of PD-1 by peritumoral lymphocytes has been reported to be an independent predictor of inferior overall survival (OS) 25,26. Galectin-1 (gal-1) expression inhibits infiltration of CD8+ effector cells, expression of TNF-related apoptosis inducing ligand (TRAIL), and Fas ligand induced apoptosis of cytotoxic T lymphocytes (CTLs) 27,28. HRS cells further shape their microenvironment by secreting immunosuppressive cytokines and chemokines, such as the chemokine thymus and activation-regulated chemokine/CCL17 (TARC), CCL5, and CCL22. These in turn, attract T helper 2 (Th2) and regulatory T (Treg) cells to the tumor microenvironment, as well as interleukin-7 (IL-7), which then induce differentiation of naïve CD4+ T cells towards FoxP3+ Treg cells 29–31. In fact, high serum levels of the chemokine TARC at diagnosis have been associated with an inferior clinical outcome 32. Tumor associated macrophages (TAM) induce signal transducer and activator of transcription (STAT) mediated suppression of T cell surveillance and cell directed cytotoxicity. Increased numbers of CD68 and CD163 expressing TAMs are also associated with inferior survival in newly diagnosed HL patients treated with standard therapy, as well as in patients following ASCT 33. Cumulatively the tumor microenvironment induces T cell exhaustion and deficient anti-tumor immunity, which plays a key role in propagating a permissive milieu for HL growth. While many of the dots of this complex network have been connected, it is still unclear how they all fit together or what is the logical road map for treating relapsed and refractory HL. At a conceptual level targeted therapies can be broadly classified as targeting: 1) HRS cell surface receptors, 2) the tumor microenvironment, 3) cell-mediated immunity (adoptive immunotherapy) and 4) signalling pathways. Figure 1 displays selected novel agents in the context of their targets.
Figure 1.
Selected Novel Agents in the Context of the Biological Targets
Targeting Molecules Expressed on HRS Cell Surface
Receptors highly expressed on the HRS cell surface, with low to absent expression on normal tissues, are optimal for targeted therapy. Trials evaluating these targets are summarized in Table 1 and 2.
Targeting CD30
CD30 is highly expressed on HRS cells, and nearly absent on normal tissue, making it an optimal target of directed therapy. It is a 120-KDa type I transmembrane glycoprotein belonging to the tumor necrosis factor (TNF) superfamily and induces signaling pathways that promote HRS cell proliferation 34. The most successful targeted therapy developed to date in HL has been brentuximab vedotin, an ADC directed against the CD30 receptor. Early clinical studies targeting CD30 with naked antibodies SGN-30 (cAC10), and MDX-060 did not demonstrate meaningful anti-tumor activity largely attributed to suboptimal antigen binding, and neutralization of anti CD30 antibodies by soluble CD30 35–37. In an effort to increase cytotoxicity, a valine–citrulline peptide linker to monomethyl auristatin E (MMAE), a synthetic analogue of the naturally occurring antimitotic agent dolastatin 10, was added to the chimeric antibody cAC10 (SGN-30) creating the ADC brentuximab vedotin. Robust anti-tumor activity reported in two phase I clinical trials led to a lot of hype regarding this agent 38,39. These data were subsequently confirmed in a phase II pivotal trial of 102 patients with heavily pretreated HL who had relapsed after ASCT 17. Overall treatment was well tolerated and ≥ grade 3 events or dose limiting toxicities (DLT) included neutropenia (20%), thrombocytopenia (8%), peripheral sensory neuropathy (8%) and anemia (6%). The response rate in this heavily pre-treated population was striking with an overall response rate (ORR) of 75%, and a CR rate of 34%. The median progression free survival (PFS) was 5.6 months, with a median duration of response (DOR) of 20.5 months 17.
These compelling data, led to FDA approval of brentuximab vedotin in 2011 for patients with relapsed/refractory HL who have failed ASCT, or two chemotherapy regimens. Data also suggest that brentuximab vedotin is active as a retreatment strategy with an ORR of 57% 40. Recently, two retrospective analyses suggest that brentuximab vedotin also provides a potential bridge to successful alloSCT 41,42. The combination of brentuximab vedotin with donor lymphocyte infusion has been shown to induce both anti-tumor immunity and sustained clinical responses in 4 patients with early relapse post alloSCT 43. Ongoing trials are evaluating brentuximab vedotin as a maintenance strategy for high risk patients after ASCT, and for relapsed disease in combination with chemotherapy or immune based therapies such as ipilimumab (Table 2).
The development and subsequent approval of brentuximab vedotin is a clear example of a novel therapy that has moved well beyond hype and offers hope for patients with relapsed and refractory HL. As a logical next step, trials evaluating its role in the frontline setting are ongoing (Tables 1 and 2). Preliminary results of a phase I trial evaluating the combination of brentuximab with adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) has been reported 44. Unfortunately, 44% (11/25) of patients experienced significant pulmonary toxicity, including two deaths on study. Subsequently with modification to exclude bleomycin (AVD), 7 of the 11 patients completed treatment without further toxicity. Notably, no pulmonary toxicity was observed in an expanded AVD-brentuximab cohort and preliminary results report a PET/CT CR of 96% 44. These data have led to a phase III frontline trial for patients with untreated advanced HL evaluating the activity of ABVD versus brentuximab-AVD. Other frontline trials include a phase I trial of ABVD followed by 6 cycles of brentuximab vedotin for patients with untreated stage I and II non-bulky HL, and a randomized trial of brentuximab vedotin in combination with etoposide, cyclophosphamide, adriamcyin, procarbazine, prednisone and brentuximab (ECAPP B) versus etoposide, cyclophosphamide, adriamcyin, doxorubicin, dacarbazine and brentuximab (ECADD B) in patients with high risk advanced stage HL. These combinations of brentuximab vedotin with standard therapy or other targeted agents continue to offer hope that in the future there may be novel treatment platforms which are well tolerated with possibly superior activity.
Other Cell Surface Targets
CD80 is a costimulatory molecule highly expressed on HRS cells and inhibits antigen-specific T cell lymphoproliferation and interferon-gamma secretion 45. Galiximab, a primatized IgG1 monoclonal antibody against CD80, has high affinity binding for CD80, and induces antibody dependent cytotoxicity (ADCC) 46. In a phase II clinical trial in patients with relapsed and refractory HL galiximab was well tolerated but had disappointing activity, with an ORR of 6.9% and a median time to progression (TTP) of 1.6 months 46,47.
CD40 is widely expressed on B and T cells and a member of the tumor necrosis factor receptor (TNFR) family which induces cell proliferation, survival, secretion of cytokines, and activation of both the classical (canonical) and alternative (non-canonical) pathways of nuclear factor Kappa B (NFκB) signaling 48. Lucatumumab (HCD122) targets both CD40+ HRS cells, and Th2/Treg signaling and has been investigated in HL (59). A phase II trial reported an ORR of 16%, all partial responses (PRs) in 18 patients with relapsed/ refractory HL 49. The therapy was generally well tolerated and reversible asymptomatic hepatotoxicity was the primary DLT.
Agents that have been investigated but are not currently in development include antibodies to the TRAIL protein, and CD25, the alpha chain of the IL-2 receptor. A phase I trial of the TRAIL-R2 antibody AMG655 in combination with bortezomib or vorinostat was suspended due to poor patient accrual. A clinical trial of the anti CD25 immunotoxin RFT5-SPMT-dgA had significant toxicity due to vascular leak syndrome and disappointing results, with only 13% of patients achieving a PR 50. A monoclonal antibody targeting IL-13 (TNX-650) is currently under investigation, however to date no clinical data have been reported.
In summary, while there has been considerable hype based on the biologic rationale of using antibodies to target differentially expressed cell surface receptors, none have matched the efficacy of brentuximab vedotin. More insightful science and combination strategies are required to truly translate to hope in the clinical setting.
Targeting the Tumor Microenvironment
Monotherapies targeting only the HRS cells are limited in their efficacy due to the major role of the microenvironment in regulating HRS function and survival 19.
Strategies targeting tumor-microenvironment interactions aim to disrupt its cellular components, or activate peritumoral T and NK cells to induce anti-tumor responses. Encouraging pre-clinical data of agents in development include: immunomodulatory drugs (lenalidomide) monoclonal antibody directed targeting of peritumoral CD20+ B cells (rituximab, almentuzumab), bispecific antibodies, such as AFM13 which simultaneously targets CD30 bearing HRS cells and CD16 on natural killer cells, selective inhibition of colony-stimulating factor-1 (CSF1R) a growth factor for tumor-associated macrophages (TAMs), the anti-CTLA-4 antibody ipilimumab, and the checkpoint inhibitors targeting PD-1 and anti-PDL-1 51,52. Ongoing trials are outlined in Table 2.
Lenalidomide is an immunomodulatory and anti-angiogenic agent, with a putative mechanism of activating cytotoxic T lymphocytes (CTLs) and NK cells against HRS cells 53. The safety and efficacy of lenalidomide as a monotherapy has been investigated in several studies. In a multicenter phase II study, 36 patients with relapsed HL were treated with 25 mg/day of lenalidomide on days 1–21 of a 28 day cycle. The ORR was 19% with moderate grade 3–4 hematologic toxicity noted;neutropenia (47%), leukopenia (29%), anemia (26%), and thrombocytopenia (18%) 54. A smaller study of 15 patients reported similar results for toxicity, as well as efficacy with an ORR of 13%. Seven additional patients had stable disease. The median TTP was 3.2 months55. Cumulatively, these studies suggest that lenalidomide has modest single agent activity in relapsed HL. There is hope that efficacy may be enhanced by combinations with chemotherapy or other HRS targeting agents with trials ongoing (Table 2).
AFM13, a bispecific tetravalent human antibody construct that simultaneously targets CD30 and CD16 on natural killer (NK cells), has been evaluated in a phase I clinical trial in relapsed/refractory HL. Patients were heavily pretreated with a median of 6 (range 3 –11) prior therapies. Of the 28 patients enrolled, 9 had received previous brentuximab vedotin, and 14 were refractory to prior therapy. AFM13 was safe and well tolerated. The most frequent adverse events included: infusion-related reactions (headache, fever, fatigue and myalgia) in 33% of patients. Moderate clinical activity (2 patients achieved PRs, 14 patients SD) was demonstrated and more mature follow-up is needed to discern potential 56.
While HRS cells rarely express CD20, the tumor microenvironment is rich in CD20 expressing B cells, and a study suggests that circulating clonotypic B cells may be the HL tumor initiating cells 57. Some studies suggest that these B cells deliver survival signals to HRS cells, and suppress T cell activation via IL-10 production 58. In contrast, other studies report that the presence of CD20 expressing B cells in the tumor microenvironment is associated with improved survival 59,60. Nonetheless, targeting CD20 with the monoclonal antibody rituximab has been actively investigated. In a pilot study 22 patients with relapsed/refractory HL were treated with single agent rituximab. The ORR was 22% and included PRs, as well as CRs 61. Interestingly, 6 of 7 patients with CD20 negative HRS cells experienced resolution of B symptoms, suggesting a possible role for CD20+ B cells in mediating the systemic cytokine response 61. Therefore it is unclear whether the activity of rituximab is due to a direct effect on HRS cells (that are occasionally CD20-positive), or a depletion of supporting B cells and peritumoral CD20+ cells. These encouraging single agent data provided the rationale to investigate rituximab in combination with chemotherapy. The safety and efficacy of rituximab and gemcitabine was investigated in 33 patients with relapsed HL. The ORR was 48% independent of HRS cell CD20 expression; however, the median failure free survival (FFS) was only 2.7 months 62. Two phase II trials have evaluated rituximab in combination with ABVD. In the first trial, 78 patients with newly diagnosed HL were treated with weekly rituximab for 6 weeks, and standard ABVD for 6 cycles 63. The combination was well tolerated with neutropenia, fatigue, and nausea the most frequent treatment related adverse events. At 68 months the event free survival (EFS) and OS, were 83% and 96% respectively. These results were superior to ABVD alone when compared to institutional historical data. A second phase II study reported similar results with a 3 year EFS and OS of 83% and 98% respectively 64. Interestingly, in this study circulating clonotypic B cells were associated with a greater frequency of relapse. Other ongoing studies are evaluating the contribution of rituximab to first line augmented bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) for patients with advanced untreated HL (HD18), and in combination with ABVD as frontline therapy for patients with advanced stage poor risk HL (Table 2).
In summary while there is reasonable hype about the rationale to target CD20, results of randomized controlled trials are required before the question of hype versus hope can be definitively answered.
CD52 is another cell surface receptor highly expressed on peritumoral B cells. Almentuzumab a humanized monoclonal anti-CD52 antibody binds to CD52 purportedly inducing cell lysis via antibody dependent cell-mediated cytotoxicity (ADCC) 65. A phase II study investigating the efficacy of single agent almentuzumab in relapsed/refractory HL was terminated due to slow patient accrual, and data has not been reported to date. The efficacy of the combination of almentuzumab with etoposide, prednisone, vincristine, cyclophosphamide, and adriamycin (EPOCH) chemotherapy in relapsed/refractory HL is currently under investigation (Table 2).
Direct immune based approaches, which reverse the anergy of peritumoral T cells and stimulate anti-tumor cytolytic activity, represent another novel strategy against relapsed/refractory HL. The association of high expression of TAMs with a short PFS, led to significant hype for evaluating their inhibition with compound PLX3397, a selective inhibitor of CSF1R (a growth factor for TAMs). Unfortunately, despite the inhibition of both CSF1R and Kit, in a phase II trial of 20 heavily pretreated HL patients PLX3397 had only modest activity, with an ORR of 5% and a median PFS of 56 days 66. Therefore the role of CSF1R in HL lymphoma biology needs to be better understood before these strategies move from hype into hope.
Lastly, immune activating strategies such as the combination of the anti-CTLA-4 antibody (ipilimumab) with brentuximab vedotin, and the anti-CD27 antibody (CDX1127) are being investigated. Checkpoint inhibitors against PD-1 expressed on peritumoral T cells, or PDL-1 expressed on HRS cell surface are also undergoing active investigation (Table 2).
In summary, it is too early in development to discern whether the data from agents targeting reactive immune cells in the tumor microenvironment, as summarized in this section, will translate to hope. Results of ongoing clinical trials over the next few years will help shed some light on the efficacy of these agents both in terms of a response rate and durability.
Adoptive Immunotherapy in HL
Adoptive immunotherapy allows the generation and transfer of T cells engineered ex vivo to target and attack tumor cells in the host, as well as immune activation of the tumor microenvironment. In relapsed/refractory Epstein Barr virus positive (EBV+) HL, an adoptive approach using ex vivo expansion of viral EBV antigen specific CTLs produced striking results albeit in a small number of patients 67,68. In this pilot study, 83% (5 of 6) patients with relapsed EBV+ HL had a clinical response, of which 4 achieved CRs sustained for more than 9 months. Other trials of adoptive immunotherapy, i.e. targeting EBV-HL through MAGE antigen, or genetically engineered T lymphocytes expressing a chimeric CD30 antigen receptor, are ongoing but have not reported data to date 69,70. Only time will tell if these innovative strategies will constitute a new domain of hope or not.
Targeting Downstream Signaling and Intracellular Survival Pathways
Several drugs target constitutively activated downstream signaling pathways that drive HRS cell proliferation, and enhance tumor cell survival. Epigenetic changes in HRS cells modulate B cell silencing, immune escape, and the transcription of genes underlying cell proliferation and survival 71. The acetylation state of proteins are modified by the opposing effects of both histone acetyltransferases (HATs) and histone deacetylases (HDACs). There are currently 4 classes of HDACs: classes I and IV are constitutive nuclear proteins that regulate cell proliferation, class II HDACS regulate genes that promote cell growth and shuttle between the nucleus and the cytoplasm, and class III HDACSs regulate chromatin structure 72. Increased expression of HDACs relative to normal tissues has been observed in HL, and in at least one study correlated with poor treatment outcome 73.
Histone deacetylase inhibitors (HDACI) modulate cellular processes and signaling pathways that are dysregulated in cancers 74,75. HDACI target tumor cells and their interaction with their local microenvironment through multiple epigenetic mechanisms including chromatin condensation and acetylation of histones affecting gene expression. Treatment of HL patients with HDACI decreases the secretion of the inhibitory cytokine CCL17 (TARC) in vitro 76. Currently, two broad classes of HDACIs are under investigation in HL: pan HDAC inhibitors that inhibit HDAC class I and II (i.e. vorinostat, and panobinostat), and selective HDACIs that preferentially inhibit class I HDACs (mocetinostat and etinostat).
Vorinostat, mocetionostat, and panobinostat have been investigated as monotherapies in relapsed/refractory HL. In a phase II study of oral vorinostat the ORR was only 4% 77. More promising reports have been reported for panibinostat in a phase II trial of 129 HL patients, all of whom had failed ASCT. The primary toxicities were hematologic. Grade 3–4 toxicities included 79% thrombocytopenia (79%), 21% anemia (21%) and neutropenia (21%). The ORR in this heavily pre-treated patient population was 27% (23% PR, 4% CR), with a median DOR of 6.9 months, and an estimated 1-year OS rate of 78%. The median PFS was ~ 6 months and 52 patients (40%) had PFS greater than 24 weeks 78. Responses were associated with a decrease in serum Tarc levels 78,79.
Mocetinostat has been evaluated in a phase 2 trial in relapsed/refractory HL. Significant toxicity was seen at the 110mg dose including grade ≥ 3 myelosuppression, fatigue, pneumonia, and in 4 patients pleural effusions (3 ≥ grade 3). The drug was better tolerated at the reduced dose of 85mg with an ORR of 21% 80.
Cumulatively, these data suggest that HDACI have activity, however the hematologic toxicity profile will likely make combination strategies with chemotherapy challenging. Currently the optimal HDACI strategy to move these drugs from the hype category to the hope category remains unclear.
HRS cells constitutively express NF-κB, in part as a result of somatic mutations in pathway members and regulators, as well as other anti-apoptotic proteins, which inhibit both the intrinsic and extrinsic pathways of apoptosis 21,23. Bortezomib, a reversible proteasome inhibitor of NFκB signaling, enhances apoptosis through down regulation of the anti-apoptotic molecules XIAP and c-FLIP and has a putative role as a chemotherapy sensitizing agent 81. Although bortezomib demonstrated anti-proliferative activity in vitro, a phase II clinical trial in relapsed/refractory HL failed to demonstrate meaningful clinical activity 82. The evidence for synergy between cytotoxic chemotherapy and bortezomib in non-Hodgkin lymphoma led to the evaluation of the combination of bortezomib and chemotherapy in relapsed/refractory HL. In a phase I trial of ifosfamide, carboplatinum and etoposide (ICE) in combination with bortezomib (BICE) given on days 1 and 4 of standard infusion, the ORR for 12 patients was 69% but significant myelosuppression was seen 83. A second study combined bortezomib given twice weekly (days 1, 4, 8, 11) in a 3 week cycle at a dose of 1 mg/m2 with gemcitabine 800mg/m2 on days 1 and 8. This combination had significantly lower activity (ORR 22%) with higher toxicity (grade 3 transaminitis) than the BICE treated patients, and was not pursued further. A study targeting NFκB with MLN4924, a small molecule inhibitor of neddylation 8, has recently been terminated due to slow accrual, and no data has been reported to date.
Other constitutively activated pathways in HRS cells include: Janus kinase-signal transducer and activator of transcription (JAK-STAT), and the phosphatidyliositol 3-kinase pathway (PI3K/AKT/mammalian target of rapamycin (MTOR) pathway). Inhibitors of JAK2 inhibitors suppress STAT phosphorylation in HL cells lines, and downregulate the expression of PDL-1 in vitro 84, however a phase I trial of the JAK2 inhibitor SB1518 did not have significant clinical activity, despite a tolerable safety profile 85.
Inhibition of MTOR has a myriad of in vitro effects including enhancement of apoptosis, cell cycle arrest, and autophagy 86,87. The clinical activity of the MTOR inhibitor everolimus was evaluated in a phase II trial in patients with relapsed/refractory HL. The ORR of this heavily pretreated patient population was 47%, with 8 patients achieving a PR, and one a CR. The median TTP was 7.2 months, with 4 responders remaining progression free at 12 months 88. Overall the therapy was reasonably well tolerated except in four patients who experienced grade ≥ 3 pulmonary toxicity. A synergy between targeting MTOR and other inhibitors of downstream signaling, such as PI3K and HDAC, has been suggested by in vitro data, and this combination is currently being evaluated. In a phase I/II study of the combination of everolimus with the HDACI panobinostat, the ORR for 13 HL patients was 46% 89. Combinations of everolimus with immunomodulatory agents, such as lenalidomide, as well as with PI3K inhibitors are currently under investigation.
Conclusion
Advances in HL biology over the past few years have yielded a plethora of novel targets and an unprecedented opportunity to develop newer therapies. These targeted therapies offer the potential to increase cure rates in patients with relapsed and refractory HL, along with the hope of decreasing long-term toxicity. The approval of brentuximab vedotin clearly offers new hope to patients with relapsed and refractory disease, and may have promise as frontline therapy. Currently there are many novel targeted therapies under clinical investigation in HL, and many more waiting to move from bench to bedside. Although these advances are exciting, it is unlikely that one size will fit all, or that any single therapy or therapeutic platform will be curative for all patients. The challenges ahead are to identify strategies that offer maximal tumor eradication with minimal systemic toxicity, and to identify subsets of patients with the highest likelihood of efficacy to a particular therapy. To accomplish this, more robust methods of risk stratification incorporating both clinical and biologic factors to identify patients at the highest risk of therapy failure are needed. This gap needs to be addressed before the full potential of novel targeted therapies can be realized and the hope of customized targeted therapies will then surpass the hype.
Key Points.
HL patients with primary refractory disease or relapse after transplant have poor outcomes and represent an unmet need.
Therapies derived from an understanding of HL biology can be broadly classified as targeting: the Hodgkin Reed Sternberg cell surface receptors, tumor microenvironment, cell mediated immunity, and intracellular signaling pathways.
Brentuximab vedotin, an antibody-drug conjugate targeting CD30, now FDA approved, offers substantial hope for improving outcomes in the treatment of HL.
Other therapies in development need longer follow-up to realize their potential.
Objectives.
Review recent advances in HL biology
Review development of novel targeted therapies in the context of HL biology
Review results of clinical trials with targeted therapies
Acknowledgments
CD is supported in part by the NYU Clinical and Translational Science Institute (CTSI) NIH/NCATS UL1 TR00038.
Footnotes
Disclosures of potential conflicts of interest
CD: Seattle Genetics, consulting and speakers bureau
RA: Seattle Genetics, research funding and advisory board, Genentech; advisory board and research funding, Millennium: The Takeda Oncology Company, research and advisory board.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancer facts and figures 2013. Atlanta, GA: American Cancer Society; 2013. [Google Scholar]
- 2.http://seer.cancer.gov/statfacts/html/hodg.html
- 3.Koontz MZ, Horning SJ, Balise R, et al. Risk of therapy-related secondary leukemia in Hodgkin lymphoma: the Stanford University experience over three generations of clinical trials. J Clin Oncol. 2013 Feb 10;31(5):592–598. doi: 10.1200/JCO.2012.44.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol. 2009 Sep 10;27(26):4239–4246. doi: 10.1200/JCO.2008.19.9174. [DOI] [PubMed] [Google Scholar]
- 5.Hodgson DC. Late effects in the era of modern therapy for Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:323–329. doi: 10.1182/asheducation-2011.1.323. [DOI] [PubMed] [Google Scholar]
- 6.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013 Feb 20;31(6):684–691. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012 May 12;379(9828):1791–1799. doi: 10.1016/S0140-6736(11)61940-5. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002 Jun 15;359(9323):2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 9.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001 Feb 1;97(3):616–623. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 10.von Tresckow B, Engert A. The emerging role of PET in Hodgkin lymphoma patients receiving autologous stem cell transplant. Expert review of hematology. 2012 Oct;5(5):483–486. doi: 10.1586/ehm.12.41. [DOI] [PubMed] [Google Scholar]
- 11.Horning SJ, Chao NJ, Negrin RS, et al. High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin’s disease: analysis of the Stanford University results and prognostic indices. Blood. 1997 Feb 1;89(3):801–813. [PubMed] [Google Scholar]
- 12.Brice P, Bouabdallah R, Moreau P, et al. Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin’s disease: analysis of 280 patients from the French registry. Societe Francaise de Greffe de Moelle. Bone Marrow Transplant. 1997 Jul;20(1):21–26. doi: 10.1038/sj.bmt.1700838. [DOI] [PubMed] [Google Scholar]
- 13.Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin’s lymphoma autografted after a first relapse. Ann Oncol. 2005 Apr;16(4):625–633. doi: 10.1093/annonc/mdi119. [DOI] [PubMed] [Google Scholar]
- 14.Josting A, Franklin J, May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin’s lymphoma registered in the database of the German Hodgkin’s lymphoma study group. J Clin Oncol. 2002 Jan 1;20(1):221–230. doi: 10.1200/JCO.2002.20.1.221. [DOI] [PubMed] [Google Scholar]
- 15.Arai S, Fanale M, Devos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013 Jun 5; doi: 10.3109/10428194.2013.798868. [DOI] [PubMed] [Google Scholar]
- 16.Thomson KJ, Peggs KS, Smith P, et al. Superiority of reduced-intensity allogeneic transplantation over conventional treatment for relapse of Hodgkin’s lymphoma following autologous stem cell transplantation. Bone Marrow Transplant. 2008 May;41(9):765–770. doi: 10.1038/sj.bmt.1705977. [DOI] [PubMed] [Google Scholar]
- 17.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Jun 20;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldinucci D, Gloghini A, Pinto A, De Filippi R, Carbone A. The classical Hodgkin’s lymphoma microenvironment and its role in promoting tumour growth and immune escape. J Pathol. 2010 Jul;221(3):248–263. doi: 10.1002/path.2711. [DOI] [PubMed] [Google Scholar]
- 19.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin’s lymphoma: increasing evidence of the importance of the microenvironment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 May 10;29(14):1812–1826. doi: 10.1200/JCO.2010.32.8401. [DOI] [PubMed] [Google Scholar]
- 20.Hsi ED. Biologic features of Hodgkin lymphoma and the development of biologic prognostic factors in Hodgkin lymphoma: tumor and microenvironment. Leukemia & lymphoma. 2008 Sep;49(9):1668–1680. doi: 10.1080/10428190802163339. [DOI] [PubMed] [Google Scholar]
- 21.Kuppers R. The biology of Hodgkin’s lymphoma. Nature reviews. Cancer. 2009 Jan;9(1):15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 22.Khan G. Epstein-Barr virus, cytokines, and inflammation: a cocktail for the pathogenesis of Hodgkin’s lymphoma? Experimental hematology. 2006 Apr;34(4):399–406. doi: 10.1016/j.exphem.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Farrell K, Jarrett RF. The molecular pathogenesis of Hodgkin lymphoma. Histopathology. 2011 Jan;58(1):15–25. doi: 10.1111/j.1365-2559.2010.03705.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim LH, Eow GI, Peh SC, Poppema S. The role of CD30, CD40 and CD95 in the regulation of proliferation and apoptosis in classical Hodgkin’s lymphoma. Pathology. 2003 Oct;35(5):428–435. doi: 10.1080/00313020310001602567. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto R, Nishikori M, Kitawaki T, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008 Mar 15;111(6):3220–3224. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 26.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010 Oct 28;116(17):3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandhi MK, Moll G, Smith C, et al. Galectin-1 mediated suppression of Epstein-Barr virus specific T-cell immunity in classic Hodgkin lymphoma. Blood. 2007 Aug 15;110(4):1326–1329. doi: 10.1182/blood-2007-01-066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juszczynski P, Ouyang J, Monti S, et al. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proceedings of the National Academy of Sciences of the United States of America. 2007 Aug 7;104(32):13134–13139. doi: 10.1073/pnas.0706017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin’s lymphoma. The American journal of pathology. 1999 Jun;154(6):1685–1691. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aldinucci D, Lorenzon D, Cattaruzza L, et al. Expression of CCR5 receptors on Reed-Sternberg cells and Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. International journal of cancer. Journal international du cancer. 2008 Feb 15;122(4):769–776. doi: 10.1002/ijc.23119. [DOI] [PubMed] [Google Scholar]
- 31.Cattaruzza L, Gloghini A, Olivo K, et al. Functional coexpression of Interleukin (IL)-7 and its receptor (IL-7R) on Hodgkin and Reed-Sternberg cells: Involvement of IL-7 in tumor cell growth and microenvironmental interactions of Hodgkin’s lymphoma. International journal of cancer. Journal international du cancer. 2009 Sep 1;125(5):1092–1101. doi: 10.1002/ijc.24389. [DOI] [PubMed] [Google Scholar]
- 32.Sauer M, Plutschow A, Jachimowicz RD, et al. Baseline serum TARC levels predict therapy outcome in patients with Hodgkin lymphoma. Am J Hematol. 2013 Feb;88(2):113–115. doi: 10.1002/ajh.23361. [DOI] [PubMed] [Google Scholar]
- 33.Tan KL, Scott DW, Hong F, et al. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood. 2012 Oct 18;120(16):3280–3287. doi: 10.1182/blood-2012-04-421057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng B, Fiumara P, Li YV, et al. MEK/ERK pathway is aberrantly active in Hodgkin disease: a signaling pathway shared by CD30, CD40, and RANK that regulates cell proliferation and survival. Blood. 2003 Aug 1;102(3):1019–1027. doi: 10.1182/blood-2002-11-3507. [DOI] [PubMed] [Google Scholar]
- 35.Ansell SM, Horwitz SM, Engert A, et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin’s lymphoma and anaplastic large-cell lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Jul 1;25(19):2764–2769. doi: 10.1200/JCO.2006.07.8972. [DOI] [PubMed] [Google Scholar]
- 36.Forero-Torres A, Leonard JP, Younes A, et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. British journal of haematology. 2009 Jul;146(2):171–179. doi: 10.1111/j.1365-2141.2009.07740.x. [DOI] [PubMed] [Google Scholar]
- 37.Blum KA, Jung SH, Johnson JL, et al. Serious pulmonary toxicity in patients with Hodgkin’s lymphoma with SGN-30, gemcitabine, vinorelbine, and liposomal doxorubicin is associated with an FcgammaRIIIa-158 V/F polymorphism. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010 Nov;21(11):2246–2254. doi: 10.1093/annonc/mdq211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. The New England journal of medicine. 2010 Nov 4;363(19):1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 39.Fanale MA, Forero-Torres A, Rosenblatt JD, et al. A Phase I Weekly Dosing Study of Brentuximab Vedotin in Patients with Relapsed/Refractory CD30-Positive Hematologic Malignancies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 Jan 1;18(1):248–255. doi: 10.1158/1078-0432.CCR-11-1425. [DOI] [PubMed] [Google Scholar]
- 40.Bartlett Nancy, Brice Pauline, Chen Robert W, Fanale Michelle A, Gopal Ajay K, Matous Jeffrey, Rosenblatt Joseph David, Grove Laurie E, Forero-Torres Andres. Retreatment with brentuximab vedotin in CD30- positive hematologic malignancies: A phase II study. J Clin Oncol; ASCO Annual Meeting Abstracts; 2012; 2012. (suppl; abstr 8027) [Google Scholar]
- 41.Chen R, Palmer JM, Thomas SH, et al. Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2012 Jun 28;119(26):6379–6381. doi: 10.1182/blood-2012-03-418673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibb A, Jones C, Bloor A, et al. Brentuximab vedotin in refractory CD30+ lymphomas: a bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK center. Haematologica. 2013 Apr;98(4):611–614. doi: 10.3324/haematol.2012.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theurich S, Malcher J, Wennhold K, et al. Brentuximab vedotin combined with donor lymphocyte infusions for early relapse of Hodgkin lymphoma after allogeneic stem-cell transplantation induces tumor-specific immunity and sustained clinical remission. J Clin Oncol. 2013 Feb 10;31(5):e59–63. doi: 10.1200/JCO.2012.43.6832. [DOI] [PubMed] [Google Scholar]
- 44.Ansell SM, Connors JM, Park SI, O’Meara MM, Younes A. Frontline Therapy with Brentuximab Vedotin Combined with ABVD or AVD in Patients with Newly Diagnosed Advanced Stage Hodgkin Lymphoma. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):798. 2012. [Google Scholar]
- 45.Fleischer J, Soeth E, Reiling N, Grage-Griebenow E, Flad HD, Ernst M. Differential expression and function of CD80 (B7-1) and CD86 (B7-2) on human peripheral blood monocytes. Immunology. 1996 Dec;89(4):592–598. doi: 10.1046/j.1365-2567.1996.d01-785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SM, Schoder H, Johnson JL, Jung SH, Bartlett NL, Cheson BD. The anti-CD80 primatized monoclonal antibody, galiximab, is well-tolerated but has limited activity in relapsed Hodgkin lymphoma: CALGB 50602 (Alliance) Leukemia & lymphoma. 2012 Nov 29; doi: 10.3109/10428194.2012.744453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nozawa Y, Wakasa H, Abe M. Costimulatory molecules (CD80 and CD86) on Reed-Sternberg cells are associated with the proliferation of background T cells in Hodgkin’s disease. Pathol Int. 1998 Jan;48(1):10–14. doi: 10.1111/j.1440-1827.1998.tb03821.x. [DOI] [PubMed] [Google Scholar]
- 48.Aldinucci D, Gloghini A, Pinto A, Colombatti A, Carbone A. The role of CD40/CD40L and interferon regulatory factor 4 in Hodgkin lymphoma microenvironment. Leuk Lymphoma. 2012 Feb;53(2):195–201. doi: 10.3109/10428194.2011.605190. [DOI] [PubMed] [Google Scholar]
- 49.Freedman AS, Kuruvilla J, Assouline SE, et al. Clinical Activity of Lucatumumab (HCD122) In Patients (pts) with Relapsed/Refractory Hodgkin or Non-Hodgkin Lymphoma Treated In a Phase Ia/II Clinical Trial ( NCT00670592) ASH Annual Meeting Abstracts. 2010 Nov 19;116(21):284. 2010. [Google Scholar]
- 50.Engert A, Diehl V, Schnell R, et al. A phase-I study of an anti-CD25 ricin A-chain immunotoxin (RFT5-SMPT-dgA) in patients with refractory Hodgkin’s lymphoma. Blood. 1997 Jan 15;89(2):403–410. [PubMed] [Google Scholar]
- 51.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012 Mar 15;18(6):1611–1618. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steidl C, Diepstra A, Lee T, et al. Gene expression profiling of microdissected Hodgkin Reed-Sternberg cells correlates with treatment outcome in classical Hodgkin lymphomaBloo. Blood. 2012 Oct 25;120(17):3530–3540. doi: 10.1182/blood-2012-06-439570. [DOI] [PubMed] [Google Scholar]
- 53.Zhu D, Corral LG, Fleming YW, Stein B. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer immunology, immunotherapy : CII. 2008 Dec;57(12):1849–1859. doi: 10.1007/s00262-008-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fehniger TA, Larson S, Trinkaus K, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood. 2011 Nov 10;118(19):5119–5125. doi: 10.1182/blood-2011-07-362475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuruvilla J, Taylor D, Wang L, Blattler C, Keating A, Crump M. Phase II Trial of Lenalidomide in Patients with Relapsed or Refractory Hodgkin Lymphoma. ASH Annual Meeting Abstracts. 2008 Nov 16;112(11):3052. 2008. [Google Scholar]
- 56.Rothe A, Younes A, Reiners KS, et al. A Phase I Study with the Bispecific Anti-CD30 x Anti-CD16A Antibody Construct AFM13 in Patients with Relapsed or Refractory Hodgkin Lymphoma. ASH Annual Meeting Abstracts. 2011 Nov 18;118(21):3709. 2011. [Google Scholar]
- 57.Jones RJ, Gocke CD, Kasamon YL, et al. Circulating clonotypic B cells in classic Hodgkin lymphoma. Blood. 2009 Jun 4;113(23):5920–5926. doi: 10.1182/blood-2008-11-189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banerjee D. Recent Advances in the Pathobiology of Hodgkin’s Lymphoma: Potential Impact on Diagnostic, Predictive, and Therapeutic Strategies. Advances in hematology. 2011:439456. doi: 10.1155/2011/439456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chetaille B, Bertucci F, Finetti P, et al. Molecular profiling of classical Hodgkin lymphoma tissues uncovers variations in the tumor microenvironment and correlations with EBV infection and outcome. Blood. 2009 Mar 19;113(12):2765–3775. doi: 10.1182/blood-2008-07-168096. [DOI] [PubMed] [Google Scholar]
- 60.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. The New England journal of medicine. 2010 Mar 11;362(10):875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Younes A, Romaguera J, Hagemeister F, et al. A pilot study of rituximab in patients with recurrent, classic Hodgkin disease. Cancer. 2003 Jul 15;98(2):310–314. doi: 10.1002/cncr.11511. [DOI] [PubMed] [Google Scholar]
- 62.Oki Y, Pro B, Fayad LE, et al. Phase 2 study of gemcitabine in combination with rituximab in patients with recurrent or refractory Hodgkin lymphoma. Cancer. 2008 Feb 15;112(4):831–836. doi: 10.1002/cncr.23237. [DOI] [PubMed] [Google Scholar]
- 63.Younes A, Oki Y, McLaughlin P, et al. Phase 2 study of rituximab plus ABVD in patients with newly diagnosed classical Hodgkin lymphoma. Blood. 2012 May 3;119(18):4123–4128. doi: 10.1182/blood-2012-01-405456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasamon YL, Jacene HA, Gocke CD, et al. Phase 2 study of rituximab-ABVD in classical Hodgkin lymphoma. Blood. 2012 May 3;119(18):4129–4132. doi: 10.1182/blood-2012-01-402792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Golay J, Manganini M, Rambaldi A, Introna M. Effect of alemtuzumab on neoplastic B cells. Haematologica. 2004 Dec;89(12):1476–1483. [PubMed] [Google Scholar]
- 66.Moskowitz CH, Younes A, de Vos S, et al. CSF1R Inhibition by PLX3397 in Patients with Relapsed or Refractory Hodgkin Lymphoma: Results From a Phase 2 Single Agent Clinical Trial. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):1638. 2012. [Google Scholar]
- 67.Bollard CM, Gottschalk S, Leen AM, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007 Oct 15;110(8):2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bollard CM, Aguilar L, Straathof KC, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin’s disease. The Journal of experimental medicine. 2004 Dec 20;200(12):1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cruz CR, Gerdemann U, Leen AM, et al. Improving T-cell therapy for relapsed EBV-negative Hodgkin lymphoma by targeting upregulated MAGE-A4. Clin Cancer Res. 2011 Nov 15;17(22):7058–7066. doi: 10.1158/1078-0432.CCR-11-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di Stasi A, De Angelis B, Rooney CM, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009 Jun 18;113(25):6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ushmorov A, Leithauser F, Sakk O, et al. Epigenetic processes play a major role in B-cell-specific gene silencing in classical Hodgkin lymphoma. Blood. 2006 Mar 15;107(6):2493–2500. doi: 10.1182/blood-2005-09-3765. [DOI] [PubMed] [Google Scholar]
- 72.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007 Aug 13;26(37):5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 73.Adams H, Fritzsche FR, Dirnhofer S, Kristiansen G, Tzankov A. Class I histone deacetylases 1, 2 and 3 are highly expressed in classical Hodgkin’s lymphoma. Expert opinion on therapeutic targets. 2010 Jun;14(6):577–584. doi: 10.1517/14728221003796609. [DOI] [PubMed] [Google Scholar]
- 74.Piekarz RL, Bates SE. Epigenetic modifiers: basic understanding and clinical development. Clin Cancer Res. 2009 Jun 15;15(12):3918–3926. doi: 10.1158/1078-0432.CCR-08-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sambucetti LC, Fischer DD, Zabludoff S, et al. Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J Biol Chem. 1999 Dec 3;274(49):34940–34947. doi: 10.1074/jbc.274.49.34940. [DOI] [PubMed] [Google Scholar]
- 76.Buglio D, Georgakis GV, Hanabuchi S, et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008 Aug 15;112(4):1424–1433. doi: 10.1182/blood-2008-01-133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirschbaum MH, Goldman BH, Zain JM, et al. A phase 2 study of vorinostat for treatment of relapsed or refractory Hodgkin lymphoma: Southwest Oncology Group Study S0517. Leukemia & lymphoma. 2012 Feb;53(2):259–262. doi: 10.3109/10428194.2011.608448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Younes A, Sureda A, Ben-Yehuda D, et al. Panobinostat in patients with relapsed/refractory Hodgkin’s lymphoma after autologous stem-cell transplantation: results of a phase II study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Jun 20;30(18):2197–2203. doi: 10.1200/JCO.2011.38.1350. [DOI] [PubMed] [Google Scholar]
- 79.Harrison SJ, Hsu AK, Neeson P, et al. Early thymus and activation-regulated chemokine (TARC) reduction and response following panobinostat treatment in patients with relapsed/refractory Hodgkin lymphoma following autologous stem cell transplant. Leuk Lymphoma. 2013 Aug 5; doi: 10.3109/10428194.2013.820287. [DOI] [PubMed] [Google Scholar]
- 80.Younes A, Oki Y, Bociek RG, et al. Mocetinostat for relapsed classical Hodgkin’s lymphoma: an open-label, single-arm, phase 2 trial. The lancet oncology. 2011 Dec;12(13):1222–1228. doi: 10.1016/S1470-2045(11)70265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kashkar H, Deggerich A, Seeger JM, et al. NF-kappaB-independent down-regulation of XIAP by bortezomib sensitizes HL B cells against cytotoxic drugs. Blood. 2007 May 1;109(9):3982–3988. doi: 10.1182/blood-2006-10-053959. [DOI] [PubMed] [Google Scholar]
- 82.Blum KA, Johnson JL, Niedzwiecki D, Canellos GP, Cheson BD, Bartlett NL. Single agent bortezomib in the treatment of relapsed and refractory Hodgkin lymphoma: cancer and leukemia Group B protocol 50206. Leukemia & lymphoma. 2007 Jul;48(7):1313–1319. doi: 10.1080/10428190701411458. [DOI] [PubMed] [Google Scholar]
- 83.Fanale M, Fayad L, Pro B, et al. Phase I study of bortezomib plus ICE (BICE) for the treatment of relapsed/refractory Hodgkin lymphoma. Br J Haematol. 2011 Jul;154(2):284–286. doi: 10.1111/j.1365-2141.2011.08618.x. [DOI] [PubMed] [Google Scholar]
- 84.Derenzini E, Lemoine M, Buglio D, et al. The JAK inhibitor AZD1480 regulates proliferation and immunity in Hodgkin lymphoma. Blood Cancer J. 2011 Dec;1(12):e46. doi: 10.1038/bcj.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Younes A, Romaguera J, Fanale M, et al. Phase I study of a novel oral Janus kinase 2 inhibitor, SB1518, in patients with relapsed lymphoma: evidence of clinical and biologic activity in multiple lymphoma subtypes. J Clin Oncol. 2012 Nov 20;30(33):4161–4167. doi: 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Witzig TE, Kaufmann SH. Inhibition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway in hematologic malignancies. Current treatment options in oncology. 2006 Jul;7(4):285–294. doi: 10.1007/s11864-006-0038-1. [DOI] [PubMed] [Google Scholar]
- 87.Rosich L, Colomer D, Roue G. Autophagy controls everolimus (RAD001) activity in mantle cell lymphoma. Autophagy. 2013 Jan;9(1):115–117. doi: 10.4161/auto.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnston PB, Inwards DJ, Colgan JP, et al. A Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010 May;85(5):320–324. doi: 10.1002/ajh.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Younes A, Copeland A, Fanale MA, et al. Safety and Efficacy of the Novel Combination of Panobinostat (LBH589) and Everolimus (RAD001) in Relapsed/Refractory Hodgkin and Non-Hodgkin Lymphoma. ASH Annual Meeting Abstracts. 2011 Nov 18;118(21):3718. [Google Scholar]