Abstract
Co-trimoxazole reduces mortality in HIV-infected adults with tuberculosis (TB), and in vitro data suggest potential antimycobacterial activity of co-trimoxazole. We aimed to evaluate whether prophylaxis with co-trimoxazole is associated with a decreased risk of incident TB in Swiss HIV Cohort Study (SHCS) participants. We determined the incidence of TB per 1,000 person-years from January 1992 to December 2012. Rates were analyzed separately in participants with current or no previous antiretroviral treatment (ART) using Poisson regression adjusted for CD4 cell count, sex, region of origin, injection drug use, and age. A total of 13,431 cohort participants contributed 107,549 person-years of follow-up: 182 patients had incident TB—132 (73%) before and 50 (27%) after ART initiation. The multivariable incidence rate ratios for cumulative co-trimoxazole exposure per year for persons with no previous ART and current ART were 0.70 (95% confidence interval [CI], 0.55 to 0.89) and 0.87 (95% CI, 0.74 to 1.0), respectively. Co-trimoxazole may prevent the development of TB among HIV-positive persons, especially among those with no previous ART.
INTRODUCTION
Although tuberculosis (TB) can be successfully treated, it remains a leading cause of death among HIV-infected persons, particularly in low-income countries. A substantial proportion of deaths among patients with TB are caused by bacterial sepsis, and rates of bacterial pneumonia are several times higher among HIV-TB-coinfected patients than in the HIV-monoinfected population. Co-trimoxazole is a combination of trimethoprim and sulfamethoxazole that is widely used to treat bacterial infections, including lower respiratory, gastrointestinal, and urinary tract infections and Pneumocystis jirovecii pneumonia (PCP). A randomized controlled trial showed that co-trimoxazole prophylaxis significantly reduced mortality in HIV-infected adults receiving TB treatment in Côte d'Ivoire (1). Other studies confirmed the effect of co-trimoxazole prophylaxis on morbidity and mortality for combination antiretroviral treatment (ART)-naive HIV-TB-coinfected (2, 3) and ART-experienced (4) persons. Furthermore, co-trimoxazole is strongly recommended as prophylaxis against PCP pneumonia in HIV-infected persons with CD4 cell counts below 200 cells/μl.
Recent in vitro studies suggested a potential antimicrobial effect of co-trimoxazole on Mycobacterium tuberculosis (5–9), whereby sulfamethoxazole seems to be the active compound in the fixed-dose combination (6). Moreover, sulfamethoxazole together with rifampin appears to have a synergistic effect against M. tuberculosis (8). Whether co-trimoxazole prophylaxis may prevent TB is uncertain. We therefore studied cumulative co-trimoxazole exposure as a determinant of incident TB among participants in the Swiss HIV Cohort Study (SHCS). Additionally, we considered total mortality as an endpoint, where we expected to find a beneficial effect of co-trimoxazole, in order to confirm adequate adjustment for various confounders.
MATERIALS AND METHODS
HIV-infected adults (≥16 years of age) who attend outpatient clinics of seven cohort centers, affiliated regional hospitals, or private practitioners collaborating with the centers are continuously enrolled in the prospective observational SHCS. The enrollment of participants is independent of social status. Health care in Switzerland is universal, with mandatory health insurance (including for immigrants and marginalized groups). Demographic, psychosocial, clinical, laboratory (immunologic and virologic parameters), and treatment information is collected at enrollment and thereafter completed every 6 months by physicians and study nurses (10). Local ethical committees approved the SHCS protocol, and written informed consent was obtained from all participants.
HIV-associated opportunistic infections, including TB events, have been documented since 1988, and data on immune reconstitution inflammatory syndrome (IRIS [including TB IRIS]) have been collected since 2005. A TB event is diagnosed if there is culture-positive evidence of Mycobacterium tuberculosis in a symptomatic patient. As part of a recent SHCS project by Fenner et al., there was an endpoint validation of TB events (11). Purified protein derivate-based tuberculin skin testing (TST) is routinely performed in the SHCS, and latent TB treatment is initiated in TST-positive cases (defined as a skin induration of ≥5 mm) according to standard guidelines. We decided to exclude patients with previous TB treatment or latent TB treatment during follow-up, since latent TB treatment could be a major source of bias (if the hypothesis is correct that the cumulative co-trimoxazole effect is based on treatment of latent TB infection).
Controlled trials identified the effectiveness of co-trimoxazole as a secondary and primary PCP prophylaxis in 1992. Therefore, SHCS participants with at least two cohort visits between 1 January 1992 and 31 December 2012, without evidence of prevalent TB and no previous TB treatment, were included in the analyses. Incident TB and all-cause mortality, respectively, were calculated as the number of events divided by the number of person-years of follow-up (PYFU). Follow-up was counted from the first visit after 1 January 1992, the earliest event, the patient's last cohort visit, or 31 December 2012.
Poisson regression was used to model the incidence of TB or all-cause mortality separately in HIV-infected persons who had never yet received or had ever previously received combination ART (subsequently denoted “no previous ART” or “current ART,” respectively). We decided to specify separate models for those with no previous ART or current ART, because of differences in who would have been prescribed co-trimoxazole, depending on ART status, and because of the potential for interactions between many covariates and ART in terms of effect on the outcomes. Because associations between co-trimoxazole intake and opportunistic infections or death are strongly confounded by CD4 cell count, which was the main determinant of prescription, we do not present univariable associations but instead present bivariable associations adjusted for the latest, time-updated square root CD4 cell count. Multivariable Poisson models for incident TB were adjusted for time-updated cumulative co-trimoxazole use, sex, region of origin, injection drug use (intravenous drug use [IDU]), and age of >40 years. Cumulative co-trimoxazole was the primary form of exposure to avoid problems with reverse causality in patients prescribed co-trimoxazole for presumptive respiratory infection that was subsequently diagnosed as TB. We also considered ramp functions in which the protective effect of co-trimoxazole increased over specified periods of time (1, 3, 6, 9, 12, 18, and 24 months) and then remained stable. Bivariable estimates of the protective effect for ramps up to 24 months did not indicate the presence of an inflection point (data not shown), which led us to use cumulative co-trimoxazole exposure in the models. Interactions between cumulative co-trimoxazole exposure and CD4 categories were analyzed with a likelihood ratio test. Participants who stopped taking co-trimoxazole were analyzed based on the cumulative co-trimoxazole received. As an alternative, ART and its interaction with co-trimoxazole use were assessed in the multivariable model for TB incidence. In a sensitivity analysis, we compared the TB incidence of patients who received co-trimoxazole with that of patients who received aerosolized pentamidine or dapsone, assuming that they have similarly advanced HIV disease and that they can serve as a control group for the co-trimoxazole group. We calculated the incidence rate ratios (IRRs) for incident TB for cumulative pentamidine and cumulative dapsone use versus cumulative co-trimoxazole use.
Cumulative co-trimoxazole exposure, as a surrogate for the time being heavily immunosuppressed, is a confounding variable for death. Therefore, we decided (i) to include current co-trimoxazole use, sex, region of origin, injection drug use, and age of >40 years in multivariable Poisson models for all-cause mortality and (ii) to fit a second Poisson model for all-cause mortality with adjustment for current and cumulative co-trimoxazole exposure.
We used Stata (version 12; StataCorp, College Station, TX) for statistical analyses.
RESULTS
From 1992 to 2012, 14,589 SHCS participants contributed data. A total of 1,158 (8%) participants were excluded because of prevalent TB within 30 days of cohort registration (n = 339) or any prior treatment of active or latent TB (n = 819), leaving 13,431 SHCS participants in analyses. Baseline characteristics are shown in (Table 1). Median age at the first visit during the study period (baseline) was 35 years (interquartile range [IQR], 30 to 42 years). A total of 3,815 (28%) individuals were women, 2,357 (18%) were from a region other than Europe, North America, or Australia, and 2,697 (20%) had prior clinical AIDS. The nadir CD4 cell count was 273 cells/μl (IQR, 129 to 448 cells/μl). TST results were available for 8,980/13,431 (67%) of subjects, and 365/8,980 (4%) had a positive test result. Participants with a positive test result were more likely to have a high CD4 cell count (median, 466 cells/μl [IQR, 310 to 637 cells/μl] versus 271 cells/μl [IQR, 131 to 440 cells/μl] in those without a positive test result). Median CD4 cell counts for Caucasians and for persons from other regions with a positive TST were 544 cells/μl (IQR, 343 to 695 cells/μl) and 336 cells/μl (IQR, 249 to 489 cells/μl), respectively. A total of 95 (2%) patients who received co-trimoxazole versus 270 (5%) who never received co-trimoxazole had positive TST results; bivariate logistic regression confirmed that this difference was fully explained by the CD4 levels at the time of TST of 207 cells/μl (IQR, 101 to 349 cells/μl) versus 437 cells/μl (IQR, 300 to 614 cells/μl), respectively (data not shown).
TABLE 1.
Baseline characteristics of 13,431 HIV-infected participants stratified by co-trimoxazole use
| Characteristica | Total | Result for participants: |
P value | |
|---|---|---|---|---|
| Never treated with co-trimoxazole | Ever treated with co-trimoxazole | |||
| Participants, no. (%) | 13,431 (100) | 8,166 (61) | 5,265 (39) | |
| Yr of baseline visit, median (IQR) | 1998 (1993–2005) | 2000 (1995–2006) | 1996 (1992–2001) | <0.001 |
| Female, no. (%) | 3,815 (100) | 2,293 (60) | 1,522 (40) | 0.299 |
| Age, median yr (IQR) | 35 (30–42) | 34 (29–41) | 35 (30–43) | <0.001 |
| Nadir CD4, median cells/μl (IQR) | 273 (129–448) | 355 (228–525) | 150 (61–274) | <0.001 |
| Nadir CD4 of <200 cells/μl, no. (%) | 4,602 (100) | 1,510 (33) | 3,092 (67) | <0.001 |
| Risk groups and respective % with nadir CD4 of <200/cells μl, no. (%) | ||||
| Heterosexuals | 4,521 (100) | 2,292 (60) | 1,829 (40) | <0.001 |
| Nadir CD4 of <200 cells/μl | 1,697 (100) | 510 (30) | 1187 (70) | <0.001 |
| IDU | 3,108 (100) | 1,591 (51) | 1,517 (49) | |
| Nadir CD4 of <200 cells/μl | 1,084 (100) | 349 (32) | 735 (68) | <0.001 |
| MSM | 5,271 (100) | 3,583 (68) | 1,688 (32) | |
| Nadir CD4 of <200 cells/μl | 1,618 (100) | 597 (37) | 1021 (63) | <0.001 |
| Other | 531 (100) | 300 (57) | 231 (44) | |
| Nadir CD4 of <200 cells/μl | 203 (100) | 54 (27) | 149 (73) | <0.001 |
| Ethnicity, no. (%) | ||||
| Caucasianb | 11,074 (100) | 6,679 (60) | 4,395 (40) | 0.012 |
| Other | 2,357 (100) | 1,487 (64) | 870 (37) | |
| CDC stage, no. (%) | ||||
| A | 8,497 (100) | 6,111 (72) | 2,386 (29) | <0.001 |
| B | 2,237 (100) | 1,131 (51) | 1,106 (49) | |
| C | 2,697 (100) | 924 (34) | 1,773 (66) | |
| Nadir CD4, median cells/μl (IQR) | 273 (129–448) | 355 (228–525) | 150 (61–274) | <0.001 |
| TST at enrollment, no. (%) | ||||
| Test performed | 8,989 (100) | 5,088 (62) | 3901 (74) | <0.001 |
| TST positive | 365 (100) | 270 (5) | 95 (2) | <0.001 |
Abbrevations: IDU, intravenous drug use; MSM, men who have sex with men; TST, tuberculin skin test.
SHCS participants from northwestern Europe, North America, and Australia.
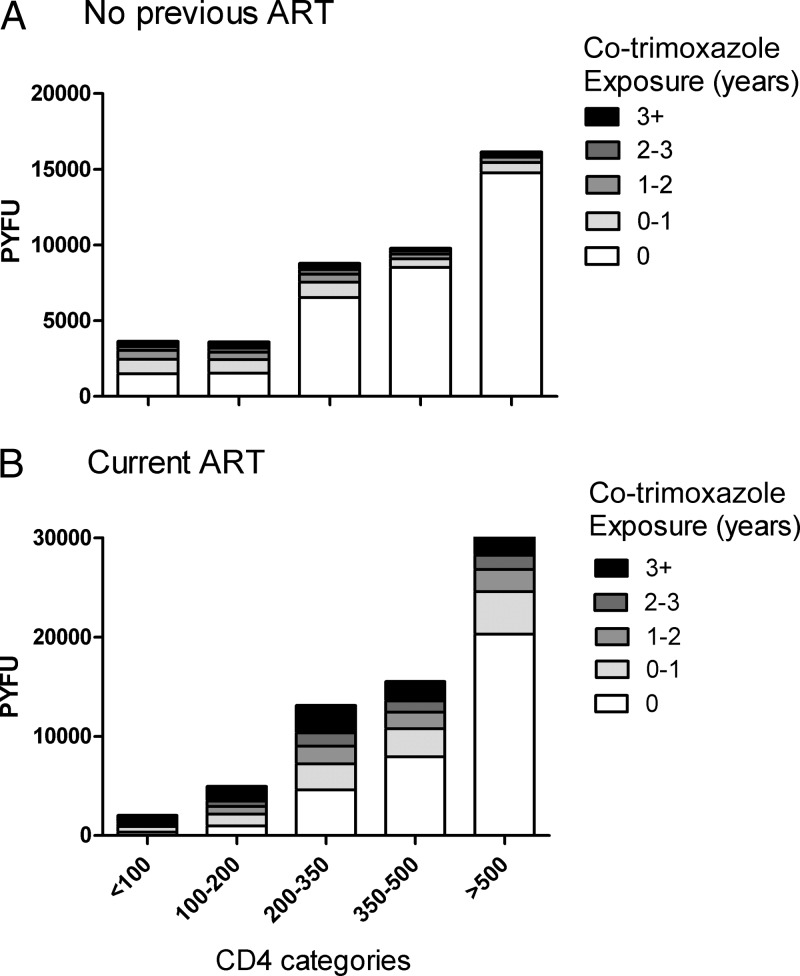
A total of 5,265 (39%) persons took co-trimoxazole at some time during the study period for median of 1.3 years (IQR, 0.50 to 2.8 years). Among participants with no previous ART, co-trimoxazole exposure was 41,891 person-years of follow-up (PYFU), and among those with current ART, co-trimoxazole exposure was 65,657 PYFU (Fig. 1). A total of 1,406/13,431 (10%) participants inhaled aerosolized pentamidine, and 639 (5%) used pyrimethamine-dapsone as second-line prophylaxis against PCP pneumonia.
FIG 1.
Cumulative previous co-trimoxazole exposure with bars classifying PYFU by latest CD4 class among patients with no previous ART (A) and those on current ART (B).
We observed 182 incident cases of TB. Incidence rates (IRs) for TB among persons with current and no previous ART were 0.76 (95% confidence interval [CI], 0.58 to 1.00) and 3.2 (95% CI, 2.7 to 3.7) per 1,000 PYFU, respectively.
Bivariable incidence rate ratios (IRRs) for cumulative co-trimoxazole exposure/year were 0.71 (95% CI, 0.56 to 0.90) for persons with no previous ART and 0.85 (95% CI, 0.72 to 1.00) for persons with current ART (Table 2). Associations remained unchanged after multivariable adjustment for CD4, region of origin, IDU, and age of >40 years at baseline, but the impact of cumulative co-trimoxazole was slightly attenuated by ART use. In a combined analysis, we included ART and its interaction with co-trimoxazole use in the multivariable model for TB incidence, also adjusted for square root CD4, region of origin, IDU, and age. There was a strong protective effect of ART (IRR, 0.31 [95% CI, 0.21 to 0.45]), and the associations with co-trimoxazole use were virtually unchanged (per year of co-trimoxazole use pre-ART, IRR, 0.69 [95% CI, 0.54 to 0.88]; during ART IRR, 0.89 [95% CI, 0.76 to 1.00]). We did not find an interaction between time since ART initiation and cumulative co-trimoxazole exposure (e.g., P = 0.66 comparing 0 to 12 months versus >12 months). Inclusion of calendar period (1992 to 1995, 1996 to 1999, 2000 to 2003, 2004 to 2007, and 2008 to 2012) in the multivariable models did not substantially change the estimate for cumulative co-trimoxazole exposure (no previous ART, IRR, 0.77 [95% CI, 0.60 to 0.98]; current ART, IRR, 0.87 [95% CI, 0.74 to 1.0]). Inclusion of both current and cumulative co-trimoxazole exposures suggested a similar protective effect of cumulative exposure (no previous ART, IRR, 0.55 [95% CI, 0.40 to 0.75]; current ART, IRR, 0.84 [95% CI, 0.70 to 0.99]) but increased risk with current co-trimoxazole use (no previous ART, IRR, 2.8 [95% CI, 1.7 to 4.4]; current ART, IRR, 1.3 [95% CI, 0.61 to 2.7]), supporting the concern about reverse causality.
TABLE 2.
Risk of incident tuberculosis in 13,431 cohort participants based on bivariable and multivariable Poisson regression analysesa
| Covariable | No previous ART (132 events during 41,891 PYFU) |
Current ART (50 events during 65,657 PFYU) |
||||||
|---|---|---|---|---|---|---|---|---|
| IRR bivariable models (95% CI)b | P value | IRR multivariable models (95% CI) | P value | IRR bivariable models (95% CI)b | P value | IRR multivariable models (95% CI) | P value | |
| Cumulative co-trimoxazole use/yr | 0.71 (0.56–0.90) | 0.005 | 0.70 (0.55–0.89) | 0.004 | 0.85 (0.72–1.00) | 0.05 | 0.87 (0.74–1.0) | 0.088 |
| Square root CD4 cells/μl | 0.90 (0.88–0.92) | <0.001 | 0.88 (0.86–0.90) | <0.001 | 0.85 (0.82–0.89) | <0.001 | 0.84 (0.80–0.87) | <0.001 |
| Region of originc | 3.4 (2.3–5.0) | <0.001 | 3.8 (2.5–5.7) | <0.001 | 5.5 (3.1–9.6) | <0.001 | 4.2 (2.3–7.6) | <0.001 |
| IDU | 1.1 (0.77–1.6) | 0.625 | 1.4 (0.95–2.1) | 0.092 | 0.31 (0.12–0.78) | 0.013 | 0.46 (0.17–1.2) | 0.113 |
| Age of >40 yrd | 0.71 (0.46–1.1) | 0.117 | 0.85 (0.54–1.3) | 0.480 | 0.54 (0.28–1.1) | 0.074 | 0.61 (0.31–1.2) | 0.155 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio; ART, antiretroviral treatment; IDU, intravenous drug use.
The IRR bivariable models were adjusted for time-updated CD4 cell count.
Region of origin other than Europe, North America, or Australia.
Age at baseline (1 January 1992 or at registration in the Swiss HIV Cohort Study, whichever is later).
In sensitivity analyses including exposure to pentamidine and/or dapsone as well as co-trimoxazole, there was an association of reduced TB incidence with cumulative co-trimoxazole use (no previous ART, IRR, 0.70 [95% CI, 0.55 to 0.87], P = 0.003; current ART, IRR, 0.86 [95% CI, 0.73 to 1.0], P = 0.075), whereas no such evidence was present for cumulative aerosolized pentamidine (no previous ART, 0.86 [95% CI, 0.63 to 1.2], P = 0.36; current ART, 0.11 [95% CI, 0.04 to 3.3], P = 0.20) and for dapsone-pyrimethamine (no previous ART, 0.83 [95% CI, 0.45 to 1.5], P = 0.55; current ART, 0.80 [95% CI, 0.38 to 1.7], P = 57).
A total of 2,447 persons died, with IRs of 10 (9 to 11) and 42 (40 to 44) per 1,000 PYFU in participants with current and no previous ART, respectively. The SHCS introduced detailed causes of death with ICD10 codes in 1999. One of the 1,016 patients who died since 1999 had miliary tuberculosis as the contributing cause of death; no other patients had TB as the primary or secondary cause of death. Bi- and multivariable models showed lower death rates associated with current co-trimoxazole use irrespective of ART (all P values were < 0.05) (Table 3). Associations after multivariable adjustment remained unchanged in an analysis that included current co-trimoxazole and cumulative co-trimoxazole exposure (all P values were < 0.05).
TABLE 3.
All-cause mortality in 13,431 cohort participants based on bivariable and multivariable Poisson regression analysesa
| Covariable | No previous ART (1,769 events during 42,229 PYFU) |
Current ART (678 events during 66,419 PYFU) |
||||||
|---|---|---|---|---|---|---|---|---|
| IRR bivariable models (95% CI)b | P value | IRR multivariable models (95% CI) | P value | IRR bivariable models (95% CI)b | P value | IRR multivariable models (95% CI) | P value | |
| Current co-trimoxazole use | 0.05 (0.04–0.07) | <0.001 | 0.05 (0.04–0.07) | <0.001 | 0.18 (0.13–0.24) | <0.001 | 0.18 (0.13–0.23) | <0.001 |
| Square root CD4 cells/μl | 0.79 (0.79–0.80) | <0.001 | 0.78 (0.77–0.78) | <0.001 | 0.87 (0.86–0.88) | <0.001 | 0.84 (0.83–0.85) | <0.001 |
| Region of originc | 0.61 (0.50–0.76) | <0.001 | 0.65 (0.52–0.80) | <0.001 | 0.35 (0.26–0.48) | <0.001 | 0.48 (0.35–0.66) | 0.001 |
| IDU | 1.1 (0.97–1.2) | 0.154 | 1.1 (1.0–1.3) | 0.013 | 1.9 (1.6–2.2) | <0.001 | 2.1 (1.8–2.5) | <0.001 |
| Age of >40 yrd | 1.4 (1.2–1.5) | <0.001 | 1.6 (1.4–1.7) | <0.001 | 1.6 (1.4–1.9) | <0.001 | 2.0 (1.7–2.3) | <0.001 |
Abbreviations: CI, confidence intervals; IRR, incidence rate ratio; ART, antiretroviral treatment; IDU, intravenous drug use.
The IRR bivariable models were adjusted for time-updated CD4 cell count.
Region of origin other than Europe, North America, or Australia.
Age at baseline (1 January 1992 or at registration in the Swiss HIV Cohort Study, whichever is later).
We found several strong associations between covariables and TB and all-cause mortality in the respective multivariable models (Tables 2 and 3). Risk of incident TB (Table 2) and all-cause mortality (Table 3) decreased as current CD4 increased irrespective of ART status; persons from regions other than Europe, North America, or Australia had higher risks of incident TB and lower risks of all-cause mortality with current and no previous ART, and IDU and older age were associated with higher risks of all-cause mortality with current and no previous ART.
DISCUSSION
We assessed incident TB and all-cause mortality and studied associations with cumulative and current co-trimoxazole exposure among 13,431 SHCS participants prospectively followed for a 20-year-period. Cumulative co-trimoxazole exposure reduced the risk for incident TB among ART-naive persons and to a lesser extent in ART-experienced persons, and current co-trimoxazole exposure reduced all-cause mortality after multivariable adjustment for CD4 cell count, region of origin other than Europe, North America, or Australia, IDU, and age of >40 years at baseline, irrespective of antiretroviral treatment.
It is difficult to compare our results with those from other cohorts because the association between co-trimoxazole prophylaxis and incident TB does not appear to have been previously investigated. Other studies estimated either the effect of ART on TB incidence (12) or the effectiveness of co-trimoxazole prophylaxis on morbidity and mortality in HIV-infected ART-naive persons (1–3) or those taking ART (4).
A recent large collaboration from Europe and the United States (12) found that TB incidence decreased after ART initiation, except in older persons and those with low CD4 cell counts. In the SHCS, we also observed a marked decrease of TB incidence by year and among persons on ART (13).
Based on studies showing that co-trimoxazole reduces morbidity and mortality among ART-naive persons with suspected or diagnosed TB (1–3), HIV-TB-coinfected persons should receive co-trimoxazole prophylaxis. However, the mechanism through which co-trimoxazole reduces mortality in these patients is unclear, and until now, many assumed that co-trimoxazole would reduce deaths due to other HIV-related opportunistic infections, invasive bacterial disease, or malaria. We found a protective effect of co-trimoxazole on all-cause mortality and incident TB among SHCS participants, suggesting that co-trimoxazole may have direct antituberculosis effects. Such speculation is supported by several in vitro studies suggesting potential activity of co-trimoxazole against M. tuberculosis (5–9) and one case report of improvement of a TB patient with co-trimoxazole treatment (5). Of note, the addition of co-trimoxazole in combination with either isoniazid or rifampin prevented the emergence of drug resistance in vitro (9).
A recently published trial with HIV-infected children receiving long-term ART in sub-Saharan Africa comparing continuing versus stopping co-trimoxazole prophylaxis found that continued co-trimoxazole prophylaxis was beneficial, with fewer hospitalizations and diagnostically confirmed malaria cases (14). Interestingly, fewer cases of other infections and also fewer cases of TB occurred in the continued co-trimoxazole group (5/376 and 15/382 TB events in the continued versus stopped co-trimoxazole group, respectively; hazard ratio, 0.33 [95% CI, 0.12 to 0.91]; P = 0.032). While most diagnoses were presumptive, reflecting challenges in pediatric TB diagnosis, and bacterial coinfections are relatively common, this adds epidemiological evidence of a protective effect of co-trimoxazole on the incidence of TB among HIV-positive persons on ART. The effect of co-trimoxazole prophylaxis was also investigated among African adults starting ART in the DART cohort (4). Current co-trimoxazole prophylaxis reduced overall mortality, but only for the first 72 weeks on ART. There were no overall or long-term benefits from co-trimoxazole for pulmonary or extrapulmonary TB (unpublished data). However, this setting is very different from that in our study: 25% of DART participants reported previous TB at enrollment, very few participants received isoniazid prophylaxis, and TB incidence was around 10-fold higher than in those on ART in the SHCS. Similar results were obtained in another trial investigating the effect of early chemoprophylaxis with co-trimoxazole on morbidity and mortality in treatment-naive HIV-infected persons in Cote d'Ivoire (15), where 11% of participants reported a history of TB at trial inclusion. Seventeen of 271 (6%) of the co-trimoxazole group and 19/270 (7%) of the placebo group were hospitalized or died from TB (P = 0.6, log rank test), but no information was available as to whether these hospitalizations/deaths were incident cases or not. Lastly, a recent study (16) found no evidence that co-trimoxazole reduced TB incidence in another high-incidence setting (South Africa). While this study is directly comparable with ours, since it excluded prevalent cases and considered only laboratory-confirmed diagnosis, it only investigated the impact of current co-trimoxazole, which both we and they suggested could be associated with residual confounding.
Strengths of our study include its statistical power because of the large number of patient-years and the prospective collection of data on incident TB and death. Additionally, we controlled for several cofactors known to be associated with incident TB or death. Several limitations should also be noted. First, Switzerland is a low-incidence country for TB, and TB incidence declined during the study period, which may limit the generalizability of our findings. This is one reason that 4% of participants had a positive TST result but received no isoniazid prophylaxis (and hence were included in the analysis), because this was not routinely provided when the lifetime risk of incident TB was categorized as very low (Swiss origin, high CD4 cells, and potential Mycobacterium bovis BCG vaccination in the past). We were not able to include TST results in our multivariable model, because TST results were only reliably obtained for 8,989/13,431 (67%) of our participants, and time-updated TST results are not available in the SHCS. Second, co-trimoxazole is routinely used among SHCS participants with CD4 cell counts below 200 cells/μl, and therefore the effects of co-trimoxazole and not being on ART are often confounded, which is why we conducted stratified analyses. Third, adherence to co-trimoxazole is not routinely assessed in the SHCS. Fourth, the SHCS does not collect information on homelessness and information on poverty, which are important social determinants of TB. However, only 0.5% of our patients were institutionalized in 2012, and the majority of intravenous drug users in Switzerland, if still substance dependent, participate in opiate substitution programs and receive methadone or even heroin in health care settings. Fifth, microbiological isolates were not stored, so we could not perform susceptibility testing for co-trimoxazole among culture-positive TB samples among SHCS participants.
In conclusion, we found epidemiological evidence of a protective effect of co-trimoxazole prophylaxis on the incidence of manifest TB among SHCS participants. Co-trimoxazole prophylaxis may hence reduce the risk of TB in ART-naive persons. Additionally, there is a trend of a protective effect even among patients on ART. The clinical utility of our observation is uncertain for the developed world. In 2013 the liberal use of ART was promoted and was clearly a higher priority than starting co-trimoxazole to reduce the risk of TB. Our findings may, however, be more relevant in resource-limited settings. Further studies, especially in low-income countries with a high burden of TB and HIV, are needed to confirm our findings.
ACKNOWLEDGMENTS
We thank all involved physicians, study nurses, and most importantly participants of the SHCS.
The members of the Swiss HIV Cohort Study and the Swiss Mother and Child HIV Study are as follows: V. Aubert, J. Barth, M. Battegay, E. Bernasconi, J. Böni, H. C. Bucher, C. Burton-Jeangros, A. Calmy, M. Cavassini, M. Egger, L. Elzi, J. Fehr, J. Fellay, P. Francioli, H. Furrer (chairman of the Clinical and Laboratory Committee), C. A. Fux, M. Gorgievski, H. Günthard (president of the SHCS), D. Haerry (deputy of the “Positive Council”), B. Hasse, H. H. Hirsch, B. Hirschel, I. Hösli, C. Kahlert, L. Kaiser, O. Keiser, C. Kind, T. Klimkait, H. Kovari, B. Ledergerber, G. Martinetti, B. Martinez de Tejada, K. Metzner, N. Müller, D. Nadal, G. Pantaleo, A. Rauch (chairman of the Scientific Board), S. Regenass, M. Rickenbach (head of the Data Center), C. Rudin (chairman of the Mother and Child substudy), P. Schmid, D. Schultze, F. Schöni-Affolter, J. Schüpbach, R. Speck, P. Taffé, P. Tarr, A. Telenti, A. Trkola, P. Vernazza, R. Weber, and S. Yerly.
B.H. had full access to all data of the study and takes responsibility for the integrity of the data and the accuracy of the data analyses. B.H., S.W., R.W., and B.L. designed the study. B.H. wrote the first draft, and B.H., S.W., R.W., and B.L. wrote the final version of the manuscript. B.L. analyzed the data. All investigators contributed to data collection and interpretation of the data, reviewed drafts of the manuscript, and approved the final manuscript.
This study has been financed in the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation. The funding source had no influence on design or conduct of the study.
B.H. has received travel grants from Astra Zeneca, Essex Chemicals, Gilead Sciences, Janssen-Cilag, and Wyeth. A.S.W. has received grants or honoraria from Abbott, Gilead Sciences, GlaxoSmithKline/Viiv Healthcare, and Tibotec. J.F. has received grants, honoraria, or travel grants from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, and Merck Sharp & Dohme. H.F. has participated in advisory boards of ViiV Healthcare, Bristol-Myers Squibb, Gilead, Merck Sharp & Dohme, Boehringer-Ingelheim, and Janssen-Cilag. H.F.'s institution has received unrestricted educational grants from Abbott, ViiV Healthcare, BMS, Roche, Gilead, Merck Sharp & Dohme, Boehringer-Ingelheim, and Janssen-Cilag. M.H. has received travel grants and speaker's honoraria from Gilead, Roche, and Celestis (Qiagen). M.B. has received travel grants, speaker's honoraria, and/or research grants from Abbott, Boehringer Ingelheim, Gilead Sciences, Hoffmann La Roche, Merck Sharp & Dohme, Tibotec, and ViiV Healthcare. R.W. has received travel grants or speaker's honoraria from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Pfizer, LaRoche, TRB Chemedica, and Tibotec and was a member of an endpoint adjudication panel of phase II and III antiretroviral treatment studies of Tibotec. B.L. has received travel grants, grants, or honoraria from Abbott, Aventis, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck Sharp & Dohme, Roche, and Tibotec. A.C., J.F., and C.D. report no conflicts of interest.
Footnotes
Published ahead of print 10 February 2014
REFERENCES
- 1.Wiktor SZ, Sassan-Morokro M, Grant AD, Abouya L, Karon JM, Maurice C, Djomand G, Ackah A, Domoua K, Kadio A, Yapi A, Combe P, Tossou O, Roels TH, Lackritz EM, De Cock KM, Coluibaly IM, Greenberg AE. 1999. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d'Ivoire: a randomised controlled trial. Lancet 353:1469–1475. 10.1016/S0140-6736(99)03465-0 [DOI] [PubMed] [Google Scholar]
- 2.Nunn AJ, Mwaba P, Chintu C, Mwinga A, Darbyshire JH, Zumla A. 2008. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ 337:a257. 10.1136/bmj.a257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimwade K, Sturm AW, Nunn AJ, Mbtha D, Zungu D, Gilks CF. 2005. Effectiveness of cotrimoxazole prophylaxis on mortality in adults with tuberculosis in rural South Africa. AIDS 19:163–168. 10.1097/00002030-200501280-00008 [DOI] [PubMed] [Google Scholar]
- 4.Walker AS, Ford D, Gilks CF, Munderi P, Ssali F, Reid A, Katabira E, Grosskurth H, Mugyenyi P, Hakim J, Darbyshire JH, Gibb DM, Babiker AG. 2010. Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet 375:1278–1286. 10.1016/S0140-6736(10)60057-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forgacs P, Wengenack NL, Hall L, Zimmermann SK, Silverman ML, Roberts GD. 2009. Tuberculosis and trimethoprim-sulfamethoxazole. Antimicrob. Agents Chemother. 53:4789–4793. 10.1128/AAC.01658-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong W, Sievers A, Leslie DE. 2010. Mycobacterium tuberculosis and sulfamethoxazole susceptibility. Antimicrob. Agents Chemother. 54:2748–2749 (Letter.) 10.1128/AAC.00029-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang TS, Kunin CM, Yan BS, Chen YS, Lee SS, Syu W., Jr 2012. Susceptibility of Mycobacterium tuberculosis to sulfamethoxazole, trimethoprim and their combination over a 12 year period in Taiwan. J. Antimicrob. Chemother. 67:633–637. 10.1093/jac/dkr501 [DOI] [PubMed] [Google Scholar]
- 8.Macingwana L, Baker B, Ngwane AH, Harper C, Cotton MF, Hesseling A, Diacon AH, van Helden P, Wiid I. 2012. Sulfamethoxazole enhances the antimycobacterial activity of rifampicin. J. Antimicrob. Chemother. 67:2908–2911. 10.1093/jac/dks306 [DOI] [PubMed] [Google Scholar]
- 9.Vilcheze C, Jacobs WR., Jr 2012. The combination of sulfamethoxazole, trimethoprim, and isoniazid or rifampin is bactericidal and prevents the emergence of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 56:5142–5148. 10.1128/AAC.00832-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoeni-Affolter F, Ledergerber B, Rickenbach M, Rudin C, Gunthard HF, Telenti A, Furrer H, Yerly S, Francioli P. 2010. Cohort profile: the Swiss HIV Cohort Study. Int. J. Epidemiol. 39:1179–1189. 10.1093/ije/dyp321 [DOI] [PubMed] [Google Scholar]
- 11.Fenner L, Gagneux S, Helbling P, Battegay M, Rieder HL, Pfyffer GE, Zwahlen M, Furrer H, Siegrist HH, Fehr J, Dolina M, Calmy A, Stucki D, Jaton K, Janssens JP, Stalder JM, Bodmer T, Ninet B, Böttger EC, Egger M, HIV Cohort Study Group, Molecular Epidemiology of Tuberculosis Study Group 2012. Mycobacterium tuberculosis transmission in a country with low tuberculosis incidence: role of immigration and HIV infection. J. Clin. Microbiol. 50:388–395. 10.1128/JCM.05392-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HIV-Causal Collaboration. 2012. Impact of antiretroviral therapy on tuberculosis incidence among HIV-positive patients in high-income countries. Clin. Infect. Dis. 54:1364–1372. 10.1093/cid/cis203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elzi L, Schlegel M, Weber R, Hirschel B, Cavassini M, Schmid P, Bernasconi E, Rickenbach M, Furrer H. 2007. Reducing tuberculosis incidence by tuberculin skin testing, preventive treatment, and antiretroviral therapy in an area of low tuberculosis transmission. Clin. Infect. Dis. 44:94–102. 10.1086/510080 [DOI] [PubMed] [Google Scholar]
- 14.Bwakura-Dangarembizi M, Kendall L, Bakeera-Kitaka S, Nahirya-Ntege P, Keishanyu R, Nathoo K, Spyer MJ, Kekitiinwa A, Lutaakome J, Mhute T, Kasirye P, Munderi P, Musiime V, Gibb DM, Walker AS, Prendergast AJ, Antiretroviral Research for Watoto (ARROW) Trial Team 2014. A randomized trial of prolonged co-trimoxazole in HIV-infected children in Africa. N. Engl. J. Med. 370:41–53. 10.1056/NEJMoa1214901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anglaret X, Chene G, Attia A, Toure S, Lafont S, Combe P, Manlan K, N′Dri-Yoman T, Salamon R, Cotrimo-CI Study Group 1999. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d'Ivoire: a randomised trial. Lancet 353:1463–1468. 10.1016/S0140-6736(98)07399-1 [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann CJ, Chaisson RE, Martinson NA. 2014. Cotrimoxazole prophylaxis and tuberculosis risk among people living with HIV. PLoS One 9:e83750. 10.1371/journal.pone.0083750 [DOI] [PMC free article] [PubMed] [Google Scholar]