Abstract
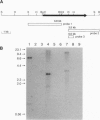
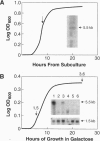
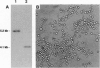
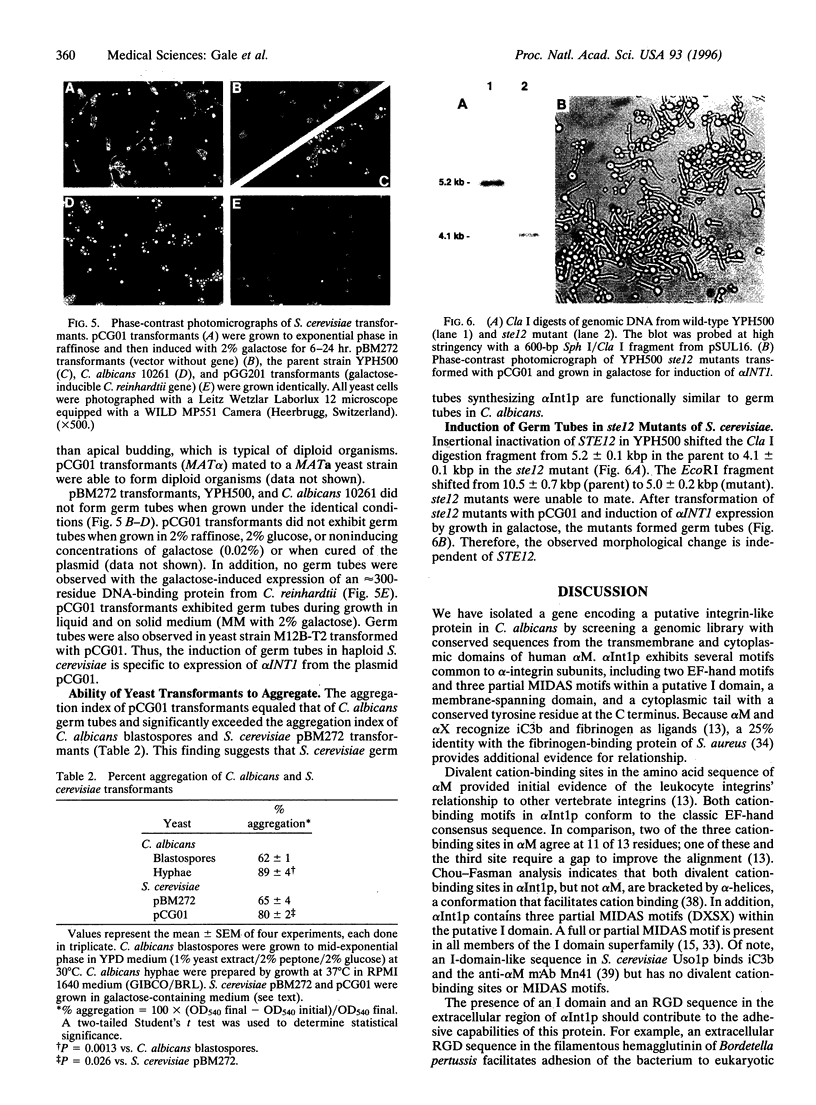
The existence of integrin-like proteins in Candida albicans has been postulated because monoclonal antibodies to the leukocyte integrins alpha M and alpha X bind to blastospores and germ tubes, recognize a candidal surface protein of approximately 185 kDa, and inhibit candidal adhesion to human epithelium. The gene alpha INT1 was isolated from a library of C. albicans genomic DNA by screening with a cDNA probe from the transmembrane domain of human alpha M. The predicted polypeptide (alpha Int1p) of 188 kDa contains several motifs common to alpha M and alpha X: a putative I domain, two EF-hand divalent cation-binding sites, a transmembrane domain, and a cytoplasmic tail with a single tyrosine residue. An internal RGD tripeptide is also present. Binding of anti-peptide antibodies raised to potential extracellular domains of alpha Int1p confirms surface localization in C. albicans blastopores. By Southern blotting, alpha INT1 is unique to C. albicans. Expression of alpha INT1 under control of a galactose-inducible promoter led to the production of germ tubes in haploid Saccharomyces cerevisiae and in the corresponding ste12 mutant. Germ tubes were not observed in haploid yeast transformed with vector alone, in transformants expressing a galactose-inducible gene from Chlamydomonas, or in transformants grown in the presence of glucose or raffinose. Transformants producing alpha Int1p bound an anti-alpha M monoclonal antibody and exhibited enhanced aggregation. Studies of alpha Int1p reveal novel roles for primitive integrin-like proteins in adhesion and in STE12-independent morphogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. E., Pringle J. R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984 Mar;98(3):934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barki M., Koltin Y., Yanko M., Tamarkin A., Rosenberg M. Isolation of a Candida albicans DNA sequence conferring adhesion and aggregation on Saccharomyces cerevisiae. J Bacteriol. 1993 Sep;175(17):5683–5689. doi: 10.1128/jb.175.17.5683-5689.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendel C. M., Hostetter M. K. Distinct mechanisms of epithelial adhesion for Candida albicans and Candida tropicalis. Identification of the participating ligands and development of inhibitory peptides. J Clin Invest. 1993 Oct;92(4):1840–1849. doi: 10.1172/JCI116775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler K. M., Baker C. J. Candida: an increasingly important pathogen in the nursery. Pediatr Clin North Am. 1988 Jun;35(3):543–563. doi: 10.1016/s0031-3955(16)36471-9. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Colombatti A., Bonaldo P., Doliana R. Type A modules: interacting domains found in several non-fibrillar collagens and in other extracellular matrix proteins. Matrix. 1993 Jul;13(4):297–306. doi: 10.1016/s0934-8832(11)80025-9. [DOI] [PubMed] [Google Scholar]
- Corbi A. L., Kishimoto T. K., Miller L. J., Springer T. A. The human leukocyte adhesion glycoprotein Mac-1 (complement receptor type 3, CD11b) alpha subunit. Cloning, primary structure, and relation to the integrins, von Willebrand factor and factor B. J Biol Chem. 1988 Sep 5;263(25):12403–12411. [PubMed] [Google Scholar]
- Corbi A. L., Miller L. J., O'Connor K., Larson R. S., Springer T. A. cDNA cloning and complete primary structure of the alpha subunit of a leukocyte adhesion glycoprotein, p150,95. EMBO J. 1987 Dec 20;6(13):4023–4028. doi: 10.1002/j.1460-2075.1987.tb02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Gaither T. A., O'Shea J. J., Rotrosen D., Lawley T. J., Wright S. A., Frank M. M., Green I. Expression of specific binding sites on Candida with functional and antigenic characteristics of human complement receptors. J Immunol. 1986 Dec 1;137(11):3577–3583. [PubMed] [Google Scholar]
- Fields S., Herskowitz I. Regulation by the yeast mating-type locus of STE12, a gene required for cell-type-specific expression. Mol Cell Biol. 1987 Oct;7(10):3818–3821. doi: 10.1128/mcb.7.10.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore B. J., Retsinas E. M., Lorenz J. S., Hostetter M. K. An iC3b receptor on Candida albicans: structure, function, and correlates for pathogenicity. J Infect Dis. 1988 Jan;157(1):38–46. doi: 10.1093/infdis/157.1.38. [DOI] [PubMed] [Google Scholar]
- Gimeno C. J., Ljungdahl P. O., Styles C. A., Fink G. R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992 Mar 20;68(6):1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- Gordon D. L., Johnson G. M., Hostetter M. K. Characteristics of iC3b binding to human polymorphonuclear leucocytes. Immunology. 1987 Apr;60(4):553–558. [PMC free article] [PubMed] [Google Scholar]
- Gustafson K. S., Vercellotti G. M., Bendel C. M., Hostetter M. K. Molecular mimicry in Candida albicans. Role of an integrin analogue in adhesion of the yeast to human endothelium. J Clin Invest. 1991 Jun;87(6):1896–1902. doi: 10.1172/JCI115214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971 Dec;69(2):265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- Heidenreich F., Dierich M. P. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985 Nov;50(2):598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Holmes A. R., Cannon R. D., Shepherd M. G. Mechanisms of aggregation accompanying morphogenesis in Candida albicans. Oral Microbiol Immunol. 1992 Feb;7(1):32–37. doi: 10.1111/j.1399-302x.1992.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Hostetter M. K., Lorenz J. S., Preus L., Kendrick K. E. The iC3b receptor on Candida albicans: subcellular localization and modulation of receptor expression by glucose. J Infect Dis. 1990 Apr;161(4):761–768. doi: 10.1093/infdis/161.4.761. [DOI] [PubMed] [Google Scholar]
- Hostetter M. K., Tao N. J., Gale C., Herman D. J., McClellan M., Sharp R. L., Kendrick K. E. Antigenic and functional conservation of an integrin I-domain in Saccharomyces cerevisiae. Biochem Mol Med. 1995 Aug;55(2):122–130. doi: 10.1006/bmme.1995.1042. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn T. E., Edwards F. F., Armstrong D. The prevalence of yeasts in clinical specimens from cancer patients. Am J Clin Pathol. 1980 Apr;73(4):518–521. doi: 10.1093/ajcp/73.4.518. [DOI] [PubMed] [Google Scholar]
- Konkel L. M., Enomoto S., Chamberlain E. M., McCune-Zierath P., Iyadurai S. J., Berman J. A class of single-stranded telomeric DNA-binding proteins required for Rap1p localization in yeast nuclei. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5558–5562. doi: 10.1073/pnas.92.12.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Köhrer K., Domdey H. Preparation of high molecular weight RNA. Methods Enzymol. 1991;194:398–405. doi: 10.1016/0076-6879(91)94030-g. [DOI] [PubMed] [Google Scholar]
- Lee J. O., Rieu P., Arnaout M. A., Liddington R. Crystal structure of the A domain from the alpha subunit of integrin CR3 (CD11b/CD18). Cell. 1995 Feb 24;80(4):631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- Liu H., Köhler J., Fink G. R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994 Dec 9;266(5191):1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- Liu H., Styles C. A., Fink G. R. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science. 1993 Dec 10;262(5140):1741–1744. doi: 10.1126/science.8259520. [DOI] [PubMed] [Google Scholar]
- Malathi K., Ganesan K., Datta A. Identification of a putative transcription factor in Candida albicans that can complement the mating defect of Saccharomyces cerevisiae ste12 mutants. J Biol Chem. 1994 Sep 16;269(37):22945–22951. [PubMed] [Google Scholar]
- Mayer C. L., Diamond R. D., Edwards J. E., Jr Recognition of binding sites on Candida albicans by monoclonal antibodies to human leukocyte antigens. Infect Immun. 1990 Nov;58(11):3765–3769. doi: 10.1128/iai.58.11.3765-3769.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt D., Francois P., Vaudaux P., Foster T. J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994 Jan;11(2):237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Hirata A., Ogawa Y., Yonehara T., Yoda K., Yamasaki M. A cytoskeleton-related gene, uso1, is required for intracellular protein transport in Saccharomyces cerevisiae. J Cell Biol. 1991 Apr;113(2):245–260. doi: 10.1083/jcb.113.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayfield E. J., Ault M. J., Keusch G. T., Brothers M. J., Nechemias C., Smith H. Infection and diabetes: the case for glucose control. Am J Med. 1982 Mar;72(3):439–450. doi: 10.1016/0002-9343(82)90511-3. [DOI] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Roberts R. L., Fink G. R. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994 Dec 15;8(24):2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- Saporito-Irwin S. M., Birse C. E., Sypherd P. S., Fonzi W. A. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol Cell Biol. 1995 Feb;15(2):601–613. doi: 10.1128/mcb.15.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Strynadka N. C., James M. N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]