Abstract
A prospective survey was conducted on 862 Enterobacteriaceae isolates with reduced susceptibility to carbapenems. The Carba NP test, UV spectrophotometry, and a DNA microarray were used to detect carbapenemase producers, and the results were compared to those from PCR and sequencing. The 172 carbapenemase producers were detected using the Carba NP test and UV spectrophotometry, whereas the DNA microarray failed to detect IMI producers. The use of the Carba NP test as a first screening, followed by the use of molecular techniques, has been determined to be an efficient strategy for identifying carbapenemase-producing Enterobacteriaceae.
TEXT
Carbapenemases have led to the ultimate evolution of resistance in Enterobacteriaceae, leaving virtually very few efficient antibiotics left for treating related infections (1, 2). The most clinically significant carbapenemases in Enterobacteriaceae are (i) Ambler class A enzymes, including KPC, IMI, and SME enzymes (1, 3, 4), (ii) metallo-β-lactamases (MBL) from the VIM, IMP, and NDM types (5, 6), and (iii) OXA-48-like enzymes (7). The detection of carbapenemase producers includes screening patients who are at risk for being carriers of carbapenemase producers, including patients who have been hospitalized abroad, as well as implementation of efficient isolation procedures for carriers; these are the main features for limiting the spread of this emerging resistance trait (8–10).
The biochemical Carba NP test, based on the detection of carbapenem hydrolysis, was recently developed (11). Molecular methods, such as simplex and multiplex PCRs, DNA hybridization, and sequencing are also used to identify carbapenemase genes.
The aim of this study was to evaluate prospectively an efficient and cost-effective strategy to detect and characterize carbapenemase-producing Enterobacteriaceae.
From June 2011 to July 2012, 862 nonduplicate clinical Enterobacteriaceae isolates of worldwide origins were tested in order to characterize the mechanisms leading to reduced susceptibility to carbapenems (Fig. 1). The isolates were identified using matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Vitek MS, bioMérieux, La Balme-les-Grottes, France). Susceptibility testing was performed by determining MIC values using the Etest (bioMérieux) on Mueller-Hinton agar plates at 37°C, and the results were recorded according to U.S. guidelines (from the CLSI), as updated in 2013 (12). All tested isolates were nonsusceptible to at least one of the three carbapenem molecules, imipenem, meropenem, or ertapenem.
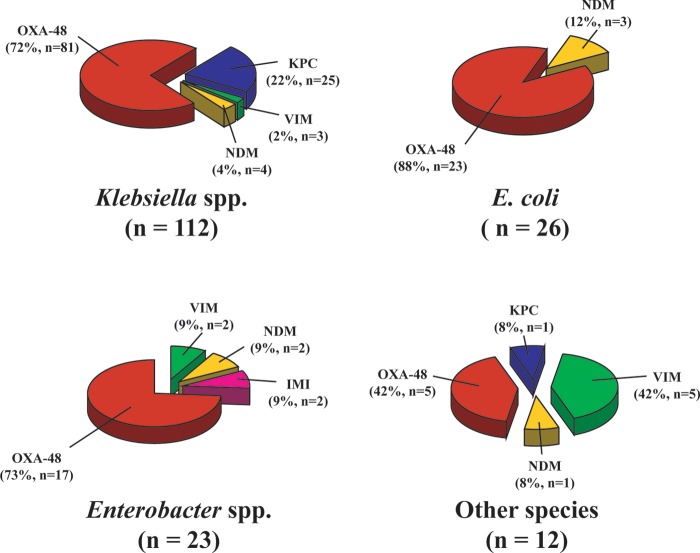
FIG 1.
Distribution of the different carbapenemase types among carbapenemase-producing Enterobacteriaceae.
The detection of the blaKPC, blaIMI, blaSME, blaGES, blaVIM, blaIMP, blaNDM, blaGIM, and blaOXA-48 carbapenemase genes was performed by simplex PCR, followed by sequencing (13). The results of PCR and sequencing were used as standards to evaluate the other detection techniques. Molecular detection of the β-lactamase genes was also performed for all carbapenemase producers (n = 173) using a DNA hybridization array approach (Check-MDR CT103 array; Check-Points, Wageningen, The Netherlands) according to the manufacturer's instructions (14).
The detection of carbapenemase production was performed by UV spectrophotometry, as previously described (15). It was also performed by using the Carba NP test (11). An improved version (faster and easier) of this test was performed with isolates grown on Mueller-Hinton agar plates (Becton, Dickinson, Le Pont-de-Chaix, France) at 37°C for 18 to 22 h, as previously described (see supplemental material) (1, 16).
Statistical analyses were performed using a χ2 test. P values of <0.05 were considered to be statistically significant.
Among the 862 enterobacterial isolates tested, the PCR-based techniques with subsequent sequencing identified 172 carbapenemase producers. Compared to the PCR-based detection method, the UV spectrophotometric method and the Carba NP test were found to be 100% sensitive and 100% specific for detecting carbapenemase producers (Table 1). Since the blaIMI gene was not included in the panel of carbapenemase genes detected by the Check-MDR CT103 array, it failed to identify the two Enterobacter strains producing the IMI-1 carbapenemase, leading to 98.8% sensitivity and 100% specificity (Table 1). The positive predictive values (PPV) were 100% for all three techniques, and the negative predictive values (NPV) were 100% for UV spectrophotometry and the Carba NP test and 99.7% for the Check-MDR CT103 array (Table 1).
TABLE 1.
Main characteristics of Carba NP test, UV spectrophotometric method, and DNA microarray for the detection of carbapenemase-producing Enterobacteriaceae
| Test parameters | Detection method characteristics |
|||
|---|---|---|---|---|
| PCR + sequencing | Carba NP test | UV spectrophotometry | DNA microarray | |
| Efficiency (%)a | ||||
| Sensitivity | 100 | 100 | 100 | 98.8 |
| Specificity | 100 | 100 | 100 | 100 |
| PPV | 100 | 100 | 100 | 100 |
| NPV | 100 | 100 | 100 | 99.7 |
| Other characteristics | ||||
| Rapidity (h) | 24–48 | <2 | 12–24 | 8–24 |
| Costb | $$ | $ | $ | $$$ |
| Expertise needsc | ++ | + | +++ | ++ |
| Complete gene identificationd | + | − | − | +/− |
PPV, positive predictive value; NPV, negative predictive value.
The number of $'s correlates with the effective (relative) price of the test.
The number of +'s correlates with the expertise and training needed to perform and interpret the test.
The + means that the technique is able to give a complete gene identification, the − means that the technique is not able to give a complete gene identification, and the +/− means that the technique is able to give a partial gene identification.
The DNA microarray was the only technique that identified additional noncarbapenemase β-lactamases. Indeed, 70% of the carbapenemase-producing Enterobacteriaceae additionally expressed at least one broad-spectrum β-lactamase, such as a plasmid-mediated cephalosporinase and/or an extended-spectrum β-lactamase (Table 2).
TABLE 2.
Molecular characterization of carbapenemase-producing Enterobacteriaceae using sequencing and DNA microarray
| Carbapenemase type(s) | Carbapenemase variant(s)a | Species | n | DNA microarray type results |
|||
|---|---|---|---|---|---|---|---|
| Acquired penicillinase(s) | ESBL(s) | Acquired cephalosporinase | Carbapenemase(s) | ||||
| KPC | KPC-2 | K. pneumoniae | 7 | TEM | None | None | KPC |
| 2 | TEM | CTX-M-1 | None | KPC | |||
| 1 | TEM | CTX-M-9 | None | KPC | |||
| 10 | TEM | SHV | None | KPC | |||
| C. freundii | 1 | TEM | None | None | KPC | ||
| KPC-3 | K. pneumoniae | 4 | TEM | None | None | KPC | |
| 1 | TEM | CTX-M-1 | None | KPC | |||
| VIM | VIM-1 | K. pneumoniae | 2 | TEM | SHV | None | VIM |
| Enterobacter cloacae | 1 | TEM | None | None | VIM | ||
| 1 | TEM | SHV | None | VIM | |||
| C. freundii | 1 | TEM | None | None | VIM | ||
| VIM-2 | C. freundii | 4 | TEM | TEM | None | VIM | |
| VIM-4 | K. pneumoniae | 1 | TEM | None | None | VIM | |
| NDM | NDM-1 | E. coli | 1 | None | CTX-M-1 | None | NDM |
| 1 | TEM | CTX-M-1 | None | NDM | |||
| 1 | TEM + SHV | CTX-M-1 | None | NDM | |||
| K. pneumoniae | 1 | None | None | CMY-2-like | NDM | ||
| 1 | None | CTX-M-1 | None | NDM | |||
| 1 | TEM | CTX-M-1 | None | NDM | |||
| E. cloacae | 1 | TEM | CTX-M-1 | None | NDM | ||
| 1 | TEM | CTX-M-1 + SHV | None | NDM | |||
| Salmonella spp. | 1 | TEM | None | DHA | NDM | ||
| IMI | IMI-1 | E. cloacae | 1 | TEM | None | None | None |
| Enterobacter asburiae | 1 | None | None | None | None | ||
| OXA-48-like | OXA-48 | E. coli | 4 | None | None | None | OXA-48 |
| 7 | TEM | None | None | OXA-48 | |||
| 1 | TEM | None | CMY-2-like | OXA-48 | |||
| 1 | None | CTX-M-1 | None | OXA-48 | |||
| 7 | TEM | CTX-M-1 | None | OXA-48 | |||
| 1 | TEM + SHV | CTX-M-1 | None | OXA-48 | |||
| 1 | TEM | CTX-M-9 | None | OXA-48 | |||
| K. pneumoniae | 5 | None | None | None | OXA-48 | ||
| 1 | TEM | None | None | OXA-48 | |||
| 1 | None | None | DHA | OXA-48 | |||
| 5 | None | CTX-M-1 | None | OXA-48 | |||
| 64 | TEM | CTX-M-1 | None | OXA-48 | |||
| 1 | TEM | CTX-M-1 | CMY-2-like | OXA-48 | |||
| 1 | None | CTX-M-9 | None | OXA-48 | |||
| E. cloacae | 1 | None | None | None | OXA-48 | ||
| 1 | None | CTX-M-1 | None | OXA-48 | |||
| 10 | TEM | CTX-M-1 | None | OXA-48 | |||
| 4 | TEM + SHV | CTX-M-1 | None | OXA-48 | |||
| Enterobacter hormaechei | 1 | TEM | CTX-M-1 | None | OXA-48 | ||
| C. freundii | 1 | TEM | SHV | None | OXA-48 | ||
| C. freundii | 1 | SHV | CTX-M-1 | None | OXA-48 | ||
| S. marcescens | 1 | None | None | None | OXA-48 | ||
| 1 | TEM | CTX-M-1 | None | OXA-48 | |||
| OXA-162 | C. freundii | 1 | None | SHV | None | OXA-48 | |
| OXA-181 | E. coli | 1 | None | CTX-M-1 | None | OXA-48 | |
| K. pneumoniae | 2 | TEM | CTX-M-1 | None | OXA-48 | ||
| NDM + OXA-48-like | NDM-1 + OXA-181 | K. pneumoniae | 1 | TEM | CTX-M-1 | None | NDM + OXA-48 |
Carbapenemase variants were obtained after sequencing.
Among the 172 carbapenemase producers, 65% were from K. pneumoniae, 15% were from Escherichia coli, 13% were Enterobacter spp., 5% were from Citrobacter freundii, 1% were from Serratia marcescens, and 1% were from Salmonella enterica (Table 2). The identified carbapenemases were of the OXA-48 (72%), KPC (15%), NDM (6%), VIM (6%), and IMI types (1%) (Table 2). The characterization of carbapenemase genes was done by sequencing, as listed in Table 2. Regardless of the enterobacterial species considered, OXA-48-like carbapenemases were predominant in our collection (Fig. 1). KPC producers were mostly identified in K. pneumoniae compared to the other enterobacterial species (96%, P < 0.001). Conversely, NDM producers were equally distributed (P > 0.05) among each type of enterobacterial species (Fig. 1).
Overall, this study showed 100% specificity and sensitivity for the Carba NP test and UV spectrophotometry to detect the production of carbapenemases (Table 1) (11). Additionally, the PPV and NPV of both techniques were also 100%. The Carba NP test was as efficient as the UV spectrophotometric method to detect carbapenemase producers but with significant advantages, since the Carba NP test is more rapid (<2 h versus 24 h for UV spectrophotometry) and does not require any specific training to use. Its cost is <$1 per tested strain, whereas the UV spectrophotometric assay and PCR-based techniques require additional equipment (a UV spectrophotometer and sonicator for the UV spectrophotometric assay and consumables, reagents, and a thermocycler for the PCR assay). On the other hand, the Check-MDR CT103 array failed to detect two IMI-1 producers, leading to 100% specificity, 98.8% sensitivity, 100% PPV, and 99.7% NPV (Tables 1 and 2). Since the Check-MDR CT103 array is designed for clinical use, it may detect the most clinically relevant carbapenemase enzymes (KPC, VIM, IMP, and OXA-48-like carbapenemases). In addition, it cannot discriminate between the different variants of a given carbapenemase. Additionally, this technique requires several successive steps (DNA extraction, ligation, PCR amplification, hybridization, and detection) requiring 8 to 24 h total. It also requires additional equipment (DNA extraction kit, thermocycler, thermomixer, and Check-Points tube reader, including the software) that costs ∼$16,000 (14). Additionally, the use of this array on a daily routine basis may be limited by its cost (∼$100) compared to UV spectrophotometry ($2 to 3), the Carba NP test ($1), and PCR-based testing ($30). However, the microarray technique may help to characterize the entire β-lactamase content of a single isolate by also detecting other broad-spectrum β-lactamase genes.
The diversity of the carbapenemases identified here mirrors the worldwide dissemination of the four main described enzymes (KPC, VIM, NDM, and OXA-48) (1). Additionally, our results further highlight the wide dissemination of the OXA-48 carbapenemase in Europe (particularly in France) accounting for 72% of the whole carbapenemases (Fig. 1) (7, 17), whereas KPC is the most widespread carbapenemase in the United States. Of note, the KPC carbapenemases are almost entirely restricted to the K. pneumoniae species (25/26). Conversely, OXA-48 and NDM were distributed among all enterobacterial species.
Since the management of patients requires the rapid identification of carbapenemase producers (regardless of its type) (18), a diagnostic strategy for the detection of carbapenemase producers in Enterobacteriaceae is proposed here (Fig. 2). This strategy is based on (i) the Carba NP test as the primary screening test for the detection of carbapenemase production, followed by (ii) a specific molecular characterization of the carbapenemase genes by simplex PCRs or the DNA microarray. The initial step (susceptibility testing and Carba NP test) may be developed in any laboratory worldwide. Molecular identification of the carbapenemase genes may be also performed locally, depending on the molecular techniques available; however, it is not required for antibiotic stewardship or infection control purposes.
FIG 2.
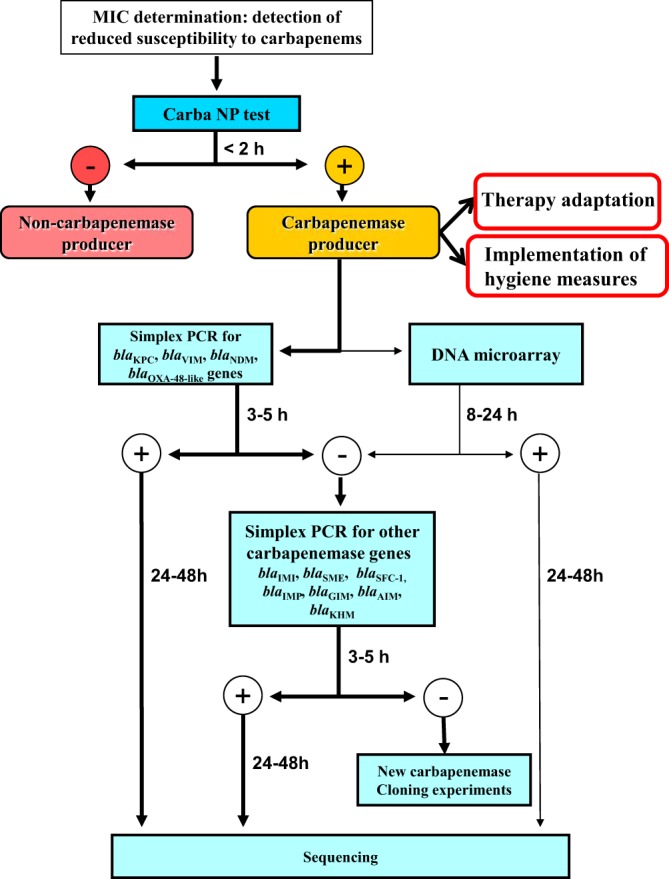
Flowchart for detection and characterization of carbapenemase producers among Enterobacteriaceae. The Carba NP test is used for rapid differentiation between carbapenemase and noncarbapenemase producers. The second step includes molecular techniques (PCRs or DNA microarray) for precise identification of carbapenemase genes. The bold arrows indicate the preferred way to identify carbapenemase genes. This second step may be followed only in university hospitals or large microbiology laboratories.
In the case of a negative result obtained with the Carba NP test, the mechanism responsible for carbapenem-decreased susceptibility is not related to the production of a carbapenemase (e.g., reduced permeability of the outer membrane associated with overexpression of chromosomal or acquired AmpC and/or extended-spectrum β-lactamases [ESBL]); therefore, no additional test is required (17). In the case of a positive result with the Carba NP test, the use of a set of five simplex PCRs (blaKPC, blaVIM, blaNDM, blaIMI, and blaOXA-48-like) may then identify all carbapenemase genes of our collection (Fig. 1 and Table 2). However, this screening may be adapted to local epidemiology, as was recently proposed for the detection of carbapenemase SME in the United States (4). The DNA microarray may be more useful for epidemiological purposes or for infection control studies, when high numbers of isolates have to be rapidly characterized (14). Additionally, this procedure may also detect potential new carbapenemases. Indeed, although molecular techniques are currently considered to be the gold standard for the detection of carbapenemase producers, they are only able to detect known carbapenemase genes. With the proposed strategy, a positive Carba NP test, followed by negative results using molecular techniques, may correspond to a novel carbapenemase that may be further characterized using cloning experiments (Fig. 2).
This is the first prospective study to evaluate at an international level the values of the different techniques for detecting carbapenemases. The strategy proposed for the detection of carbapenemase producers presents several advantages for treating infected patients and for the isolation of carriers. Indeed, it will lead to the rapid identification of carbapenemase producers (<2 h) using the Carba NP test, allowing for better antibiotic stewardship (18). This strategy may also have a significant impact on preventing the development of nosocomial outbreaks by acting rapidly on the management of carriers (through isolation and cohorting) as demonstrated for KPC outbreaks, at least in Israel (19). Finally, since the first step of this strategy, which includes susceptibility testing, and the Carba NP test are based on cheap techniques, it may be followed worldwide and therefore contribute to limiting the spread of what has been recently termed the new Red Plague (20).
Supplementary Material
ACKNOWLEDGMENTS
This work was partially funded by a grant from the INSERM (UMR914), by the Université of Fribourg, Switzerland, and by grants from the European Community (no. R-GNOSIS, FP7/HEALTH-F3-2011-282512, MAGIC-BULLET, and FP7/HEALTH-F3-2001-278232).
An international patent form for the Carba NP test has been filed on behalf of INSERM Transfert (Paris, France).
Footnotes
Published ahead of print 27 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01239-13.
REFERENCES
- 1.Nordmann P, Dortet L, Poirel L. 2012. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol. Med. 18:263–272. 10.1016/j.molmed.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 2.Schwaber MJ, Carmeli Y. 2008. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA 300:2911–2913. 10.1001/jama.2008.896 [DOI] [PubMed] [Google Scholar]
- 3.Nordmann P, Cuzon G, Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228–236. 10.1016/S1473-3099(09)70054-4 [DOI] [PubMed] [Google Scholar]
- 4.Bush K, Pannell M, Lock JL, Queenan AM, Jorgensen JH, Lee RM, Lewis JS, Jarrett D. 2013. Detection systems for carbapenemase gene identification should include the SME serine carbapenemase. Int. J. Antimicrob. Agents 41:1–4. 10.1016/j.ijantimicag.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 5.Nordmann P, Poirel L, Walsh TR, Livermore DM. 2011. The emerging NDM carbapenemases. Trends Microbiol. 19:588–595. 10.1016/j.tim.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 6.Walsh TR, Toleman MA, Poirel L, Nordmann P. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306–325. 10.1128/CMR.18.2.306-325.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J. Antimicrob. Chemother. 67:1597–1606. 10.1093/jac/dks121 [DOI] [PubMed] [Google Scholar]
- 8.Mitka M. 2013. Indian public health leaders move to reduce antimicrobial resistance. JAMA 309:531–532. 10.1001/jama.2013.297 [DOI] [PubMed] [Google Scholar]
- 9.Infectious Diseases Society of America (IDSA), Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, Gerding D, Lynfield R, Reller LB, Rex J, Schwartz D, Septimus E, Tenover FC, Gilbert DN. 2011. Combating antimicrobial resistance: policy recommendations to save lives. Clin. Infect. Dis. 52(Suppl 5):S397–S428. 10.1093/cid/cir153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh TR, Toleman MA. 2011. The emergence of pan-resistant Gram-negative pathogens merits a rapid global political response. J. Antimicrob. Chemother. 67:1–3. 10.1093/jac/dkr378 [DOI] [PubMed] [Google Scholar]
- 11.Nordmann P, Poirel L, Dortet L. 2012. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 18:1503–1507. 10.3201/eid1809.120355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement. CLSI M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 13.Poirel L, Dortet L, Bernabeu S, Nordmann P. 2011. Genetic features of blaNDM-1-positive Enterobacteriaceae. Antimicrob. Agents Chemother. 55:5403–5407. 10.1128/AAC.00585-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuzon G, Naas T, Bogaerts P, Glupczynski Y, Nordmann P. 2012. Evaluation of a DNA microarray for the rapid detection of extended-spectrum β-lactamases (TEM, SHV and CTX-M), plasmid-mediated cephalosporinases (CMY-2-like, DHA, FOX, ACC-1, ACT/MIR and CMY-1-like/MOX) and carbapenemases (KPC, OXA-48, VIM, IMP and NDM). J. Antimicrob. Chemother. 67:1865–1869. 10.1093/jac/dks156 [DOI] [PubMed] [Google Scholar]
- 15.Bernabeu S, Poirel L, Nordmann P. 2012. Spectrophotometry-based detection of carbapenemase producers among Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 74:88–90. 10.1016/j.diagmicrobio.2012.05.021 [DOI] [PubMed] [Google Scholar]
- 16.Dortet L, Bréchard L, Poirel L, Nordmann P. 2014. Comparison of diverse growing media for further detection of carbapenemase production using the Carba NP test. J. Med. Microbiol., in press [Google Scholar]
- 17.Dortet L, Cuzon G, Nordmann P. 2013. Dissemination of carbapenemase-producing Enterobacteriaceae in France, 2012. J. Antimicrob. Chemother., in press. 10.1093/jac/dkt433 [DOI] [PubMed] [Google Scholar]
- 18.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob. Agents Chemother. 56:2108-2113. 10.1128/AAC.06268-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwaber MJ, Lev B, Israeli A, Solter E, Smollan G, Rubinovitch B, Shalit I, Carmeli Y, Israel Carbapenem-Resistant Enterobacteriaceae Working Group 2011. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin. Infect. Dis. 52:848–855. 10.1093/cid/cir025 [DOI] [PubMed] [Google Scholar]
- 20.Looke DF, Gottlieb T, Jones CA, Paterson DL. 2013. Gram-negative resistance: can we combat the coming of a new “Red Plague”? Med. J. Aust. 198:243–244. 10.5694/mja13.10190 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.