Abstract
We investigated the in vitro effects of four alkyl-galactofuranoside derivatives, i.e., octyl-β-d-galactofuranoside (compound 1), 6-amino-β-d-galactofuranoside (compound 2), 6-N-acetamido-β-d-galactofuranoside (compound 3), and 6-azido-β-d-galactofuranoside (compound 4), on Leishmania donovani. Their mechanism of action was explored using electron paramagnetic resonance spectroscopy (EPR) and nuclear magnetic resonance (NMR), and ultrastructural alterations were analyzed by transmission electron microscopy (TEM). Compound 1 showed the most promising effects by inhibiting promastigote growth at a 50% inhibitory concentration (IC50) of 8.96 ± 2.5 μM. All compounds exhibit low toxicity toward human macrophages. Compound 1 had a higher selectivity index than the molecule used for comparison, i.e., miltefosine (159.7 versus 37.9, respectively). EPR showed that compound 1 significantly reduced membrane fluidity compared to control promastigotes and to compound 3. The furanose ring was shown to support this effect, since the isomer galactopyranose had no effect on parasite membrane fluidity or growth. NMR showed a direct interaction of all compounds (greatest with compound 1, followed by compounds 2, 3, and 4, in descending order) with the promastigote membrane and with octyl-galactopyranose and octanol, providing evidence that the n-octyl chain was primarily involved in anchoring with the parasite membrane, followed by the putative crucial role of the furanose ring in the antileishmanial activity. A morphological analysis of compound 1-treated promastigotes by TEM revealed profound alterations in the parasite membrane and organelles, but this was not the case with compound 3. Quantification of annexin V binding by flow cytometry confirmed that compound 1 induced apoptosis in >90% of promastigotes. The effect of compound 1 was also assessed on intramacrophagic amastigotes and showed a reduction in amastigote growth associated with an increase of reactive oxygen species (ROS) production, thus validating its promising effect.
INTRODUCTION
The World Health Organization (WHO) ranks leishmaniasis as the second most important protozoan parasitic disease after malaria for its grave morbidity, high mortality, and global distribution, involving 88 countries or territories to which it is endemic. The estimated incidence is as high as 2 million new cases per year, including 0.5 million cases of visceral leishmaniasis (VL) and l.5 million of cutaneous leishmaniasis, with 350 million people at risk (1). The recommendations for the treatment of leishmaniasis have recently been actualized (1, 2). Conventional drugs used for the treatment of VL (antimonials and amphotericin B) require prolonged administration, have toxicity risks, and are currently facing challenges of resistance in certain regions of endemicity, such as India (3). New therapeutic agents, such as liposomal amphotericin B and miltefosine, have demonstrated their efficacy in large field clinical trials, but their widespread use is limited by adverse events, cost, and intravenous use or teratogenicity, respectively. Combining therapies of the existing drugs remains another solution but carries the risk of cumulative toxicity (4, 5). Therefore, there is a need to investigate novel cost-effective therapeutic approaches (6, 7), all the more because currently no reliable human vaccine is available (8).
The external structure of the Leishmania parasite presents attractive targets for generating future antileishmanial drugs, particularly lipophosphoglycan (LPG), which is the main molecular structure covering the parasite surface and is known to be responsible for its survival and virulence (9). It has already been demonstrated that attenuated virulence or death of the parasite is due to alterations in the LPG structure (10, 11). The core structure of LPG, which is common to all Leishmania species, consists of a hexasaccharide containing a nonacetylated glucosamine unit and two mannopyranose entities linked to a rare β-d-galactofuranose residue and ends with two galactopyranoses. The whole is attached to inositol, which allows linkage with the phospholipid anchor, and one of the mannoses is substituted by a glucose-1-phosphate (12).
The presence of rare galactofuranoses is a characteristic shared by some microorganisms, including trypanosomatids, like Leishmania and Trypanosoma cruzi, as well as mycobacteria and Aspergillus (13). Indeed, it is well documented that galactofuranose (Galf) and Galf-containing glycans play a vital role in the life cycle and pathogenesis of the parasite and in mediating important host-parasite interactions (14). The complete absence of Galf in mammals makes it an interesting target for development of new chemotherapeutic strategies, particularly against leishmaniasis (13, 15). Our group and other teams previously reported an inhibitory effect of octyl galactofuranosides (16) or bicatenary thiogalactofuranosides (17) on mycobacterial strains. These results led us to investigate such effects on Leishmania, since the glycocalyx of this microorganism shares some common characteristics with mycobacteria. Therefore, four different synthetic octyl galactofuranoside derivatives were synthesized and analyzed for their ability to interfere with Leishmania donovani promastigote survival or growth. Additional experiments were conducted to explain their mode of interaction with the parasite membrane and to characterize parasite damage, using electron paramagnetic resonance (EPR), nuclear magnetic resonance (NMR), transmission electron microscopy (TEM), and annexin V binding. The effects of the most powerful compounds were verified with intramacrophagic amastigotes.
MATERIALS AND METHODS
Chemistry.
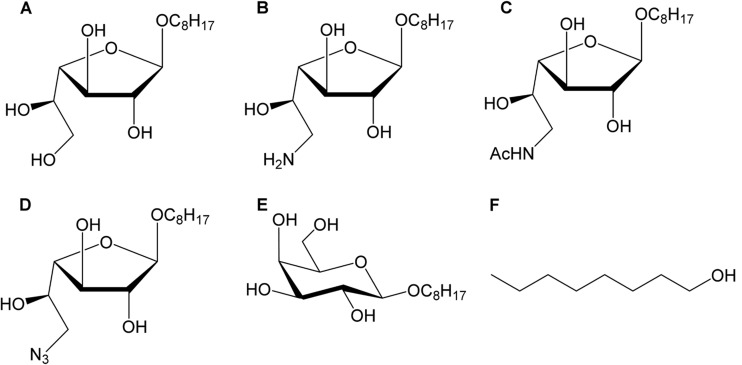
The different galactofuranose derivatives were obtained using highly specific synthesis pathways. Starting with octyl galactofuranoside 1, the three other target molecules were synthesized as previously described (18) (Fig. 1). The synthesis relied on a common intermediate, the octyl-6-azido-6-2,3-di-O-benzoyl-6-deoxy-galactofuranoside (compound 4b). First, reduction of compound 4b provided 6-amino derivative 2b, which can be either deprotected or acetylated to give compounds 2 and 3b, respectively. Subsequent cleavage of the ester groups of compound 3b gave compound 3 in 30% yields starting with compound 4b. In parallel, direct deprotection of the benzoyl protecting groups of compound 4b under basic conditions provided the free octyl-6-azido-galactofuranoside (compound 4). Details about the synthesis can be provided upon request. The four resulting products, namely, octyl-β-d-galactofuranoside (compound 1), 6-amino-galactofuranoside (compound 2), 6-N-acetamido-β-d-galactofuranoside (compound 3), and 6-azido-galactofuranoside (compound 4), were then tested (19). In some experiments, two other compounds were used as controls, i.e., octyl-β-d-galactopyranoside (compound 5) (Galp-Oct) and octanol (compound 6) (Fig. 1). Miltefosine (hexadecylphosphocholine [HePC]) was used as a reference molecule with proven efficacy against visceral leishmaniasis (20).
FIG 1.
Structures of Galf derivatives evaluated against Leishmania. (A) Octyl-β-d-galactofuranoside/compound 1; (B) 6-amino-galactofuranoside/compound 2; (C) 6-N-acetamido-β-d-galactofuranoside/compound 3; (D) 6-azido-galactofuranoside/compound 4; control compounds, like (E) octyl-β-d-galactopyranoside/compound 5; and (F) octanol/compound 6.
Cell cultures. (i) Leishmania donovani promastigotes.
The L. donovani strain was isolated from a human patient positively diagnosed with VL and was typed by the Centre National de Référence des Leishmanioses (Montpellier, France) as MHOM/SD/97/LEM3427, Zym MON-18. Schneider's drosophila medium (Sigma-Aldrich) supplemented with 10% fetal calf serum (FCS) (Invitrogen) was used to amplify parasites from amastigotes freshly isolated from mice and to maintain serial passages of L. donovani promastigotes at 27°C. L. donovani cultures at 3 to 5 days (exponential growth phase) were used to determine the antiparasitic effect of the different derivatives of galactofuranose. For cell infection experiments, promastigote cultures at stationary phase were used.
(ii) THP-1-derived human macrophages.
The THP-1 (human acute monocytic leukemia) cell line was differentiated into macrophages after overnight stimulation with phorbol-12-myristate-13-acetate (PMA) as described earlier (21). The cell line was maintained at 37°C with 5% CO2 using RPMI 1640 medium (Gibco) supplemented with 10% decomplemented fetal calf serum (FCS), 100 IU/ml penicillin, and 100 μg/ml streptomycin.
(iii) Human blood monocyte-derived macrophages.
Human blood monocyte-derived macrophages (HMØ) were obtained by purifying monocytes from peripheral blood mononuclear cells obtained from blood buffy coats (supplied by Etablissement Français du Sang, Rennes, France), as described earlier (22). Briefly, granulocyte-macrophage colony-stimulating factor (GM-CSF)-mediated differentiation of monocytes was conducted for 6 days to obtain primary human macrophages; cells were cultured in RPMI 1640 medium supplemented with 10% decomplemented FCS. The culture conditions were similar to those mentioned for THP-1 macrophages.
Macrophage cytotoxicity assay.
The cytotoxicity test was performed with macrophages derived from THP-1 and HMØ by an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay as described earlier (23). Briefly, the THP-1-derived macrophages were matured overnight (96-well plate) with the culture conditions mentioned above at a final density of 105 macrophages/well. The medium was removed, and fresh medium was added with the final concentrations of different Galf derivatives (5 μM to 3,200 μM) and HePC (3 μM to 2,457 μM). The macrophages were further incubated for 24 h before proceeding with MTT. Briefly, the medium was removed carefully and replaced by 100 μl of MTT (Molecular Probes, CA) solution at 0.5 mg/ml according to the manufacturer's protocol. The plates were incubated for 2 h at 37°C; afterwards, the medium was removed, and 100 μl of dimethyl sulfoxide (DMSO) was added in each well. Absorbance was read at 540 nm using a SpectroStar Nano instrument (BMG Labtech, USA) to determine the amount of formazan production, which determines the relative cell viability. Data were analyzed using the MARS data analysis software program. The cytotoxicity concentration for 50% of cells (CC50) for Galf derivative-treated conditions was determined by setting the optical density of the control to 100%. All conditions were performed in triplicate.
Promastigote growth inhibition assay.
The in vitro antiparasitic effect of the synthesized galactofuranose derivatives was evaluated on promastigotes by using a culture microtitration method as previously described (19, 24, 25). Briefly, L. donovani promastigote parasites exponentially propagated (day 3 of culture) were incubated with different concentrations of Galf derivatives (1 μM to 3,200 μM) and HePC (0.9 μM to 2,457 μM) under sterile conditions. The concentration of parasites was set at 105/ml (10,000 promastigotes/well), and each condition was tested in triplicate. After 48 h of incubation, serial 2-fold dilutions of the suspensions were performed in 96-well microplates using Schneider's drosophila culture medium, to reach the dilution limit of residual viable parasites. Subsequently, the plates were incubated for 3 additional days and were examined using an inverted microscope at a magnification of ×200 or ×400. Evaluation of parasite survival was assessed by determining the presence or absence of mobile promastigotes in each well, and evaluation of parasite growth was done by obtaining the last dilution titer for which the well contained at least one parasite; comparison was done between treated and untreated conditions. The effects of galactofuranose derivatives on L. donovani promastigote growth were expressed as the reduction percentage of parasite growth obtained for each drug concentration, by comparison to untreated wells, and the 50% inhibitory concentration (IC50) was determined. The selectivity index (SI) was calculated as CC50/IC50, as described earlier (26). Each experiment was repeated at least thrice.
Promastigote cytotoxicity assay.
A commercial cell cytotoxicity assay (CellTox Green; Promega) was also used to determine the IC50 of compounds. This assay is based on the intensity of fluorescence detection after binding of a fluorescent dye to DNA of cells with impaired membrane integrity. Fluorescence intensity is proportional to cell death. The experiment was performed following the manufacturer's instructions. Briefly, serial dilutions (160 μM to 0.156 μM) of compounds were incubated with 75 × 103 parasites (1.5 × 106/ml) in a black 96-well microplate for 24 h. Then, 100 μl of CellTox reagent diluted to 1/500 was added per well, placed for 1 min on a rotating agitator, and then incubated for 15 min at room temperature (RT). Fluorescence was detected on a fluorocytometer (Gemini) using 485- to 500-nm (excitation) and 520- to 530-nm (emission) filters. Results were acquired by using SoftMax Pro Gemini XS software and converted to Excel format. Experiments were performed in quadriplate and repeated twice.
STDD-NMR.
The quantification of molecular interaction of L. donovani promastigotes (whole cells) and compounds 1, 2, 3, 4, 5, and 6 were analyzed by saturation transfer double difference (STDD)-NMR. Galactose was used as a negative control. Parasite cultures at exponential growth stage (3 to 5 days) were harvested by centrifugation at 2,500 × g for 15 min at 4°C. The supernatant was discarded, and the pellet was resuspended in phosphate-buffered saline (PBS) buffer (pH 7). The same steps were repeated twice using deuterated PBS (previously exchanged with D2O; pH = 7.5 to 7.8). Each sample tube was filled with an amount of L. donovani ranging from 2 × 108 to 2.7 × 108 cells per tube. The product tested was introduced at a concentration of 1.5 mg/ml and completed to 500 μl of deuterated PBS. This concentration of 1.5 mg/ml was chosen to ensure observing properly the effects and avoiding a too-long acquisition time, which could lead to cell degradation. All samples were checked by microscopy after the experiments to ensure that these conditions did not affect cell integrity during data acquisition.
STD-NMR experiments were recorded at 298 K on a Bruker Advance III 400-MHz spectrometer. The on-resonance irradiation and the off-resonance of the cell suspension were performed at 7 ppm and +100 ppm, respectively; the resulting difference between both spectra constitutes the saturation transfer difference (STD) spectrum. Acquisition of an STD-NMR spectrum for each compound was made in the absence of cells as a reference in order to verify the absence of STD effect under these experimental conditions. The STD-NMR reference spectrum of the cell suspension without compound was used for the double-difference filter. The final STDD spectra were obtained by manually subtracting the cell STD spectrum from each of the (cells plus compound) spectra. Intensities of all STD effects were calculated through integrals over the respective signals in a 1H NMR reference spectrum. The largest STD effect in each spectrum was set to 100%, and relative intensities were determined. Hence, sufficient comparisons of relative STD effects between the different compounds were possible, but absolute binding intensities could not be determined.
EPR.
The fluidity of the L. donovani promastigote membrane was determined by a spin-labeling method using electron paramagnetic resonance (EPR), as described previously (27). L. donovani promastigotes were incubated with the obtained IC50 of different Galf derivatives (compounds 1, 2, and 3) or with controls (compounds 5, 6, and HePC) for 48 h at 27°C. Standard controls having membrane fluidifying and stabilizing effects, namely, Tween 20 and ursodeoxycholic acid (UDCA), respectively, were used (27, 28). To investigate the reversibility and specificity of the rigidifying effect observed with compound 1, L. donovani promastigotes were initially incubated with compound 1 for 48 h and then washed using PBS and treated with Tween 20 or UDCA for 30 min before EPR analysis. Afterwards, the parasites were washed twice with PBS and were spin labeled with 32 μg/ml 5-doxylstearic acid (DSA) (Sigma-Aldrich), a fatty acid exhibiting a stable nitroxide radical ring at the C-5 position. After three washing steps with PBS, the resulting promastigote sample was transferred to a disposable glass capillary. The EPR spectra of untreated and treated labeled samples were acquired at room temperature on a Brucker Elexsys EPR spectrometer operating at 3,509-G center field, 20-mW microwave power, 9.86-GHz microwave frequency, 1.77-G modulation amplitude, and 100-kHz modulation frequency. The fluidity of the labeled membrane was quantified by calculating the order parameter S according to equations described previously (29). The S value is inversely proportional to the membrane fluidity; an increase in the value of S is interpreted as a decrease in membrane fluidity and vice versa. All conditions were performed in triplicate and obtained from three identical experiments.
TEM.
L. donovani promastigotes were incubated with two different galactofuranose derivatives (compounds 1 and 3) at 125 μg/ml (5 × 108 promastigotes/sample) for 48 h at 27°C. Afterwards, the parasites were washed twice with PBS and then centrifuged and fixed for 4 h with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2), rinsed, and then postfixed for 3 h with 1.5% OsO4 in the same buffer. Samples were washed and dehydrated in a graded series of increasing concentrations of ethanol (50, 70, 80, 90, and 100% [vol/vol]). Pure ethanol was then exchanged by propylene oxide, and samples were gradually infiltrated with increasing concentrations (30, 50, 70, and 100% [vol/vol]) of epoxy resin (mixed with propylene oxide) for a minimum of 3 h per step. Samples were left overnight in pure epoxy resin. Infiltration was continued the next day with 2,4,6-Tris dimethylaminomethyl phenol-30 (DMP30)-epoxy resin for 3 h. Samples were finally embedded in a new mix of DMP30-epoxy resin and polymerized at 60°C for 24 h. Ultrathin sections (80 nm) were then cut with a UCT Leica ultramicrotome, placed on grids, poststained for 30 min with uranyl acetate and then for 20 min with lead citrate, and viewed with a Jeol 1400 TEM supplied with a Gatan Orius camera.
Analysis of PS externalization by flow cytometry.
Apoptosis-like cell death is characterized by the exposure of phosphatidylserine (PS) at the cell membrane. PS externalization was determined according to the manufacturer's protocol using the commercial Dead Cell apoptosis kit (Molecular Probes), with annexin V-Alexa Fluor 488 and propidium iodide (PI). Annexin V binds to the cell by exploiting its high Ca2+-dependent affinity for PS, whereas PI labeling is observed following necrosis. Briefly, L. donovani promastigotes were incubated with or without compound 1 or 2 at their respective IC50s for 24 h, washed twice using PBS, and resuspended in annexin binding buffer at 1 × 107 parasites/100 μl. Stauroporine (STS) was used as a positive control for apoptosis and incubated with parasites at a final concentration of 2.5 μM. Five microliters of annexin V solution and 1 μl PI at 100 μg/ml were added, and the mixture was incubated for 15 min at room temperature, following the manufacturer's instructions. Afterwards, 400 μl of annexin binding buffer was added, and the fluorescence of the stained parasites was analyzed using a Beckman Coulter FC500 cytometer; fluorescence emission was quantified using CXP analysis software.
Confocal microscopy analysis of annexin V binding.
L. donovani promastigotes (107) were treated with compound 1 at IC50 for 24 h or left untreated, later washed once using PBS, and labeled for morphological visualization using carboxy fluorescein diacetate succinimidyl ester (CFSE) (Invitrogen) during 5 min at a 2 μM concentration for 107 parasites/ml. Then, parasites were labeled with annexin V-phycoerythrin (PE) (apoptosis detection kit; BD Pharmingen) according to the manufacturer's instructions. After a washing step with PBS, parasites were observed by using a Leica TCS SP5 microscope (Leica Microsystems). Images were processed using LAS-AF software (Leica Microsystems).
Amastigote growth inhibition assay.
Human macrophages (THP-1) were cultured in 8-well Labtech slides and infected for 24 h with untreated L. donovani promastigotes at a 20:1 promastigote/macrophage ratio. Wells were washed 5 times using culture medium to remove all extracellular promastigotes and were incubated with either compound 1, 2, or 3 at 80 μM or HePC at 7.66 μM for 48 h at 37°C. The cells were washed once with medium and stained with May-Grünwald Giemsa (MGG) stain, and the percentage of infected macrophages was determined (presence of intracellular parasites) via microscopic examination using a 100× oil immersion lens. A minimum of 200 macrophages were counted in different locations of each stained well for both treated and untreated conditions. Data were analyzed by setting values of multiplying amastigotes (>2 parasites/cell) to 100% for untreated wells and comparing with values obtained with treated conditions. All conditions were done in triplicate and repeated at least three times.
Detection of ROS production.
To determine the generation of reactive oxygen species (ROS) in L. donovani-infected macrophages, we used the cell-permeating probes dihydrorhodamine 123 (DHR) and dihydroethidium (DHE) for measuring the production of peroxide and superoxide species, respectively. The techniques were optimized as described previously (30) with some modifications. DHR 123 (Molecular Probes) was used to detect increased fluorescence after passive diffusion across the parasite membrane, where it reacts with hydrogen peroxide in the presence of peroxidases from cationic rhodamine 123 emitting green fluorescence, which localizes in the mitochondria. DHE (Molecular Probes) forms a red fluorescent product (ethidium) upon reaction with superoxide anions. HMØ were infected with L. donovani (10:1) for 24 h, followed by treatment with 80 μM compound 1 or 2 for 24 h. Lipopolysaccharide (LPS) was used as a control to stimulate HMØ ROS production. Cells were washed with PBS and then centrifuged and incubated with either DHR or DHE in a 1-ml volume for 60 min and 20 min, respectively. Cells were washed once with cold PBS, and fluorescence was measured by flow cytometry using a Beckman Coulter FC500 cytometer. Quantification of fluorescence emission was analyzed using CXP analysis software.
Statistical analysis.
Data were expressed as arithmetic means ± standard errors of the means (SEM) and compared using the Mann-Whitney test. Statistical analysis was made using the GraphPad Prism v5 software program (GraphPad Software, USA). Statistically significant results are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
RESULTS
Inhibition of L. donovani promastigote growth by Galf derivatives in vitro.
Several Galf derivatives showed inhibition of parasite growth, notably compounds 1 and 2, with IC50s of 8.96 ± 2.5 μM and 41.6 ± 8.3 μM, respectively. Contrasting with that, compounds 3 and 4 showed limited or no effect on parasite growth even at high concentrations compared to results under the control untreated condition (Table 1). Overall, the efficacies of the four compounds could be ranked as compound 1 being the most effective, followed by compounds 2, 3, and 4, in decreasing order of effectiveness. To gain further insight into the mechanism of action of Galf derivatives and particularly to investigate whether the strong effects shown by compound 1 were due to the octyl chain or to the sugar ring structure, we subsequently tested its structural isomer compound 5 (n-octyl-β-d-galactopyranoside) and compound 6 (octyl chain alone). We found no effects on the growth of L. donovani promastigotes using optimal concentrations of compound 5 or 6. In parallel, L. donovani promastigotes were cultured with HePC, and the calculated IC50 was 3.38 μM under experimental conditions similar to those for other Galf derivatives (Table 1). The determination of IC50 using the CellTox Green assay confirmed that the best antileishmanial activity was obtained with compound 1 (IC50 = 2.4 μM; 95% confidence interval [CI], 0.39 to 14.7 μM) and suggested that this compound had an effect on membrane integrity. Indeed, the principle of this assay relies on the intracellular penetration of a dye and fluorescence detection after binding to DNA, which can occur only in the case of membrane alterations. Results with this assay led to a somewhat similar IC50 for compound 2 but with a huge unreliable confidence interval (95% CI = 0.3 to 39.7) and did not yield any result for the remaining compounds or for HePC (data not shown).
TABLE 1.
In vitro IC50s of various galactofuranose derivatives for L. donovani promastigote growth and determination of CC50s toward human macrophages
| Compound | IC50 (μM)a (mean ± SEM) | CC50 (μM)a (mean ±SEM) |
Selectivity index (CC50/IC50) |
||
|---|---|---|---|---|---|
| HMØ | THP-1 | HMØ | THP-1 | ||
| 1 | 8.96 ± 2.5 | 1,431.4 ± 0.4 | 1,508.4 ± 0.03 | 159.7 | 168.3 |
| 2 | 41.6 ± 8.3 | 736.6 ± 0.1 | 804.6 ± 0.08 | 17.7 | 19.3 |
| 3 | 800 ± 0 | 1,595.4 ± 0.4 | 1,608.8 ± 0.03 | 1.99 | 2.01 |
| 4 | 1,360 ± 16.6 | 747.4 ± 0.3 | ND | 0.55 | ND |
| HePC | 3.38 ± 0 | 128.1 ± 0.3 | 146.1 ± 0.03 | 37.9 | 43.2 |
Each concentration (serial dilutions) was tested in triplicate in three independent experiments. IC50, 50% inhibitory concentration; CC50, cytotoxic concentration; HMØ; blood-derived human macrophages; ND, not determined.
Galf derivatives have low cytotoxic effect on human macrophages.
The MTT assay showed that Galf derivatives did not present any cytotoxic effects against either THP-1 or HMØ at optimum biological activity concentrations. The CC50 of compounds 1, 2, 3, and 4 toward HMØ was high, ranging from 1,431.3 ± 0.4 μM to 747.3 ± 0.3 μM, whereas it was 128.1 ± 0.3 μM for HePC (Table 1). The selectivity index (SI) was calculated for the Galf derivatives; the most significant SI was obtained for compound 1, which was 4.2 times better than that of HePC with blood-derived human macrophages (159.7 versus 37.9, respectively). Similar results were obtained with the THP-1 cell line (Table 1). Due to its higher IC50, the SI of compound 2 was lower than that of HePC.
STDD-NMR spectroscopy demonstrates molecular interaction between L. donovani promastigotes and galactofuranose derivatives.
STD-NMR nowadays constitutes a reliable and cheap tool for observing interactions between ligands and their biological receptors or ligands (31). In order to determine the level of interaction between the galactofuranose derivatives and Leishmania donovani membrane assemblages, we used a second generation of STD-NMR, termed saturation transfer double difference (STDD-NMR), which can be an effective tool when using whole cells (32, 33). The double STD (STDD) spectrum is obtained from the difference between the STD spectrum of a sample containing cells and their presumed ligand and the STD spectrum obtained with the sole cells, thus eliminating noise due to other molecular components present in the cell surface.
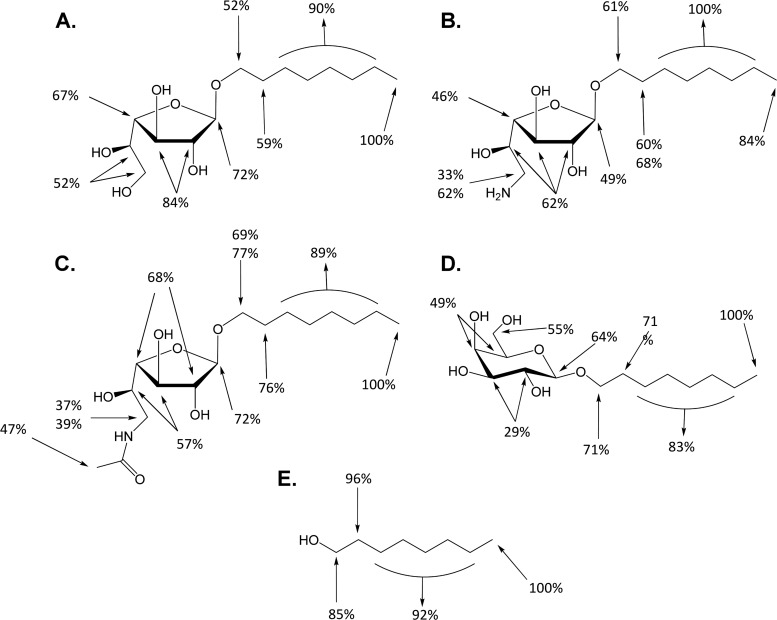
Octyl galactofuranosides were all tested at a concentration of 1.5 mg/ml, below the compound 1 critical micelle concentration, which is 1.78 mg/ml, thus avoiding a micellar state. Such concentrations were previously shown to be needed to measure an interaction between a compound and the membrane (34). The resultant effects were expressed as percentages, giving 100% to the highest signal, which is in all cases the signal for the terminal protons of the alkyl chain. Compounds 1, 2, and 3 showed similar results, namely, a strong STD effect on the alkyl chain and relative close intensities for all the other sugar ring protons (Fig. 2). Although interaction intensities of the sugar ring were similar for the three above-cited compounds, they were somewhat higher for the compound 1 sugar motif (52% to 84%) than for compound 2 (33% to 62%) and compound 3 (37% to 72%) (Fig. 2). Compound 4 did not yield any workable spectrum despite repeated experiments.
FIG 2.
Quantification of molecular interactions of Galf derivatives with the promastigote membrane by STDD-nuclear magnetic resonance. Interaction results obtained for compound 1 (A), compound 2 (B), compound 3(C), compound 5(D), and compound 6 (E) are shown. The terminal protons of the alkyl chain yielded the highest STD effect in each spectrum and thus was set to 100%, and the relative intensities of other molecular structures were determined. Data are representative of two independent experiments.
To correlate these assays to the in vitro experiments, STDD spectra of compound 5 (n-octyl- galactopyranoside) and compound 6 (n-octanol) were also set up and showed also a similar pattern of interaction for the alkyl chain (high transfer saturation) but a lower one for each of the galactopyranose sugar ring protons (29% to 55% interaction only) (Fig. 2). A negative control using galactose, which led to no STD effect at all, confirmed that the observed signals were not an artifact.
Compounds 1 and 2 exert a rigidifying effect on the L. donovani promastigote membrane.
To further explore the consequences of this molecular interaction of galactofuranose derivatives with L. donovani promastigotes, we evaluated their effect on membrane fluidity using the EPR technique. Three Galf derivatives yielding distinctive effects on parasite growth in vitro were selected, i.e., compounds 1, 2, and 3. A significant increase in the value of S (order parameter S) in parasites treated with compound 1 at the IC50 was obtained (S = 0.64 ± 0.004 versus 0.604 ± 0.002 in treated and untreated control parasites, respectively; P < 0.0001) (Fig. 3A). Since S is inversely proportional to the membrane fluidity, compound 1 appears to significantly rigidify the L. donovani phospholipidic membrane. Similar results were obtained for compound 2, with an S value of 0.63 ± 0.001 (P < 0.001). In contrast, when parasites were treated with the least-effective compound, compound 3, no significant difference in the value of the order parameter was observed (S = 0.584 ± 0.0006; P value is not significant [ns]), indicating that this compound had no effect on promastigote membrane fluidity. Two other compounds, 5 and 6, which showed interaction by NMR, which was lower, but no antiparasitic effects, were also analyzed for their effects on membrane fluidity. Neither significantly modified membrane fluidity (Fig. 3A).
FIG 3.
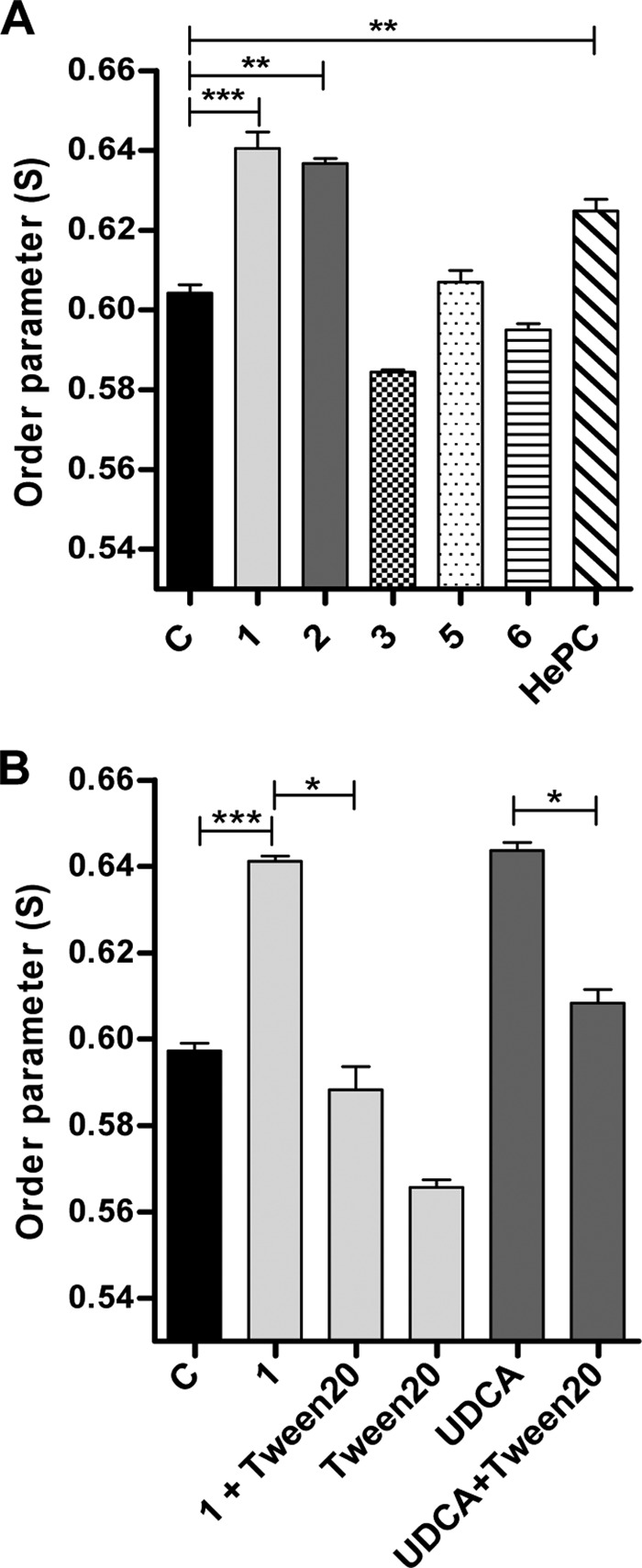
Analysis of membrane fluidity by electron paramagnetic resonance. Parasites were incubated with or without the Galf derivatives for 2 days and then incubated with spin label 5-DSA. (A) Treatment with compounds 1 and 2 at their respective IC50 and with compounds 3, 5, and 6 at >400 μM concentrations; lane C represents untreated control parasites. (B) Reversal of rigidifying effect of compound 1 after treatment with Tween 20 and similar effects observed after treatment with the rigidifying control UDCA followed by Tween 20. Data are expressed as means ±SEM and are representative of three independent experiments; each condition was tested in triplicate. Significance of results is expressed as follows: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
To further validate the rigidifying effect of compound 1, we verified the effect of treatment with Tween 20 and ursodeoxycholic acid (UDCA). Results showed the reversal of the rigidifying effect of compound 1, with an S value of 0.641 ± 0.001 and an S value of 0.588 ± 0.005 (P < 0.05) before and after Tween 20 treatment, respectively, yielding results similar to those for parasite treatment with Tween 20 alone (S = 0.566 ± 0.002; P = ns). Additionally, UDCA was added to the L. donovani promastigotes for 1 h of incubation as a stabilizing control (28) and confirmed a rigidifying effect at a level similar to that obtained with compound 1 (S = 0.643 ± 0.002). This effect due to UDCA could be also reversed by treatment with Tween 20 (S = 0.608 ± 0.003; P < 0.05) (Fig. 3B).
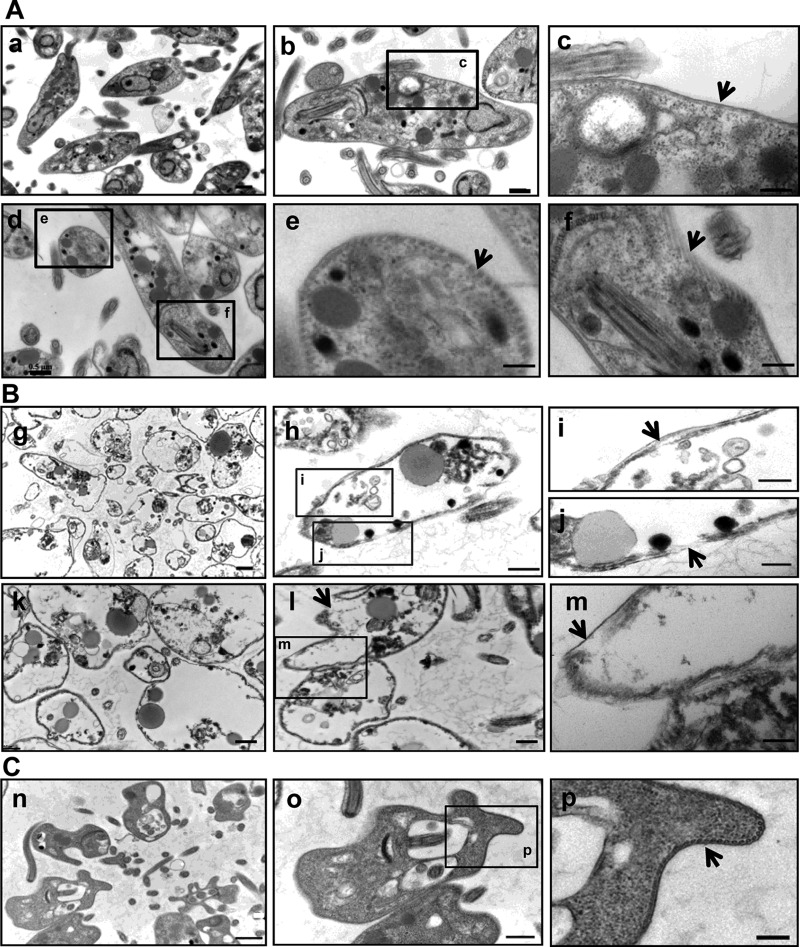
Transmission electron microscopy analysis of L. donovani promastigotes treated with compound 1 reveals profound structural alterations.
We investigated the nature and extent of damage to L. donovani promastigotes caused by compound 1, using TEM. Compound 3, which had little effect on parasite growth, was used as a control. Untreated promastigotes presented well-defined, intact cellular structural details (Fig. 4A), notably on higher magnification (×80.000). In particular, the surface remained intact, which is essential for the viability of promastigotes, and the subpellicular microtubules structure is intact, as shown in longitudinal or cross-sectional views (Fig. 4A, panels c, e, and f). Conversely, after 48 h of incubation with compound 1, about 90% of parasites showed severe alterations (Fig. 4B, panels g and k), extending from the phospholipidic membrane to inside organelles (Fig. 4B, panels h and l). Structural abnormalities included prominent surface changes, including the detachment of the plasma membrane from the layer of subpellicular microtubules (Fig. 4B, panels i and m) or a complete interruption of the plasma membrane (Fig. 4B, panels j and l) leading to extensive damage of cytoplasmic organelles, such as the cell nucleus, kinetoplast, mitochondria, and storage compartments, and a leakage of damaged organelles outside the cell. Beside these striking effects observed with compound 1, compound 3 did not lead to major alterations of membrane integrity but rather induced some deformations (Fig. 4C), which had no visible consequences for intracellular organelles, thus probably explaining the lack of effect on parasite survival and growth.
FIG 4.
Ultrastructural analysis of L. donovani promastigotes by transmission electron microscopy. (A) Untreated promastigotes with intact cytoplasmic organelles (a and d), intact plasma membrane (arrow) (b and c), or intact subpellicular microtubules (arrow) (e and f). (B) Promastigotes treated with compound 1, showing a high proportion of altered parasites (g and k), partial (h and i, arrow) or complete (m, arrow) detachment of the plasma membrane from the layer of subpellicular microtubules or complete disruption of the plasma membrane (j and l, arrow), or destruction of numerous intracellular organelles. (C) Compound 3-treated promastigotes, showing deformation of the plasma membrane (n and o) but no membrane rupture (p, arrow). Data are representative of two independent experiments. Scale bars represent 1 μm (a, g, and n) at magnification ×12,000 (12K), 0.5 μm (b, d, h, k, and o) at magnification 25K, or 0.2 μm (c, e, f, i, j, m, and p) at magnification 80K.
Compound 1 induces apoptosis in L. donovani promastigotes.
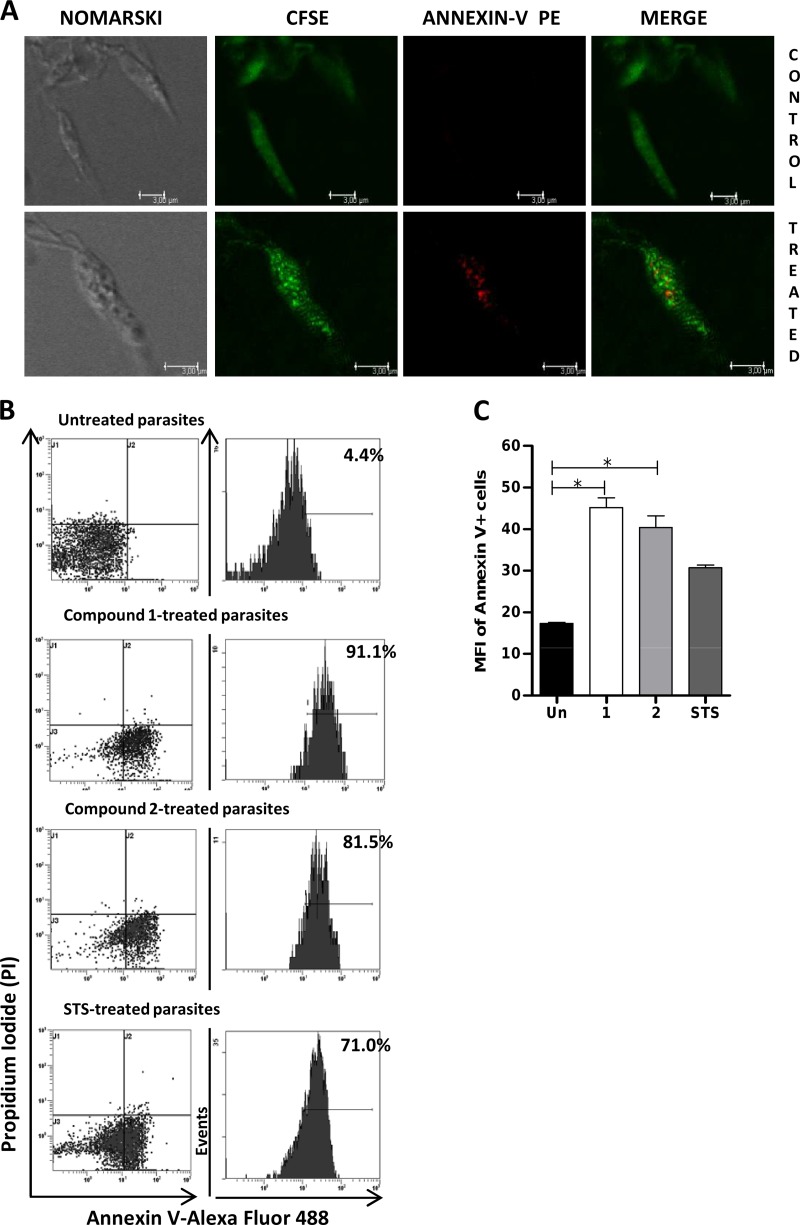
A confocal analysis of promastigotes labeled with annexin V and CFSE revealed an increase in surface binding of annexin V after treatment with compound 1 (Fig. 5A) compared to that for untreated parasites. Flow cytometry analysis of promastigotes treated with compound 1 or 2 showed a dramatic increase in annexin V binding, with 91.1% and 81.5% of labeled cells, respectively, compared to that for untreated parasites (4.4%). The mean fluorescence intensity (MFI) of annexin V+ parasites was significantly higher than that of untreated ones (P < 0.05) (Fig. 5B and C). In contrast, PI labeling was very low in control parasites and did not increase after treatment, confirming that parasite damage induced apoptosis and that death was not due to necrosis.
FIG 5.
Quantification of apoptotic promastigotes. (A) Confocal microscopy examination of L. donovani promastigotes incubated with compound 1 at its IC50 (lower panel) or left untreated (upper panel) and then labeled with CFSE and annexin V-PE. Representative slides from triplicate experiments are shown. (B) Flow cytometry analysis of L. donovani promastigotes labeled with annexin V-AF488 and PI. Staurosporine (STS) was used as a positive control for apoptosis. The left panels show dot plots from control or treated parasites, and right panels show the quantification of annexin V-positive cells. Each panel is representative of triplicate experiments. (C) Mean fluorescence intensity (MFI) of annexin V+ promastigotes, treated with compound 1 (1), compound 2 (2), or STS or left untreated (Un). Results are expressed as means ± SEM of data from triplicate experiments, and significance is expressed as follows: *, P < 0.05.
Compound 1 controls intramacrophagic amastigote growth through stimulation of ROS production.
To examine the capacity of Galf derivatives to control the intramacrophagic amastigote multiplication, THP-1-derived macrophages were infected with L. donovani promastigotes for 24 h and then treated with the different compounds for 48 h. Compounds 1 and 2 significantly reduced amastigote multiplication (P < 0.05), whereas only moderate effects were observed with compound 3 (Fig. 6). HePC used as a control showed effective inhibition of amastigotes multiplication inside infected macrophages in a manner similar to that of compound 1 (Fig. 6).
FIG 6.
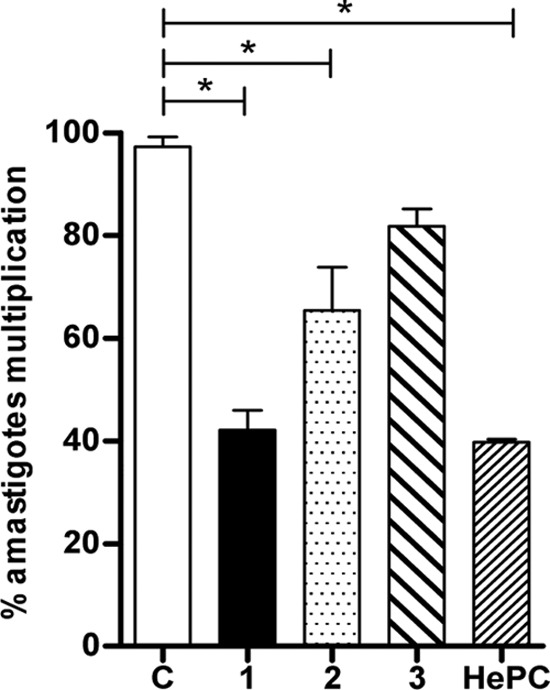
Inhibition of intramacrophagic L. donovani amastigote multiplication after treatment with Galf derivatives. THP-1 macrophages were infected by L. donovani promastigotes for 24 h and then treated for 48 h with different compounds. Results are presented as percentages of infected macrophages with multiplying amastigotes, determined by comparison to the values of untreated control wells (lane C) arbitrarily set to 100%. Significant results are shown as “*” (P < 0.05). Data are representative of three independent experiments.
To investigate the mechanism by which macrophages control amastigote growth, we quantified ROS production by flow cytometry. The MFI of DHR+ infected macrophages was significantly increased in parasites treated with compounds 1 and 2, but to a greater extent with compound 2 (P < 0.05 and P < 0.01, respectively) (Fig. 7A), indicating peroxide production in these cells. Similarly, superoxide detection was increased in infected macrophages treated with compounds 1 and 2 (P < 0.05) (Fig. 7B) compared to that for untreated macrophages.
FIG 7.
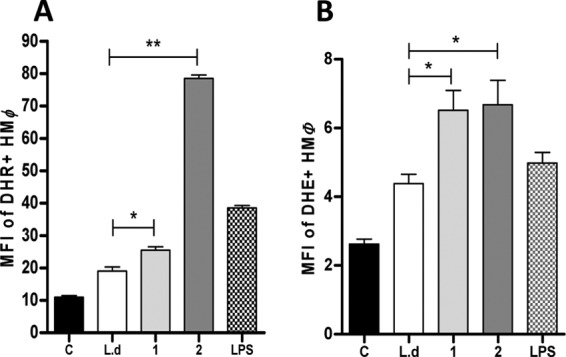
Quantification of reactive oxygen species (ROS) production by macrophages. Human macrophages (HMØ) were infected (lanes L.d, 1, 2, and LPS) or not (lane C) with L. donovani and then treated overnight with compound 1 (1) or compound 2 (2) or LPS or left untreated (L.d). (A) peroxide levels were measured using the specific fluorescent dye dihydrorhodamine (DHR). (B) Superoxide detection was quantified using dihydroxyethidium (DHE) staining. Significant results are shown as “*” (P < 0.05). Data are representative of two independent experiments.
DISCUSSION
Recent first-line drugs against leishmaniasis include miltefosine, paromomycin, and liposomal amphotericin B, which present multiple shortcomings, such as resistance and side effects (35, 36). Therefore, novel therapeutic approaches, particularly those aimed at exploring new horizons for the development of efficient antileishmanial molecules, are required (37–39). Current advances in our understanding of the biosynthesis of Galf-containing glycoconjugates have opened a new field of research, since these compounds are found at the surface of several widespread human pathogens, including Leishmania (13). Interestingly, galactose is also present in mammalian cells but exclusively in the hexopyranosyl form, thus making furanosides a specific target for intervention therapies (40).
Since UDP-Galf is essential for the biosynthesis of all glycoconjugates containing a Galf moiety, we first generated UDP-Galf derivatives with the aim of interfering with LPG synthesis or with Mycobacterium smegmatis growth, with promising results (19, 41). However, synthesis of UDP-Galf derivatives is a complex process, and further experiments are currently limited by the quantities of compounds synthesized.
In this work, we focused on synthetic alkyl galactofuranoside derivatives that fulfill two main criteria: (i) the presence of a C-6 alterable galactofuranose ring linked to a lipophilic C8 tail, and (ii) their availability in large quantities. We demonstrated that activity of Galf derivatives against L. donovani promastigotes varied according to the group substituted at the C-6 position. Compound 1 showed the strongest effect on promastigote growth, whereas compound 2 had a moderate IC50 and compounds 3 and 4 were found to be poorly active. Additionally, we demonstrated that the activity of these furanoside compounds was specific by incubating parasites with a structural isomer of compound 1, i.e., compound 5 (Galp-Oct), and with the octyl chain, i.e., compound 6, solely. Both of them had no biological effect, implying that the activity is conferred by the furanoside ring. Our IC50 results using HePC on promastigotes are in coherence with previously published data (26, 42), thus allowing reliable comparison of efficacy between our molecules and a reference treatment.
The uniqueness of Galf derivatives relies in their low cell toxicity. In our hands, the CC50 values obtained for THP-1 and HMØ were 6- to 12-fold lower than those with HePC, which showed cytotoxic levels similar to those described earlier by others (26, 43). Compound 1 had the most interesting SI, which was 4-fold higher than that of HePC.
Regarding the contrasting results obtained with the various C-6-modified octyl galactofuranosides, the poor inhibition observed with compound 3 (6-N3-) and compound 4 (6-NHAc-) suggests that the presence of a polar moiety at this terminal position is important to obtain an inhibitory effect on L. donovani promastigotes. Besides, we could rule out an effect due to the inherent auto-organization property of alkyl glycosides, which could possibly facilitate compound penetration into the membrane since the concentrations tested were well below the critical micelle concentration value (1.78 mg/ml for compound 1), and thus its activity was not due to a surfactant property. In addition, according to Weniger et al. (44), the biological activity of any drug cannot be attributed to cytotoxicity when its SI is ≥10, which was the case here.
These first observations suggested a specific interaction with the parasite membrane which could decrease parasite growth or modify cell recognition and prompted us to explore more deeply the properties of these compounds. To confirm the hypothesis that the products directly interact with L. donovani membrane assemblages, we proceeded to the setting up of STDD-NMR with whole cells. This technique yielded spectra showing that all of them could interact with the membrane through the octyl chain. We can therefore stress the fact that this part of the molecules is the closest to the membrane and could probably act as a lipophilic anchor or could possibly be recognized by enzymes presenting a hydrophobic pocket. Additionally, a moderately stronger interaction of the furanoside ring than of the pyranoside ring was observed by NMR, emphasizing the role of the specific sugar structure in the biological effects observed on Leishmania survival and infectivity.
Since molecular interaction through the octyl chain seems essential to anchor the molecule but unlikely to support the specificity of the biological effect, we used EPR to further investigate the potential role of the furanose form on the promastigote membrane. It appeared that compounds 1 and 2 had a significant rigidifying effect on the promastigote membrane, similar to the UDCA stiffening control, an effect which was not observed with compound 3 or 5, both of which otherwise had little biological effect on parasite growth. Huge membrane and intracellular damages induced by compound 1 but not by the poorly efficient compound 3 were evidenced using TEM. In other studies reporting the effect of drugs like HePC and diterpenoid alkaloids, parasite death seems to result from intracellular damages rather than primary membrane damage (37, 45). This is consistent with the results of our cytotoxicity assay using CellTox green dye, which can efficiently enter cells and bind to DNA only in the presence of membrane alterations. In our hands, this assay was reliable to determine an IC50 only for compound 1, which reinforces the finding that this drug can alter the parasite membrane. The initial membrane damage could then lead to promastigote apoptosis, as suggested by the high level of annexin V-positive parasites after treatment with compound 1, and subsequently to intracellular damages. These results definitely confirm that compound 1 is a potent drug against the promastigote stage of L. donovani.
Finally, since the ultimate goal of anti-Leishmania drug development is to combat the amastigote stage, we verified the effect of the most effective compounds, 1 and 2, on a model of human macrophages infected with L. donovani. Both compounds reduced intracellular amastigote growth, though compound 2 was efficient to a lesser degree. As a first attempt to explore the mode of action against amastigotes, we characterized ROS production by infected macrophages, and we obtained evidence that both compounds stimulate peroxide and superoxide production. Since an oxidative burst is the hallmark of an efficient antileishmanial response, as observed in vivo (46), this could explain the enhanced parasite control in vitro. Further explorations are needed to determine the mechanism of the effect on amastigotes more accurately, but these primary results are promising and are in agreement with a very recent article in which the authors showed an immunomodulatory effect of galactofuranosyl glycosides on macrophages (47).
In conclusion, we demonstrated for the first time a significant biological effect of two octyl galactofuranoside derivatives on L. donovani promastigotes and amastigotes, particularly compound 1, octyl-β-d-galactofuranoside, whose activity is supported by the furanose motif and seems to tightly interact with the parasite membrane. Thus, alkyl galactofuranosides could be an attractive basis for further drug development against this neglected disease.
ACKNOWLEDGMENTS
This work was supported by a grant of Agence Nationale pour la Recherche (ANR BLANC 7181). R.D. thanks Ministère de l'Enseignement Supérieur et de la Recherche for a grant. We also acknowledge the European Union (FEDER), the Region Bretagne, the Conseil Général d'Ille-et-Vilaine, Rennes Métropole, and the French Ministère de l'Enseignement Supérieur et de la Recherche (MESR) for financial support of the project Membratox.
We acknowledge Jean-Paul Guégan (ENSCR) for his valuable help in NMR experiment acquisition.
We have no transparency issues to declare.
Footnotes
Published ahead of print 27 January 2014
REFERENCES
- 1.WHO. 2010. Control of the leishmaniases. World Health Organ. Tech. Rep. Ser. 2010(959):xii–xiii, 1–186, back cover [PubMed] [Google Scholar]
- 2.Buffet PA, Rosenthal E, Gangneux JP, Lightburne E, Couppie P, Morizot G, Lachaud L, Marty P, Dedet JP. 2011. Therapy of leishmaniasis in France: consensus on proposed guidelines. Presse Med. 40:173–184 (In French.) 10.1016/j.lpm.2010.09.023 [DOI] [PubMed] [Google Scholar]
- 3.Croft SL, Sundar S, Fairlamb AH. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111–126. 10.1128/CMR.19.1.111-126.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangneux JP, Dullin M, Sulahian A, Garin YJ, Derouin F. 1999. Experimental evaluation of second-line oral treatments of visceral leishmaniasis caused by Leishmania infantum. Antimicrob. Agents Chemother. 43:172–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. 2007. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 5:873–882. 10.1038/nrmicro1748 [DOI] [PubMed] [Google Scholar]
- 6.Matlashewski G, Arana B, Kroeger A, Battacharya S, Sundar S, Das P, Sinha PK, Rijal S, Mondal D, Zilberstein D, Alvar J. 2011. Visceral leishmaniasis: elimination with existing interventions. Lancet Infect. Dis. 11:322–325. 10.1016/S1473-3099(10)70320-0 [DOI] [PubMed] [Google Scholar]
- 7.Mondal S, Bhattacharya P, Ali N. 2010. Current diagnosis and treatment of visceral leishmaniasis. Expert Rev. Anti Infect. Ther. 8:919–944. 10.1586/eri.10.78 [DOI] [PubMed] [Google Scholar]
- 8.Mutiso JM, Macharia JC, Kiio MN, Ichagichu JM, Rikoi H, Gicheru MM. 2013. Development of Leishmania vaccines: predicting the future from past and present experience. J. Biomed. Res. 27:85–102. 10.7555/JBR.27.20120064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novozhilova NM, Bovin NV. 2010. Structure, functions, and biosynthesis of glycoconjugates of Leishmania spp. cell surface. Biochemistry 75:686–694. 10.1134/S0006297910060027 [DOI] [PubMed] [Google Scholar]
- 10.Kleczka B, Lamerz AC, van Zandbergen G, Wenzel A, Gerardy-Schahn R, Wiese M, Routier FH. 2007. Targeted gene deletion of Leishmania major UDP-galactopyranose mutase leads to attenuated virulence. J. Biol. Chem. 282:10498–10505. 10.1074/jbc.M700023200 [DOI] [PubMed] [Google Scholar]
- 11.Spath GF, Epstein L, Leader B, Singer SM, Avila HA, Turco SJ, Beverley SM. 2000. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc. Natl. Acad. Sci. U. S. A. 97:9258–9263. 10.1073/pnas.160257897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConville MJ, Schnur LF, Jaffe C, Schneider P. 1995. Structure of Leishmania lipophosphoglycan: inter- and intra-specific polymorphism in Old World species. Biochem. J. 310(Part 3):807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tefsen B, Ram AF, van Die I, Routier FH. 2012. Galactofuranose in eukaryotes: aspects of biosynthesis and functional impact. Glycobiology 22:456–469. 10.1093/glycob/cwr144 [DOI] [PubMed] [Google Scholar]
- 14.de Lederkremer RM, Colli W. 1995. Galactofuranose-containing glycoconjugates in trypanosomatids. Glycobiology 5:547–552. 10.1093/glycob/5.6.547 [DOI] [PubMed] [Google Scholar]
- 15.Oppenheimer M, Valenciano AL, Sobrado P. 2011. Biosynthesis of galactofuranose in kinetoplastids: novel therapeutic targets for treating leishmaniasis and Chagas' disease. Enzyme Res. 2011:415976. 10.4061/2011/415976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legentil L, Audic JL, Daniellou R, Nugier-Chauvin C, Ferrieres V. 2011. Studies of a furanoside as antimycobacterial agent loaded into a biodegradable PBAT/sodium caseinate support. Carbohydr. Res. 346:1541–1545. 10.1016/j.carres.2011.04.025 [DOI] [PubMed] [Google Scholar]
- 17.Davis CB, Hartnell RD, Madge PD, Owen DJ, Thomson RJ, Chong AK, Coppel RL, von Itzstein M. 2007. Synthesis and biological evaluation of galactofuranosyl alkyl thioglycosides as inhibitors of mycobacteria. Carbohydr. Res. 342:1773–1780. 10.1016/j.carres.2007.04.027 [DOI] [PubMed] [Google Scholar]
- 18.Ferrières V, Bertho J-N, Plusquellec D. 1998. A convenient synthesis of alkyl D-glycofuranosiduronic acids and alkyl D-glycofuranosides from unprotected carbohydrates. Carbohydr. Res. 311:25–35. 10.1016/S0008-6215(98)00197-9 [DOI] [Google Scholar]
- 19.Dureau R, Robert-Gangneux F, Gangneux JP, Nugier-Chauvin C, Legentil L, Daniellou R, Ferrieres V. 2010. Synthetic UDP-furanoses inhibit the growth of the parasite Leishmania. Carbohydr. Res. 345:1299–1305. 10.1016/j.carres.2010.02.020 [DOI] [PubMed] [Google Scholar]
- 20.Rahman M, Ahmed BN, Faiz MA, Chowdhury MZ, Islam QT, Sayeedur R, Rahman MR, Hossain M, Bangali AM, Ahmad Z, Islam MN, Mascie-Taylor CG, Berman J, Arana B. 2011. Phase IV trial of miltefosine in adults and children for treatment of visceral leishmaniasis (kala-azar) in Bangladesh. Am. J. Trop. Med. Hyg. 85:66–69. 10.4269/ajtmh.2011.10-0661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwende H, Fitzke E, Ambs P, Dieter P. 1996. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 59:555–561 [PubMed] [Google Scholar]
- 22.van Grevenynghe J, Rion S, Le Ferrec E, Le Vee M, Amiot L, Fauchet R, Fardel O. 2003. Polycyclic aromatic hydrocarbons inhibit differentiation of human monocytes into macrophages. J. Immunol. 170:2374–2381 [DOI] [PubMed] [Google Scholar]
- 23.Tominaga H, Ishiyama M, Ohseto F, Sasamoto K, Hamamoto T, Suzuki K, Watanabe M. 1999. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 36:47–50. 10.1039/A809656B [DOI] [Google Scholar]
- 24.Gangneux JP, Sulahian A, Garin YJ, Derouin F. 1996. Lipid formulations of amphotericin B in the treatment of experimental visceral leishmaniasis due to Leishmania infantum. Trans. R. Soc. Trop. Med. Hyg. 90:574–577. 10.1016/S0035-9203(96)90330-2 [DOI] [PubMed] [Google Scholar]
- 25.Buffet PA, Sulahian A, Garin YJ, Nassar N, Derouin F. 1995. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob. Agents Chemother. 39:2167–2168. 10.1128/AAC.39.9.2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morais-Teixeira E, Damasceno QS, Galuppo MK, Romanha AJ, Rabello A. 2011. The in vitro leishmanicidal activity of hexadecylphosphocholine (miltefosine) against four medically relevant Leishmania species of Brazil. Mem. Inst. Oswaldo Cruz 106:475–478. 10.1590/S0074-02762011000400015 [DOI] [PubMed] [Google Scholar]
- 27.Sergent O, Pereira M, Belhomme C, Chevanne M, Huc L, Lagadic-Gossmann D. 2005. Role for membrane fluidity in ethanol-induced oxidative stress of primary rat hepatocytes. J. Pharmacol. Exp. Ther. 313:104–111. 10.1124/jpet.104.078634 [DOI] [PubMed] [Google Scholar]
- 28.Guldutuna S, Deisinger B, Weiss A, Freisleben HJ, Zimmer G, Sipos P, Leuschner U. 1997. Ursodeoxycholate stabilizes phospholipid-rich membranes and mimics the effect of cholesterol: investigations on large unilamellar vesicles. Biochim. Biophys. Acta 1326:265–274. 10.1016/S0005-2736(97)00030-8 [DOI] [PubMed] [Google Scholar]
- 29.Ogura R, Sugiyama M, Sakanashi T, Ninomiya T. 1988. ESR spin-labeling method of determining membrane fluidity in biological materials—tissue culture cells, cardiac mitochondria, erythrocytes and epidermal cells. Kurume Med. J. 35:171–182. 10.2739/kurumemedj.35.171 [DOI] [PubMed] [Google Scholar]
- 30.Carvalho L, Luque-Ortega JR, Lopez-Martin C, Castanys S, Rivas L, Gamarro F. 2011. The 8-aminoquinoline analogue sitamaquine causes oxidative stress in Leishmania donovani promastigotes by targeting succinate dehydrogenase. Antimicrob. Agents Chemother. 55:4204–4210. 10.1128/AAC.00520-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhunia A, Bhattacharjya S, Chatterjee S. 2012. Applications of saturation transfer difference NMR in biological systems. Drug Discov. Today 17:505–513. 10.1016/j.drudis.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 32.Claasen B, Axmann M, Meinecke R, Meyer B. 2005. Direct observation of ligand binding to membrane proteins in living cells by a saturation transfer double difference (STDD) NMR spectroscopy method shows a significantly higher affinity of integrin αIIbβ3 in native platelets than in liposomes. J. Am. Chem. Soc. 127:916–919. 10.1021/ja044434w [DOI] [PubMed] [Google Scholar]
- 33.Pereira A, Pfeifer TA, Grigliatti TA, Andersen RJ. 2009. Functional cell-based screening and saturation transfer double-difference NMR have identified haplosamate A as a cannabinoid receptor agonist. ACS Chem. Biol. 4:139–144. 10.1021/cb800264k [DOI] [PubMed] [Google Scholar]
- 34.Sylla B, Guégan J-P, Wieruszeski J-M, Nugier-Chauvin C, Legentil L, Daniellou R, Ferrières V. 2011. Probing β-(1→3)-d-glucans interactions with recombinant human receptors using high-resolution NMR studies. Carbohydr. Res. 346:1490–1494. 10.1016/j.carres.2011.03.038 [DOI] [PubMed] [Google Scholar]
- 35.Bhargava P, Singh R. 2012. Developments in diagnosis and antileishmanial drugs. Interdiscip. Perspect. Infect. Dis. 2012:626838. 10.1155/2012/626838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luque-Ortega JR, de la Torre BG, Hornillos V, Bart JM, Rueda C, Navarro M, Amat-Guerri F, Acuna AU, Andreu D, Rivas L. 2012. Defeating Leishmania resistance to miltefosine (hexadecylphosphocholine) by peptide-mediated drug smuggling: a proof of mechanism for trypanosomatid chemotherapy. J. Control Release 161:835–842. 10.1016/j.jconrel.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 37.Sanchez-Moreno M, Gomez-Contreras F, Navarro P, Marin C, Ramirez-Macias I, Olmo F, Sanz AM, Campayo L, Cano C, Yunta MJ. 2012. In vitro leishmanicidal activity of imidazole- or pyrazole-based benzo[g]phthalazine derivatives against Leishmania infantum and Leishmania braziliensis species. J. Antimicrob. Chemother. 67:387–397. 10.1093/jac/dkr480 [DOI] [PubMed] [Google Scholar]
- 38.Ramirez-Macias I, Marin C, Salas JM, Caballero A, Rosales MJ, Villegas N, Rodriguez-Dieguez A, Barea E, Sanchez-Moreno M. 2011. Biological activity of three novel complexes with the ligand 5-methyl-1,2,4-triazolo[1,5-a]pyrimidin-7(4H)-one against Leishmania spp. J. Antimicrob. Chemother. 66:813–819. 10.1093/jac/dkq537 [DOI] [PubMed] [Google Scholar]
- 39.Coimbra ES, Libong D, Cojean S, Saint-Pierre-Chazalet M, Solgadi A, Le Moyec L, Duenas-Romero AM, Chaminade P, Loiseau PM. 2010. Mechanism of interaction of sitamaquine with Leishmania donovani. J. Antimicrob. Chemother. 65:2548–2555. 10.1093/jac/dkq371 [DOI] [PubMed] [Google Scholar]
- 40.Pedersen LL, Turco SJ. 2003. Galactofuranose metabolism: a potential target for antimicrobial chemotherapy. Cell. Mol. Life Sci. 60:259–266. 10.1007/s000180300021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peltier P, Belanova M, Dianiskova P, Zhou R, Zheng RB, Pearcey JA, Joe M, Brennan PJ, Nugier-Chauvin C, Ferrieres V, Lowary TL, Daniellou R, Mikusova K. 2010. Synthetic UDP-furanoses as potent inhibitors of mycobacterial galactan biogenesis. Chem. Biol. 17:1356–1366. 10.1016/j.chembiol.2010.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vermeersch M, da Luz RI, Tote K, Timmermans JP, Cos P, Maes L. 2009. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: practical relevance of stage-specific differences. Antimicrob. Agents Chemother. 53:3855–3859. 10.1128/AAC.00548-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foucher AL, Rachidi N, Gharbi S, Blisnick T, Bastin P, Pemberton IK, Spath GF. 2013. Apoptotic marker expression in the absence of cell death in staurosporine-treated Leishmania donovani. Antimicrob. Agents Chemother. 57:1252–1261. 10.1128/AAC.01983-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weniger B, Robledo S, Arango GJ, Deharo E, Aragon R, Munoz V, Callapa J, Lobstein A, Anton R. 2001. Antiprotozoal activities of Colombian plants. J. Ethnopharmacol. 78:193–200. 10.1016/S0378-8741(01)00346-4 [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez P, Marin C, Rodriguez-Gonzalez I, Hitos AB, Rosales MJ, Reina M, Diaz JG, Gonzalez-Coloma A, Sanchez-Moreno M. 2005. In vitro activity of C20-diterpenoid alkaloid derivatives in promastigotes and intracellular amastigotes of Leishmania infantum. Int. J. Antimicrob. Agents 25:136–141. 10.1016/j.ijantimicag.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 46.Robert-Gangneux F, Drogoul AS, Rostan O, Piquet-Pellorce C, Cayon J, Lisbonne M, Herbelin A, Gascan H, Guiguen C, Samson M, Gangneux JP. 2012. Invariant NKT cells drive hepatic cytokinic microenvironment favoring efficient granuloma formation and early control of Leishmania donovani infection. PLoS One 7:e33413. 10.1371/journal.pone.0033413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sassaki GL, Rattmann YD, Santana-Filho AP, Riter DS, Iagher F, Trindade ES, da Silva MD, Santos AR, de Souza LM, Iacomini M, Gorin PA. 2013. Galactofuranosyl glycosides: immunomodulatory effects on macrophages and in vivo enhancement of lethality on sepsis. Chem. Biol. Interact. 205:29–37. 10.1016/j.cbi.2013.05.014 [DOI] [PubMed] [Google Scholar]