Abstract
Ceftaroline, the active metabolite of the prodrug ceftaroline fosamil, is a cephalosporin with broad-spectrum in vitro activity against Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant Streptococcus pneumoniae (MDRSP), and common Gram-negative pathogens. This study investigated the in vivo activity of ceftaroline fosamil compared with clindamycin, linezolid, and vancomycin in a severe pneumonia model due to MRSA-producing Panton-Valentine leukocidin (PVL). A USA300 PVL-positive clone was used to induce pneumonia in rabbits. Infected rabbits were randomly assigned to no treatment or simulated human-equivalent dosing with ceftaroline fosamil, clindamycin, linezolid, or vancomycin. Residual bacterial concentrations in the lungs and spleen were assessed after 48 h of treatment. PVL expression was measured using a specific enzyme-linked immunosorbent assay (ELISA). Ceftaroline, clindamycin, and linezolid considerably reduced mortality rates compared with the control, whereas vancomycin did not. Pulmonary and splenic bacterial titers and PVL concentrations were greatly reduced by ceftaroline, clindamycin, and linezolid. Ceftaroline, clindamycin, and linezolid were associated with reduced pulmonary tissue damage based on significantly lower macroscopic scores. Ceftaroline fosamil, clindamycin, and, to a lesser extent, linezolid were efficient in reducing bacterial titers in both the lungs and spleen and decreasing macroscopic scores and PVL production compared with the control.
INTRODUCTION
Staphylococcus aureus is often a cause of complicated skin and skin structure infections, bacteremia, pneumonia, and other serious human infections (1). Community-acquired methicillin-resistant S. aureus (CA-MRSA) emerged as a significant health threat in the mid-1990s (1). Although CA-MRSA is the causative pathogen in a relatively small percentage of patients with community-acquired pneumonia (CAP), it manifests as a severe necrotizing pneumonia, is associated with high mortality if not suspected early, and frequently occurs in younger patients with longer life expectancies (2, 3). The USA300 clone is one of the most common causes of CA-MRSA infections in the United States (1, 4–6). USA300 has been associated with invasive disease, including severe necrotizing pneumonia, bacteremia, endocarditis, and osteomyelitis, and its incidence has significantly increased in the United States, from 12% in 2004 to 38% in 2006 (1, 7). The prevalence of the USA300 clone and that of other CA-MRSA clones is also increasing in Europe, with contrasting prevalences between studies and countries (8, 9).
A large percentage of CA-MRSA harbors the Panton-Valentine leukocidin (PVL) toxin genes (10). The PVL toxin is associated with severe necrotizing infections, and necrotizing pneumonia caused by PVL-producing S. aureus is associated with high mortality rates (10, 11). Between 60% and 100% of MRSA strains in the United States encode the PVL toxin, and the majority of these strains are USA300 (6, 12).
Current guidelines from the Infectious Diseases Society of America (IDSA) recommend the use of vancomycin, linezolid, or clindamycin for treating CA-MRSA pneumonia (13). However, resistance to CA-MRSA therapies, including clindamycin and tetracycline, has been seen with certain isolates (14). Newer antibiotics that are active against PVL-producing MRSA may thus be appropriate for treating severe necrotizing pneumonia. Ceftaroline, the active metabolite of the prodrug ceftaroline fosamil, is a cephalosporin with broad-spectrum in vitro bactericidal activity against Gram-positive organisms, including MRSA and multidrug-resistant Streptococcus pneumoniae (MDRSP), and common Gram-negative pathogens (15–18). Ceftaroline has demonstrated bactericidal in vitro activity against resistant S. aureus, including vancomycin-intermediate and -resistant and daptomycin-nonsusceptible isolates (19, 20). Ceftaroline fosamil is currently approved for use in the United States for community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) (21). Ceftaroline fosamil also received European Commission approval for CAP and complicated skin and soft tissue infections (cSSTI) in August 2012 (22).
The rabbit is the preferred model for studying the role of PVL in necrotizing pneumonia because PVL produces similar effects on human and rabbit neutrophils (23). Furthermore, rabbits are more susceptible to the cytotoxic effects of PVL than rodents (10, 24). The present study was designed to evaluate the in vivo efficacy of ceftaroline compared with vancomycin, linezolid, or clindamycin in a rabbit model of severe PVL-positive CA-MRSA pneumonia using simulated human-equivalent dosing.
(These data were presented in part at the 21st European Congress of Clinical Microbiology and Infectious Diseases/27th International Congress of Chemotherapy and Infection 2011, Milan, Italy [poster P1502].)
MATERIALS AND METHODS
Animals.
Immunocompetent male New Zealand rabbits (body weight, 2.8 to 3 kg) were used in the present study. The animals were placed in individual cages and were fed ad libitum with water and feed, according to the current recommendations of the European Institute of Health. The experimental protocol was approved by the local ethics committee for animal experimentation (protocol no. 0810; C2EA, Dijon, France).
Bacterial strains, growth conditions, and antibiotics.
A USA300 PVL-positive clone was obtained from Frank DeLeo, National Institutes of Health (Hamilton, MT, USA) (25). Bacteria were grown on casein hydrolysate and yeast extract (CCY) (Becton, Dickinson, Le Pont de Claix, France) as culture medium to optimize the PVL levels. Bacterial stocks were kept at −80°C in brain heart infusion (BHI) broth (bioMérieux, Marcy l'Etoile, France) supplemented with 15% (vol/vol) glycerol. Stock cultures were replenished every month with isolates recovered from untreated infected rabbits to maintain virulence.
For in vitro studies, antibiotics were reconstituted according to the manufacturer's instructions. For in vivo rabbit studies, reconstitution was modified to simulate human dosing. The commercial forms of vancomycin (Mylan, Saint Priest, France) and clindamycin (Pfizer, Paris, France) were reconstituted with sterile saline. For linezolid (Pfizer, Paris, France), the commercial form was not concentrated enough for simulated human dosing, and thus, the pure substance was reconstituted in sterile saline. Ceftaroline fosamil (Forest Laboratories, Inc., New York, NY, USA; lot FMD-CEF-030) was used to prepare the dosage solution in 1.9% arginine according to the procedure provided by Cerexa, Inc. (Oakland, CA, USA).
The MICs of vancomycin, linezolid, clindamycin, and ceftaroline for the studied USA300 isolate were 1, 1, 0.25, and 1 mg/liter, respectively.
Preparation of the inoculum.
Before each animal experiment, the staphylococcal strain from 1 frozen aliquot was cultured on agar plates (Chapman, bioMérieux, Marcy l'Etoile, France) and incubated for 24 h at 37°C. Five colonies were inoculated into 9 ml of CCY and incubated for 10 h at 37°C with agitation. Based on pilot experiments, a final inoculum of 3 × 109 CFU/ml in 0.5 ml saline was selected to induce persistent pneumonia and to enable detection of relatively large quantities of PVL in the lungs. Viable bacterial counts were determined using optical density measurements in reference to a standard curve and then confirmed by using successive dilution cultures and plating on agar.
Experimental pneumonia model.
Production of bacteremic pneumonia in immunocompetent rabbits and installation of double central venous catheters were performed as previously described (26). Briefly, bacterial pneumonia was induced by endobronchial challenge with 0.5 ml saline containing 3 × 109 CFU/ml of the test strain 24 h after jugular catheterization. Animals were randomly assigned 5 h after bacterial challenge to the control group (no treatment) or an antibiotic regimen.
Antibiotics were delivered through a central venous catheter with changing infusion rates. A computer-controlled system delivered vancomycin, linezolid, clindamycin, or ceftaroline fosamil to mimic the pharmacokinetic (PK) parameters observed in healthy humans as follows: (i) intravenous (i.v.) vancomycin dosed continuously to simulate a steady-state concentration of 30 mg/liter, (ii) 600 mg linezolid given i.v. twice daily, (iii) 600 mg clindamycin given i.v. 3 times daily, and (iv) 600 mg ceftaroline fosamil given i.v. twice daily. Antibiotic treatment was continued for 48 h.
Antibiotic concentrations in serum.
For each animal, antibiotic concentrations were determined from iterative blood samples obtained through a second catheter approximately 8 to 10 times during the 48 h (approximately 1.5 ml per sample; the total volume of blood samples during the experiment was less than 10% of the total blood volume of the animal). Samples were centrifuged for 10 min at 10,000 × g, and the serum was removed. Ceftaroline concentrations were determined in triplicate by a disk plate bioassay method with antibiotic medium II (Difco Laboratories, Detroit, MI, USA) and Bacillus subtilis as the indicator organism. The standards were prepared in 0.7% saline water, and the linearity of the standard curves used for disk plate bioassays was at least 0.98 (coefficient of correlation [r2]). Vancomycin concentrations were determined by an immunofluorescence polarization method. Linezolid and clindamycin concentrations were determined by high-performance liquid chromatography (HPLC). The limits of detection were 0.25, 0.3, 0.03, and 0.03 mg/liter for ceftaroline, vancomycin, linezolid, and clindamycin, respectively. Protein binding of each antibiotic was determined by an ultrafiltration method in rabbit plasma. After centrifugation through 10,000-molecular-weight-cutoff filters (Vivaspin 2 Hydrosart; Sartorius Stedim, France), serum ultrafiltrates were analyzed for antibiotic determination. The amount of antibiotic able to pass through the filter represented the unbound portion of drug in plasma. The amount of adsorption on the ultrafiltration membrane and the amount of the nonspecific binding were also evaluated.
Pharmacokinetic analyses.
PK data were analyzed using Kinetica software (Innaphase, Philadelphia, PA, USA). Compartmental and noncompartmental approaches were used to describe the different PK parameters.
Evaluation of infection and PVL production.
Five control rabbits were culled at 5 h postinfection to assess the bacterial burden in the lungs at the start of therapy. All untreated (control) rabbits died within the first 24 h and were included in the analysis. Treated rabbits that survived over the experiment period were sacrificed 2 h after the end of the 48-hour antibiotic infusion. The spleen and pulmonary lobes were weighed and homogenized in 5 ml sterile saline (MiniMix; Intersciences, Saint Nom, France). Bacteria were counted in a sample of this crude homogenate by plating 10-fold dilutions on Chapman agar plates and incubating the plates for 24 h at 37°C. The bacterial concentrations in each pulmonary lobe and in the spleen were calculated after adjusting for the weight of the lobe or spleen. The threshold value was 1 log10 CFU/g. For statistical comparisons of the differences between the pulmonary bacterial densities, culture-negative lobes were considered to contain 1 log10 CFU/g. For each rabbit, the mean pulmonary bacterial concentration was calculated according to each lobar bacterial concentration with lobar weight [e.g., mean concentration = Σ(lobar concentration × lobar weight)/Σ(lobar weights)].
Macroscopic pulmonary injury scores were calculated according to a macroscopic scoring grid, as described by Piroth and associates (27). The total macroscopic score is the sum of the scores for individual pulmonary lobes, with 2 points added for purulent pleural effusion (scoring grid: 0, normal; 1, scar; 2, slight congestion; 3, red congestion; 4, gray congestion; 5, yellowish congestion).
The PVL concentration was also determined in the supernatants of lung homogenates by using a specific solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) (28).
Evaluation of PVL mRNA expression in vitro.
Cultures of S. aureus LAC USA300 were performed in Mueller-Hinton (MH) broth supplemented with calcium (50 mg/liter) and magnesium (12.5 mg/liter) at 37°C with gyratory shaking (300 rpm). At the late exponential growth phase (2 McFarland standard), one-quarter of the MIC of each antibiotic (oxacillin, ceftaroline, clindamycin, linezolid, and vancomycin) was added to the glass tubes. Cultures with or without antibiotics were incubated at 37°C with shaking for 6 h.
Aliquots of 2 ml of each culture were centrifuged at 10,000 × g for 10 min, and the pellets were first washed with 0.5 ml of Tris buffer (10 mM), centrifuged at 10,000 × g for 10 min, and then adjusted to an optical density at 600 nm (OD600) of 0.75 in Tris buffer (10 mM). One and a half milliliters of adjusted and washed bacterial suspension was centrifuged at 10,000 × g for 10 min, and the pellets were treated with lysostaphin (Sigma) at a final concentration of 200 mg/liter. The total RNA of the pellets was then purified using a Qiagen RNeasy Plus Mini Kit according to the manufacturer's instructions. The amount of RNA recovered was assessed with a NanoDrop spectrophotometer, and 1 μg of total RNA was reverse transcribed using a Promega reverse transcription (RT) system with random primers, as recommended by the provider. The resulting cDNA was used as a template for real-time amplification (LightCycler 2.0; Roche), using gyrB- and pvl-specific primers (29). The amounts of pvl-specific amplicons were determined by quantitative PCR (qPCR) relative to an internal standard (gyrB). The expression levels of pvl genes were expressed as n-fold variations of the pvl or gyrB copy number in the presence of antibiotics relative to the pvl or gyrB copy number of the growth control. All qPCR data were analyzed using the relative expression software tool REST 2009 version 2.0.13 (30).
Statistical analysis.
Animals that received a bacterial inoculum between 1.6 × 109 CFU/ml and 6.3 × 109 CFU/ml were included in the statistical analysis. Animals whose PK monitoring was not adequate (e.g., the catheter was occluded) were excluded. The maximal area under the concentration-time curve from 0 to 24 h (AUC0-24) variation tolerated was ±20% around the expected values. For evaluation of antibacterial efficacy, only rabbits that received at least a 30-hour treatment were included in the statistical analysis.
Statistical analysis was performed with GraphPad Prism software. Quantitative variables were compared using a Mann-Whitney U test or analysis of variance and post hoc analysis using Bonferroni's test. Percentages were compared using Fisher's exact test. Quantitative relationships between antimicrobial efficacy and each of the PK/pharmacodynamic (PD) parameters were determined using a maximum-effect (Emax) model (Hill formula) with SigmaPlot software (version 9.0). Death rates were recorded daily, and the survival curves were constructed using the Kaplan-Meier method and compared using the log rank test. A P value of <0.05 was considered significant for all tests performed.
RESULTS
Pharmacokinetic simulation of human-equivalent i.v. treatments.
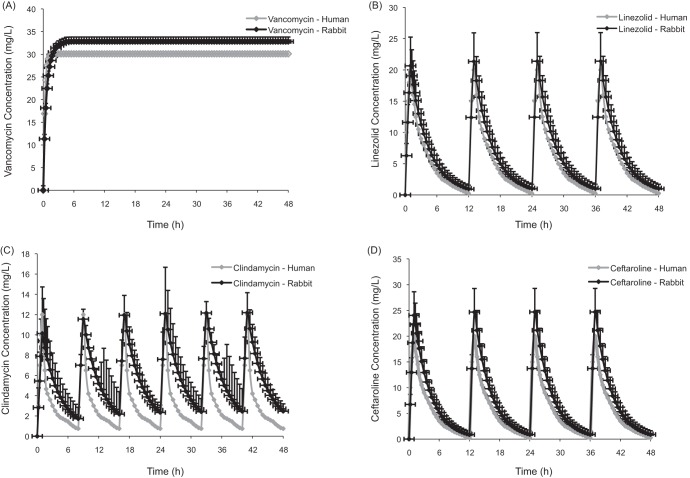
Serum drug concentrations obtained after simulated human dosing in rabbits with vancomycin, linezolid, clindamycin, and ceftaroline fosamil are shown in Fig. 1. The corresponding PK parameters (maximum drug concentration in serum [Cmax], minimum drug concentration in serum [Cmin], drug concentration at steady-state [CSS], and AUC0-24) are presented in Table 1. Human and rabbit PK parameters were comparable in this study, with the exception of the Cmin of clindamycin, which was higher in rabbits than in humans.
FIG 1.
Total concentration-time profiles for vancomycin continuous infusion (A), linezolid at 600 mg twice daily (B), clindamycin at 600 mg 3 times daily (C), and ceftaroline fosamil at 600 mg twice daily (D) in humans and rabbits. The data are presented as means ± SD.
TABLE 1.
Pharmacokinetics (mean) of a model of human therapy in rabbits
| PK parameter | Subject | Valuea |
|||
|---|---|---|---|---|---|
| Vancomycin | Linezolid | Clindamycin | Ceftaroline fosamil | ||
| Cmax (mg/liter) | Rabbit | 13.7 | 11 | 24 | |
| Human | 15.6 | 12 | 21 | ||
| Cmin (mg/liter) | Rabbit | 2.3 | 1.4 | 0.8 | |
| Human | 2.6 | 0.75 | 0.25 | ||
| Css (mg/liter) | Rabbit | 32.82 | |||
| Human | 30 | ||||
| AUC0-24 (mg · h/liter) | Rabbit | 382 | 122 | 99 | 166 |
| Human | 352 | 138 | 68 | 131 | |
Using vancomycin in continuous infusion (equivalent to a CSS of 30 mg/liter for 48 h), linezolid (equivalent to 600 mg i.v. twice daily for 48 h), clindamycin (equivalent to 600 mg i.v. 3 times daily for 48 h), or ceftaroline fosamil (equivalent to 600 mg i.v. twice daily for 48 h) (expressed as the total drug fraction).
Mortality rates.
There were 74 rabbits used in this experiment; 8 rabbits were excluded because the inoculum or PK was inadequate. Of the 66 remaining, 41 were included in the analyses, which included the “early” control group (n = 6) and the animals that survived longer than 30 h of treatment (n = 35) (Table 2). Because of the rapid mortality (<30 h) in all or most of the control (8/8) and vancomycin (9/12) treatment groups, an “early-death” group was formed, which included rabbits that did not survive beyond 30 h of treatment. The vancomycin early-death group was studied separately in the statistical analysis because a high number of vancomycin-treated rabbits died before 24 h. Survival rates after treatment with antibiotic regimens are shown in Fig. 2. Death occurred within 12 to 15 h postinoculation for 100% of the untreated rabbits. Although vancomycin did not reduce the mortality rate, linezolid, clindamycin, and ceftaroline significantly reduced mortality compared with the control (P < 0.0001). Nine out of 12 vancomycin-treated rabbits (75%) died within 24 h of treatment.
TABLE 2.
Numbers of animals used in the study
| Treatment | No. of rabbits tested | No. of early deaths (<30 h) | No. that survived to 48 h |
|---|---|---|---|
| Control without treatment | 8 | 8 | 0 |
| 5-h control (scheduled autopsy at 5 h postinoculation) | 6 | 6 | 0 |
| Vancomycin | 12 | 9 | 3 |
| Linezolid | 14 | 5 | 9 |
| Clindamycin | 12 | 1 | 11 |
| Ceftaroline fosamil | 12 | 2 | 10 |
FIG 2.
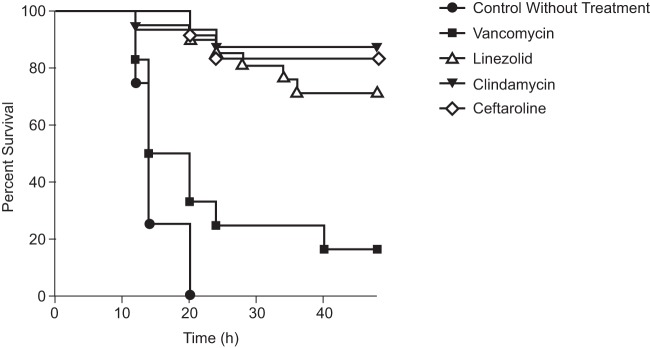
Survival rates of rabbits after treatment with vancomycin equivalent to a continuous infusion in humans (CSS = 30 mg/liter), linezolid equivalent to 600 mg i.v. twice daily, clindamycin equivalent to 600 mg i.v. 3 times daily, or ceftaroline fosamil equivalent to 600 mg i.v. twice daily in the USA300 MRSA pneumonia model.
Effects of antibiotics on reducing bacterial loads and in vivo production of PVL using simulated human dosing in the rabbit model of experimental PVL-positive CA-MRSA pneumonia.
At the start of therapy, mean pulmonary bacterial concentrations were 7.3 ± 0.5 (mean ± standard deviation [SD]) log10 CFU/g. All rabbits in the control group were considered bacteremic, indicating the infection diffused from the pulmonary to the systemic compartment, with high bacterial concentrations in the spleen of 5.1 ± 0.8 log10 CFU/g. Bacterial counts in the lungs and spleen were significantly reduced by all treatment regimens compared with the control group (P = 0.0001 for each group, except P = 0.001 for vancomycin at 48 h) (Table 3). The greatest reduction of bacterial counts in lung tissue was seen in both the ceftaroline and the clindamycin groups, with bacterial counts posttreatment of 2.9 ± 1.4 log10 CFU/g and 3.1 ± 1.6 log10 CFU/g, respectively. The greatest reduction of bacterial counts in the spleen was seen in the clindamycin group, with a bacterial count posttreatment of 1.1 ± 0.2 log10 CFU/g, which was comparable to the ceftaroline group (bacterial count posttreatment, 1.4 ± 0.7 log10 CFU/g). There was no significant difference in macroscopic scores between control and vancomycin-treated rabbits (Table 3). Significantly lower macroscopic scores were noted after treatment with ceftaroline (P = 0.0001), clindamycin (P = 0.001), and linezolid (P = 0.001) compared with control animals, which correlated with reduced tissue damage (Table 3). Rabbits treated with vancomycin did not have significantly reduced PVL in lungs compared with controls, which correlated with the high mortality rate and tissue damage observed in the vancomycin group (Table 3). Treatment with ceftaroline, linezolid, and clindamycin significantly reduced PVL in lungs compared with controls (P < 0.01) (Table 3).
TABLE 3.
Organism titers in lungs and spleen, macroscopic score, number of PVL-positive lobes, and PVL concentrations in lungs of untreated and antibiotic-treated rabbits infected with USA300 MRSA (means ± SD)
| Treatment (no. of rabbits) | Organism titer (log10 CFU/g) |
Global macroscopic score | No. of PVL-positive lobes | Pulmonary PVL concn (μg/g) | |
|---|---|---|---|---|---|
| Lung | Spleen | ||||
| Control without treatment (8) | 8.3 ± 0.7 | 5.1 ± 0.8 | 26.4 ± 3.5 | 3.4 ± 1.6 | 0.094 ± 0.116 |
| Vancomycin | |||||
| <24 h (9) | 7.3 ± 0.5 | 4.1 ± 1.5 | 25.6 ± 4 | 4 ± 1.9 | 0.090 ± 0.116 |
| at 48 h (3) | 5.3 ± 1.1a | 2.5 ± 1.3a | 27 ± 7.9 | 2.7 ± 0.6 | 0.058 ± 0.089 |
| Linezolid (9) | 4.8 ± 1.3b | 2.4 ± 1.3b | 19.4 ± 4.9a | 1.1 ± 0.6 | 0.0185 ± 0.0215c |
| Clindamycin (11) | 3.1 ± 1.6b,d | 1.1 ± 0.2b,d | 17.5 ± 8.5a | 0.5 ± 0.7 | 0.002 ± 0.002c |
| Ceftaroline fosamil (10) | 2.9 ± 1.4b,d | 1.4 ± 0.7b,d | 15.5 ± 3.8b | 1.1 ± 0.7 | 0.004 ± 0.005c |
P = 0.001 compared to control.
P = 0.0001 compared to control.
P < 0.05 compared to control.
P < 0.05 compared to linezolid.
Antimicrobial effects on PVL mRNA expression in vitro.
In a qPCR analysis of the effects of oxacillin, ceftaroline, clindamycin, linezolid, and vancomycin on PVL mRNA expression during the early stationary phase, oxacillin induced a (28 ± 2.82)-fold increase, whereas clindamycin resulted in a (4.7 ± 0.64)-fold decrease in the PVL mRNA level (Fig. 3). Ceftaroline, linezolid, and vancomycin did not alter the PVL mRNA level relative to the untreated control.
FIG 3.

Antimicrobial effects on PVL mRNA expression. S. aureus LAC USA300 MRSA cultures were grown with or without antibiotics at one-quarter of the MIC. After 6 h of incubation, aliquots of cultures were used for total RNA extraction and subsequent RT-PCR as described in Materials and Methods. The results are expressed as n-fold differences in the pvl/gyrB ratio in the presence of antibiotics relative to the pvl/gyrB ratio for the strain grown without antibiotics. These results represent the differences in the PVL mRNA levels detected in the presence of different antibiotics relative to the PVL mRNA level of the growth control. The values are means and SD (2 repeated different experiments). a, significantly different from the control (isolate grown without antibiotic).
DISCUSSION
CA-MRSA is an increasing concern in patients with CAP because of the association with poor outcomes (3). Ceftaroline has been shown to have a strong affinity for multiple penicillin-binding proteins (PBPs) in S. aureus, including PBP2a, which is responsible for methicillin resistance (31, 32). Previous in vitro studies have demonstrated that ceftaroline has bactericidal activity against CA-MRSA, methicillin-susceptible S. aureus (MSSA), health care-associated MRSA (HA-MRSA), heteroresistant vancomycin-intermediate S. aureus (hVISA), vancomycin-intermediate S. aureus (VISA), vancomycin-resistant S. aureus (VRSA), and daptomycin-nonsusceptible S. aureus (19, 20) isolates. The in vivo efficacy of ceftaroline was previously demonstrated against MRSA in a rabbit osteomyelitis model (33) and a rabbit endocarditis model (34) and against S. aureus (MRSA and MSSA) in a PVL-negative murine pneumonia model (35).
In the present study, the rabbit model is advantageous, because severe disease can be induced in the immunocompetent animals and computer-driven infusion pumps can simulate pharmacokinetics reflective of human dosing. However, this study was limited to only 1 pneumonia model and 1 strain of MRSA. In this model, clindamycin, ceftaroline, and, to a lesser extent, linezolid were effective in reducing the mortality rate of necrotizing staphylococcal pneumonia. Bacterial burdens in the lungs and spleen were significantly lower in linezolid-, clindamycin-, and ceftaroline-treated rabbits. A 48-hour vancomycin treatment also reduced the bacterial burden compared with the control group, but the early-mortality rate was very high in this group, as recently described by Diep and colleagues (36).
Linezolid, clindamycin, and ceftaroline reduced the lung injury in this model, whereas vancomycin did not significantly reduce the tissue damage compared with the control group. In addition, linezolid, clindamycin, and ceftaroline effectively reduced the PVL concentration in the lungs, whereas vancomycin did not significantly reduce it, and this correlated with tissue damage and a high mortality rate in this group. Further testing may be necessary to ascertain the direct relationship between decreased lung concentrations of PVL and reduction in tissue damage. These findings do suggest that alternatives to vancomycin may be more appropriate in PVL-producing MRSA infections. Although clindamycin significantly decreased PVL levels, this reduction was likely a result of decreased pvl mRNA expression as seen in vitro, suggesting a negative regulation effect on gene transcription. In contrast, ceftaroline does not alter PVL mRNA expression in vitro, and the strong reduction of the pvl level in infected animals is thus likely to be a result of a direct decrease in bacterial counts. Despite its powerful known bactericidal effect, ceftaroline did not increase PVL concentrations within the lung, thus suggesting that antibiotics with a specific antitoxinic effect are not mandatory in the setting of PVL-positive MRSA pneumonia if a large reduction of the inoculum is rapidly achieved.
MRSA isolates that are resistant to current antibiotics are emerging, confirming the need for newer antibiotic options. Evidence of the development of clindamycin-resistant isolates is increasing (14, 37). In addition, the role of vancomycin in treating severe MRSA infections may be diminishing. In in vitro, in vivo, and clinical studies, vancomycin has been shown to be less efficacious than comparators (36, 38–41). Additionally, vancomycin is associated with poorer outcomes in patients with MSSA infections than β-lactam antibiotics (42, 43). This is important, because MSSA can also carry the PVL genes, and thus, newer antibiotics, such as ceftaroline, with good coverage of MSSA and activity against PVL-positive isolates are necessary. A double-blind study comparing ceftaroline fosamil with the combination of ceftriaxone and vancomycin in the treatment of patients at risk for MRSA pneumonia is currently enrolling patients (NCT01645735).
Conclusions.
In this rabbit model of necrotizing staphylococcal pneumonia, human-equivalent dosing with clindamycin, ceftaroline fosamil, and, to a lesser extent, linezolid demonstrated efficacy in bacterial reduction in both the lungs and spleen and also reduction of lung injury or tissue damage. The bactericidal effect of ceftaroline is associated with a strong reduction in the concentration of pulmonary PVL without apparent effect on pvl expression. This study provides strong support for clinical evaluation of ceftaroline fosamil for use in treating PVL-positive CA-MRSA.
ACKNOWLEDGMENTS
We acknowledge Jerome Etienne for fruitful discussions.
Scientific Therapeutics Information, Inc., provided editorial assistance, which was funded by Forest Research Institute, Inc. This study was supported by Cerexa, Inc. (Oakland, CA, USA), a wholly owned subsidiary of Forest Laboratories, Inc. (New York, NY, USA). Cerexa, Inc. was involved in the design and decision to present these results. Cerexa, Inc. had no involvement in the collection, analysis, or interpretation of data.
D.C.-B.'s institution has received grants from Cerexa and Pfizer. D.H.'s institution has received grants from Cerexa and Pfizer. S.D.S.'s institution has received grants from Cerexa and Pfizer. D.L.'s institution has received grants from Cerexa and Pfizer. D.B. is an employee of Cerexa, Inc., a wholly owned subsidiary of Forest Laboratories, Inc. Forest Laboratories, Inc. sponsored this study. D.B. holds stock and stock options in Forest Laboratories, Inc. C.B., O.D., and P.G. have no conflicts of interest. P.-E.C. has served as a board member and a consultant for Astellas; has received grants from Pfizer; has been on the speakers' bureaus for Astellas, Novartis, and Pfizer; has received payment for manuscript preparation from Thermofisher Scientific; and has received payment for development of educational presentations from Astellas. L.P. has served on the boards of Bristol Myers Squibb and Gilead; has served as a consultant for Janssen-Cilag, MSD, Roche, Schering Plough, and Viiv Healthcare; and has been paid for expert testimony by Boehringer; his institution has received grants from Roche; he has been a member of the speakers' bureaus for Abbott, Bristol Myers Squibb, MSD, Pfizer, and Viiv Healthcare; and he has received payment for travel expenses for an international congress (CROI). G.L. has been a consultant for Novartis, has received grants from Pfizer, has served on the speakers' bureau for Pfizer, and has received payment for development of educational presentations from bioMérieux. F.V.'s institution has received payment for board membership for F.V. to serve on the board of bioMérieux and as a consultant for Astra Zeneca, Novartis, and Pfizer, and his institution has received payment from bioMérieux for F.V. to receive grants and patents. P.C. has no conflict of interest.
Footnotes
Published ahead of print 6 January 2014
REFERENCES
- 1.O'Hara FP, Amrine-Madsen H, Mera RM, Brown ML, Close NM, Suaya JA, Acosta CJ. 2012. Molecular characterization of Staphylococcus aureus in the United States 2004–2008 reveals the rapid expansion of USA300 among inpatients and outpatients. Microb. Drug Resist. 18:555–561. 10.1089/mdr.2012.0056 [DOI] [PubMed] [Google Scholar]
- 2.Hidron AI, Low CE, Honig EG, Blumberg HM. 2009. Emergence of community-acquired meticillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. Lancet Infect. Dis. 9:384–392. 10.1016/S1473-3099(09)70133-1 [DOI] [PubMed] [Google Scholar]
- 3.Wunderink RG. 2013. How important is methicillin-resistant Staphylococcus aureus as a cause of community-acquired pneumonia and what is best antimicrobial therapy? Infect. Dis. Clin. North Am. 27:177–188. 10.1016/j.idc.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 4.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144:309–317. 10.7326/0003-4819-144-5-200603070-00005 [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core surveillance (ABCs) MRSA Investigators 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- 6.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Costello AJ, Kroeger JS, Biek D, Critchley IA, Diekema DJ, Doern GV. 2011. Activity of ceftaroline and epidemiologic trends in Staphylococcus aureus isolates collected from 43 medical centers in the United States in 2009. Antimicrob. Agents Chemother. 55:4154–4160. 10.1128/AAC.00315-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tenover FC, Goering RV. 2009. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J. Antimicrob. Chemother. 64:441–446. 10.1093/jac/dkp241 [DOI] [PubMed] [Google Scholar]
- 8.Marchese A, Gualco L, Maioli E, Debbia E. 2009. Molecular analysis and susceptibility patterns of meticillin-resistant Staphylococcus aureus (MRSA) strains circulating in the community in the Ligurian area, a northern region of Italy: emergence of USA300 and EMRSA-15 clones. Int. J. Antimicrob. Agents 34:424–428. 10.1016/j.ijantimicag.2009.06.016 [DOI] [PubMed] [Google Scholar]
- 9.Schaumburg F, Köck R, Mellmann A, Richter L, Hasenberg F, Kriegeskorte A, Friedrich AW, Gatermann S, Peters G, von Eiff C, Becker K. 2012. Population dynamics among methicillin-resistant Staphylococcus aureus isolates in Germany during a 6-year period. J. Clin. Microbiol. 50:3186–3192. 10.1128/JCM.01174-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, Gardner DJ, Fan X, Tseng CW, Liu GY, Badiou C, Etienne J, Lina G, Matthay MA, DeLeo FR, Chambers HF. 2010. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. U. S. A. 107:5587–5592. 10.1073/pnas.0912403107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillet Y, Vanhems P, Lina G, Bes M, Vandenesch F, Floret D, Etienne J. 2007. Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton-Valentine leukocidin. Clin. Infect. Dis. 45:315–321. 10.1086/519263 [DOI] [PubMed] [Google Scholar]
- 12.David MZ, Daum RS. 2010. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 23:616–687. 10.1128/CMR.00081-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak JM, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–55. 10.1093/cid/ciq146 [DOI] [PubMed] [Google Scholar]
- 14.McDougal LK, Fosheim GE, Nicholson A, Bulens SN, Limbago BM, Shearer JE, Summers AO, Patel JB. 2010. Emergence of resistance among USA300 methicillin-resistant Staphylococcus aureus isolates causing invasive disease in the United States. Antimicrob. Agents Chemother. 54:3804–3811. 10.1128/AAC.00351-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs MR, Good CE, Windau AR, Bajaksouzian S, Biek D, Critchley IA, Sader HS, Jones RN. 2010. Activity of ceftaroline against recent emerging serotypes of Streptococcus pneumoniae in the United States. Antimicrob. Agents Chemother. 54:2716–2719. 10.1128/AAC.01797-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGee L, Biek D, Ge Y, Klugman M, du Plessis M, Smith AM, Beall B, Whitney CG, Klugman KP. 2009. In vitro evaluation of the antimicrobial activity of ceftaroline against cephalosporin-resistant isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 53:552–556. 10.1128/AAC.01324-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mushtaq S, Warner M, Ge Y, Kaniga K, Livermore DM. 2007. In vitro activity of ceftaroline (PPI-0903M, T-91825) against bacteria with defined resistance mechanisms and phenotypes. J. Antimicrob. Chemother. 60:300–311. 10.1093/jac/dkm150 [DOI] [PubMed] [Google Scholar]
- 18.Sader HS, Fritsche TR, Jones RN. 2008. Antimicrobial activities of ceftaroline and ME1036 tested against clinical strains of community-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 52:1153–1155. 10.1128/AAC.01351-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saravolatz L, Pawlak J, Johnson L. 2010. In vitro activity of ceftaroline against community-associated methicillin-resistant, vancomycin-intermediate, vancomycin-resistant, and daptomycin-nonsusceptible Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 54:3027–3030. 10.1128/AAC.01516-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhanel GG, Rossnagel E, Nichol K, Cox L, Karlowsky JA, Zelenitsky S, Noreddin AM, Hoban DJ. 2011. Ceftaroline pharmacodynamic activity versus community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus, heteroresistant vancomycin-intermediate S. aureus, vancomycin-intermediate S. aureus and vancomycin-resistant S. aureus using an in vitro model. J. Antimicrob. Chemother. 66:1301–1305. 10.1093/jac/dkr110 [DOI] [PubMed] [Google Scholar]
- 21.Forest Pharmaceuticals, Inc. 2012. Teflaro (ceftaroline fosamil) prescribing information. Forest Pharmaceuticals, Inc., St. Louis, MO [Google Scholar]
- 22.AstraZeneca AB. 2012. Zinforo; summary of product characteristics. AstraZeneca AB, Sodertalje, Sweden [Google Scholar]
- 23.Löffler B, Hussain M, Grundmeier M, Brück M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. 2010. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 6:e1000715. 10.1371/journal.ppat.1000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipinska U, Hermans K, Meulemans L, Dumitrescu O, Badiou C, Duchateau L, Haesebrouck F, Etienne J, Lina G. 2011. Panton-Valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: a rabbit model. PLoS One 6:e22864. 10.1371/journal.pone.0022864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, Long RD, Dorward DW, Gardner DJ, Lina G, Kreiswirth BN, DeLeo FR. 2006. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J. Infect. Dis. 194:1761–1770. 10.1086/509506 [DOI] [PubMed] [Google Scholar]
- 26.Croisier-Bertin D, Piroth L, Charles P-E, Larribeau A, Biek D, Ge Y, Chavanet P. 2011. Ceftaroline versus ceftriaxone in a highly penicillin-resistant pneumococcal pneumonia rabbit model using simulated human dosing. Antimicrob. Agents Chemother. 55:3557–3563. 10.1128/AAC.01773-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piroth L, Martin L, Coulon A, Lequeu C, Duong M, Buisson M, Portier H, Chavanet P. 1999. Development of a new experimental model of penicillin-resistant Streptococcus pneumoniae pneumonia and amoxicillin treatment by reproducing human pharmacokinetics. Antimicrob. Agents Chemother. 43:2484–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badiou C, Dumitrescu O, George N, Forbes AR, Drougka E, Chan KS, Ramdani-Bouguessa N, Meugnier H, Bes M, Vandenesch F, Etienne J, Hsu LY, Tazir M, Spiliopoulou I, Nimmo GR, Hulten KG, Lina G. 2010. Rapid detection of Staphylococcus aureus Panton-Valentine leukocidin in clinical specimens by enzyme-linked immunosorbent assay and immunochromatographic tests. J. Clin. Microbiol. 48:1384–1390. 10.1128/JCM.02274-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumitrescu O, Choudhury P, Boisset S, Badiou C, Bes M, Benito Y, Wolz C, Vandenesch F, Etienne J, Cheung AL, Bowden MG, Lina G. 2011. Beta-lactams interfering with PBP1 induce Panton-Valentine leukocidin expression by triggering sarA and rot global regulators of Staphylococcus aureus. Antimicrob. Agents Chemother. 55:3261–3271. 10.1128/AAC.01401-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. 10.1093/nar/30.9.e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosowska-Shick K, McGhee PL, Appelbaum PC. 2010. Affinity of ceftaroline and other β-lactams for penicillin-binding proteins from Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob. Agents Chemother. 54:1670–1677. 10.1128/AAC.00019-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moisan H, Pruneau M, Malouin F. 2010. Binding of ceftaroline to penicillin-binding proteins of Staphylococcus aureus and Streptococcus pneumoniae. J. Antimicrob. Chemother. 65:713–716. 10.1093/jac/dkp503 [DOI] [PubMed] [Google Scholar]
- 33.Jacqueline C, Amador G, Caillon J, Le Mabecque V, Batard E, Miègeville AF, Biek D, Ge Y, Potel G, Hamel A. 2010. Efficacy of the new cephalosporin ceftaroline in the treatment of experimental methicillin-resistant Staphylococcus aureus acute osteomyelitis. J. Antimicrob. Chemother. 65:1749–1752. 10.1093/jac/dkq193 [DOI] [PubMed] [Google Scholar]
- 34.Jacqueline C, Caillon J, Le Mabecque V, Miègeville AF, Hamel A, Bugnon D, Ge JY, Potel G. 2007. In vivo efficacy of ceftaroline (PPI-0903), a new broad-spectrum cephalosporin, compared with linezolid and vancomycin against methicillin-resistant and vancomycin-intermediate Staphylococcus aureus in a rabbit endocarditis model. Antimicrob. Agents Chemother. 51:3397–3400. 10.1128/AAC.01242-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhalodi AA, Crandon JL, Biek D, Nicolau DP. 2012. Efficacy of ceftaroline fosamil in a staphylococcal murine pneumonia model. Antimicrob. Agents Chemother. 56:6160–6165. 10.1128/AAC.01078-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diep BA, Afasizheva A, Le HN, Kajikawa O, Matute-Bello G, Tkaczyk C, Sellman B, Badiou C, Lina G, Chambers HF. 12 April 2013. Effects of linezolid on suppressing in vivo production of staphylococcal toxins and improving survival outcomes in a rabbit model of methicillin-resistant Staphylococcus aureus necrotizing pneumonia. J. Infect. Dis. 10.1093/infdis/jit129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohapatra TM, Shrestha B, Pokhrel BM. 2009. Constitutive and inducible clindamycin resistance in Staphylococcus aureus and their association with meticillin-resistant S. aureus (MRSA): experience from a tertiary care hospital in Nepal. Int. J. Antimicrob. Agents 33:187–189. 10.1016/j.ijantimicag.2008.08.009 [DOI] [PubMed] [Google Scholar]
- 38.Docobo-Pérez F, López-Rojas R, Domínguez-Herrera J, Jiménez-Mejias ME, Pichardo C, Ibáñez-Martínez J, Pachón J. 2012. Efficacy of linezolid versus a pharmacodynamically optimized vancomycin therapy in an experimental pneumonia model caused by methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 67:1961–1967. 10.1093/jac/dks142 [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Olondris P, Rigol M, Soy D, Guerrero L, Agusti C, Quera MA, Li Bassi G, Esperatti M, Luque N, Liapikou M, Filella X, Marco F, de la Bellacasa JP, Torres A. 2012. Efficacy of linezolid compared to vancomycin in an experimental model of pneumonia induced by methicillin-resistant Staphylococcus aureus in ventilated pigs. Crit. Care Med. 40:162–168. 10.1097/CCM.0b013e31822d74a2 [DOI] [PubMed] [Google Scholar]
- 40.Vidaillac C, Leonard SN, Rybak MJ. 2009. In vitro activity of ceftaroline against methicillin-resistant Staphylococcus aureus and heterogeneous vancomycin-intermediate S. aureus in a hollow fiber model. Antimicrob. Agents Chemother. 53:4712–4717. 10.1128/AAC.00636-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. 2012. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin. Infect. Dis. 54:621–629. 10.1093/cid/cir895 [DOI] [PubMed] [Google Scholar]
- 42.Deresinski S. 2007. Counterpoint: vancomycin and Staphylococcus aureus—an antibiotic enters obsolescence. Clin. Infect. Dis. 44:1543–1548. 10.1086/518452 [DOI] [PubMed] [Google Scholar]
- 43.Kollef MH. 2007. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin. Infect. Dis. 45(Suppl 3):S191–S195. 10.1086/519470 [DOI] [PubMed] [Google Scholar]