Abstract
Purpose
Although breast cancer is a growing health problem in sub-Saharan Africa, reasons for its increased occurrence remain unclear.
Methods
We reviewed published literature to determine the magnitude of the increase in breast cancer, associated risk factors (including for breast cancer subtypes), and ways to reduce incidence and mortality.
Results
Some of the increased breast cancer occurrence likely reflects that women are living longer and adopting lifestyles that favor higher incidence rates. However, a greater proportion of breast cancers occur among premenopausal women as compared to elsewhere, which may reflect unique risk factors. Breast cancers diagnosed among African women reportedly include a disproportionate number of poor prognosis tumors, including hormone receptor negative, triple negative and core basal phenotype tumors. However, it is unclear how lack of standardized methods for tissue collection, fixation and classification contribute to these rates. Given appropriate classifications, it will be of interest to compare rates with other populations and to identify risk factors that relate to specific tumor subtypes. This includes not only risk factors that have been recognized in other populations but some that may play unique roles among African women, such as genetic factors, microbiomata, xenoestrogens, hair relaxers and skin lighteners.
Conclusions
With limited opportunities for effective treatment, a focus is needed on identifying etiologic factors that may be amenable to intervention. It will also be essential to understand reasons why women delay seeking care after the onset of symptoms and for there to be educational campaigns about the importance of early detection.
Breast cancer is a growing health problem in sub-Saharan Africa [1, 2], with it now having surpassed cervical cancer as the leading cause of death in many countries [3]. Some of the increased occurrence likely reflects that women are living longer and adopting lifestyles that favor higher incidence rates (e.g., delays in childbearing, decreased total fertility, more obesity) [4]. However, a greater proportion of breast cancers occur among premenopausal women as compared to Westernized countries, possibly reflecting unique risk factors. The problem of breast cancer in Africa is compounded by the lack standardized diagnostic and treatment programs and that many women delay seeking treatment for symptoms, with a large proportion of the diagnosed cancer being ones that are not amenable to treatment. This includes a disproportionate number of poor prognosis tumors, including hormone receptor negative tumors. Coupling this with the paucity of treatment facilities and resources, it is apparent that a greater focus is needed on primary and secondary means of prevention, particularly since the early ages at onset of many breast cancers result in high associated disability and years of life lost [5].
Although breast cancer is becoming more common and many of the diagnosed cancers among African women have a poor prognosis, little is known about associated risk factors, especially ones that may be modifiable. Further, it has not been clarified why so many women present with poor prognosis and/or advanced diseases and what interventions could lead to earlier access to care and improved survival.
Demographic trends
In sub-Saharan Africa, breast cancer is responsible for one in four diagnosed cancers and one in five cancer deaths in women [6]. Despite its emerging public health importance, incidence rates are still generally low in Africa, presumably below 35 per 100,000 women in most countries (as compared to over 90–120 per 100,000 women in most European or North American countries). Precise incidence figures in Africa, however, are lacking given the absence of cancer registration in most countries. Recent GLOBOCAN data [7] estimate that in 2012 94,000 women developed breast cancer and 48,000 died from it in sub-Saharan Africa.
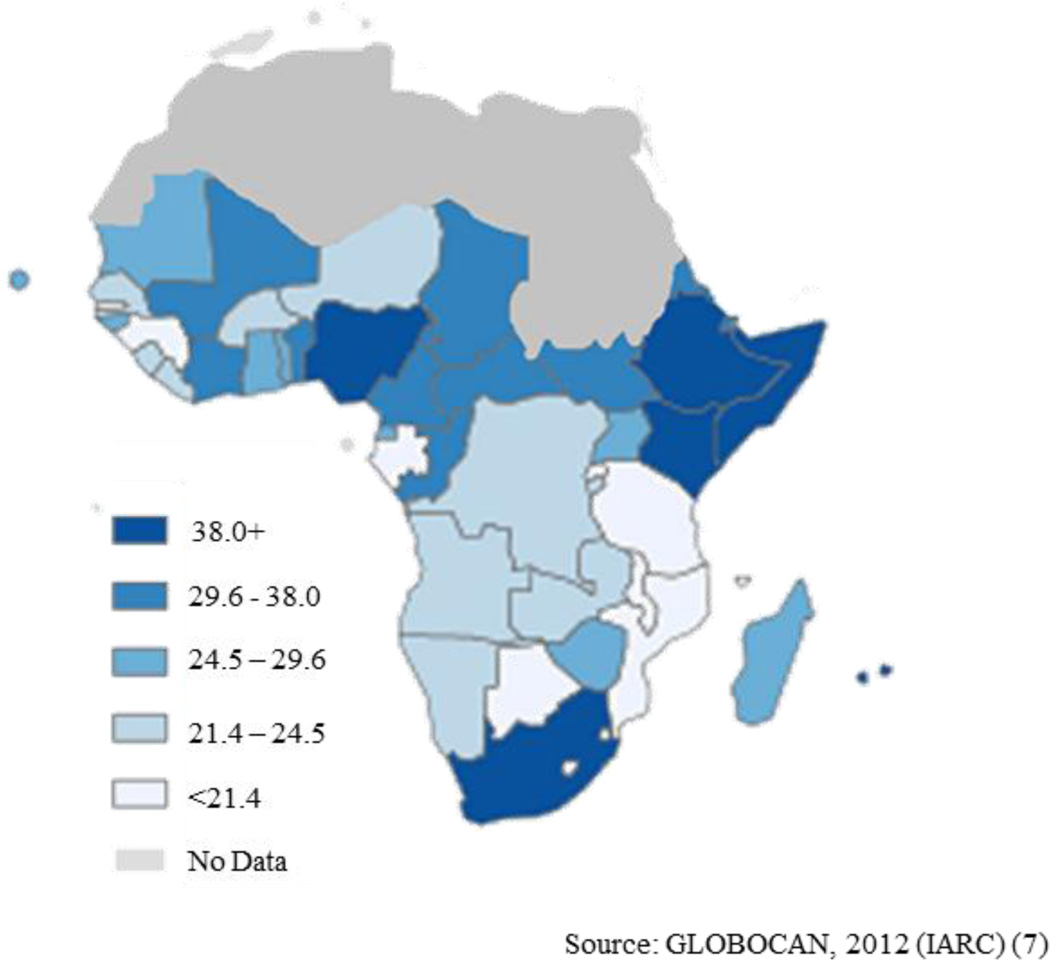
There is substantial variation in estimated breast cancer incidence across different African regions (Figure 1). Estimates of age-standardized incidence rates (per 100,000 women) are 30.4 in eastern Africa, 26.8 in middle Africa, 38.6 in western Africa, and 38.9 in southern Africa [7]. The rates in southern Africa may reflect better reporting, as well as the predominance of women of Anglo-European background and/or higher standards of living than women in other parts of Africa. Studies have documented that breast cancer is more common in urban than rural settings [8]; however, this may reflect some misreporting of primary places of residence since women with urban addresses often can gain better health care access.
Figure 1.
Estimated age-standardized breast cancer incidence rate per 100,000
There have been many reports of growing incidence, although largely based on imprecise incidence rates. Across sub-Saharan Africa, there are only four population-based registries (Kampala, Uganda; Harare, Zimbabwe; Blantyre, Malawi, and PROMEC, South Africa) cited in volume X of the World Health Organization’s (WHO) Cancer in Five Continents [9]. In 2012, the African Cancer Registry Network (AFCRN) was inaugurated and currently encompasses registries from twenty-five countries that are assessed as being at least 75% complete. Estimated country-specific incidence rates by registry data quality are shown in Table 1.
Table 1.
Incidence of breast cancer in sub-Saharan Africa by data quality
| Population | Numbers | Crude Rate | ASR | Cumulative Risk |
|---|---|---|---|---|
| High Quality Regional (coverage lower than 10%) * | ||||
| Zimbabwe | 1136 | 17.2 | 28.5 | 3.20 |
| Uganda | 2420 | 13.6 | 27.5 | 2.91 |
| Malawi | 762 | 9.6 | 16.8 | 1.75 |
| National Data (rates) † | ||||
| Mauritius | 533 | 80.1 | 64.2 | 6.98 |
| South Africa | 9815 | 38.4 | 41.5 | 4.33 |
| Namibia | 213 | 17.9 | 24.4 | 2.67 |
| Botswana | 155 | 15.3 | 19.9 | 2.10 |
| Swaziland | 44 | 7.1 | 10.5 | 1.02 |
| The Gambia | 40 | 4.3 | 9.8 | 0.53 |
| Regional Data (rates) * | ||||
| Nigeria | 27304 | 33.2 | 50.4 | 5.02 |
| Ethiopia | 12956 | 29.8 | 41.8 | 4.06 |
| Kenya | 4465 | 20.9 | 38.3 | 4.32 |
| Cameroon | 2625 | 25.6 | 35.2 | 3.30 |
| Congo | 424 | 20.1 | 31.7 | 3.58 |
| Mali | 1349 | 16.5 | 29.8 | 3.12 |
| Niger | 1171 | 14.2 | 23.8 | 2.39 |
| Zambia | 824 | 11.9 | 22.4 | 2.41 |
| Tanzania | 2732 | 11.5 | 19.4 | 2.12 |
| Guinea | 471 | 9.1 | 14.5 | 1.60 |
| Mozambique | 1095 | 8.7 | 14.5 | 1.78 |
| Frequency Data § | ||||
| Cote d Ivoire | 2248 | 22.2 | 33.7 | 3.43 |
| Benin | 910 | 19.2 | 30.2 | 3.10 |
| Togo | 603 | 19.0 | 27.2 | 2.74 |
| Ghana | 2260 | 18.0 | 25.6 | 2.72 |
| Burkina Faso | 1144 | 13.0 | 22.7 | 2.28 |
| Gabon | 98 | 12.6 | 16.1 | 1.64 |
| Rwanda | 576 | 10.0 | 15.9 | 1.64 |
| No Data‖ | ||||
| Somalia | 1260 | 25.5 | 40.6 | 4.23 |
| Eritrea | 660 | 23.3 | 35.9 | 3.52 |
| Chad | 1274 | 21.4 | 34.1 | 3.37 |
| South Sudan | 1114 | 20.6 | 31.8 | 3.34 |
| Central African Republic | 504 | 21.7 | 31.4 | 3.22 |
| Madagascar | 1799 | 16.4 | 26.6 | 2.98 |
| Guinea-Bissau | 140 | 17.6 | 26.0 | 2.55 |
| Mauritania | 323 | 17.9 | 25.8 | 2.56 |
| Equatorial Guinea | 74 | 20.5 | 25.2 | 2.45 |
| Cape Verde | 59 | 23.2 | 25.1 | 2.45 |
| Sierra Leone | 470 | 15.0 | 24.3 | 2.53 |
| Liberia | 320 | 15.2 | 24.1 | 2.52 |
| Angola | 1328 | 13.1 | 23.5 | 2.55 |
| Burundi | 696 | 15.6 | 23.5 | 2.52 |
| DRC | 4578 | 13.1 | 23.5 | 2.58 |
| Senegal | 869 | 13.2 | 22.4 | 2.38 |
| Comoros | 40 | 10.4 | 17.4 | 1.95 |
| Lesotho | 76 | 6.8 | 9.0 | 0.90 |
one cancer registry covering part of a country is used as representative of the country profile or estimated as the weighted average of the local rates
most recent rates applied to 2012 population
age/sex specific rates for "all cancers" were partitioned using data on relative frequency of different cancers (by age and sex).
the rates are those of neighbouring countries or registries in the same area
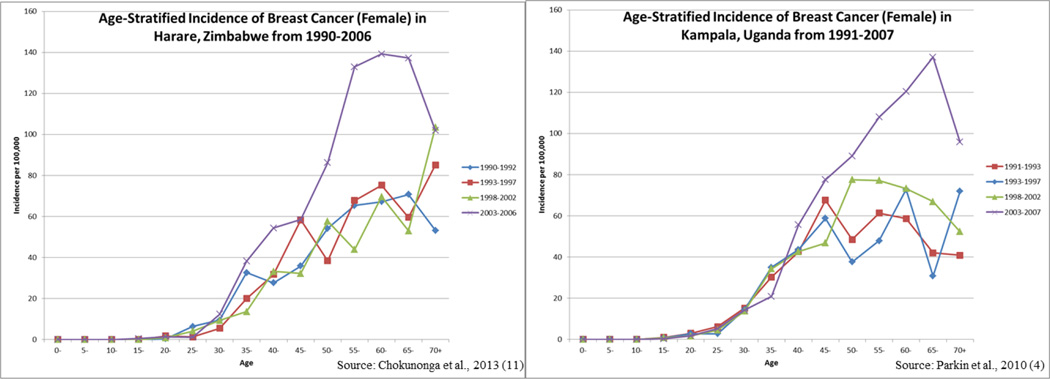
Recent incidence data from registries in Kampala, Uganda [4], Harare, Zimbabwe [10], the Gambia [11] and Mali-Bamako [11] provide substantial support for the notion of an increasing breast cancer incidence in sub-Saharan Africa. In Zimbabwe, a 4.5% annual increase in breast cancer incidence over the period 1991–2010 has been noted [10]. Two of the registries (the Gambia and Mali) reported the greatest rate of increase for women under age 55 years [11], but time trends may have been influenced by reporting problems. In the two registries that have contributed to Cancer in Five Continents over an extended period of time (Uganda and Zimbabwe), the largest increases in incidence over time were seen among the post-menopausal age groups [4, 10] (Figure 2), suggesting that trends may have been driven by changes in traditional risk factors, including declines in fertility and increasing rates of obesity.
Figure 2.
Comparison of breast cancer incidence in Zimbabwe and Uganda from 1990–2007.
Despite incomplete cancer registration in Africa, it has been estimated by GLOBOCAN that by the year 2050 that there may be a doubling in the number of incident cases from present estimates [7]. The majority of these new cases are predicted to occur among women under 65 years. The average age of diagnosis of breast cancers among African women tends to be young, with estimates that a majority of cancers develop among women 50 years or younger [11]—a considerably younger age than seen in Caucasian populations. Although this is likely due in part to the fact that fewer African women live past 65 years of age compared to women in developed countries [1], this may not entirely explain the younger breast cancer ages. The fact that African-American women also tend to develop breast cancers at younger ages than Caucasian women in the U.S. [12] suggests that there may be additional factors involved, including either genetic or environmental factors or an interplay of the two. That African women may develop unique breast tumor subtypes could also be an important contributory factor to the unusual age distribution noted in Africa.
Role of previously identified risk factors
Only a few well-designed epidemiologic studies of breast cancer have been conducted in African countries, and most investigations have had limited sample sizes and restricted power to detect associations. These have primarily been hospital-based case-control studies, raising concerns regarding the validity of the results given uncertain hospital referral patterns and the fact that some women with breast cancer never make it to hospital. Further, the risk factors that have been examined have primarily been limited to a few reproductive and anthropometric variables.
Reproductive factors have been the risk factor most commonly evaluated, with discrepant results across studies, possibly owing to small sample sizes and/or to differences in disease subtypes studied. An early study by Parkin [13] suggested that multiparity was a risk factor for breast cancers diagnosed prior to 45 years of age, but an apparent protective factor for later onset cancers. This corresponds with more recent findings from another African study [14] as well as findings from a study of African-American women [15]. Given that African as well as African-American women tend to begin childbearing at young ages, it has been hypothesized that findings could reflect the observed short-term transient increase of pregnancy on breast cancer risk that translates into reduced risks as women age [16]. Later ages at first birth might be expected to be associated with reduced risks for premenopausal onset cancers, as noted in one investigation [11]. In other studies, late age at first birth was noted to be a risk factor [17] or unrelated to risk [14, 18].
The well accepted inverse relationship of age at menarche to breast cancer risk has been observed in one investigation [14], but another study showed late menarche to be a risk factor in postmenopausal women [11]. At least one study showed significant risk elevations associated with irregular menstrual periods [19]. A strong inverse relation of breastfeeding to breast cancer risk has been noted, with risk decreasing 7% for every 12 months of breastfeeding [14]. However, other studies have shown only weak [18] or non-substantial [20] risk relationships.
In several studies, higher socioeconomic status (as measured by such factors as education, income and housing) has been linked with higher breast cancer risks [17, 21]. Adult literacy rates, as reported by the Word Bank [22], show some correlation to breast cancer incidence (using data from GLOBOCAN, restricted to countries with adequate reporting [7]) but there are outliers, possibly reflecting imprecise measures (Figure 3). Women in higher socioeconomic classes may have a high prevalence of breast cancer risk factors (e.g., late age at first birth, early menarche, late menopause, obesity and heavy alcohol consumption) [23], although studies have not specifically evaluated this. It is also possible that disease ascertainment is more accurate among women of higher socioeconomic status.
Figure 3.
Age-standardized breast cancer incidence by female adult literacy rate for sub-Saharan African countries
The effects on breast cancer risk of certain contraceptives has been of interest, especially given injectable progestogen-only contraceptives, which are widely used in certain countries, including South Africa [24]. In a large hospital-based case-control study, significant increases in breast cancer risk were found among women who had used either oral or injectable contraceptives within the previous 10 years [24]. These risks declined with increasing time since last use and were unrelated to duration of use.
Several studies have assessed relations with anthropometric factors. In the largest study, involving 1,233 Nigerian breast cancer cases [25], body mass index (BMI) was inversely related to risk. However, given the typical late presentation of disease among African women, such a finding must be cautiously interpreted and may reflect weight loss due to illness. In the African setting, it may be more appropriate to focus on other anthropometric measures that are less prone to cachexia. Several studies have reported positive relations of either waist circumference [26] or waist-to-hip ratios (WHR) [26, 27] for both premenopausal and postmenopausal breast cancers. Hip circumference has been noted to be inversely related to risk [26]. In one of these studies, the relation with WHR persisted after adjustment for obesity [25]. Tallness has also been found to be a significant risk factor for breast cancer in two Nigerian studies [17, 25]. Although height has also been related to breast cancer risk in non-African populations, the relation in Africa appears somewhat stronger, prompting the suggestion that energy intake during childhood may play a distinctive etiologic role in Africans [25].
Findings regarding the potential role on breast cancer risk of various anthropometric parameters have led to an interest in the role of nutritional factors. Although case-control studies are not considered optimal for assessing dietary factors, one study suggested that a low ratio of polyunsaturated/saturated fatty acid intake might be a protective factors for the development of breast cancers in Tanzania; this factor appeared more important than total fat intake [28].
In the limited number of epidemiologic studies conducted in Africa, only a few of the environmental risk factors that might underlie incidence patterns have been addressed. Data are largely lacking regarding relationships with developmental history, physical activity, medical history, drug exposures, environmental contaminants, and most dietary factors, including alcohol consumption—all factors that have been shown to impact breast cancer risk in non-African populations. Further, although a few studies have examined biologic factors that might underlie some of these risk factors [17, 29, 30], there are many more biomarkers worthy of consideration, including a wide variety of genetic, hormonal and immunologic markers.
Role of potential novel factors
Studies of breast cancer in Africa present unique opportunities for evaluating novel risk factors that may play important roles in this part of the world. This includes the role of infectious agents, environmental and occupational factors, and certain cosmetic exposures.
In terms of infectious agents, the great diversity of microbiomes in Africa may allow for unique insights into an exposure that is receiving increasing attention as a risk factor for many diseases [31], including hormonally-related ones. Microbiomes are of interest for breast cancer given that estrogens can be deconjugated by bacterial β-glucuronidases in the distal gut, allowing reabsorption of liberated estrogens that have been excreted by the liver as bile salts.
African studies may also allow further assessment of the role of immune-compromised states, of interest given increasing recognition for a role of inflammatory markers in the etiology of breast cancer. For some of these diseases, there may be concomitant exposures that require consideration. Malaria is a common infectious disease in many sub-Saharan African countries, and there have been long-standing programs aimed at its eradication through wide-spread use of insecticides, including various xenoestrogens, such as dichlorodiphenyltrichloroethane (DDT). Although the relationship of environmental estrogens to breast cancer risk has been controversial [32], the exposures involved with most studies in non-African countries have tended to be at much lower levels than in Africa. Given the long-standing use of insecticides in Africa and the fact that DDT and other insecticides can alter hormone levels, it has been proposed that this exposure might be the cause of increasing rates of hormone receptor positive breast cancers in Africa [33].
Several lifestyle factors that are unique among African women are also worthy of pursuit in relation to breast cancer risk. This includes hair relaxers, which have widespread usage among African women [34]. Hair relaxers can cause burns and lesions in the scalp, facilitating entry of relaxer constituents into the body, including hormonally active compounds, such as phthalates, which may be listed under such terms as fragrances or perfumes. One study, which evaluated the effects of hair straighteners among African-Americans, found no appreciable associations with either frequency of use, duration of use or number of burns [35]. However, a recent evaluation in this same cohort found striking relationships of all of these exposures with uterine leiomyomata, another hormonally-responsive disease [36].
Skin lighteners are commonly used by African women [37, 38]. The most widely used creams consist of hydroquinones, but additionally steroids and a mixture of steroids and hydroquinones are used [37]. Particular attention is warranted regarding the age at initiation and frequency of use of skin lighteners, as well as where on the body specific agents are applied.
Family history of breast cancer has not been well investigated in African studies, although several studies have shown it to be an important etiologic factor [17, 39]. The proportion of women reporting a family history was, however, quite low, most likely reflecting that diagnostic information is often not widely discussed with patients or their families. Thus, data with regards to family history are likely underestimates of the true prevalence.
Recent genome-wide association studies (GWAS), primarily in populations of European descent, have identified more than 70 regions of the genome associated with breast cancer risk [40, 41]. Genetic studies in African and other ancestral populations have the potential to identify causal variants in fine-mapping studies as well as variants that are more common and/or specific to risk in African women. There has been one GWAS in women of African ancestry that suggests that common variants associated with breast cancer are distinct from those identified in European populations [42].
Tumor characteristics and etiologic heterogeneity
The few studies that have addressed breast cancer risk factors among African women have mainly focused on breast cancer as a single disease entity. However, it is well recognized that breast cancer is a heterogeneous disease, with some clinical characteristics being distinctively correlated with certain risk predictors [43, 44]. Further investigations are needed to assess risk factors for specific breast cancer subtypes, including hormone receptor negative, triple negative [absence of markers for estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth receptor 2 (HER2)], and core basal phenotype tumors.
A number of studies have assessed the clinical characteristics of breast cancers diagnosed in Africa, with the larger investigations summarized in Table 2 [45–58]. The average age at diagnosis in most studies was late 40’s, about a decade younger than in the US or other Western populations (http://seer.cancer.gov/statfacts/html/breast.html). The majority of studies report a high frequency of poorly differentiated tumors, although there has been considerable variation across studies, with rates ranging from 16–83%. Tumors tend to be large, with the vast majority being >2 cm. In addition, the majority of studies showed greater than 70% of patients had node positive or Stage III tumors, likely reflecting a combination of a lack of organized screening/detection programs and potentially more aggressive tumor presentation. Consistent with the reported high prevalence of poorly differentiated and early-onset tumors, many of the tumors have been reported as hormone receptor negative. However, reported rates of both ER and PR negativity have varied substantially across studies, with the respective rates ranging from 36–79% and 30–87%. Fewer studies have reported on HER2 status, but tumors have largely been classified as not expressing this marker. As a result, the rates of triple negative cancers have been high, with a number of studies showing that the majority of African women are diagnosed with such tumors.
Table 2.
Clinical Characteristics of Breast Cancers Diagnosed at Selected African Hospitals
| Author, year | Location | Years of diagnosis |
Number of breast cancer patients |
Average age at diagnosis (yrs) |
Tissue Sample Collection Method |
% Grade Poorly Differentiated |
Mean Tumor Size (cm) |
% Nodal Status Positive |
% stage III+ |
% ER− | % PR− | % HER2− | % ER− /PR− /HER2− (triple negative) |
% basal- like |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nyagol, 2003 | Nairobi, Kenya | 2002–2004 | 158 | 47 | mastectomy | 66 | 4.5 | 79 | 69 | --- | --- | --- | 28 | --- |
| Ikpatt, 2003 | Calabar, Nigeria | 1995–2000 | 129 | 43 | surgery | 57 | 4.8 | 80 | 54 | 76 | 86 | --- | --- | --- |
| Gukas, 20051 | Jos, Nigeria | --- | 178 | 44 | ---- | 71 | --- | --- | 58 | 75 | 72 | --- | --- | --- |
| Awadelkarim, 20072 | Khartoum, Sudan | 2004–2005 | 114 | 52 | ---- | 68 | 4.8 | 90 | 38 | 36 | 33 | 83 | --- | 10 |
| Adebamowo, 2007 | Ibadan, Nigeria | 2004–2005 | 192 | ---- | core needle biopsy |
16 | --- | --- | 75 | 27 | 30 | 80 | ---- | 16 |
| Bird, 20083 | Kijabe, Kenya | 2001–2007 | 129 | 48 | core needle biopsy and surgery |
50 | 6.8 | 72 | 60 | 76 | --- | 74 | 44 | --- |
| Yarney, 20084 | Accra, Ghana | 2004–2007 | 74 | --- | surgery | --- | --- | --- | --- | 57 | 82 | 81 | --- | --- |
| Huo, 2009 | 6 sites in Nigeria and Senegal | 2007–2009 | 378 | 45 | TMA | 83 | 4.4 | 72 | --- | 76 | 80 | 83 | 55 | 27 |
| Stark, 2010 | Kumasi, Ghana | 2001–2007 | 75 | 48 | ---- | 76 | 3.2 | --- | 76 | 76 | 67 | 96 | 82 | --- |
| Burson, 20105 | Dar es Salaam, Tanzania | 2007–2009 | 488 | 49 | surgery | --- | 69.1%>5 cm | --- | 91 | 76 | 66 | --- | --- | --- |
| Ly, 2012 | Bamoko, Mali | 2008–2011 | 114 | 46 | core needle biopsy |
78 | 90%>5 cm | 89 | 78 | 61 | 72 | 82 | 46 | |
| Ohene-Yeboa, 20126 | Kumasi, Ghana | 2004–2009 | 330 | 49 | fine needle aspiration, core, excision, and incisional biopsies |
54 | --- | --- | 85 | 53 | 87 | 80 | 43 | --- |
| Agboola, 2012 | Lagos, Nigeria | 2002–2008 | 308 | ---- | TMA | --- | 91.2% > 2 cm | 92 | --- | 79 | 72 | 82 | 48 | 38 |
| McCormack, 20137 | Soweto, South Africa | 2006–2012 | 1218 | 55 | core needle biopsy |
42 | --- | --- | 54 | 35 | 47 | 74 | 20 | --- |
Tumor analysis only done on 36 patients
Included male breast cancers; node status for 25% cases only
Only 34 tested for Her2
Only 74/610 patients evaluated and only 53 with reports on Her2
Only collected recptor data on 57 patients but 49.1% ER−/PR−
Only 20% of specimens tested for hormone receptor status
90% of women were black; % ER- and PR- significantly higher in black than non-black patients
Although a number of African studies have indicated high rates of hormone receptor negativity, it is unclear to what extent the absence of markers reflects issues related to tissue collection and processing, leading to inaccurate immunohistochemistry (IHC) results [1]. Problems include poor quality specimens from large and necrotic tumors, prolonged delay before fixation, questionable quality of fixation materials, prolonged stay in fixative, poor laboratory techniques, and limited quality assurance/quality control practices. The rate of ER negativity was found to only be 27% in a Nigerian study that used core needle biopsies, which are less prone to delays in fixation that can influence IHC results [45]. In addition, two recent investigations (each involving over 1,200 cases) that have used standardized methods for collection, processing and classification of tumors have reported rates of triple negative cancers closer to 20% [51, 58].
Access to care
Many women delay seeking medical attention until their tumors are quite advanced. Reasons for this delay include a lack of knowledge surrounding cancer diagnosis and treatment, fear of surgery, non-acceptance of hospital treatment and/or preferences for alternative care, and challenges to receiving treatment [59–61]. It has also been well documented that patients often delay timely attention to symptoms. In Nigeria, there was a mean delay of 11.2 months between the onset of symptoms and presentation, and 39% presented with fungating tumors [62]. Delays in seeking treatment may reflect a sense of hopelessness and fatalism [63], particularly given that many women’s experience with breast cancer has involved a death by a close family member or friend. Fear of mastectomy remains a prominent barrier to timely treatment [60, 64], particularly given that husbands often leave their wives following such surgery [65]. These delays are unfortunate, as it has been estimated that earlier detection methods could increase survival rates in sub-Saharan Africa for one third of cancer patients [3].
Cancer awareness is low in most African countries [66]. A survey from the Union for International Cancer Control (UICC) showed that approximately 25% of Africans surveyed believed that cancer had no cure and only 36% believed cancer was a major health issue [67]. In a low-resource community in South Africa over 80% of the women were unaware of the warning signs of breast cancer [68]. In another South African study, over one third of women were unaware about tests for breast cancer, with lack of knowledge being more common among older and rural women [69]. In a Cameroonian study, 13% of women surveyed believed breast cancer was preventable by vaccination, but only 37% recognized the efficacy of breast examination [70]. Education is clearly an important component of breast cancer prevention; a Nigerian study found that participants with higher education were 3.6 times more likely to practice breast selfexamination (BSE) than those with lower education levels [66].
In many cases, effective treatment is hindered by women initially seeking care from traditional healers [59, 63, 71, 72]. Cancer is often viewed as a disease of the spirit, and women often only seek conventional care when traditional treatment has failed to result in the desired effects [73]. A study of breast cancer patients in Cameroon indicated that 55% went to traditional healers before presenting for medical consultation [74]. In Enugu, Nigera, 17.5% of patients first sought aid from traditional healers, and this was significantly associated with greater than three months delay to presentation at a modern health facility [71]. Studies have documented that use of traditional healers is linked with substantial delays in patients coming to hospital [71, 75], but there may also be other reasons underlying the delays. One study in Ghana concluded that dealing with the causes of delayed presentation appears more important than attempts to screen for breast cancer, since patients identified through community screening still present late [64].
There are also logistical issues affecting access to care, including transportation problems. One South African study showed that women living long distances from a medical center have a greater likelihood of being diagnosed with advanced tumors [76]. There are also economic issues to consider as many of the costs associated with cancer care (including those associated with biopsies, pathology and chemotherapy) are not covered by insurance. In a Nigerian study, the inability to afford the costs of treatment was found to be the single most important contributor to patients not completing treatment regimens [77].
In addition to problems with personal resources, there are limited facilities for detection and treatment of cancer in most African countries. Mammography facilities are sparse and this may not be the best approach for detection among young women who have dense breasts [78]. A cost-effectiveness study assessing breast cancer interventions in Ghana found that clinical breast examination in combination with treatment at all stages was more cost-effective than mammography screening for women aged 40–69 years [79]. While radiotherapy units have increased by almost a third in the previous decade, the supply only meets 18% of the demonstrated need [80]. As shown in Figure 4, there are only a limited number of radiotherapy centers across Africa, with some countries only having one machine and others having none [81]. Chemotherapeutic agents are also often not available [77] and, even when available, targeted use may be hindered by unreliable access to IHC agents and inaccurate classification of tumors [82].
Figure 4.
External beam radiotherapy machines in Africa 2010
Summary and Conclusions
Given the absence of cancer registration, it is difficult to quantitate the extent to which breast cancer incidence in Africa is increasing; however, the burden of disease is clearly increasing given enhanced longevity and trends towards more women having adopting lifestyle factors that favor higher disease rates. As in developed countries, major contributors to disease occurrence undoubtedly include delays in childbearing, reduced fertility and increases in obesity. The role of other risk factors, including some more novel ones, remains less clear and additional epidemiologic investigations are needed. It will be important for these investigations to identify factors that are associated with poor prognosis breast cancer subtypes--including hormone receptor negative and core basal phenotype tumors that reportedly predominate among African women. Further studies, however, are needed to define the true prevalence of these tumors since many previous studies may have been plagued by pathologic classification problems.
Given the substantial delays in many women seeking care, it will also be important for there to be effective campaigns to better educate women about the importance of early detection and access to care [79]. There have been a number of recent campaigns to provide information about the early signs of breast cancer and to encourage women to participate in BSE, but these have often been ad hoc and not part of a systematic national control strategy, leading to limited coverage. In addition to more systematic efforts, assessments are needed to determine the effectiveness of campaign programs in terms of women actually practicing BSE [66, 83] and seeking prompt medical attention when problems are detected. Since many women initially seek treatment from traditional healers, it will be important to integrate these healers into overall health care systems [72].
Although the number of treatment facilities must increase to counter the rising breast cancer incidence in sub-Saharan African, this undoubtedly will be a long process [84]. Therefore, more attention is needed on primary and secondary preventive efforts. Several epidemiologic studies are ongoing, which hopefully will identify risk factors that may be amenable to intervention. It will also be important for these studies to understand the barriers to women seeking medical care early enough for treatment to be effective.
Acknowledgments
This research was supported in part by funds from the intramural research program of the National Cancer Institute, National Institutes of Health.
None of the authors has a financial relationship with the organization that sponsored the research.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Reference List
- 1.Akarolo-Anthony SN, Ogundiran TO, Adebamowo CA. Emerging breast cancer epidemic: evidence from Africa. Breast Cancer Res. 2010;12(Suppl 4):S8. doi: 10.1186/bcr2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sylla BS, Wild CP. A million africans a year dying from cancer by 2030: what can cancer research and control offer to the continent? Int J Cancer. 2012;130:245–250. doi: 10.1002/ijc.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Forman D, et al. Cancer burden in Africa and opportunities for prevention. Cancer. 2012;118:4372–4384. doi: 10.1002/cncr.27410. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Nambooze S, Wabwire-Mangen F, Wabinga HR. Changing cancer incidence in Kampala, Uganda, 1991–2006. Int J Cancer. 2010;126:1187–1195. doi: 10.1002/ijc.24838. [DOI] [PubMed] [Google Scholar]
- 5.Soerjomataram I, Lortet-Tieulent J, Parkin DM, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 7.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet} Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 8.Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H. Part I: Cancer in Indigenous Africans--burden, distribution, and trends. Lancet Oncol. 2008;9:683–692. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- 9.Forman D, Bray F, Brewster DH, et al. Cancer Incidence in Five Continents, Vol. X (electronic version) Lyon, IARC: 2013. [Google Scholar]
- 10.Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM, Parkin DM. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. Int. J Cancer. 2013;133:721–729. doi: 10.1002/ijc.28063. [DOI] [PubMed] [Google Scholar]
- 11.Sighoko D, Kamate B, Traore C, et al. Breast cancer in pre-menopausal women in West Africa: Analysis of temporal trends and evaluation of risk factors associated with reproductive life. Breast. 2013;22:828–835. doi: 10.1016/j.breast.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Brinton LA, Sherman ME, Carreon JD, Anderson WF. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst. 2008;100:1643–1648. doi: 10.1093/jnci/djn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkin DM, Vizcaino AP, Skinner ME, Ndhlovu A. Cancer patterns and risk factors in the African population of southwestern Zimbabwe, 1963–1977. Cancer Epidemiol. Biomarkers Prev. 1994;3:537–547. [PubMed] [Google Scholar]
- 14.Huo D, Adebamowo CA, Ogundiran TO, et al. Parity and breastfeeding are protective against breast cancer in Nigerian women. Br J Cancer. 2008;98:992–996. doi: 10.1038/sj.bjc.6604275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer JR, Wise LA, Horton NJ, Adams-Campbell LL, Rosenberg L. Dual effect of parity on breast cancer risk in African-American women. J Natl Cancer Inst. 2003;95:478–483. doi: 10.1093/jnci/95.6.478. [DOI] [PubMed] [Google Scholar]
- 16.Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331:5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- 17.Okobia M, Bunker C, Zmuda J, et al. Case-control study of risk factors for breast cancer in Nigerian women. Int J Cancer. 2006;119:2179–2185. doi: 10.1002/ijc.22102. [DOI] [PubMed] [Google Scholar]
- 18.Jordan I, Hebestreit A, Swai B, Krawinkel MB. Breast cancer risk among women with long-standing lactation and reproductive parameters at low risk level: a case-control study in Northern Tanzania. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1255-7. [DOI] [PubMed] [Google Scholar]
- 19.Adebamowo CA, Ogundiran TO, Adenipekun AA, et al. Obesity and height in urban Nigerian women with breast cancer. Ann Epidemiol. 2003;13:455–461. doi: 10.1016/s1047-2797(02)00426-x. [DOI] [PubMed] [Google Scholar]
- 20.Coogan PF, Rosenberg L, Shapiro S, Hoffman M. Lactation and breast carcinoma risk in a South African population. Cancer. 1999;86:982–989. doi: 10.1002/(sici)1097-0142(19990915)86:6<982::aid-cncr13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256–1264. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1256::aid-cncr13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.The World Bank. African Development Indicators 2012/13 [electronic] Washington, DC; 2013. [Google Scholar]
- 23.Sitas F, Parkin DM, Chirenje M, Stein L, Abratt R, Wabinga H. Part II: Cancer in Indigenous Africans--causes and control. Lancet Oncol. 2008;9:786–795. doi: 10.1016/S1470-2045(08)70198-0. [DOI] [PubMed] [Google Scholar]
- 24.Urban M, Banks E, Egger S, et al. Injectable and oral contraceptive use and cancers of the breast, cervix, ovary, and endometrium in black South African women: case-control study. PLoS Med. 2012;9:e1001182. doi: 10.1371/journal.pmed.1001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogundiran TO, Huo D, Adenipekun A, et al. Case-control study of body size and breast cancer risk in Nigerian women. Am J Epidemiol. 2010;172:682–690. doi: 10.1093/aje/kwq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogundiran TO, Huo D, Adenipekun A, et al. Body fat distribution and breast cancer risk: findings from the Nigerian breast cancer study. Cancer Causes Control. 2012;23:565–574. doi: 10.1007/s10552-012-9916-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okobia MN, Bunker CH, Zmuda JM, et al. Anthropometry and breast cancer risk in Nigerian women. Breast J. 2006;12:462–466. doi: 10.1111/j.1075-122X.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- 28.Jordan I, Hebestreit A, Swai B, Krawinkel MB. Dietary patterns and breast cancer risk among women in northern Tanzania: a case-control study. Eur J Nutr. 2013;52:905–915. doi: 10.1007/s00394-012-0398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okobia MN, Bunker CH, Garte SJ, et al. Leptin receptor Gln223Arg polymorphism and breast cancer risk in Nigerian women: a case control study. BMC Cancer. 2008;8:338. doi: 10.1186/1471-2407-8-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okobia MN, Bunker CH, Garte SJ, et al. Cytochrome P450 1B1 Val432Leu polymorphism and breast cancer risk in Nigerian women: a case control study. Infect Agent Cancer. 2009;4(Suppl 1):S12. doi: 10.1186/1750-9378-4-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mai V, Draganov PV. Recent advances and remaining gaps in our knowledge of associations between gut microbiota and human health. World J Gastroenterol. 2009;15:81–85. doi: 10.3748/wjg.15.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kortenkamp A. Breast cancer, oestrogens and environmental pollutants: a re-evaluation from a mixture perspective. Int J Androl. 2006;29:193–198. doi: 10.1111/j.1365-2605.2005.00613.x. [DOI] [PubMed] [Google Scholar]
- 33.Dey S, Soliman AS, Merajver SD. Xenoestrogens may be the cause of high and increasing rates of hormone receptor positive breast cancer in the world. Med Hypotheses. 2009;72:652–656. doi: 10.1016/j.mehy.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 34.Etemesi BA. Impact of hair relaxers in women in Nakuru, Kenya. Int J Dermatol. 2007;46(Suppl 1):23–25. doi: 10.1111/j.1365-4632.2007.03458.x. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg L, Boggs DA, Adams-Campbell LL, Palmer JR. Hair relaxers not associated with breast cancer risk: evidence from the black women's health study. Cancer Epidemiol Biomarkers Prev. 2007;16:1035–1037. doi: 10.1158/1055-9965.EPI-06-0946. [DOI] [PubMed] [Google Scholar]
- 36.Wise LA, Palmer JR, Reich D, Cozier YC, Rosenberg L. Hair relaxer use and risk of uterine leiomyomata in african-american women. Am J Epidemiol. 2012;175:432–440. doi: 10.1093/aje/kwr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nnoruka E, Okoye O. Topical steroid abuse: its use as a depigmenting agent. J Natl Med Assoc. 2006;98:934–939. [PMC free article] [PubMed] [Google Scholar]
- 38.Olumide YM, Akinkugbe AO, Altraide D, et al. Complications of chronic use of skin lightening cosmetics. Int J Dermatol. 2008;47:344–353. doi: 10.1111/j.1365-4632.2008.02719.x. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg L, Kelly JP, Shapiro S, Hoffman M, Cooper D. Risk factors for breast cancer in South African women. S Afr Med J. 2002;92:447–448. [PubMed] [Google Scholar]
- 40.Garcia-Closas M, Couch FJ, Lindstrom S, et al. Genome-wide association studies identify four ER negative-specific breast cancer risk loci. Nat Genet. 2013;45:392. doi: 10.1038/ng.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michailidou K, Hall P, Gonzalez-Neira A, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45:353–352. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen F, Chen GK, Stram DO, et al. A genome-wide association study of breast cancer in women of African ancestry. Hum Genet. 2013;132:39–48. doi: 10.1007/s00439-012-1214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Closas M, Brinton LA, Lissowska J, et al. Established breast cancer risk factors by clinically important tumour characteristics. Br J Cancer. 2006;95:123–129. doi: 10.1038/sj.bjc.6603207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adebamowo CA, Famooto A, Ogundiran TO, Aniagwu T, Nkwodimmah C, Akang EE. Immunohistochemical and molecular subtypes of breast cancer in Nigeria. Breast Cancer Res Treat. 2008;110:183–188. doi: 10.1007/s10549-007-9694-5. [DOI] [PubMed] [Google Scholar]
- 46.Agboola AJ, Musa AA, Wanangwa N, et al. Molecular characteristics and prognostic features of breast cancer in Nigerian compared with UK women. Breast Cancer Res Treat. 2012;135:555–569. doi: 10.1007/s10549-012-2173-7. [DOI] [PubMed] [Google Scholar]
- 47.Awadelkarim KD, Arizzi C, Elamin EO, et al. Pathological, clinical and prognostic characteristics of breast cancer in Central Sudan versus Northern Italy: implications for breast cancer in Africa. Histopathology. 2008;52:445–456. doi: 10.1111/j.1365-2559.2008.02966.x. [DOI] [PubMed] [Google Scholar]
- 48.Bird PA, Hill AG, Houssami N. Poor hormone receptor expression in East African breast cancer: evidence of a biologically different disease? Ann Surg Oncol. 2008;15:1983–1988. doi: 10.1245/s10434-008-9900-7. [DOI] [PubMed] [Google Scholar]
- 49.Burson AM, Soliman AS, Ngoma TA, et al. Clinical and epidemiologic profile of breast cancer in Tanzania. Breast Dis. 2010;31:33–41. doi: 10.3233/BD-2009-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gukas ID, Jennings BA, Mandong BM, et al. Clinicopathological features and molecular markers of breast cancer in Jos, Nigeria. West Afr J Med. 2005;24:209–213. doi: 10.4314/wajm.v24i3.28220. [DOI] [PubMed] [Google Scholar]
- 51.Huo D, Ikpatt F, Khramtsov A, et al. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol. 2009;27:4515–4521. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikpatt OF, Ndoma-Egba R. Oestrogen and progesterone receptors in Nigerian breast cancer: relationship to tumour histopathology and survival of patients. Cent Afr J Med. 2003;49:122–126. [PubMed] [Google Scholar]
- 53.Ly M, Antoine M, Dembele AK, et al. High incidence of triple-negative tumors in sub-saharan Africa: a prospective study of breast cancer characteristics and risk factors in Malian women seen in a Bamako university hospital. Oncology. 2012;83:257–263. doi: 10.1159/000341541. [DOI] [PubMed] [Google Scholar]
- 54.Nyagol J, Nyong'o A, Byakika B, et al. Routine assessment of hormonal receptor and her-2/neu status underscores the need for more therapeutic targets in Kenyan women with breast cancer. Anal Quant Cytol Histol. 2006;28:97–103. [PubMed] [Google Scholar]
- 55.Ohene-Yeboah M, Adjei E. Breast cancer in Kumasi, Ghana. Ghana. Med J. 2012;46:8–13. [PMC free article] [PubMed] [Google Scholar]
- 56.Stark A, Kleer CG, Martin I, et al. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer. 2010;116:4926–4932. doi: 10.1002/cncr.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yarney J, Vanderpuye V, Clegg Lamptey JN. Hormone receptor and HER-2 expression in breast cancers among Sub-Saharan African women. Breast J. 2008;14:510–511. doi: 10.1111/j.1524-4741.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 58.McCormack VA, Joffe M, van den Berg E, et al. Breast cancer receptor status and stage at diagnosis in over 1,200 consecutive public hospital patients in Soweto, South Africa: a case series. Breast Cancer Res. 2013;15:R84. doi: 10.1186/bcr3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clegg-Lamptey JN, Dakubo JC, Attobra YN. Psychosocial aspects of breast cancer treatment in Accra, Ghana. East Afr Med J. 2009;86:348–353. doi: 10.4314/eamj.v86i7.54152. [DOI] [PubMed] [Google Scholar]
- 60.Ibrahim NA, Oludara MA. Socio-demographic factors and reasons associated with delay in breast cancer presentation: a study in Nigerian women. Breast. 2012;21:416–418. doi: 10.1016/j.breast.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Yarney J, Donkor A, Opoku SY, et al. Characteristics of users and implications for the use of complementary and alternative medicine in Ghanaian cancer patients undergoing radiotherapy and chemotherapy: a cross- sectional study. BMC Complement Altern Med. 2013;13:16. doi: 10.1186/1472-6882-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adesunkanmi AR, Lawal OO, Adelusola KA, Durosimi MA. The severity, outcome and challenges of breast cancer in Nigeria. Breast. 2006;15:399–409. doi: 10.1016/j.breast.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 63.De Ver DT, Bogale S, Hobden C, et al. A mixed-method assessment of beliefs and practice around breast cancer in Ethiopia: implications for public health programming and cancer control. Glob Public Health. 2011;6:719–731. doi: 10.1080/17441692.2010.510479. [DOI] [PubMed] [Google Scholar]
- 64.Clegg-Lamptey J, Dakubo J, Attobra YN. Why do breast cancer patients report late or abscond during treatment in ghana? A pilot study. Ghana Med J. 2009;43:127–131. [PMC free article] [PubMed] [Google Scholar]
- 65.Odigie VI, Tanaka R, Yusufu LM, et al. Psychosocial effects of mastectomy on married African women in Northwestern Nigeria. Psychooncology. 2010;19:893–897. doi: 10.1002/pon.1675. [DOI] [PubMed] [Google Scholar]
- 66.Okobia MN, Bunker CH, Okonofua FE, Osime U. Knowledge, attitude and practice of Nigerian women towards breast cancer: a cross-sectional study. World J Surg. Oncol. 2006;4:11. doi: 10.1186/1477-7819-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Machlin A, Wakefield M, Spittal M, Hill D, editors. UICC Special Report. Cancer related beliefs and behaviours in eight geographic regions. 2009. [Google Scholar]
- 68.Maree J, Wright S, Lu X. Breast Cancer Risks and Screening Practices among Women Living in a Resource Poor Community in Tshwane, South Africa. Breast J. 2013;19:453–454. doi: 10.1111/tbj.12143. [DOI] [PubMed] [Google Scholar]
- 69.Pillay AL. Rural and urban South African women's awareness of cancers of the breast and cervix. Ethn Health. 2002;7:103–114. doi: 10.1080/1355785022000038588. [DOI] [PubMed] [Google Scholar]
- 70.Suh MA, Atashili J, Fuh EA, Eta VA. Breast self-examination and breast cancer awareness in women in developing countries: a survey of women in Buea, Cameroon. BMC Res Notes. 2012;5:627. doi: 10.1186/1756-0500-5-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ezeome ER. Delays in presentation and treatment of breast cancer in Enugu, Nigeria. Niger J Clin Pract. 2010;13:311–316. [PubMed] [Google Scholar]
- 72.O'Brien KS, Soliman AS, Annan K, Lartey RN, Awuah B, Merajver SD. Traditional herbalists and cancer management in Kumasi, Ghana. J Cancer Educ. 2012;27:573–579. doi: 10.1007/s13187-012-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Opoku SY, Benwell M, Yarney J. Knowledge, attitudes, beliefs, behaviour and breast cancer screening practices in Ghana, West Africa. Pan Afr Med J. 2012;11:28. [PMC free article] [PubMed] [Google Scholar]
- 74.Kemfang Ngowa JD, Yomi J, Kasia JM, Mawamba Y, Ekortarh AC, Vlastos G. Breast Cancer Profile in a Group of Patients Followed up at the Radiation Therapy Unit of the Yaounde General Hospital, Cameroon. Obstet Gynecol Int. 2011;2011:143506. doi: 10.1155/2011/143506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clegg-Lamptey J, Hodasi W. A study of breast cancer in korle bu teaching hospital: assessing the impact of health education. Ghana Med J. 2007;41:72–77. doi: 10.4314/gmj.v41i2.55305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dickens C, Joffe M, Jacobson JS, et al. Quantifying the delay in stage at diagnosis associated with greater residential distance from hospital in over 1000 breast cancer patients diagnosed at a South African public hospital. Cancer Epidemiol Biomarkers Prev. (In press). [Google Scholar]
- 77.Adisa AO, Gukas ID, Lawal OO, Adesunkanmi AR. Breast cancer in Nigeria: is non-adherence to chemotherapy schedules a major factor in the reported poor treatment outcome? Breast J. 2010;16:206–207. doi: 10.1111/j.1524-4741.2009.00883.x. [DOI] [PubMed] [Google Scholar]
- 78.Galukande M, Kiguli-Malwadde E. Rethinking breast cancer screening strategies in resource-limited settings. Afr Health Sci. 2010;10:89–92. [PMC free article] [PubMed] [Google Scholar]
- 79.Zelle SG, Nyarko KM, Bosu WK, et al. Costs, effects and cost-effectiveness of breast cancer control in Ghana. Trop Med Int Health. 2012;17:1031–1043. doi: 10.1111/j.1365-3156.2012.03021.x. [DOI] [PubMed] [Google Scholar]
- 80.Barton MB, Frommer M, Shafiq J. Role of radiotherapy in cancer control in low-income and middle-income countries. Lancet Oncol. 2006;7:584–595. doi: 10.1016/S1470-2045(06)70759-8. [DOI] [PubMed] [Google Scholar]
- 81.Abdel-Wahab M, Bourque JM, Pynda Y, et al. Status of radiotherapy resources in Africa: an International Atomic Energy Agency analysis. Lancet Oncol. 2013;14:e168–e175. doi: 10.1016/S1470-2045(12)70532-6. [DOI] [PubMed] [Google Scholar]
- 82.Kingham TP, Alatise OI, Vanderpuye V, et al. Treatment of cancer in sub-Saharan Africa. Lancet Oncol. 2013;14:e158–e167. doi: 10.1016/S1470-2045(12)70472-2. [DOI] [PubMed] [Google Scholar]
- 83.Olugbenga-Bello A, Oladele EA, Bello TO, Ojo JO, Oguntola AS. Awareness and breast cancer risk factors: perception and screening practices among females in a tertiary institution in Southwest Nigeria. Niger Postgrad Med J. 2011;18:8–15. [PubMed] [Google Scholar]
- 84.Stefan DC, Elzawawy AM, Khaled HM, et al. Developing cancer control plans in Africa: examples from five countries. Lancet Oncol. 2013;14:e189–e195. doi: 10.1016/S1470-2045(13)70100-1. [DOI] [PubMed] [Google Scholar]