Abstract
In chronic schistosomiasis, hepatic fibrosis is linked to the portal hypertension that causes morbidity in Schistosoma mansoni infection. Silymarin (SIL) is a hepatoprotective and antioxidant medicament largely prescribed against liver diseases that has previously been shown to prevent fibrosis during acute murine schistosomiasis. Here we employed silymarin to try to reverse established hepatic fibrosis in chronic schistosomiasis. Silymarin or vehicle was administered to BALB/c mice every 48 h, starting on the 40th (80 days of treatment), 70th (50 days), or 110th (10 days) day postinfection (dpi). All mice were sacrificed and analyzed at 120 dpi. Treatment with silymarin reduced liver weight and granuloma sizes, reduced the increase in alanine aminotransferase and aspartate aminotransferase levels, and reduced the established hepatic fibrosis (assessed by hydroxyproline contents and picrosirius staining). Treatment with silymarin also reduced the levels of interleukin-13 (IL-13) in serum and increased the gamma interferon (IFN-γ)/IL-13 ratio. There was a linear correlation between IL-13 levels in serum and hydroxyproline hepatic content in both infected untreated and SIL-treated mice, with decreased IL-13 levels corresponding to decreased hydroxyproline hepatic contents. Treatment with either SIL or N-acetylcysteine reduced both proliferation of fibroblast cell lines and basal/IL-13-induced production of collagen I, indicating that besides inhibiting IL-13 production during infection, SIL antioxidant properties most likely contribute to inhibition of collagen production downstream of IL-13. These results show that silymarin interferes with fibrogenic cytokines, reduces established fibrosis, and inhibits downstream effects of IL-13 on fibrogenesis, indicating the drug as a safe and cheap treatment to liver fibrotic disease in schistosomiasis.
INTRODUCTION
Schistosomiasis is a chronic disease of high prevalence and wide distribution around the world (1) caused by worms that parasitize the vascular system. The morbidity in schistosomiasis is associated with the arrival of worm eggs to the liver and the stimulation of a granulomatous reaction (2). Chronic liver pathology is closely associated to the nature of the host inflammatory response. The immunological progression of this disease is frequently divided in distinct phases: prepostural acute Th1 phase, postural acute Th2 phase, and chronic Th2 downmodulated phase (3). The control of this Th response throughout the chronic phase may be associated with the reduction of morbidity in schistosomiasis. During acute murine infection, administration of antioxidant drugs such as curcumin (4), resveratrol (5), n-acetylcysteine (6), artemether (7), and silymarin (8) is used to reduce morbidity and prevent hepatic fibrosis. The reversal of established hepatic fibrosis at the chronic stage is directly linked to the portal hypertension, the major cause of morbidity in Schistosoma mansoni infection, and was not attained in any of these previous works. Currently, there is no medical treatment to reverse the hepatic fibrosis once it is established.
Silymarin is composed mainly of flavonolignans (9). It is a reactive oxygen species (ROS) scavenger (10) that inhibits lipid peroxidation (11), stimulates glutathione synthesis (12), induces superoxide dismutase (13), and has iron-chelating activities (14). Silymarin is therefore an antioxidant and also a reputed hepatoprotective drug (15). We have previously demonstrated that silymarin administration reduces fibrosis deposition in the liver during acute S. mansoni infection. This reduction is associated with a decrease in granuloma sizes (8).
Several works have focused on the role of cytokines in promoting liver collagen deposition during S. mansoni infection. The cytokine interleukin-13 (IL-13) directly stimulates collagen synthesis by fibroblasts (16), and blocking studies established that IL-13 is the primary fibrogenic factor in S. mansoni infection (17). Additionally, IL-13 blockade greatly reduces fibrosis during chronic infection, although it fails to affect the overall granulomatous response (18). The role of IL-4 in fibrogenesis is controversial (17, 19) and possibly mistaken as fibrogenic due to impaired IL-13 responses in IL-4-deficient mice (18, 19). On the other hand, gamma interferon (IFN-γ) exhibits marked antifibrotic activity during S. mansoni infection (20, 21).
The development of new drugs to be used against schistosomiasis is of great relevance (22). Although praziquantel can reduce fibrosis (23), drugs that could accelerate or amplify this role would be very important. Here we assessed whether silymarin administered during chronic schistosomiasis could reverse the established hepatic fibrosis. We also evaluated the levels of profibrogenic IL-13 and IL-4 and antifibrogenic IFN-γ in serum in order to get insight into silymarin's mechanism of action. Silymarin reduced the levels of IL-13 and IL-4 in serum, increased the IFN-γ/IL-13 ratio, and diminished hepatic fibrosis in chronic schistosomiasis. Additionally, we studied the effects of silymarin upon basal and IL-13-stimulated collagen I production by fibroblasts and found that silymarin inhibits both, besides inhibiting proliferation of fibroblast cell lines. These results indicate silymarin as a promising antifibrotic drug to be tested in clinical studies.
MATERIALS AND METHODS
Animals, drug, and infection.
Adult BALB/c female mice (7 to 8 weeks of age) were infected with 60 cercariae of Schistosoma mansoni strain BH by the cutaneous route, reaching the chronic phase at 120 days postinfection (dpi). Briefly, silymarin (batch number 107K0762; silybin content, 47%; Sigma-Aldrich, USA) is composed mainly of flavonolignans from the fruit of Silybum marianum, including silicristin (22.6%), silydianin (9.06%), silybin A (21.3%), silybin B (34.9%), isosilybin A (8.26%), and isosilybin B (3.91%), as determined by high-pressure liquid chromatography (HPLC) (24). Silymarin was suspended in 1% carboxymethylcellulose (CMC) (Sigma-Aldrich, USA) (25, 26) to avoid quick precipitation and administered every 48 h at 10 mg kg−1 of body weight intraperitoneally (i.p.) as previously described (8). Noninfected controls (N) and infected (I) mice were divided randomly in seven groups of 8 animals: nontreated (N and I), treated during 80 days with CMC (I+Veh 80D), or treated with silymarin for 80 days (N+SIL 80D and I+SIL 80D), 50 days (I+SIL 50D), or 10 days (I+SIL 10D). The groups treated during 80 days, 50 days, and 10 days started treatment at the 40th dpi, 70th dpi, or 110th dpi, respectively. Animals from all groups were maintained under controlled temperature and light conditions, fed a balanced diet and sterile water ad libitum, and submitted to euthanasia under anesthesia at 120 dpi. Procedures were approved and conducted in accordance with guidelines for care and use of laboratory animals (CEUA) of the Centro de Ciências da Saúde-UFRJ (protocol number DBFCICB032, 2009), which conform to the National Institutes of Health (Bethesda, MD, USA) guidelines.
Parasitological parameters.
Hepatic and intestinal tissues were digested as described by Cheever (27). Briefly, tissues were maintained in 4% KOH at room temperature for approximately 12 h, followed by 1 h of incubation at 37°C. Eight independent samples were counted.
ALT and AST levels.
The alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in serum, markers of hepatocellular damage, were established by colorimetric assay using a commercial kit from Labtest Diagnóstica S.A. (Lagoa Santa, MG, Brazil).
Histopathological analysis.
Transversal sections of all liver lobes were collected, fixed in 4% buffered formaldehyde solution, and embedded in paraffin. Five-micrometer sections were stained with hematoxylin and eosin (H&E) or phosphomolybdic acid-picrosirius red staining (PMA-PSR) (28) and read by bright-field microscopy. The area of hepatic granuloma was determined in histological sections from 20 to 30 granulomas per animal, containing central eggs, randomly chosen. The granuloma area was manually delimited in H&E images, and the collagen area was determined in PMS-PSR images, which were captured by a charge-coupled-device (CCD) camera using bright-field microscopy and automatically processed with ImageJ 1.45 software. All evaluations were performed by two different blinded observers.
Hydroxyproline.
Hydroxyproline quantification was determined as described by Stegemann and Stalder (29). Briefly, livers were maintained in acetone at room temperature until complete dehydration, followed by hydrochloric acid hydrolyzation for overnight incubation at 107°C. Colorimetric assay was then performed using chloramine-T buffer (Sigma, USA), Ehrlich's reagent (Sigma, USA), and perchloric acid (Merck).
Cytokine assay.
IL-13, IL-4, and IFN-γ levels in serum were measured at 120 dpi by a sandwich enzyme-linked immunosorbent assay technique with capture and detection antibodies according to the instructions of the manufacturer (R&D, USA). Recombinant cytokines were used as standards.
Fibroblast proliferation and viability assays.
Mouse embryonic fibroblasts (MEFs) and murine hepatic stellate cell line (GRX) were cultured at 37°C in 5% CO2 and 95% air atmosphere maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), whereas cell line L929 was routinely cultivated in RPMI with 10% FBS.
For lactate dehydrogenase (LDH) and cell proliferation assay using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), 2 × 103 L929 and MEF cells/well or 1 × 104 GRX cells/well were seeded in 96-well plates for 12, 24, 48, and 72 h. The cell membrane integrity was evaluated by quantifying LDH via a cytotoxicity detection kit, according to the manufacturer's instructions (Doles, Brazil). The cell proliferation assay was based on cleavage of the tetrazolium salt MTT (0.5 mg/ml) after incubation for 4 h, using dimethyl sulfoxide (DMSO) to dissolve formazan crystals quantified by a spectrophotometer with absorbance of 590 nm.
Immunofluorescence.
Cells were plated onto 24-well plates at a density of 1 × 105 cells per well and cultured for 7 days in the presence of SIL (50 μM), N-acetylcysteine (NAC) (10 mM), and/or recombinant IL-13 (rIL-13) (50 ng/ml). Cell cultures were subsequently rinsed with PBS, fixed with 4% paraformaldehyde for 4 h, permeabilized with Triton–0.03% PBS, and blocked with 10% BSA for 30 min. Then, cells were washed with Triton–0.03% PBS and incubated overnight with anti-collagen I, N-terminal (1:500, batch number 310154; Sigma-Aldrich) at 4°C. The cells were washed five times for 10 min, incubated with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit secondary antibody (1:200) at room temperature for 2 h, and then washed with PBS five times.
A Leica microscope inverted DMI6000 B connected to a Leica DFC 360Fx camera was used to assess immunofluorescence. Four pictures were captured for each group with high magnification (×400), and the same area was manually selected and analyzed (75,025.39 μm2). The fluorescence intensity was automatically determined using Leica Application Suite software (Advanced Fluorescence Lite, LAS AF Version 2.6.0).
Statistical analysis.
Statistical analysis was performed by analysis of variance (ANOVA) with Tukey's posttest. P values of <0.05 were considered significant.
RESULTS
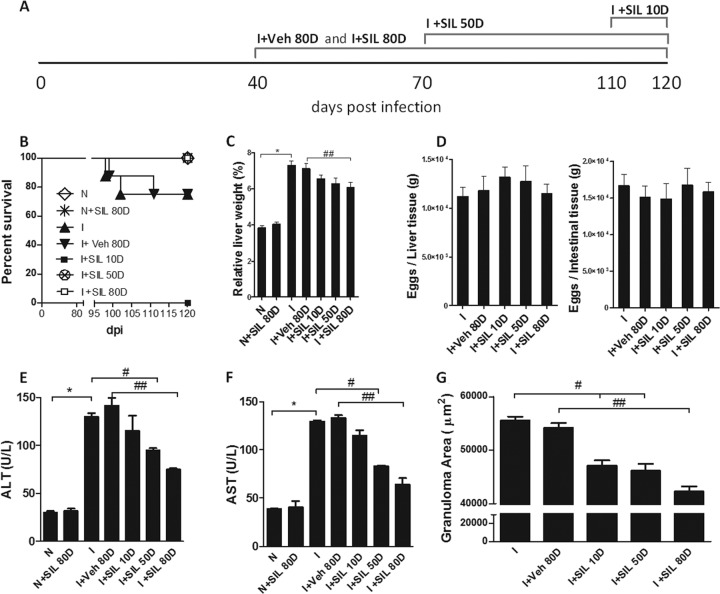
Silymarin (SIL) or vehicle (Veh, 1% carboxymethylcellulose) was administered to BALB/c mice every 48 h, starting at 40 dpi and extending through an 80-day period (I+Veh 80D and I+SIL 80D), starting at 70 dpi and extending through a 50-day period (I+SIL 50D), or starting at 110 dpi and extending through a 10-day period (I+SIL 10D). Noninfected mice were also treated with silymarin for 80 days as a control (N+SIL 80D). All mice were sacrificed and analyzed at 120 dpi.
All silymarin-treated mice survived chronic infection, while some infected nontreated (I, n = 2) or Veh-treated mice (I+Veh 80D, n = 2) died, but this phenomenon was not statistically significant (Fig. 1B). The characteristic hepatomegaly that accompanies S. mansoni infection was partially reduced by treatment with silymarin (Fig. 1C). Hepatic and intestinal tissues were digested, and no parasitological differences were observed between groups (Fig. 1D). The levels of the hepatic lesion markers ALT and AST in serum were decreased in mice treated with silymarin (Fig. 1E and F). Treatment with silymarin also reduced the sizes of the granulomas (Fig. 1G). These data demonstrate that silymarin protects mice from liver disease during chronic schistosomiasis.
FIG 1.
Silymarin reduced mortality and liver morbidity in chronic S. mansoni infection. Mice were left untreated (I) or treated with carboxymethylcellulose (I+Veh 80D) or silymarin (10 mg kg−1) for 10 days (I+SIL 10D), 50 days (I+SIL 50D), or 80 days (I+SIL 80D). Noninfected groups were used as controls (N and N+SIL 80D). (A) Schematic image describing the experimental design; (B) survival curve; (C) liver weights in relation to total animal weight; (D) equal distribution of tissue eggs; (E) ALT levels in sera; (F) AST levels in sera. (G) Granuloma areas were evaluated on histological sections (5 μm) of hepatic tissue stained with H&E; all granulomas containing a central viable egg were measured. Results are expressed as means + standard errors (SE) (n = 8). *, P < 0.05 for N versus I comparison; #, P < 0.05 for I versus I+SIL 50D and I+SIL 10D; ##, P < 0.05 for I+Veh 80D versus I+SIL 80D. Results are representative of two similar experiments.
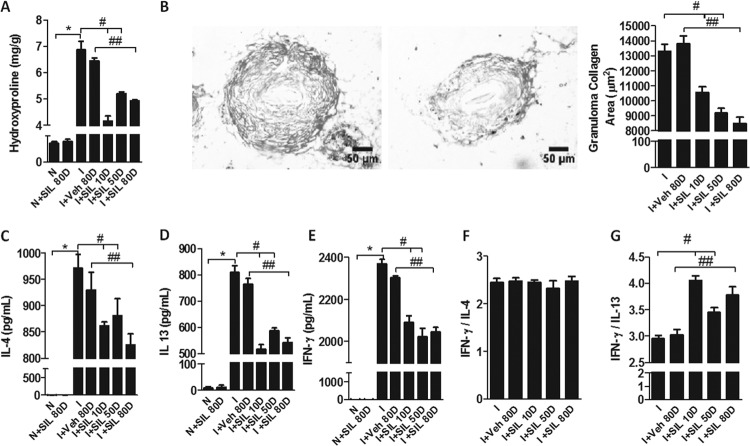
Treatment with silymarin greatly reduced the hydroxyproline content of the livers, indicating that it reverses hepatic fibrosis at the chronic stage (Fig. 2A). These results were confirmed by the reduced collagen area of the granulomas that was observed in silymarin-treated mice and illustrated by the two images in Fig. 2B. The levels of the profibrogenic cytokines IL-4 and IL-13 in serum were reduced in silymarin-treated compared to vehicle-treated or nontreated mice (Fig. 2C and D). Treatment with silymarin also reduced the levels of the antifibrogenic cytokine IFN-γ in serum (Fig. 2E), but the IFN-γ/IL-13 ratio was increased in silymarin-treated mice, indicating that silymarin favors a rather antifibrogenic profile (Fig. 2G). The IFN-γ/IL-4 ratio remained unchanged (Fig. 2F). Note that even when silymarin treatment was started late after infection (at 110 dpi) and extended through a short period (10 days), it was capable of reversing fibrosis and decreasing IL-13 amounts in serum, indicating that silymarin probably acts on late stages of fibrogenesis instead of on the establishment of a Th2 response.
FIG 2.
Silymarin reduced fibrosis and profibrogenic cytokines in chronic S. mansoni infection. (A) Biochemical quantification of hydroxyproline. (B) Examples of images from histological sections (5 μm) of hepatic tissue stained with picrosirius from I+Veh 80D (left) and I+SIL 80D (right) used to evaluate the granuloma collagen areas. (C to E) Concentrations of IL-4 (C), IL-13 (D), and IFN-γ (E) in serum. (F) IFN-γ/IL-4 relation; (G) IFN-γ/IL-13 ratio. Results were expressed as means + SE. *, P < 0.05 for N versus I comparison; #, P < 0.05 for I versus I+SIL 50D and I+SIL 10D; ##, P < 0.05 for I+Veh 80D versus I+SIL 80D.
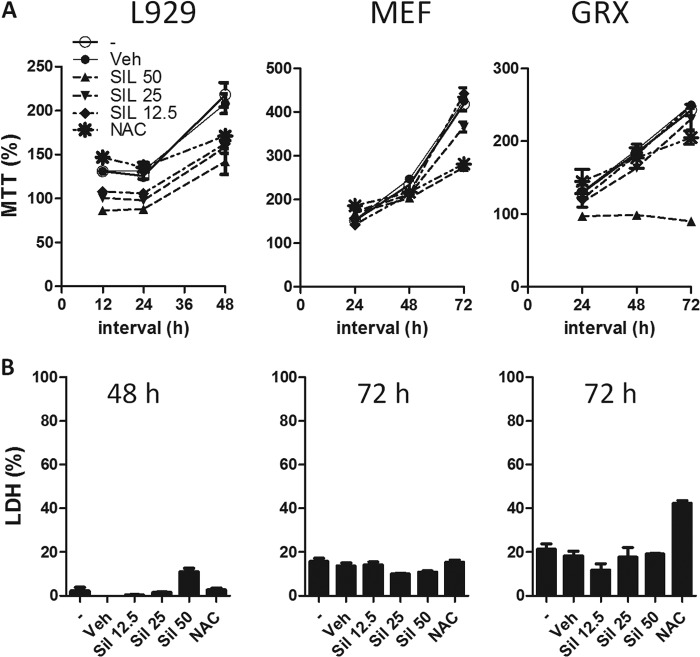
We next assessed whether silymarin could alter the proliferation of fibroblasts/hepatic stellate cells (HSC), the cells responsible for collagen deposition in the S. mansoni granulomatous reaction. To this purpose, we studied the proliferation of L929 cells (mouse fibroblastic lineage), mouse embryonic fibroblasts (MEFs), and hepatic stellate cells (GRX) by MTT assays. Silymarin was capable of inhibiting the proliferation of all three cell types at 50 μM (Fig. 3A) and caused a low decrease in viability, assessed by LDH (Fig. 3B). N-acetylcysteine, a drug capable of replenishing glutathione and helping scavenge reactive oxygen species, was also capable of inhibiting proliferation of these lineages, though at later time points and with a somewhat reduced efficiency. These results indicate that silymarin acts through its antioxidant properties to inhibit fibroblast proliferation.
FIG 3.
Silymarin inhibits fibroblast proliferation. (A) L929 murine fibroblasts, murine embryonic fibroblasts (MEFs), and GRX cells (hepatic stellate cell lineage) were incubated with SIL (12.5, 25, or 50 μM), NAC (10 mM), or vehicle (dimethyl sulfoxide [DMSO]) or left untreated for 12, 24, 48, or 72 h. Cell proliferation was assayed by MTT as described in Materials and Methods. (B) Viability of cell cultures at the later time point was assessed by LDH assay. Data represent means + SE for triplicate values. Results are representative of two similar experiments.
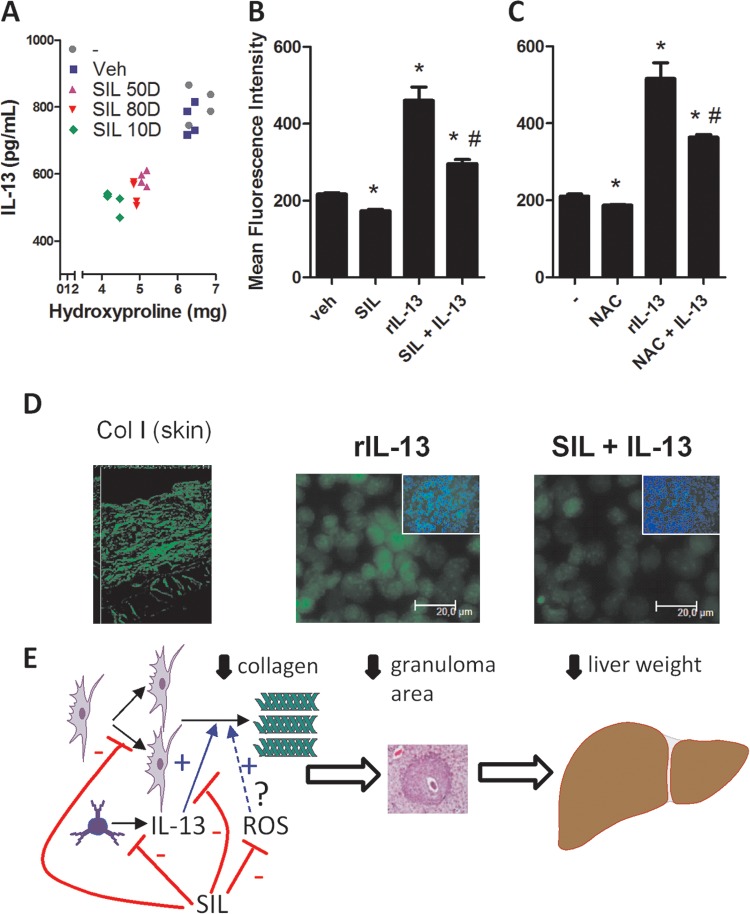
We studied the correlation between IL-13 amounts in serum and hepatic hydroxyproline contents in nontreated and silymarin-treated mice by Pearson's analysis. The reduction of IL-13 amounts in serum produced by silymarin treatment was linearly correlated with the reduction of hydroxyproline hepatic content in mice (Fig. 4A). In fact, treatment did not modify the original correlation between hydroxyproline and IL-13 found in infected nontreated mice. These data suggest that at least most of silymarin's antifibrogenic effects are exerted through the reduction that it causes in IL-13 levels. The cytokine IL-13 is thought to exert its fibrogenic effects by promoting collagen deposition by fibroblasts and HSC. To assess whether silymarin also acted downstream of IL-13 to inhibit fibrosis, we studied the production of collagen I by confluent L929 cell cultures after incubation with recombinant IL-13 (rIL-13), silymarin, or silymarin + rIL-13 for a week. The cytokine rIL-13 induced great amounts of collagen I, while silymarin alone was capable of inhibiting basal production of collagen I (Fig. 4B). When administered together, silymarin was capable of inhibiting rIL-13-induced collagen I production (Fig. 4B). A similar profile was found when NAC was used instead of silymarin, indicating that the antioxidant properties of silymarin are involved in its inhibitory effects upon fibrosis (Fig. 4C). Representative immunostaining of L929 is shown in Fig. 4D, along with a positive anti-collagen I-labeled skin control to ensure the specific staining pattern.
FIG 4.
Inhibition of fibrogenesis by silymarin correlates with decreased IL-13 serum amounts and reduced IL-13-induced collagen I production by fibroblasts. (A) Analysis of the correlation between IL-13 serum amounts and hepatic hydroxyproline contents in infected nontreated or treated mice. Samples examined for hydroxyproline and IL-13 came from the same pool of mice. The Pearson analysis resulted in an r value of 0.93 (significant linear correlation, P < 0.0001). (B) Quantification of collagen I production by L929 cells treated with rIL-13 (50 ng/ml), SIL (50 μM), or rIL-13+SIL for a week. (C) Experiment similar to that described for panel B, except that NAC (10 mM) was used instead of SIL. Collagen I immunofluorescence staining was analyzed as described in Materials and Methods. *, P < 0.05 compared to vehicle (Veh) in panel B or untreated (−) in panel C; #, P < 0.05, compared to rIL-13. (D) Illustrative confluent cell cultures as in panel C assessed for collagen I production by immunofluorescence microscopy. Insets represent collagen I and DAPI (4′,6-diamidino-2-phenylindole) simultaneously stained cell cultures. Skin was used as a specific labeling control (left). (E) Based on our results, we postulate that SIL acts pleiotropically to inhibit fibrogenesis by decreasing IL-13 levels, fibroblast proliferation, and IL-13-dependent and basal (possibly ROS-dependent) collagen I production.
Together, our results allow us to postulate that silymarin has pleiotropic effects on fibrogenesis, reducing IL-13 amounts in serum, fibroblast proliferation, and IL-13-induced collagen I deposition by fibroblasts (Fig. 4E).
DISCUSSION
Silymarin is a natural product that has been used as a hepatoprotective medicament since the time of ancient Greece (30). It prevents apoptotic and necrotic cell death in the liver (31) and retards the progression of alcohol-induced hepatic fibrosis (32). Silymarin is the natural product of most widespread use in the treatment of liver diseases (33), and it is sold over the counter all over the world (34). Its low toxicity encouraged researchers to use silymarin in long-term tests in chronic and severe liver conditions in humans, and its efficacy in the prevention of liver fibrosis and stimulation of liver regeneration was observed in alcoholic and nonalcoholic fatty liver diseases and in drug- and chemical-induced hepatic toxicity (35). In viral hepatitis C patients, high-dosage studies were performed with silymarin, and silymarin did not show any toxicity (36) and still decreased progression from fibrosis to cirrhosis (37). Furthermore, the coadministration of silymarin with darunavir/ritonavir seems to be safe in HIV-infected patients (38). There are no known collateral effects of silymarin that could raise doubt on the safety of this drug in schistosomiasis, but still, our study in mice raised no concern on the safety of silymarin in schistosomiasis, representing a first step toward future human tests.
Schistosoma mansoni causes a rather silent infection in humans until the parasite has accomplished oviposition, by 6 to 8 weeks after infection, when it becomes symptomatic (39). Some eggs laid in the mesenteric vessels are carried by the blood flow and become trapped in the liver. Once the eggs have reached the liver, they can no longer be eliminated, and they promote a granulomatous reaction that isolates the eggs from the hepatic parenchyma. Collagen is deposited around the eggs by myofibroblasts. However, the fibrosis and vascular damage alter the blood flow in the liver, producing portal hypertension (40, 41). An inflamed, enlarged, and fibrotic liver was associated with increased AST and ALT levels in serum, which are markers of hepatic injury. Here, we have showed that the treatment with silymarin at the chronic phase of infection is capable of reducing ALT and AST levels in serum, hepatomegaly, and hepatic granuloma size. These results indicate that silymarin interferes with the overall hepatic disease, reducing hepatic injury and fibrosis and ameliorating morbidity.
The immune response associated with schistosomiasis is markedly different in its various phases (3). We have previously demonstrated that silymarin reduces granuloma area and collagen deposition during acute S. mansoni infection (8). It has been previously demonstrated that other antioxidants also have the ability to prevent schistosomiasis-induced fibrosis when administered in the acute phase (4, 5). El-Lakkany and coworkers (2012), treating infected mice with silymarin from 84 to 126 dpi, observed a slight decrease in the parasite burden and then in fibrosis, but the effect was smaller than that of praziquantel alone and possibly secondary to the decrease in burden (42). Herein, we did not observe alterations in the deposition of eggs in liver and intestinal tissues, which could suggest that there is no difference in parasite burden.
As fibrosis results from an active process that involves continuous collagen synthesis and degradation, one might expect that a drug that could interfere with this balance could reverse hepatic fibrosis. Here we showed that silymarin administered at the chronic stage of schistosomiasis could reverse chronic hepatic fibrosis and morbidity, even when administered for a short period late at the chronic stage. The reduction in hepatic fibrosis was associated with a decrease in the levels of fibrogenic IL-13 in serum and an increase in the IFN-γ/IL-13 ratio. As the short-course treatment (10 days, 5 doses) with silymarin started late after infection (110 dpi) was also able to reverse hepatic fibrosis to the same extent as that achieved with the long-course treatment (80 days, 40 doses), we believe that the treatment did not interfere with the Th polarization. Instead, the reduction of the secretion of effector cytokines or the direct effects of silymarin upon collagen secretion most likely contributed to reversing fibrosis.
Recently, silymarin was demonstrated to be capable of reducing transforming growth factor β1 (TGF-β1) at the acute phase and the number of mast cells in mice infected with S. mansoni (42). The authors associated the silymarin-induced reduction of fibrosis with a reduction in the levels of TGF-β1 in serum. However, TGF-β, a fibrogenic cytokine that is usually induced by IL-13 (43, 44), does not seem to be involved in the fibrogenic response to S. mansoni infection, as its blockade slightly reduces granuloma sizes but does not affect liver fibrosis (45, 46).
The collagen deposition that accompanies the granulomatous reaction is thought to be the result of fibrogenic cytokines signaling to myofibroblasts (47). In fact, blockade of IL-13 has been demonstrated to reduce established hepatic fibrosis (17), while IL-13 knockout mice (48) and mice transgenic for a soluble IL-13 receptor that blocks IL-13 actions (49) present reduced amounts of collagen in granulomas. Here we showed that treatment with antioxidant silymarin reduces the levels of IL-13 in serum. Though silymarin also reduced the levels of IL-4 and IFN-γ in plasma, the IFN-γ/IL-13 ratio was increased in silymarin-treated mice, while the IFN-γ/IL-4 ratio remained unchanged, indicating that the reduction of fibrosis better correlates with an increased IFN-γ/IL-13 ratio, as previously shown by others (18). Silymarin can thus represent a cheaper alternative to anti-IL-13 blockade in fibrotic diseases.
Human skin fibroblasts treated with silibinin have a reduction in type I collagen expression, which indicates that silibinin has the potential to prevent fibrotic skin changes (50). Moreover, fibroblast cell lines obtained from pulmonary infection with S. mansoni (51) as well as HSC (52), the two main cell types implicated in fibrogenic responses during the S. mansoni granulomatous reaction, react to rIL-13 with increased production of collagen I (53). Here we studied the direct effects of silymarin on collagen I production and fibroblast proliferation, phenomena involved in the genesis of hepatic fibrosis. Our results show that silymarin inhibits both fibroblast proliferation and IL-13-induced or basal collagen I production, indicating that besides reducing IL-13 levels, silymarin is capable of directly inhibiting IL-13 signaling responses. Nevertheless, the relevance of these direct antifibrogenic responses in S. mansoni-infected mice treated with silymarin is most likely to be small, since IL-13 amounts in serum linearly correlate with hydroxyproline hepatic content in silymarin-treated mice.
Oxidative stress is associated with fibrogenesis. It is known that lymphocytes from gp91phox−/− mice (deficient in the superoxide production that accompanies the respiratory burst) produce less IL-13 under allergic sensitization (54), indicating that IL-13 production is under ROS control and that antioxidants are able to reduce liver fibrosis (55, 56) in many instances. We have shown here that IL-13-related liver fibrosis can also be reduced by silymarin, and we speculate that its antioxidant effects control both IL-13 production and downstream fibrogenic events.
The decrease in IL-13 amounts in serum and the increase in the IFN-γ/IL-13 ratio show that silymarin interferes with fibrogenic cytokines, a phenomenon that we have showed to be correlated with reduced collagen content. Treatment with silymarin partially reduced hepatic tissue lesions and decreased hepatomegaly, granuloma sizes, ALT and AST levels, and the established hepatic fibrosis. In light of the attested safety of silymarin in long-term treatments, as well as its broad efficacy as a hepatoprotective medicament, we believe that the murine study performed here should be followed by baboon studies (a successful primate model of schistosomiasis [57]) in order to pave the way for future clinical studies. The pleiotropic effects of silymarin on fibrogenesis that we showed here, besides its widespread use in hepatic diseases and its low cost, indicate that silymarin is a promising candidate to be tested as a treatment to S. mansoni infection sequelae.
ACKNOWLEDGMENTS
We are grateful to the Laboratório de Malacologia (FIOCRUZ-RJ) for the S. mansoni cercariae.
We declare that we have no conflict of interests.
Footnotes
Published ahead of print 21 January 2014
REFERENCES
- 1.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6:411–425. 10.1016/S1473-3099(06)70521-7 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 2003. Action against worms. PPC Newsl. 1:1–6 [Google Scholar]
- 3.Pearce EJ, MacDonald AS. 2002. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2:499–511. 10.1038/nri843 [DOI] [PubMed] [Google Scholar]
- 4.Allam G. 2009. Immunomodulatory effects of curcumin treatment on murine schistosomiasis mansoni. Immunobiology 214:712–727. 10.1016/j.imbio.2008.11.017 [DOI] [PubMed] [Google Scholar]
- 5.El-Agamy DS, Shebl AM, Said SA. 2011. Prevention and treatment of Schistosoma mansoni-induced liver fibrosis in mice. Inflammopharmacology 19:307–316. 10.1007/s10787-011-0092-6 [DOI] [PubMed] [Google Scholar]
- 6.Seifel-Din SH, Al-Hroob AM, Ebeid FA. 2011. Schistosoma mansoni: N-acetylcysteine downregulates oxidative stress and enhances the antischistosomal activity of artemether in mice. Exp. Parasitol. 128:230–235. 10.1016/j.exppara.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 7.Abdul-Ghani R, Loutfy N, Sheta M, Hassan A. 2011. Artemether shows promising female schistosomicidal and ovicidal effects on the Egyptian strain of Schistosoma mansoni after maturity of infection. Parasitol. Res. 108:1199–1205. 10.1007/s00436-010-2163-9 [DOI] [PubMed] [Google Scholar]
- 8.Mata-Santos HA, Lino FG, Rocha CC, Paiva CN, Castelo Branco MT, Pyrrho AS. 2010. Silymarin treatment reduces granuloma and hepatic fibrosis in experimental schistosomiasis. Parasitol. Res. 107:1429–1434. 10.1007/s00436-010-2014-8 [DOI] [PubMed] [Google Scholar]
- 9.He Q, Osuchowski MF, Johnson VJ, Sharma RP. 2002. Physiological responses to a natural antioxidant flavonoid mixture, silymarin, in BALB/c mice: I induction of transforming growth factor beta1 and c-myc in liver with marginal effects on other genes. Planta Med. 68:676–679. 10.1055/s-2002-33801 [DOI] [PubMed] [Google Scholar]
- 10.Comoglio A, Leonarduzzi G, Carini R, Busolin D, Basaga H, Albano E, Tomasi A, Poli G, Morazzoni P, Magistretti MJ. 1990. Studies on the antioxidant and free radical scavenging properties of IdB 1016 a new flavanolignan complex. Free Radic. Res. Commun. 11:109–115. 10.3109/10715769009109673 [DOI] [PubMed] [Google Scholar]
- 11.Valenzuela A, Lagos C, Schmidt K, Videla LA. 1985. Silymarin protection against hepatic lipid peroxidation induced by acute ethanol intoxication in the rat. Biochem. Pharmacol. 34:2209–2212. 10.1016/0006-2952(85)90421-6 [DOI] [PubMed] [Google Scholar]
- 12.Valenzuela A, Aspillaga M, Vial S, Guerra R. 1989. Selectivity of silymarin on the increase of the glutathione content in different tissues of the rat. Planta Med. 55:420–422. 10.1055/s-2006-962056 [DOI] [PubMed] [Google Scholar]
- 13.Muzes G, Deak G, Lang I, Nekam K, Gergely P, Feher J. 1991. Effect of the bioflavonoid silymarin on the in vitro activity and expression of superoxide dismutase (SOD) enzyme. Acta Physiol. Hung 78:3–9 [PubMed] [Google Scholar]
- 14.Koksal E, Gulcin I, Beyza S, Sarikaya O, Bursal E. 2009. In vitro antioxidant activity of silymarin. J. Enzyme Inhib. Med. Chem. 24:395–405. 10.1080/14756360802188081 [DOI] [PubMed] [Google Scholar]
- 15.Luper S. 1998. A review of plants used in the treatment of liver disease: part 1. Altern. Med. Rev. 3:410–421 [PubMed] [Google Scholar]
- 16.Wynn TA. 2011. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 208:1339–1350. 10.1084/jem.20110551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. 1999. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Invest. 104:777–785. 10.1172/JCI7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiaramonte MG, Cheever AW, Malley JD, Donaldson DD, Wynn TA. 2001. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology 34:273–282. 10.1053/jhep.2001.26376 [DOI] [PubMed] [Google Scholar]
- 19.Cheever AW, Williams ME, Wynn TA, Finkelman FD, Seder RA, Cox TM, Hieny S, Caspar P, Sher A. 1994. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J. Immunol. 153:753–759 [PubMed] [Google Scholar]
- 20.Czaja MJ, Weiner FR, Takahashi S, Giambrone MA, van der Meide PH, Schellekens H, Biempica L, Zern MA. 1989. Gamma-interferon treatment inhibits collagen deposition in murine schistosomiasis. Hepatology 10:795–800. 10.1002/hep.1840100508 [DOI] [PubMed] [Google Scholar]
- 21.Wynn TA, Cheever AW. 1995. Cytokine regulation of granuloma formation in schistosomiasis. Curr. Opin. Immunol. 7:505–511. 10.1016/0952-7915(95)80095-6 [DOI] [PubMed] [Google Scholar]
- 22.Caffrey CR, Secor WE. 2011. Schistosomiasis: from drug deployment to drug development. Curr. Opin. Infect. Dis. 24:410–417. 10.1097/QCO.0b013e328349156f [DOI] [PubMed] [Google Scholar]
- 23.Liang YJ, Luo J, Yuan Q, Zheng D, Liu YP, Shi L, Zhou Y, Chen AL, Ren YY, Sun KY, Sun Y, Wang Y, Zhang ZS. 2011. New insight into the antifibrotic effects of praziquantel on mice in infection with Schistosoma japonicum. PLoS One 6:e20247. 10.1371/journal.pone.0020247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen Z, Dumas TE, Schrieber SJ, Hawke RL, Fried MW, Smith PC. 2008. Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab. Dispos. 36:65–72. 10.1124/dmd.107.017566 [DOI] [PubMed] [Google Scholar]
- 25.Channabasavaraj KP, Badami S, Bhojraj S. 2008. Hepatoprotective and antioxidant activity of methanol extract of Ficus glomerata. J. Nat. Med. 62:379–383. 10.1007/s11418-008-0245-0 [DOI] [PubMed] [Google Scholar]
- 26.Huber A, Thongphasuk P, Erben G, Lehmann WD, Tuma S, Stremmel W, Chamulitrat W. 2008. Significantly greater antioxidant anticancer activities of 2,3-dehydrosilybin than silybin. Biochim. Biophys. Acta 1780:837–847. 10.1016/j.bbagen.2007.12.012 [DOI] [PubMed] [Google Scholar]
- 27.Cheever AW. 1968. Conditions affecting the accuracy of potassium hydroxide digestion techniques for counting Schistosoma mansoni eggs in tissues. Bull. World Health Organ. 39:328–331 [PMC free article] [PubMed] [Google Scholar]
- 28.Dolber PC, Spach MS. 1993. Conventional and confocal fluorescence microscopy of collagen fibers in the heart. J. Histochem. Cytochem. 41:465–469. 10.1177/41.3.7679127 [DOI] [PubMed] [Google Scholar]
- 29.Stegemann H, Stalder K. 1967. Determination of hydroxyproline. Clin. Chim. Acta 18:267–273. 10.1016/0009-8981(67)90167-2 [DOI] [PubMed] [Google Scholar]
- 30.Rainone F. 2005. Milk thistle. Am. Fam. Physician 72:1285–1288 http://www.aafp.org/afp/2005/1001/p1285.html [PubMed] [Google Scholar]
- 31.Patel N, Joseph C, Corcoran GB, Ray SD. 2010. Silymarin modulates doxorubicin-induced oxidative stress, Bcl-xL and p53 expression while preventing apoptotic and necrotic cell death in the liver. Toxicol. Appl. Pharmacol. 245:143–152. 10.1016/j.taap.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 32.Lieber CS, Leo MA, Cao Q, Ren C, DeCarli LM. 2003. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J. Clin. Gastroenterol. 37:336–339. 10.1097/00004836-200310000-00013 [DOI] [PubMed] [Google Scholar]
- 33.Crocenzi FA, Roma MG. 2006. Silymarin as a new hepatoprotective agent in experimental cholestasis: new possibilities for an ancient medication. Curr. Med. Chem. 13:1055–1074. 10.2174/092986706776360950 [DOI] [PubMed] [Google Scholar]
- 34.Anthony K, Subramanya G, Uprichard S, Hammouda F, Saleh M. 2013. Antioxidant and anti-hepatitis C viral activities of commercial milk thistle food supplements. Antioxidants 2:23–36. 10.3390/antiox2010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feher J, Lengyel G. 2012. Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Curr. Pharm. Biotechnol. 13:210–217. 10.2174/138920112798868818 [DOI] [PubMed] [Google Scholar]
- 36.Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ, Berman J, Liu QY, Doo E, Fried MW. 2010. Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J. Clin. Pharmacol. 50:434–449. 10.1177/0091270009347475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freedman ND, Curto TM, Morishima C, Seeff LB, Goodman ZD, Wright EC, Sinha R, Everhart JE. 2011. Silymarin use and liver disease progression in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis trial. Aliment. Pharmacol. Ther. 33:127–137. 10.1111/j.1365-2036.2010.04503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molto J, Valle M, Miranda C, Cedeno S, Negredo E, Clotet B. 2012. Effect of milk thistle on the pharmacokinetics of darunavir-ritonavir in HIV-infected patients. Antimicrob. Agents Chemother. 56:2837–2841. 10.1128/AAC.00025-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neva FA, Brown HW. 1994. Basic clinical parasitology, 6th ed. Appleton & Lange, Norwalk, CT [Google Scholar]
- 40.Cheever AW, Andrade ZA. 1967. Pathological lesions associated with Schistosoma mansoni infection in man. Trans. R. Soc. Trop. Med. Hyg. 61:626–639. 10.1016/0035-9203(67)90125-3 [DOI] [PubMed] [Google Scholar]
- 41.El Scheich T, Hofer L, Kaatano G, Foya J, Odhiambo D, Igogote J, Lwambo N, Ekamp H, Karst K, Haussinger D, Richter J. 2012. Hepatosplenic morbidity due to Schistosoma mansoni in schoolchildren on Ukerewe Island, Tanzania. Parasitol. Res. 110:2515–2520. 10.1007/s00436-011-2793-6 [DOI] [PubMed] [Google Scholar]
- 42.El-Lakkany NM, Hammam OA, El-Maadawy WH, Badawy AA, Ain-Shoka AA, Ebeid FA. 2012. Anti-inflammatory/anti-fibrotic effects of the hepatoprotective silymarin and the schistosomicide praziquantel against Schistosoma mansoni-induced liver fibrosis. Parasit. Vectors 5:9. 10.1186/1756-3305-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. 2006. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat. Med. 12:99–106. 10.1038/nm1332 [DOI] [PubMed] [Google Scholar]
- 44.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA. 2001. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J. Exp. Med. 194:809–821. 10.1084/jem.194.6.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herbert DR, Orekov T, Perkins C, Finkelman FD. 2008. IL-10 and TGF-beta redundantly protect against severe liver injury and mortality during acute schistosomiasis. J. Immunol. 181:7214–7220 http://www.jimmunol.org/content/181/10/7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. 2004. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J. Immunol. 173:4020–4029 http://www.jimmunol.org/content/173/6/4020 [DOI] [PubMed] [Google Scholar]
- 47.Kisseleva T, Brenner DA. 2006. Hepatic stellate cells and the reversal of fibrosis. J. Gastroenterol. Hepatol. 21(Suppl 3):S84–S87. 10.1111/j.1440-1746.2006.04584.x [DOI] [PubMed] [Google Scholar]
- 48.Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. 2000. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 164:2585–2591 http://www.jimmunol.org/content/164/5/2585 [DOI] [PubMed] [Google Scholar]
- 49.Chiaramonte MG, Mentink-Kane M, Jacobson BA, Cheever AW, Whitters MJ, Goad ME, Wong A, Collins M, Donaldson DD, Grusby MJ, Wynn TA. 2003. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J. Exp. Med. 197:687–701. 10.1084/jem.20020903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho JW, Il KJ, Lee KS. 2013. Downregulation of type I collagen expression in silibinin-treated human skin fibroblasts by blocking the activation of Smad2/3-dependent signaling pathways: potential therapeutic use in the chemoprevention of keloids. Int. J. Mol. Med. 31:1148–1152. 10.3892/ijmm.2013.1303 [DOI] [PubMed] [Google Scholar]
- 51.Jakubzick C, Choi ES, Kunkel SL, Joshi BH, Puri RK, Hogaboam CM. 2003. Impact of interleukin-13 responsiveness on the synthetic and proliferative properties of Th1- and Th2-type pulmonary granuloma fibroblasts. Am. J. Pathol. 162:1475–1486. 10.1016/S0002-9440(10)64280-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugimoto R, Enjoji M, Nakamuta M, Ohta S, Kohjima M, Fukushima M, Kuniyoshi M, Arimura E, Morizono S, Kotoh K, Nawata H. 2005. Effect of IL-4 and IL-13 on collagen production in cultured LI90 human hepatic stellate cells. Liver Int. 25:420–428. 10.1111/j.1478-3231.2005.01087.x [DOI] [PubMed] [Google Scholar]
- 53.Hams E, Aviello G, Fallon PG. 2013. The schistosoma granuloma: friend or foe? Front. Immunol. 4:89. 10.3389/fimmu.2013.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sevin CM, Newcomb DC, Toki S, Han W, Sherrill TP, Boswell MG, Zhu Z, Collins RD, Boyd KL, Goleniewska K, Huckabee MM, Blackwell TS, Peebles RS., Jr 2013. Deficiency of gp91phox inhibits allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 49:396–402. 10.1165/rcmb.2012-0442OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi JH, Jin SW, Kim HG, Khanal T, Hwang YP, Lee KJ, Choi CY, Chung YC, Lee YC, Jeong HG. 2013. Platycodi Radix attenuates dimethylnitrosamine-induced liver fibrosis in rats by inducing Nrf2-mediated antioxidant enzymes. Food Chem. Toxicol. 56:231–239. 10.1016/j.fct.2013.02.033 [DOI] [PubMed] [Google Scholar]
- 56.Galicia-Moreno M, Rodriguez-Rivera A, Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. 2009. N-acetylcysteine prevents carbon tetrachloride-induced liver cirrhosis: role of liver transforming growth factor-beta and oxidative stress. Eur. J. Gastroenterol. Hepatol. 21:908–914. 10.1097/MEG.0b013e32831f1f3a [DOI] [PubMed] [Google Scholar]
- 57.Farah IO, Nyindo M, King CL, Hau J. 2000. Hepatic granulomatous response to Schistosoma mansoni eggs in BALB/c mice and olive baboons (Papio cynocephalus anubis). J. Comp. Pathol. 123:7–14. 10.1053/jcpa.1999.0378 [DOI] [PubMed] [Google Scholar]